Unraveling the Role of Natural Sediments in sII Mixed Gas Hydrate Formation: An Experimental Study
Abstract
1. Introduction
2. Results and Discussion
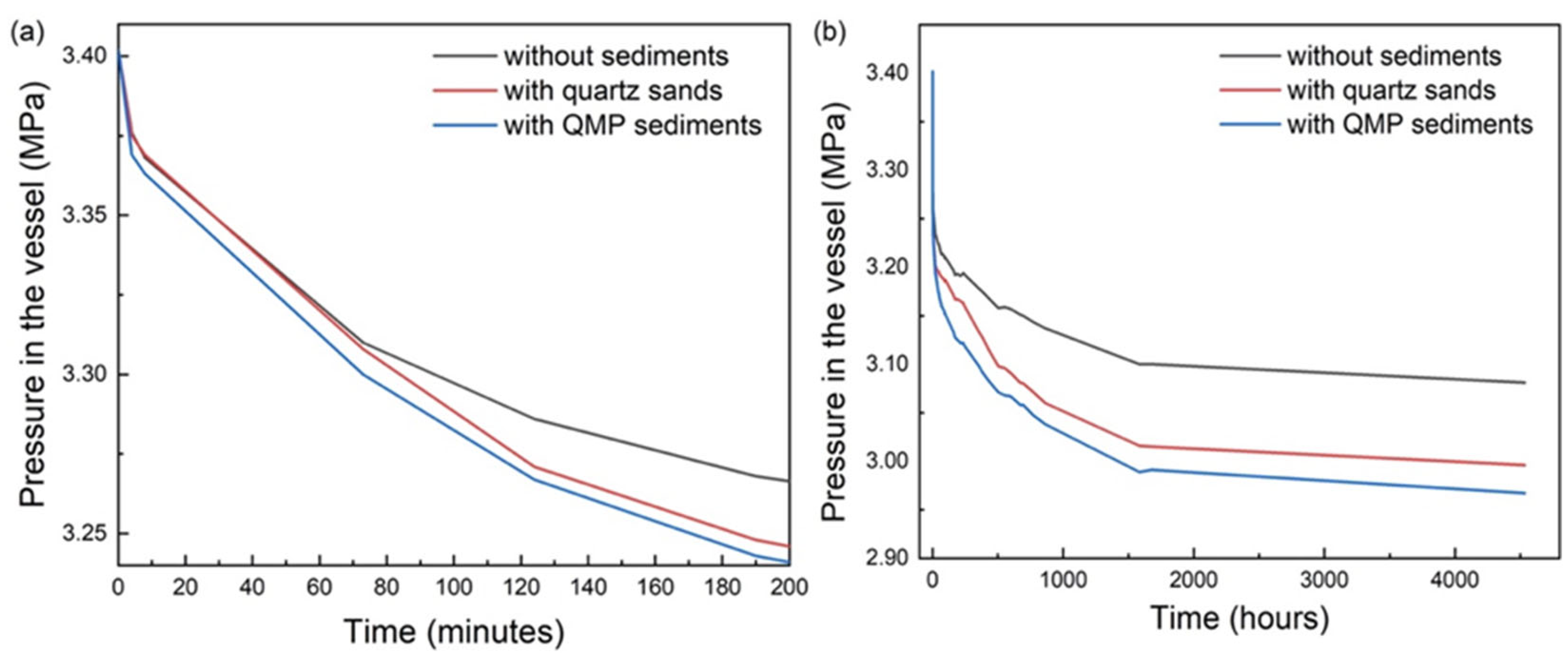
2.1. Influence of Sediments on the Formation Kinetics of Mixed Gas Hydrates
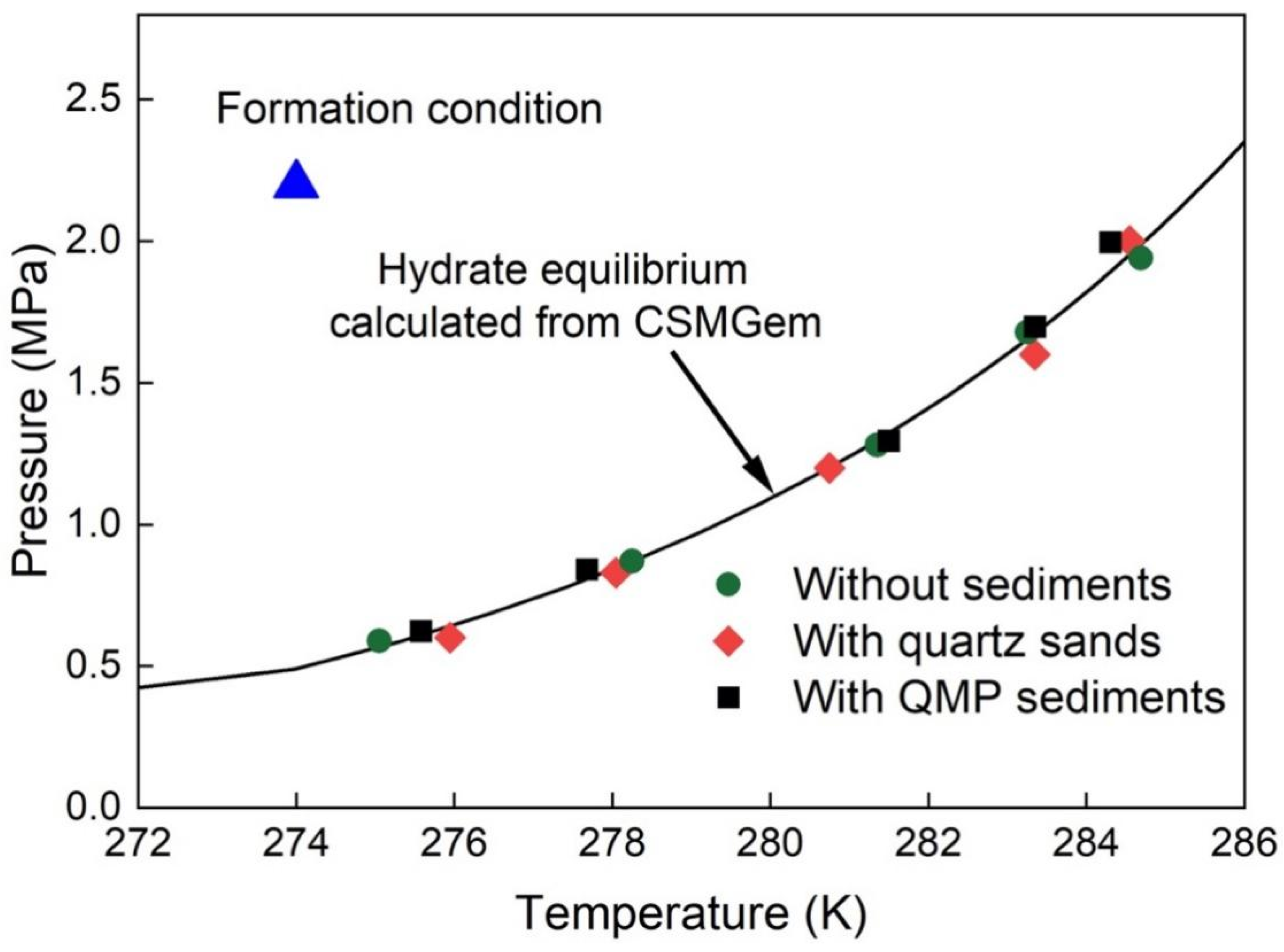
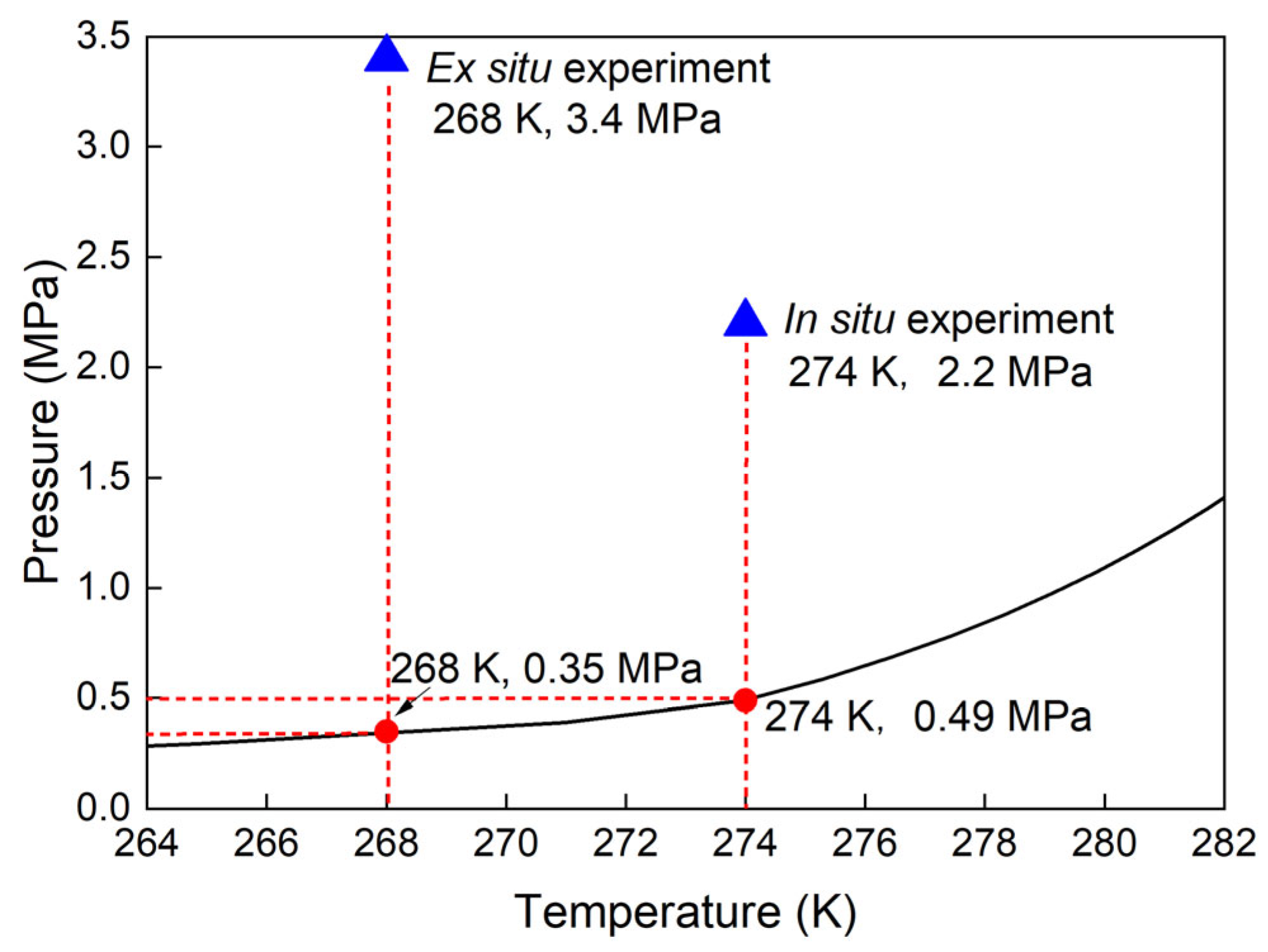
2.2. Effect of the Sediments on the Thermodynamic Properties of Mixed Gas Hydrates
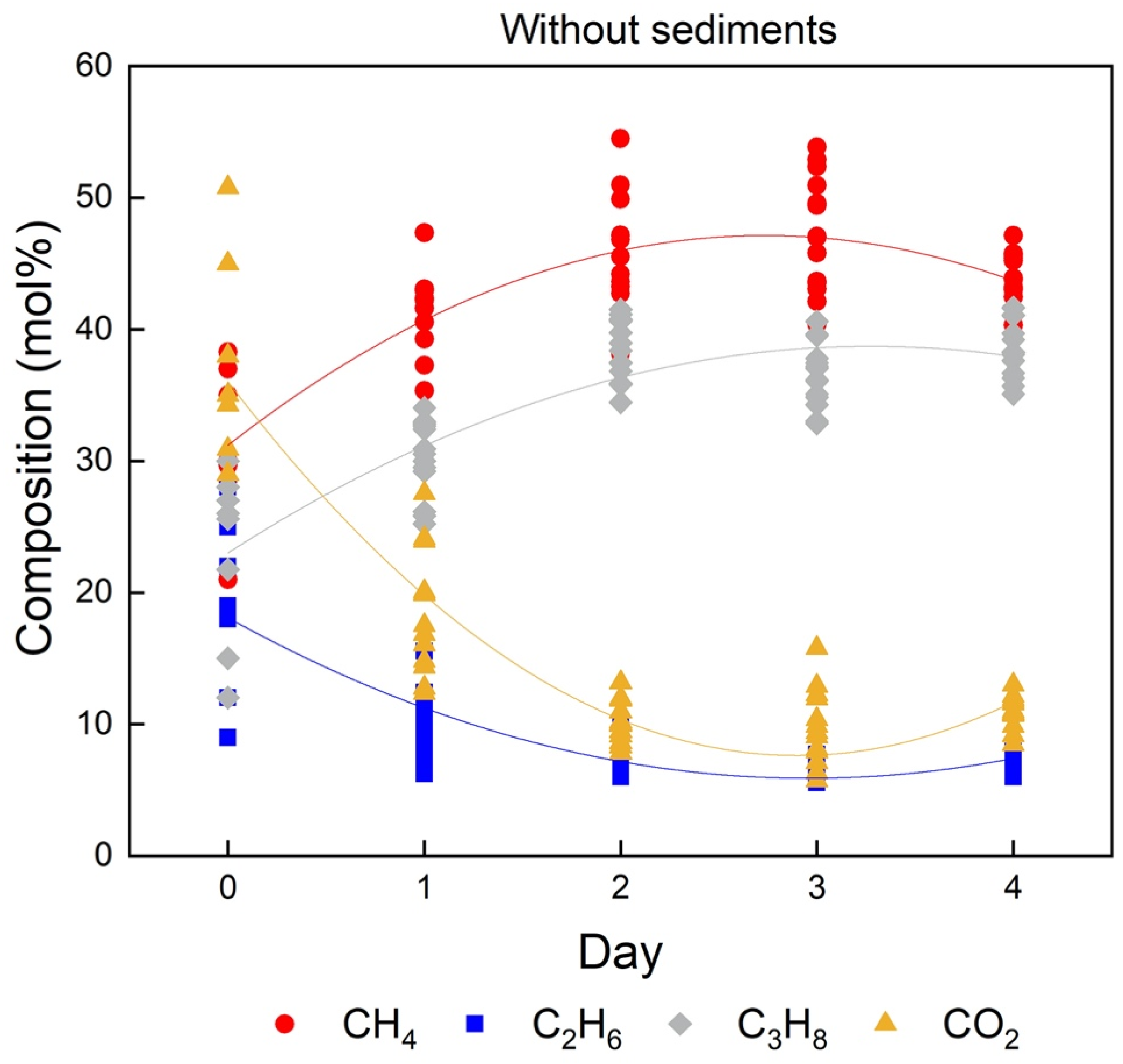
2.3. Effect of the Sediments on the Composition of Mixed Gas Hydrates
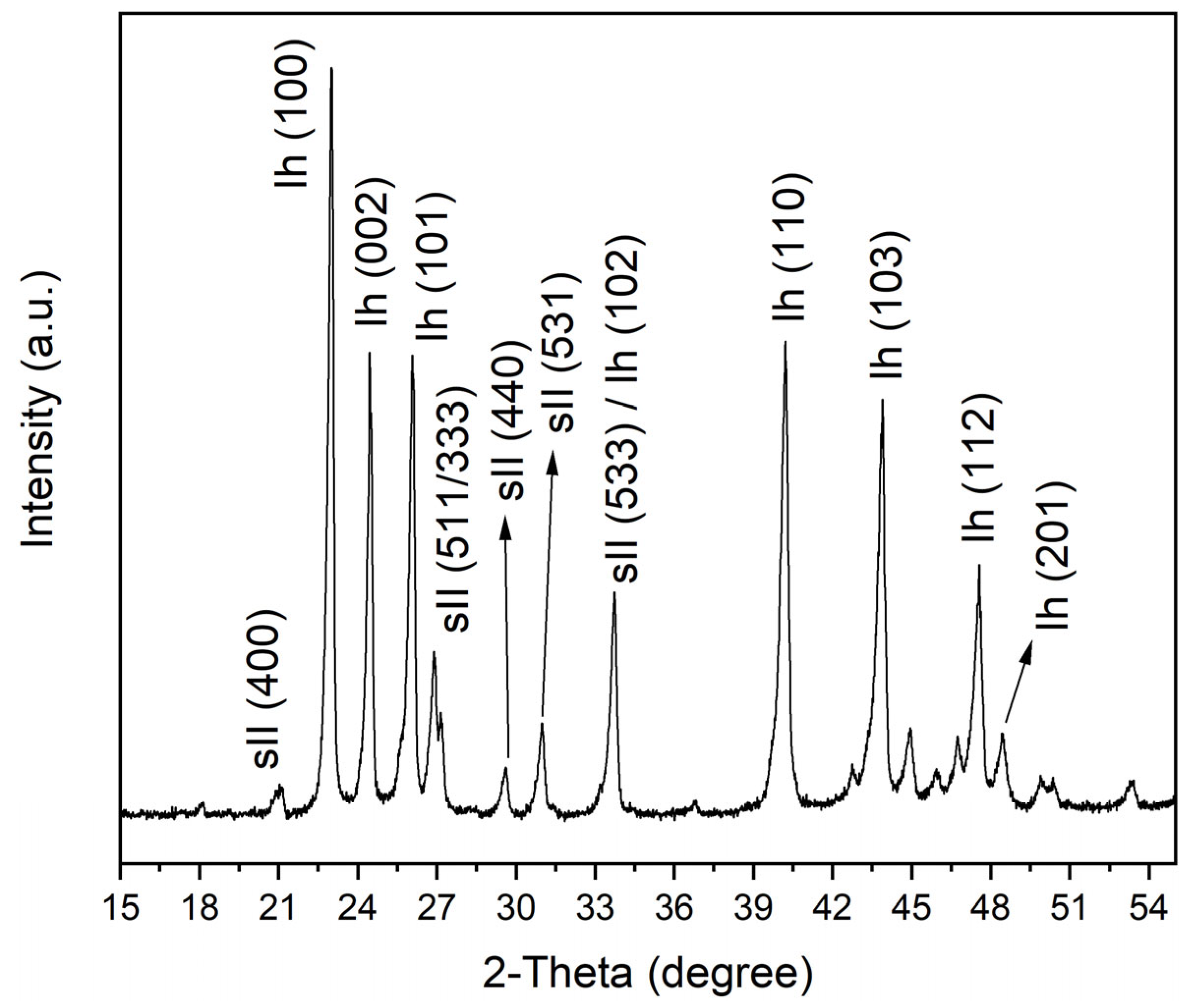
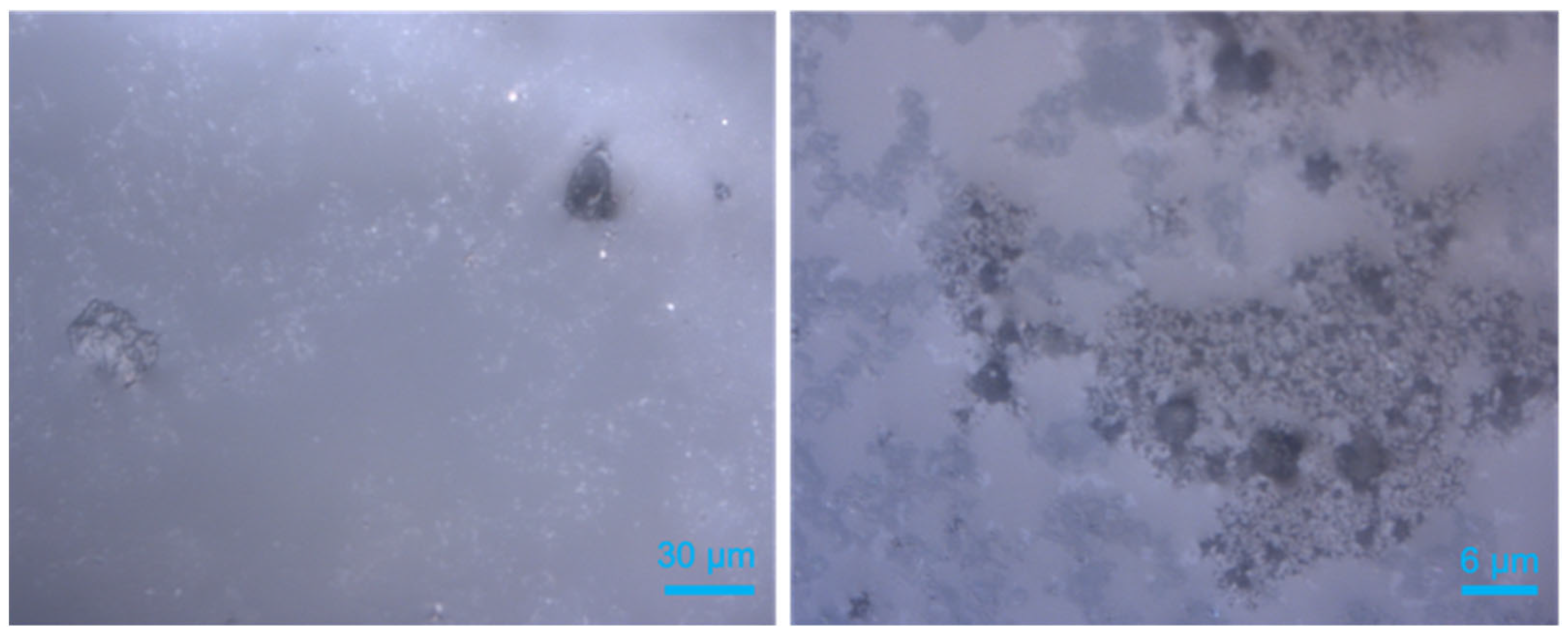
2.4. Coexisting Phases Formed with the Presence of QMP Sediments
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhu, Y.; Zhang, Y.; Wen, H.; Lu, Z.; Jia, Z.; Li, Y.; Li, Q.; Liu, C.; Wang, P.; Guo, X. Gas Hydrates in the Qilian Mountain Permafrost, Qinghai, Northwest China. Acta Geol. Sin. Ed. 2010, 84, 1–10. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, Y.; Zhang, Y.; Wen, H.; Li, Y.; Liu, C. Gas Hydrate Occurrences in the Qilian Mountain Permafrost, Qinghai Province, China. Cold Reg. Sci. Technol. 2011, 66, 93–104. [Google Scholar] [CrossRef]
- Dallimore, S.R.; Collett, T.S. Summary and Implications Ofthe Mallik 2002 Gas Hydrate Production ResearchWell Program. In Scientific Results from theMallik2002GasHydrate Production Research Well Program, Mackenzie Delta, Northwest Territories, Canada; Dallimore, S.R., Collett, T.S., Eds.; Geological Survey of Canada: Ottawa, ON, Canada, 2005; pp. 1–36. [Google Scholar]
- Wang, P.K.; Zhu, Y.H.; Lu, Z.Q.; Huang, X.; Pang, S.J.; Zhang, S. Gas Hydrate Stability Zone Migration Occurred in the Qilian Mountain Permafrost, Qinghai, Northwest China: Evidences from Pyrite Morphology and Pyrite Sulfur Isotope. Cold Reg. Sci. Technol. 2014, 98, 8–17. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, Y.; Liu, H.; Zhang, Y.; Jin, C.; Huang, X.; Wang, P. Gas Source for Gas Hydrate and Its Significance in the Qilian Mountain Permafrost, Qinghai. Mar. Pet. Geol. 2013, 43, 341–348. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, Y.; Wang, P.; Guo, X. Hydrocarbon Gas Composition and Origin of Core Gas from the Gas Hydrate Reservoir in Qilian Mountain Permafrost. Geol. Bull. China 2011, 30, 1851–1856. [Google Scholar]
- Cheng, B.; Xu, J.; Lu, Z.; Li, Y.; Wang, W.; Yang, S.; Liu, H.; Wang, T.; Liao, Z. Hydrocarbon Source for Oil and Gas Indication Associated with Gas Hydrate and Its Significance in the Qilian Mountain Permafrost, Qinghai, Northwest China. Mar. Pet. Geol. 2018, 89, 202–215. [Google Scholar] [CrossRef]
- Lu, Z.; Zhai, G.; Zhu, Y.; Zhang, Y.; Li, Y.; Wang, W.; Wang, T.; Liu, H.; Tang, S.; Tan, P. Fault Control of Gas Hydrate Accumulation in Qilian Mountain Permafrost. Int. J. Offshore Polar Eng. 2016, 26, 199–205. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Wei, Y.; Yang, C.; Nie, J.; Cao, D. Gas Migration for Terrestrial Gas Hydrates in the Juhugeng Mining Area of Muli Basin, Qilian Mountains, Northwest China. Energy Explor. Exploit. 2020, 38, 989–1013. [Google Scholar] [CrossRef]
- Liu, C.; Meng, Q.; He, X.; Li, C.; Ye, Y.; Lu, Z.; Zhu, Y.; Li, Y.; Liang, J. Comparison of the Characteristics for Natural Gas Hydrate Recovered from Marine and Terrestrial Areas in China. J. Geochem. Explor. 2015, 152, 67–74. [Google Scholar] [CrossRef]
- Xia, N.; Liu, C.; Ye, Y.; Meng, Q.; Lin, X.; He, X. Study on Determination Method of Natural Gas Hydrates by Micro-Laser Raman Spectroscopy. Rock Miner. Anal. 2011, 30, 416–422. [Google Scholar]
- Schicks, J.M. Der Einfluss molekularer Eigenschaften von Gasen auf die Hydratbildungsprozesse, die thermodynamischen Eigenschaften und die Reaktionen von einfachen und gemischten Gashydraten. Ph.D. Thesis, Deutsches GeoForschungsZentrum, Potsdam, Germany, 2013. [Google Scholar]
- Schicks, J.M.; Luzi-Helbing, M. Kinetic and Thermodynamic Aspects of Clathrate Hydrate Nucleation and Growth. J. Chem. Eng. Data 2015, 60, 269–277. [Google Scholar] [CrossRef]
- Kida, M.; Khlystov, O.; Zemskaya, T.; Takahashi, N.; Minami, H.; Sakagami, H.; Krylov, A.; Hachikubo, A.; Yamashita, S.; Shoji, H.; et al. Coexistence of Structure I and II Gas Hydrates in Lake Baikal Suggesting Gas Sources from Microbial and Thermogenic Origin. Geophys. Res. Lett. 2006, 33, L24603. [Google Scholar] [CrossRef]
- Klapp, S.A.; Bohrmann, G.; Kuhs, W.F.; Mangir Murshed, M.; Pape, T.; Klein, H.; Techmer, K.S.; Heeschen, K.U.; Abegg, F. Microstructures of Structure I and II Gas Hydrates from the Gulf of Mexico. Mar. Pet. Geol. 2010, 27, 116–125. [Google Scholar] [CrossRef]
- Aminnaji, M.; Anderson, R.; Tohidi, B. Experimental Measurement of Multiple Hydrate Structure Formation in Binary and Ternary Natural Gas Analogue Systems by Isochoric Equilibrium Methods. Energy Fuels 2021, 35, 9341–9348. [Google Scholar] [CrossRef]
- Pan, M.; Schicks, J.M. Influences of Gas Supply Changes on the Formation Process of Complex Mixed Gas Hydrates. Molecules 2021, 26, 3039. [Google Scholar] [CrossRef] [PubMed]
- Clennell, M.B.; Hovland, M.; Booth, J.S.; Henry, P.; Winters, W.J. Formation of Natural Gas Hydrates in Marine Sediments 1. Conceptual Model of Gas Hydrate Growth Conditioned by Host Sediment Properties. J. Geophys. Res. Earth 1999, 104, 22985–23003. [Google Scholar] [CrossRef]
- Heeschen, K.U.; Abendroth, S.; Priegnitz, M.; Spangenberg, E.; Thaler, J.; Schicks, J.M. Gas Production from Methane Hydrate: A Laboratory Simulation of the Multistage Depressurization Test in Mallik, Northwest Territories, Canada. Energy Fuels 2016, 30, 6210–6219. [Google Scholar]
- Cha, S.B.; Oaur, H.; Wildman, T.R.; Sloan, E.D. A Third-Surface Effect on Hydrate Formation. J. Phys. Chem. 1988, 92, 6492–6494. [Google Scholar]
- Lu, Z.Q.; Zhu, Y.H.; Zhang, Y.Q.; Wen, H.J.; Li, Y.H.; Wang, P.K. Estimation Method of Gas Hydrate Resources in the Qilian Mountain Permafrost Area, Qinghai, China—A Case of the Drilling Area. Geol. Bull. China 2010, 29, 1310–1318. [Google Scholar]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Heeschen, K.U.; Schicks, J.M.; Oeltzschner, G. The Promoting Effect of Natural Sand on Methane Hydrate Formation: Grain Sizes and Mineral Composition. Fuel 2016, 181, 139–147. [Google Scholar] [CrossRef]
- Torres, M.E.; Tréhu, A.M.; Cespedes, N.; Kastner, M.; Wortmann, U.G.; Kim, J.H.; Long, P.; Malinverno, A.; Pohlman, J.W.; Riedel, M.; et al. Methane Hydrate Formation in Turbidite Sediments of Northern Cascadia, IODP Expedition 311. Earth Planet. Sci. Lett. 2008, 271, 170–180. [Google Scholar] [CrossRef]
- Winters, W.J.; Dallimore, S.R.; Collett, T.S.; Jenner, K.A.; Katsube, J.T.; Cranston, R.E.; Wright, J.F.; Nixon, F.M.; Uchida, T. Relation between Gas Hydrate and Physical Properties at the Mallik 2L-38 Research Well in the Mackenzie Delta. Ann. N. Y. Acad. Sci. 2000, 912, 94–100. [Google Scholar] [CrossRef]
- Østergaard, K.K.; Anderson, R.; Llamedo, M.; Tohidi, B. Hydrate Phase Equilibria in Porous Media: Effect of Pore Size and Salinity. Terra Nov. 2002, 14, 307–312. [Google Scholar] [CrossRef]
- Uchida, T.; Takeya, S.; Chuvilin, E.M.; Ohmura, R.; Nagao, J.; Yakushev, V.S.; Istomin, V.A.; Minagawa, H.; Ebinuma, T.; Narita, H.; et al. Decomposition of Methane Hydrates in Sand, Sandstone, Clays, and Glass Beads. J. Geophys. Res. Solid Earth 2004, 109, 1–12. [Google Scholar] [CrossRef]
- Kleinberg, R.L.; Flaum, C.; Griffin, D.D.; Brewer, P.G.; Malby, G.E.; Peltzer, E.T.; Yesinowski, J.P. Deep Sea NMR: Methane Hydrate Growth Habit in Porous Media and Its Relationship to Hydraulic Permeability, Deposit Accumulation, and Submarine Slope Stability. J. Geophys. Res. Solid Earth 2003, 108, 2508. [Google Scholar] [CrossRef]
- Wang, X.; Schultz, A.J.; Halpern, Y. Kinetics of Methane Hydrate Formation from Polycrystalline Deuterated Ice. J. Phys. Chem. A 2002, 106, 7304–7309. [Google Scholar] [CrossRef]
- Mizuno, Y.; Hanafusa, N. Studies of Surface Properties of Ice Using Nuclear Magnetic Resonance. J. Phys. Colloq. 1987, 48, 511–517. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.S.; Chen, Z.Y.; Li, G.; Wang, Y. Effects of Particle and Pore Sizes on the Formation Behaviors of Methane Hydrate in Porous Silica Gels. J. Nat. Gas Sci. Eng. 2016, 35, 1463–1471. [Google Scholar] [CrossRef]
- Blackwell, V.R. Formation Processes of Clathrate Hydrates of Carbon Dioxide and Methane; California Institute of Technology: Pasadena, CA, USA, 1998. [Google Scholar]
- Uchida, T.; Ebinuma, T.; Takeya, S.; Nagao, J.; Narita, H. Effects of Pore Sizes on Dissociation Temperatures and Pressures of Methane, Carbon Dioxide, and Propane Hydrates in Porous Media. J. Phys. Chem. A 2002, 106, 820–826. [Google Scholar] [CrossRef]
- Lu, H.; Kawasaki, T.; Ukita, T.; Moudrakovski, I.; Fujii, T.; Noguchi, S.; Shimada, T.; Nakamizu, M.; Ripmeester, J.; Ratcliffe, C. Particle Size Effect on the Saturation of Methane Hydrate in Sediments—Constrained from Experimental Results. Mar. Pet. Geol. 2011, 28, 1801–1805. [Google Scholar] [CrossRef]
- Malagar, B.R.C.; Lijith, K.P.; Singh, D.N. Formation & Dissociation of Methane Gas Hydrates in Sediments: A Critical Review. J. Nat. Gas Sci. Eng. 2019, 65, 168–184. [Google Scholar] [CrossRef]
- Bhawangirkar, D.R.; Nair, V.C.; Prasad, S.K.; Sangwai, J.S. Natural Gas Hydrates in the Krishna-Godavari Basin Sediments under Marine Reservoir Conditions: Thermodynamics and Dissociation Kinetics Using Thermal Stimulation. Energy Fuels 2021, 35, 8685–8698. [Google Scholar] [CrossRef]
- Schicks, J.M.; Pan, M.; Giese, R.; Poser, M.; Aminatulmimi, N.I.; Luzi-Helbing, M.; Bleisteiner, B.; Lenz, C. A New High-Pressure Cell for Systematic in Situ Investigations of Micro-Scale Processes in Gas Hydrates Using Confocal Micro-Raman Spectroscopy. Rev. Sci. Instrum. 2020, 91, 115103. [Google Scholar] [PubMed]
- Walrafen, G.E.; Yang, W.H.; Chu, Y.C. Raman Evidence for the Clathratelike Structure of Highly Supercooled Water. ACS Symp. Ser. 1997, 676, 287–308. [Google Scholar] [CrossRef]
- Uchida, T.; Takeya, S.; Kamata, Y.; Ohmura, R.; Narita, H. Spectroscopic Measurements on Binary, Ternary, and Quaternary Mixed-Gas Molecules in Clathrate Structures. Ind. Eng. Chem. Res. 2007, 46, 5080–5087. [Google Scholar] [CrossRef]
- Beeskow-Strauch, B.; Schicks, J.M. The Driving Forces of Guest Substitution in Gas Hydrates-A Laser Raman Study on CH4-CO2 Exchange in the Presence of Impurities. Energies 2012, 5, 420–437. [Google Scholar] [CrossRef]
- Jacobson, L.C.; Hujo, W.; Molinero, V. Nucleation Pathways of Clathrate Hydrates: Effect of Guest Size and Solubility. J. Phys. Chem. B 2010, 114, 13796–13807. [Google Scholar] [CrossRef]
- Schicks, J.M.; Luzi-Helbing, M. Cage Occupancy and Structural Changes during Hydrate Formation from Initial Stages to Resulting Hydrate Phase. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 528–536. [Google Scholar]
- Sa, J.H.; Kwak, G.H.; Lee, B.R.; Park, D.H.; Han, K.; Lee, K.H. Hydrophobic Amino Acids as a New Class of Kinetic Inhibitors for Gas Hydrate Formation. Sci. Rep. 2013, 3, 2428. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, P.; Pang, S.; Zhang, S.; Xiao, R. A Review of the Resource and Test Production of Natural Gas Hydrates in China. Energy Fuels 2021, 35, 9137–9150. [Google Scholar] [CrossRef]
- Li, B.; Sun, Y.H.; Guo, W.; Shan, X.L.; Wang, P.K.; Pang, S.J.; Jia, R.; Zhang, G.B. The Mechanism and Verification Analysis of Permafrost-Associated Gas Hydrate Formation in the Qilian Mountain, Northwest China. Mar. Pet. Geol. 2017, 86, 787–797. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, M.; Mayanna, S.; Schleicher, A.M.; Spangenberg, E.; Schicks, J.M. Reservoir Formation Damage during Hydrate Dissociation in Sand-Clay Sediment from Qilian Mountain Permafrost, China. Appl. Energy 2020, 263, 114619. [Google Scholar] [CrossRef]
- Collett, T.S.; Johnson, A.H.; Knapp, C.C.; Boswell, R. Natural Gas Hydrates: A Review. In Natural Gas Hydrates—Energy Resource Potential and Associated Geologic Hazards; Boswell, R.M., Knapp, C., Johnson, A., Collett, T., Eds.; AAPG Memoir: Tulsa, OK, USA, 2009; pp. 146–219. [Google Scholar]
- Beeskow-Strauch, B.; Schicks, J.M.; Spangenberg, E.; Erzinger, J. The Influence of SO2 and NO2 Impurities on CO2 Gas Hydrate Formation and Stability. Chem. Eur. J. 2011, 17, 4376–4384. [Google Scholar] [CrossRef] [PubMed]
- Kleeberg, R.; Bergmann, J. Quantitative Röntgenphasenanalyse Mit Den Rietveld- Programmen BGMN Und AUTOQUANT in Der Täglichen Laborpraxis. Ber. DTTG Greifswald. 1998, 6, 237–250. [Google Scholar]
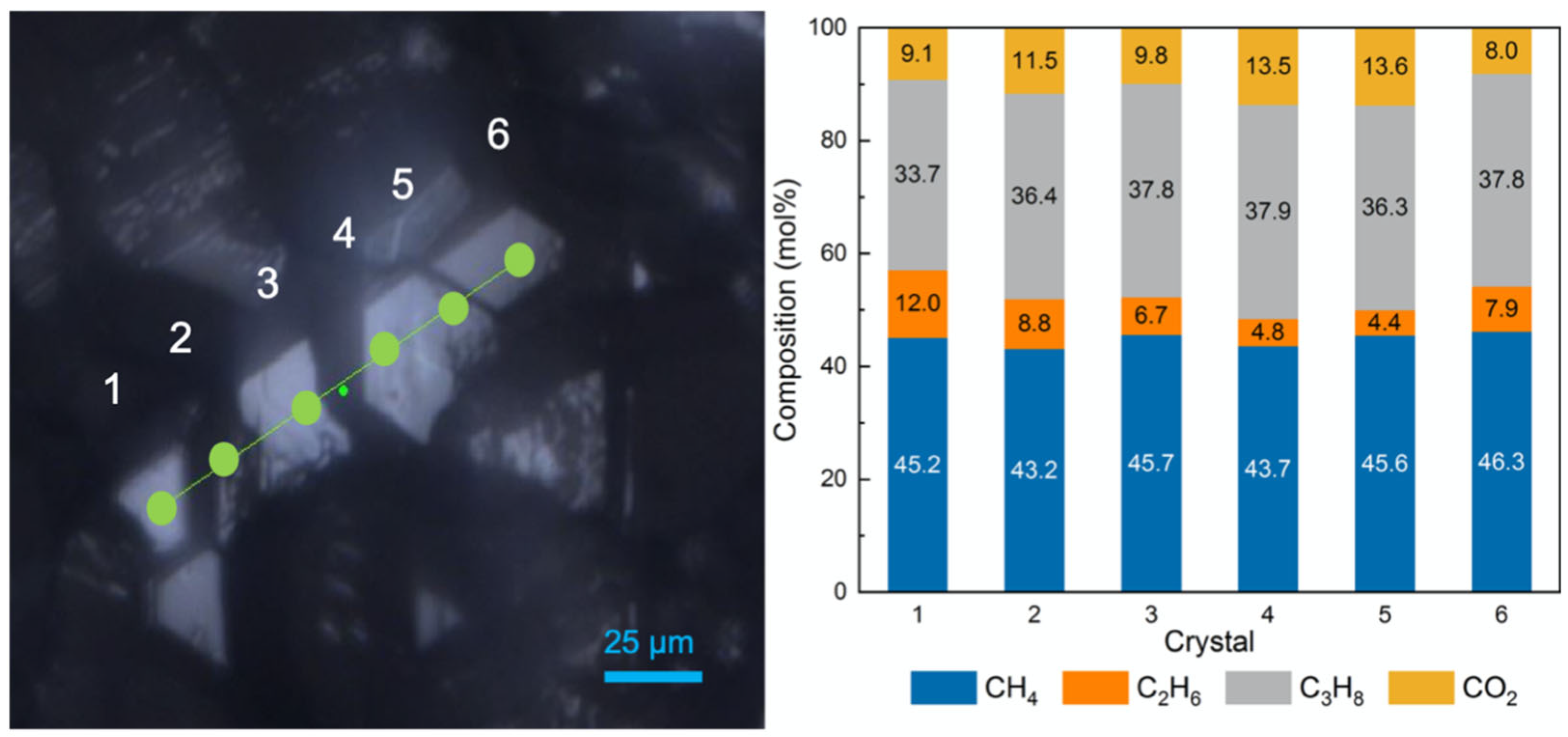
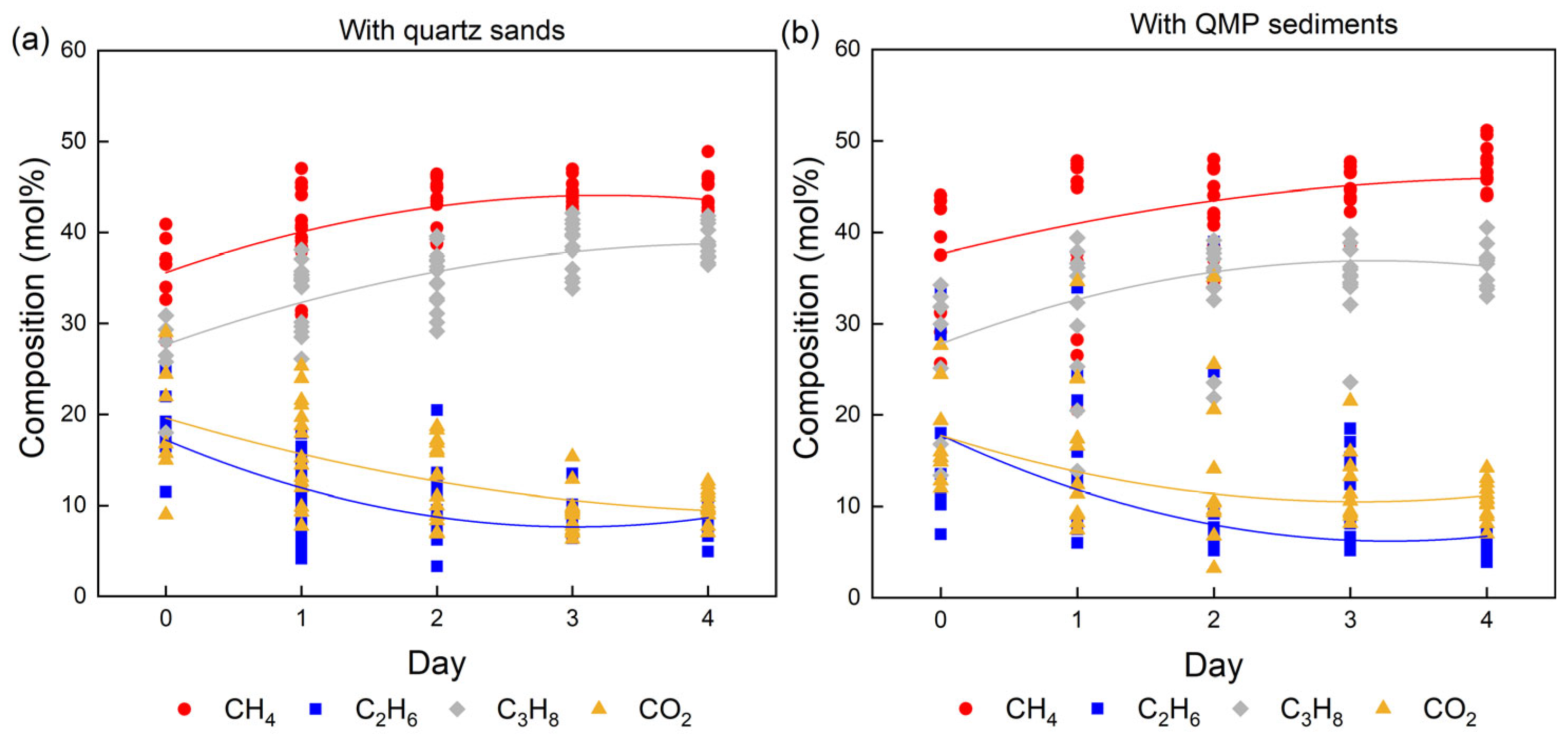
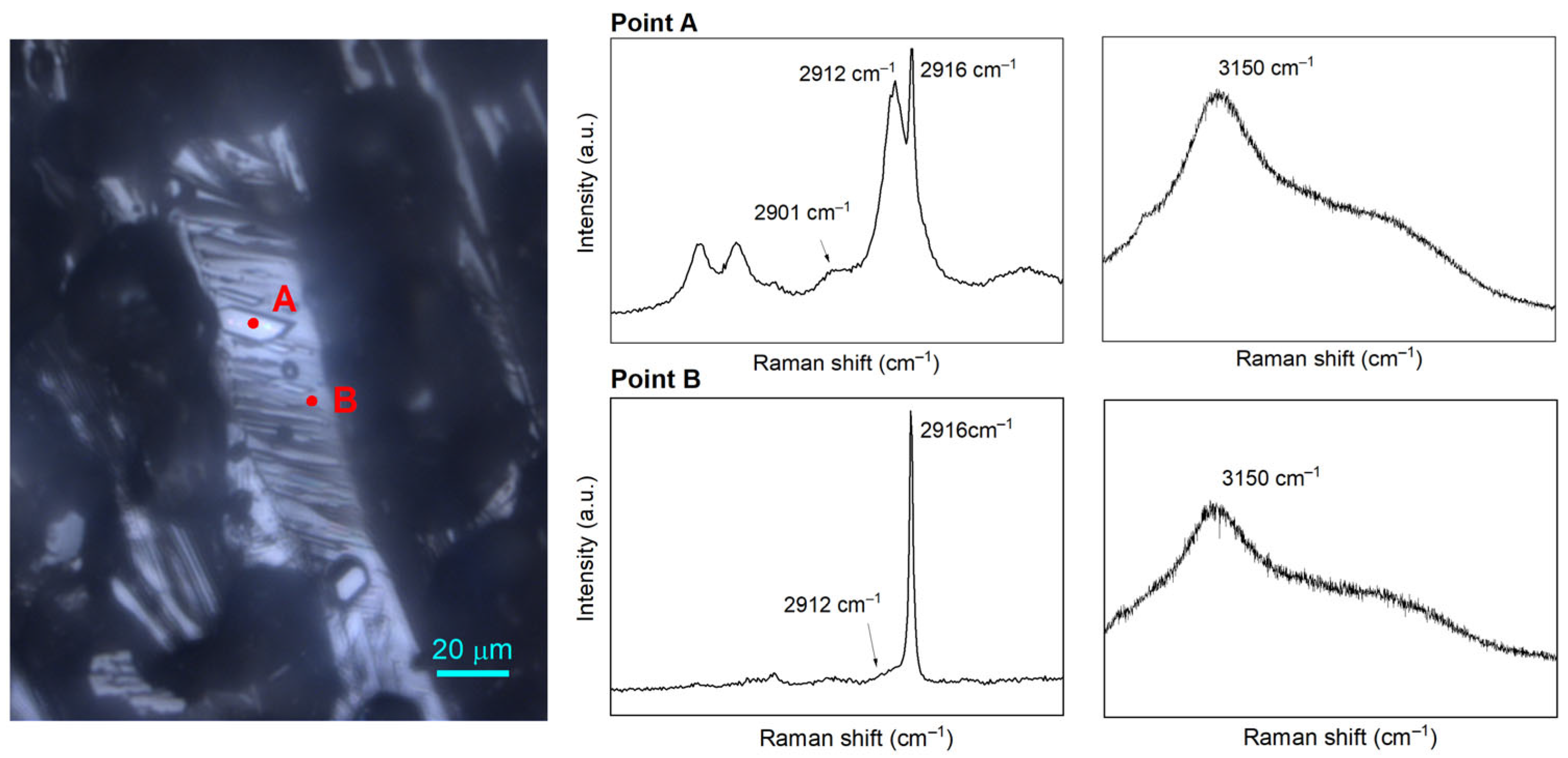
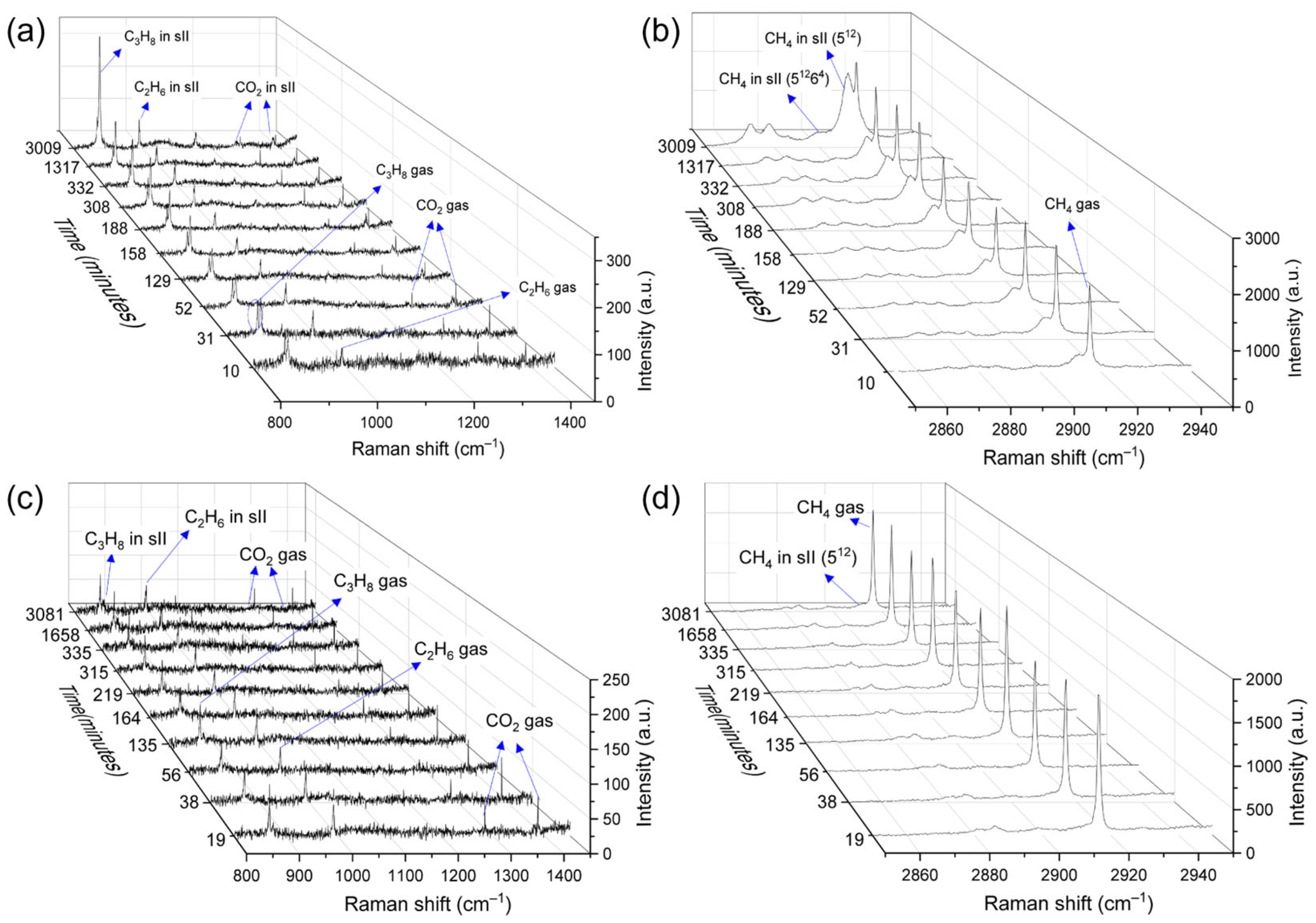
| No. | Sediments | P (MPa) | T (K) | Average mol% | |||
|---|---|---|---|---|---|---|---|
| CH4 | C2H6 | C3H8 | CO2 | ||||
| Original gas | 63 | 15 | 15 | 7 | |||
| Test 1 | / | 2.2 | 274 | 47.41 | 19.06 | 19.08 | 14.45 |
| Test 2 | / | 2.2 | 274 | 47.40 | 20.39 | 17.05 | 15.6 |
| Test 3 | QMP sediments | 2.2 | 274 | 47.55 | 20.26 | 17.32 | 14.87 |
| Test 4 | QMP sediments | 2.2 | 274 | 47.53 | 19.64 | 19.11 | 13.72 |
| Test 5 | Quartz sands | 2.2 | 274 | 45.16 | 20.24 | 19.45 | 15.15 |
| Test 6 | Quartz sands | 2.2 | 274 | 45.41 | 20.11 | 18.99 | 15.49 |
| Average gas composition | 46.74 | 19.95 | 18.55 | 14.81 | |||
| Calculated composition of hydrate phase (CSMGem) | 58.7 | 3.9 | 32.0 | 5.4 | |||
| Guest Component | ||||
|---|---|---|---|---|
| CH4 | C2H6 | C3H8 | CO2 | |
| Without sediment (Day 0) | 31.6 ± 5.9 | 15.2 ± 5.1 | 26.0 ± 6.3 | 37.9 ± 7.3 |
| Without sediment (Day 4) | 43.6 ± 2.3 | 7.0 ± 0.5 | 38.6 ± 2.2 | 10.8 ± 1.2 |
| Quartz sand (Day 0) | 35.5 ± 4.4 | 18.6 ± 4.3 | 27.0 ± 4.5 | 18.9 ± 6.7 |
| Quartz sand (Day 4) | 43.7 ± 2.3 | 8.0 ± 1.4 | 38.7 ± 1.7 | 10.0 ± 1.6 |
| QMP sediment (Day 0) | 35.0 ± 7.5 | 21.1 ± 10.5 | 25.3 ± 8.4 | 18.5 ± 6.4 |
| QMP sediment (Day 4) | 47.1 ± 2.3 | 5.8 ± 1.0 | 36.4 ± 2.1 | 10.7 ± 2.0 |
| Component | Vibrational Mode | Cavity Type/Gas Phase | νmeasured (cm−1) | νliterature (cm−1) | References |
|---|---|---|---|---|---|
| CH4 | C–H stretching | Gas phase | 2916 | 2916 | [39] |
| sII 512 | 2912 | 2912 | [2] | ||
| sII 51264 | 2901 | 2901 | |||
| C2H6 | C–C stretching | Gas phase | 993 | 993 | [2] |
| sII 51264 | 991 | 992 | |||
| C3H8 | C–C stretching | Gas phase | 869 | 869 | [39] |
| sII 51264 | 877 | 877 | |||
| CO2 | C–O stretching | Gas phase | 1285 | 1285 | [40] |
| sII 51264 | 1274 | 1275 | |||
| overtone bending | Gas phase | 1387 | 1388 | [40] | |
| sII 51264 | 1381 | 1381 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, M.; Schicks, J.M. Unraveling the Role of Natural Sediments in sII Mixed Gas Hydrate Formation: An Experimental Study. Molecules 2023, 28, 5887. https://doi.org/10.3390/molecules28155887
Pan M, Schicks JM. Unraveling the Role of Natural Sediments in sII Mixed Gas Hydrate Formation: An Experimental Study. Molecules. 2023; 28(15):5887. https://doi.org/10.3390/molecules28155887
Chicago/Turabian StylePan, Mengdi, and Judith M. Schicks. 2023. "Unraveling the Role of Natural Sediments in sII Mixed Gas Hydrate Formation: An Experimental Study" Molecules 28, no. 15: 5887. https://doi.org/10.3390/molecules28155887
APA StylePan, M., & Schicks, J. M. (2023). Unraveling the Role of Natural Sediments in sII Mixed Gas Hydrate Formation: An Experimental Study. Molecules, 28(15), 5887. https://doi.org/10.3390/molecules28155887







