Curcumin–Sodium Alginate and Curcumin–Chitosan Conjugates as Drug Delivery Systems: An Interesting Rheological Behaviour
Abstract
1. Introduction
2. Results and Discussion
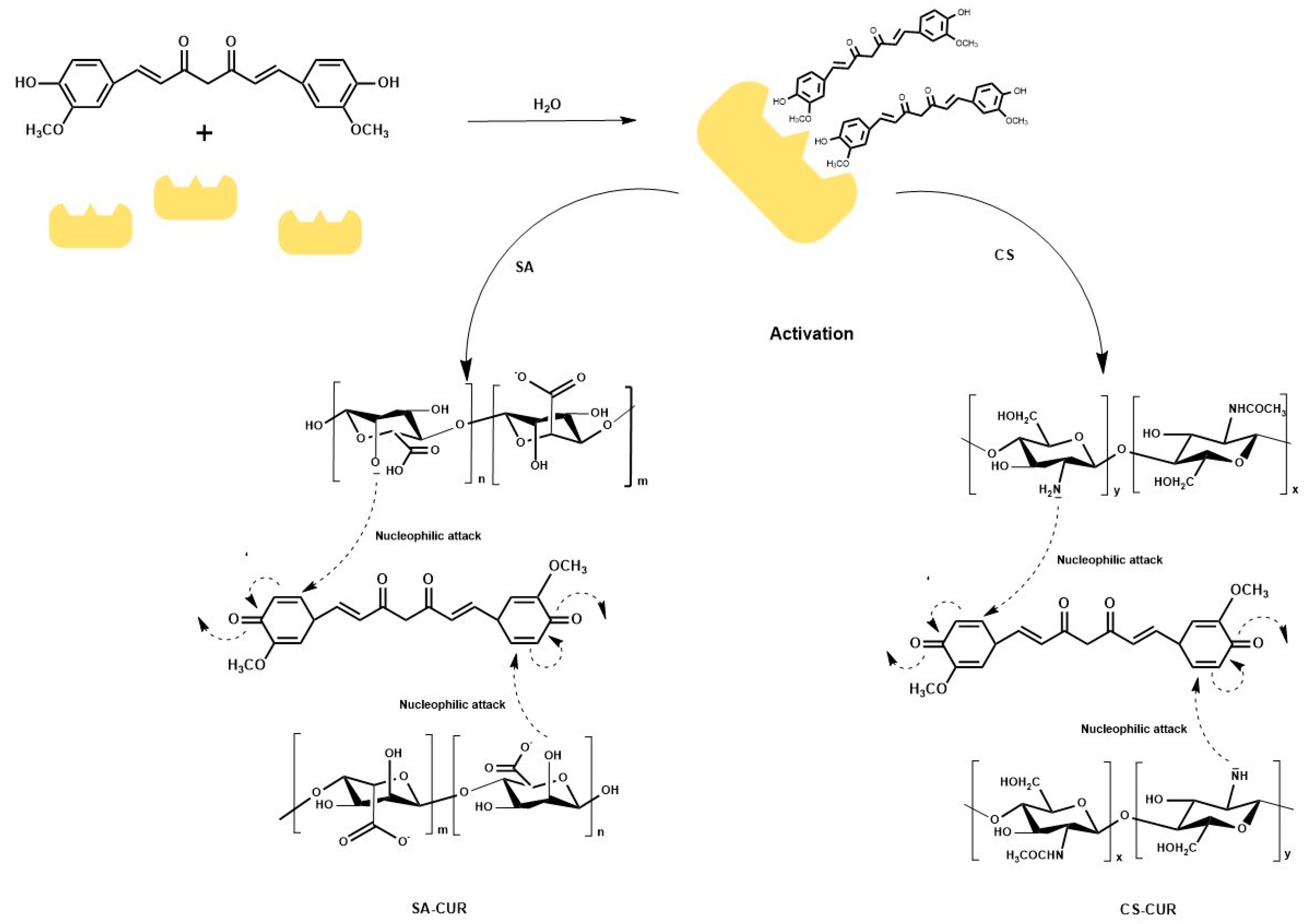
2.1. Synthesis of Alginate and Chitosan Conjugates
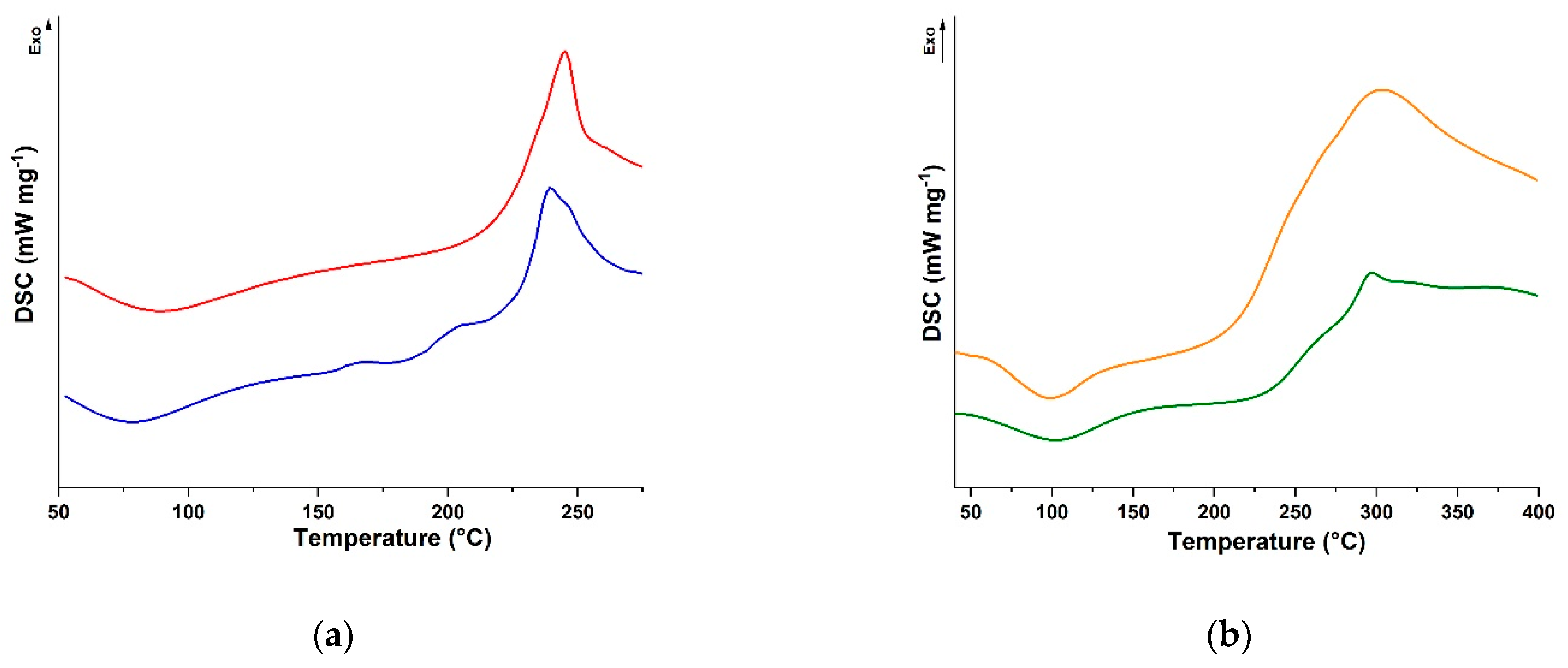
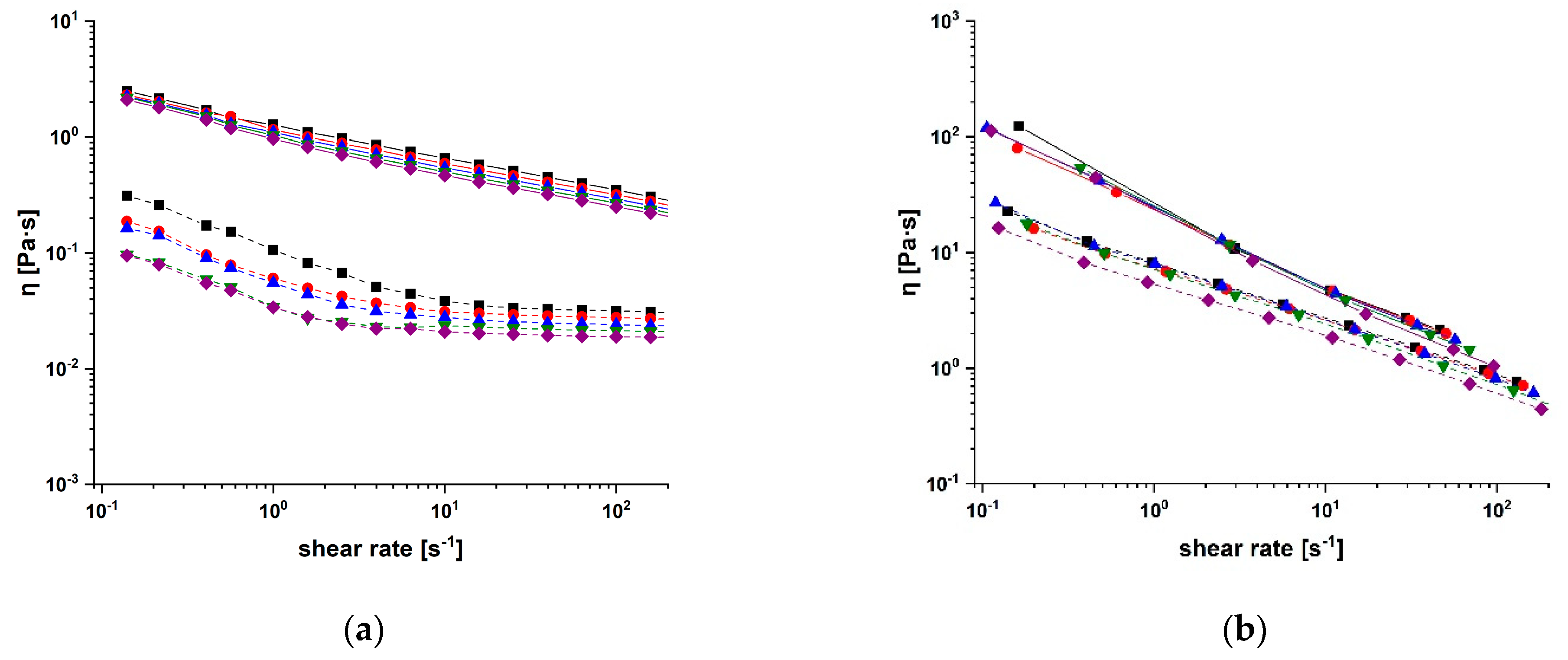
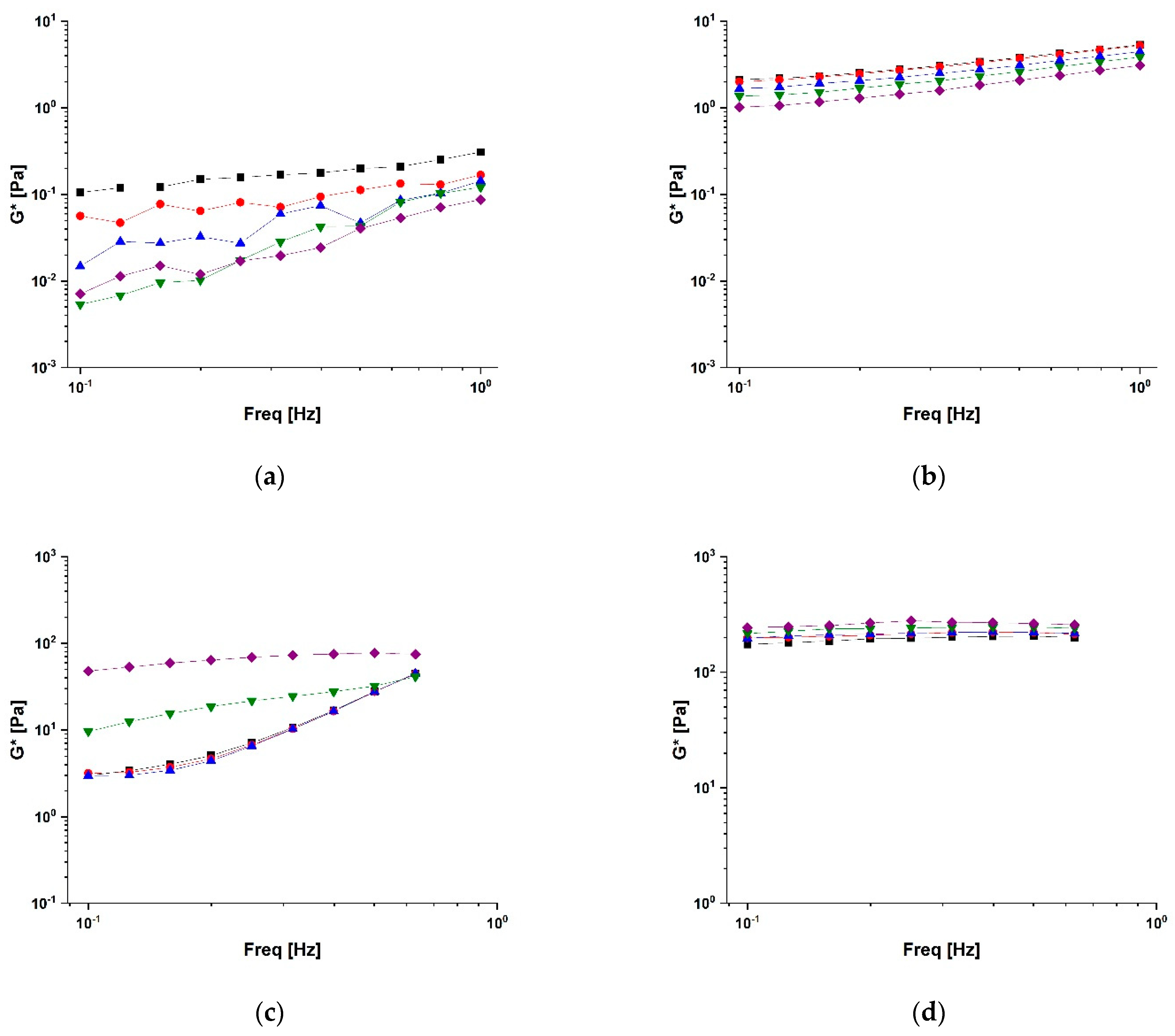
2.2. Rheological Characterisation of Conjugates
2.3. Drug Delivery Performance
3. Materials and Methods
3.1. Laccase Immobilisation
3.2. Synthesis of Bioconjugates
3.3. Characterisation Procedure
3.4. In Vistro Drug Release
3.5. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sarathi, P.; Padhi, S. Insight of the various in silico screening techniques developed for assortment of cocrystal formers and their thermodynamic characterization. Drug Dev. Ind. Pharm. 2021, 47, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Juster, H.R.; Distlbacher, T.; Steinbichler, G. Viscosity Analysis of a Polymer-Based Drug Delivery System Using Open-Source CFD Methods and High-Pressure Capillary Rheometry. Int. Polym. Process. 2014, 29, 570–578. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Wang, C.-P.J.; Byun, M.J.; Kim, S.-N.; Park, W.; Park, H.H.; Kim, T.-H.; Lee, J.S.; Park, C.G. Biomaterials as therapeutic drug carriers for inflammatory bowel disease treatment. J. Control Release 2022, 345, 1–19. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Cao, L.; Parakhonskiy, B.V.; Skirtach, A.G. Hard, Soft, and Hard-and-Soft Drug Delivery Carriers Based on CaCO3 and Alginate Biomaterials: Synthesis, Properties, Pharmaceutical Applications. Pharmaceutics 2022, 14, 909. [Google Scholar] [CrossRef]
- Qu, F.; Geng, R.; Liu, Y.; Zhu, J. Advanced nanocarrier- and microneedle-based transdermal drug delivery strategies for skin diseases treatment. Theranostics 2022, 12, 3372–3406. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Zhang, Z.; Lee, L.T.O.; Peng, L.; Lu, L.G.; He, X.; Zhang, X.J. Amphiphilic Dendrimer Doping Enhanced Ph-Sensitivity of Liposomal Vesicle for Effective Co-Delivery toward Synergistic Ferroptosis-Apoptosis Therapy of Hepatocellular Carcinoma. Adv. Healthc. Mater. 2023, 12, 2202663. [Google Scholar] [CrossRef]
- Ying, K.; Zhu, Y.; Wan, J.; Zhan, C.; Wang, Y.; Xie, B.; Xu, P.; Pan, H.; Wang, H. Macrophage membrane-biomimetic adhesive polycaprolactone nanocamptothecin for improving cancer-targeting efficiency and impairing metastasis. Bioact. Mater. 2023, 20, 449–462. [Google Scholar] [CrossRef]
- Hassan, M.A. A Long Acting Ophthalmic Gel Formulations of Atenolol. Drug Dev. Ind. Pharm. 2007, 33, 1192–1198. [Google Scholar] [CrossRef]
- Corazza, E.; di Cagno, M.P.; Bauer-Brandl, A.; Abruzzo, A.; Cerchiara, T.; Bigucci, F.; Luppi, B. Drug delivery to the brain: In situ gelling formulation enhances carbamazepine diffusion through nasal mucosa models with mucin. Eur. J. Pharm. Sci. 2022, 179, 106294. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; Hamed, R.; Abdo, H.; Swellmeen, L.; Basheer, H.A.; Wahdan, W.; Abu Kwiak, A.D. Formulation and Evaluation of Azithromycin-Loaded Niosomal Gel: Optimization, in Vitro Studies, Rheological Characterization, and Cytotoxicity Study. ACS Omega 2022, 7, 39782–39793. [Google Scholar] [CrossRef] [PubMed]
- Rananaware, P.; Pandit, P.; Naik, S.; Mishra, M.; Keri, R.S.; Brahmkhatri, V.P. Anti-amyloidogenic property of gold nanoparticle decorated quercetin polymer nanorods in pH and temperature induced aggregation of lysozyme. RSC Adv. 2022, 12, 23661–23674. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, L.; Le, Y.; Chen, J.-F.; Wang, J.; Yun, J. Synergistic effect of PEGylated resveratrol on delivery of anticancer drugs. Int. J. Pharm. 2016, 498, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Saranya, T.S.; Rajan, V.K.; Biswas, R.; Jayakumar, R.; Sathianarayanan, S. Synthesis, characterisation and biomedical applications of curcumin conjugated chitosan microspheres. Int. J. Biol. Macromol. 2018, 110, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Montané, X.; Kowalczyk, O.; Reig-Vano, B.; Bajek, A.; Roszkowski, K.; Tomczyk, R.; Pawliszak, W.; Giamberini, M.; Mocek-Płóciniak, A.; Tylkowski, B. Current Perspectives of the Applications of Polyphenols and Flavonoids in Cancer Therapy. Molecules 2020, 25, 3342. [Google Scholar] [CrossRef]
- Mouthuy, P.-A.; Snelling, S.J.; Dakin, S.G.; Milković, L.; Gašparović, A.; Carr, A.J.; Žarković, N. Biocompatibility of implantable materials: An oxidative stress viewpoint. Biomaterials 2016, 109, 55–68. [Google Scholar] [CrossRef]
- Li, W.; Fang, K.X.; Yuan, H.; Li, D.R.; Li, H.C.; Chen, Y.; Luo, X.Y.; Zhang, L.; Ye, X.C. Acid-Induced Poria Cocos Alkali-Soluble Polysaccharide Hydrogel: Gelation Behaviour, Characteristics, and Potential Application in Drug Delivery. Int. J. Biol. Macromol. 2023, 242, 124383. [Google Scholar] [CrossRef]
- Rong, L.; Liu, Y.; Fan, Y.; Xiao, J.; Su, Y.; Lu, L.; Peng, S.; Yuan, W.; Zhan, M. Injectable nano-composite hydrogels based on hyaluronic acid-chitosan derivatives for simultaneous photothermal-chemo therapy of cancer with anti-inflammatory capacity. Carbohydr. Polym. 2023, 310, 120721. [Google Scholar] [CrossRef]
- Silva, A.K.A.; Letourneur, D.; Chauvierre, C. Polysaccharide Nanosystems for Future Progress in Cardiovascular Pathologies. Theranostics 2014, 4, 579–591. [Google Scholar] [CrossRef]
- Kurczewska, J. Recent Reports on Polysaccharide-Based Materials for Drug Delivery. Polymers 2022, 14, 4189. [Google Scholar] [CrossRef] [PubMed]
- Vittorio, O.; Cojoc, M.; Curcio, M.; Spizzirri, U.G.; Hampel, S.; Nicoletta, F.P.; Iemma, F.; Dubrovska, A.; Kavallaris, M.; Cirillo, G. Polyphenol Conjugates by Immobilized Laccase: The Green Synthesis of Dextran-Catechin. Macromol. Chem. Phys. 2016, 217, 1488–1492. [Google Scholar] [CrossRef]
- Voli, F.; Valli, E.; Lerra, L.; Kimpton, K.; Saletta, F.; Giorgi, F.M.; Mercatelli, D.; Rouaen, J.R.C.; Shen, S.; Murray, J.E.; et al. Intratumoral Copper Modulates Pd-L1 Expression and Influences Tumor Immune Evasion. Cancer Res. 2020, 80, 4129–4144. [Google Scholar] [CrossRef]
- Cirillo, G.; Pantuso, E.; Curcio, M.; Vittorio, O.; Leggio, A.; Iemma, F.; De Filpo, G.; Nicoletta, F.P. Alginate Bioconjugate and Graphene Oxide in Multifunctional Hydrogels for Versatile Biomedical Applications. Molecules 2021, 26, 1355. [Google Scholar] [CrossRef] [PubMed]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef]
- Yang, H.; Du, Z.; Wang, W.; Song, M.; Sanidad, K.; Sukamtoh, E.; Zheng, J.; Tian, L.; Xiao, H.; Liu, Z.; et al. Structure—Activity Relationship of Curcumin: Role of the Methoxy Group in Anti-inflammatory and Anticolitis Effects of Curcumin. J. Agric. Food Chem. 2017, 65, 4509–4515. [Google Scholar] [CrossRef]
- Zhu, J.; Sanidad, K.Z.; Sukamtoh, E.; Zhang, G. Potential roles of chemical degradation in the biological activities of curcumin. Food Funct. 2017, 8, 907–914. [Google Scholar] [CrossRef]
- Cikrikci, S.; Mozioglu, E.; Yilmaz, H. Biological Activity of Curcuminoids Isolated from Curcuma Longa. Rec. Nat. Prod. 2008, 2, 19–24. [Google Scholar]
- Sohn, S.-I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin—An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef]
- Golchin, A.; Hosseinzadeh, S.; Staji, M.; Soleimani, M.; Ardeshirylajimi, A.; Khojasteh, A. Biological behavior of the curcumin incorporated chitosan/poly(vinyl alcohol) nanofibers for biomedical applications. J. Cell. Biochem. 2019, 120, 15410–15421. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of Curcumin, a Component of Golden Spice, and Its Miraculous Biological Activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [PubMed]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-Based Hydrogels as Drug Delivery Vehicles in Cancer Treatment and Their Applications in Wound Dressing and 3d Bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Roquero, D.M.; Katz, E. “Smart” alginate hydrogels in biosensing, bioactuation and biocomputing: State-of-the-art and perspectives. Sens. Actuators Rep. 2022, 4, 100095. [Google Scholar] [CrossRef]
- Tomić, S.L.; Radić, M.M.B.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-Based Hydrogels and Scaffolds for Biomedical Applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent Advances of Chitosan Formulations in Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 10975. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Pérez-Álvarez, L.; Silván, U.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Photocrosslinkable and self-healable hydrogels of chitosan and hyaluronic acid. Int. J. Biol. Macromol. 2022, 216, 291–302. [Google Scholar] [CrossRef]
- Lerra, L.; Farfalla, A.; Sanz, B.; Cirillo, G.; Vittorio, O.; Voli, F.; Le Grand, M.; Curcio, M.; Nicoletta, F.P.; Dubrovska, A.; et al. Graphene Oxide Functional Nanohybrids with Magnetic Nanoparticles for Improved Vectorization of Doxorubicin to Neuroblastoma Cells. Pharmaceutics 2019, 11, 3. [Google Scholar] [CrossRef]
- di Luca, M.; Curcio, M.; Valli, E.; Cirillo, G.; Voli, F.; Butini, M.E.; Farfalla, A.; Pantuso, E.; Leggio, A.; Nicoletta, F.P.; et al. Combining antioxidant hydrogels with self-assembled microparticles for multifunctional wound dressings. J. Mater. Chem. B 2019, 7, 4361–4370. [Google Scholar] [CrossRef]
- Vittorio, O.; Le Grand, M.; Makharza, S.A.; Curcio, M.; Tucci, P.; Iemma, F.; Nicoletta, F.P.; Hampel, S.; Cirillo, G. Doxorubicin synergism and resistance reversal in human neuroblastoma BE(2)C cell lines: An in vitro study with dextran-catechin nanohybrids. Eur. J. Pharm. Biopharm. 2018, 122, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Nicoletta, F.P.; Curcio, M.; Spizzirri, U.G.; Picci, N.; Iemma, F. Enzyme immobilization on smart polymers: Catalysis on demand. React. Funct. Polym. 2014, 83, 62–69. [Google Scholar] [CrossRef]
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.K. From Protein Engineering to Immobilization: Promising Strategies for the Upgrade of Industrial Enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. [Google Scholar] [CrossRef]
- Miletic, N.; Nastasovic, A.; Loos, K. Immobilization of Biocatalysts for Enzymatic Polymerizations: Possibilities, Advantages, Applications. Bioresour. Technol. 2012, 115, 126–135. [Google Scholar] [CrossRef]
- Curcio, M.; Cirillo, G.; Tucci, P.; Farfalla, A.; Bevacqua, E.; Vittorio, O.; Iemma, F.; Nicoletta, F.P. Dextran-Curcumin Nanoparticles as a Methotrexate Delivery Vehicle: A Step Forward in Breast Cancer Combination Therapy. Pharmaceuticals 2020, 13, 2. [Google Scholar] [CrossRef]
- Yu, G.; Yan, X.; Han, C.; Huang, F. Characterization of supramolecular gels. Chem. Soc. Rev. 2013, 42, 6697–6722. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Arıca, M.Y. Immobilization of laccase onto poly(glycidylmethacrylate) brush grafted poly(hydroxyethylmethacrylate) films: Enzymatic oxidation of phenolic compounds. Mater. Sci. Eng. C 2009, 29, 1990–1997. [Google Scholar] [CrossRef]
- Madhavi, V.; Lele, S.S. Laccase: Properties and Applications. Bioresources 2009, 4, 1694–1717. [Google Scholar] [CrossRef]
- Silva, C.; Matamá, T.; Kim, S.; Padrão, J.; Prasetyo, E.N.; Kudanga, T.; Nyanhongo, G.S.; Guebitz, G.M.; Casal, M.; Cavaco-Paulo, A. Antimicrobial and antioxidant linen via laccase-assisted grafting. React. Funct. Polym. 2011, 71, 713–720. [Google Scholar] [CrossRef]
- Nonappa, N.; Kolehmainen, E. Solid state NMR studies of gels derived from low molecular mass gelators. Soft Matter 2016, 12, 6015–6026. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain Mobility, Thermal, and Mechanical Properties of Poly(ethylene furanoate) Compared to Poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Tan, Y.T.F.; Peh, K.K.; Al-Hanbali, O. Effect of Carbopol and Polyvinylpyrrolidone on the Mechanical, Rheological, and Release Properties of Bioadhesive Polyethylene Glycol Gels. Aaps Pharmscitech. 2000, 1, 24. [Google Scholar] [CrossRef]
- Keshavarz, M.; Kaffashi, B. The ability of retention, drug release and rheological properties of nanogel bioadhesives based on cellulose derivatives. Pharm. Dev. Technol. 2013, 19, 952–959. [Google Scholar] [CrossRef]
- Hamed, R.; Al Baraghthi, T.; Alkilani, A.Z.; Abu-Huwaij, R. Correlation between Rheological Properties and in Vitro Drug Release from Penetration Enhancer-Loaded Carbopola (R) Gels. J. Pharm. Innov. 2016, 11, 339–351. [Google Scholar] [CrossRef]
- Martínez-Ruvalcaba, A.; Chornet, E.; Rodrigue, D. Dynamic Rheological Properties of Concentrated Chitosan Soltions. Appl. Rheol. 2004, 14, 140–147. [Google Scholar] [CrossRef]
- Coppola, L.; Gianferri, R.; Nicotera, I.; Oliviero, C.; Ranieri, G.A. Structural changes in CTAB/H2O mixtures using a rheological approach. Phys. Chem. Chem. Phys. 2004, 6, 2364–2372. [Google Scholar] [CrossRef]
- Bertolo, M.R.; Martins, V.C.; Horn, M.M.; Brenelli, L.B.; Plepis, A.M. Rheological and antioxidant properties of chitosan/gelatin-based materials functionalized by pomegranate peel extract. Carbohydr. Polym. 2019, 228, 115386. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.E.; Coppola, L.; Gaudio, D.; Nicotera, I.; Oliviero, C. Shear Rheology and Phase Behaviour of Sodium Oleate/Water Mixtures. Colloids Surf. A-Physicochem. Eng. Asp. 2007, 297, 95–104. [Google Scholar] [CrossRef]
- Sobral, P.J.D.A.; Gebremariam, G.; Drudi, F.; Pinheiro, A.C.D.A.S.; Romani, S.; Rocculi, P.; Rosa, M.D. Rheological and Viscoelastic Properties of Chitosan Solutions Prepared with Different Chitosan or Acetic Acid Concentrations. Foods 2022, 11, 2692. [Google Scholar] [CrossRef] [PubMed]
- Timmons, J.; Falzone, G.; Balonis, M.; Bauchy, M.; Sant, G. Anomalous variations in the viscous activation energy of suspensions induced by fractal structuring. J. Colloid Interface Sci. 2018, 530, 603–609. [Google Scholar] [CrossRef]
- Dodero, A.; Vicini, S.; Alloisio, M.; Castellano, M. Rheological properties of sodium alginate solutions in the presence of added salt: An application of Kulicke equation. Rheol. Acta 2020, 59, 365–374. [Google Scholar] [CrossRef]
- Razmkhah, S.; Razavi, S.M.A.; Mohammadifar, M.A. Dilute Solution, Flow Behavior, Thixotropy and Viscoelastic Characterization of Cress Seed (Lepidium Sativum) Gum Fractions. Food Hydrocoll. 2017, 63, 404–413. [Google Scholar] [CrossRef]
- Gabriele, D.; de Cindio, B.; D’Antona, P. A weak gel model for foods. Rheol. Acta 2001, 40, 120–127. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Perez, A.F.; Cardoza, I.L.; Baluyot-Reyes, N.; Ba, Y. Mucoadhesive and Rheological Studies on the Co-Hydrogel Systems of Poly(Ethylene Glycol) Copolymers with Fluoroalkyl and Poly(Acrylic Acid). Polymers 2021, 13, 1956. [Google Scholar] [CrossRef]
- Shalviri, A.; Raval, G.; Prasad, P.; Chan, C.; Liu, Q.; Heerklotz, H.; Rauth, A.M.; Wu, X.Y. pH-Dependent doxorubicin release from terpolymer of starch, polymethacrylic acid and polysorbate 80 nanoparticles for overcoming multi-drug resistance in human breast cancer cells. Eur. J. Pharm. Biopharm. 2012, 82, 587–597. [Google Scholar] [CrossRef]
- Oberlintner, A.; Bajić, M.; Kalčíková, G.; Likozar, B.; Novak, U. Biodegradability study of active chitosan biopolymer films enriched with Quercus polyphenol extract in different soil types. Environ. Technol. Innov. 2020, 21, 101318. [Google Scholar] [CrossRef]
- Pang, Y.; Xi, F.; Luo, J.; Liu, G.; Guo, T.; Zhang, C. An alginate film-based degradable triboelectric nanogenerator. RSC Adv. 2018, 8, 6719–6726. [Google Scholar] [CrossRef]
- Chellat, F.; Tabrizian, M.; Dumitriu, S.; Chornet, E.; Rivard, C.-H.; Yahia, L. Study of biodegradation behavior of chitosan-xanthan microspheres in simulated physiological media. J. Biomed. Mater. Res. 2000, 53, 592–599. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Hushmandi, K.; Mirzaei, S.; Bokaie, S.; Bigham, A.; Makvandi, P.; Rabiee, N.; Thakur, V.K.; Kumar, A.P.; Sharifi, E.; et al. Chitosan-based nanoscale systems for doxorubicin delivery: Exploring biomedical application in cancer therapy. Bioeng. Transl. Med. 2023, 8, e10325. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release. 3. Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Laracuente, M.-L.; Yu, M.H.; McHugh, K.J. Zero-order drug delivery: State of the art and future prospects. J. Control Release 2020, 327, 834–856. [Google Scholar] [PubMed]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. (Ed.) 5—Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. ISBN 9780081000922. [Google Scholar] [CrossRef]
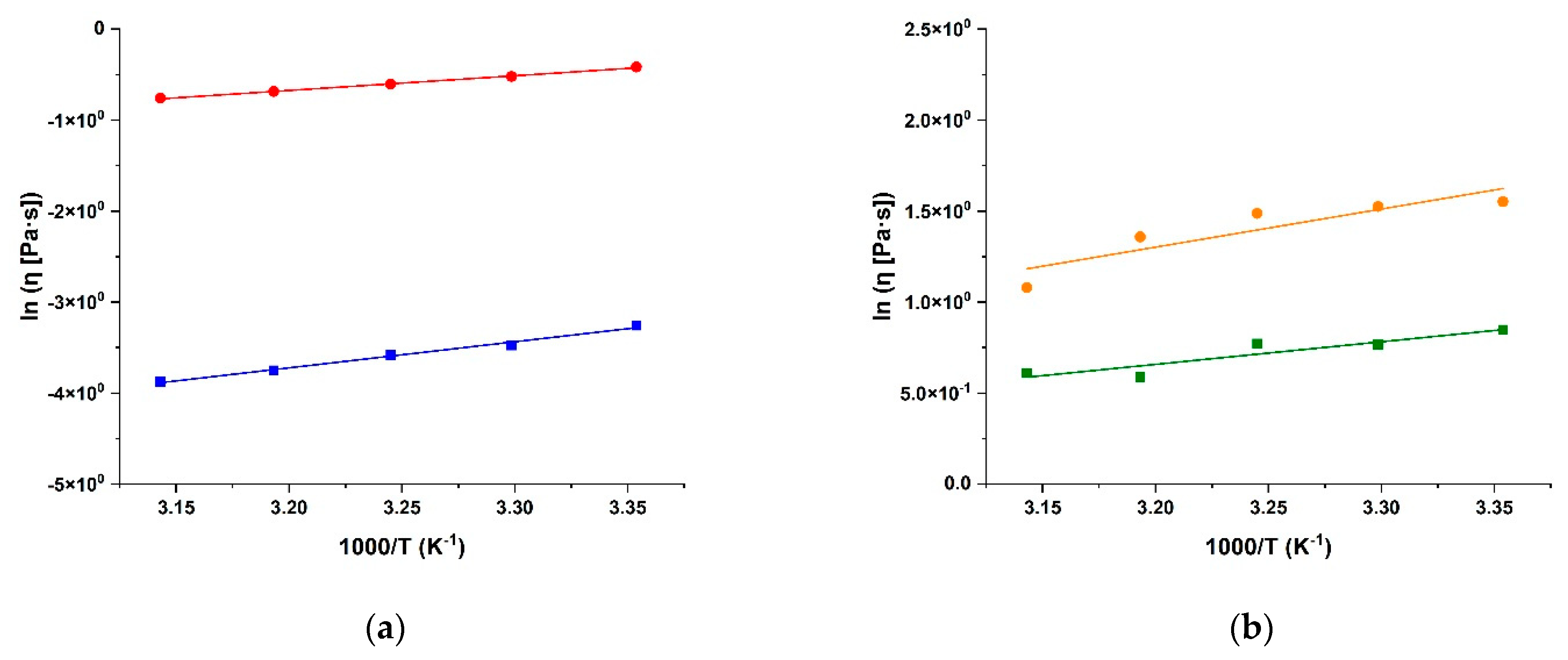

| Sample | R2 | η0 (Pa s) | Ea (kJ mol−1) |
|---|---|---|---|
| SA | 0.9903 | (2.5 ± 0.1) 10−6 | 23.8 ± 0.1 |
| SA-CUR | 0.9968 | (2.9 ± 0.1) 10−3 | 10.3 ± 0.1 |
| CS | 0.9460 | (2.6 ± 0.1) 10−2 | 13.4 ± 0.1 |
| CS-CUR | 0.9081 | (4.4 ± 0.1) 10−2 | 17.4 ± 0.1 |
| Sample | Temperature | R2 | A (±0.1) | z (±0.1) |
|---|---|---|---|---|
| SA | 25 | 0.9831 | 0.3 | 1.6 |
| 30 | 0.9819 | 0.2 | 1.4 | |
| 35 | 0.9471 | 0.1 | 1.2 | |
| 40 | 0.9754 | 0.1 | 1.2 | |
| 45 | 0.9527 | 0.1 | 1.2 | |
| SA-CUR | 25 | 0.9946 | 5.6 | 2.1 |
| 30 | 0.9943 | 4.7 | 2.1 | |
| 35 | 0.9958 | 4.0 | 2.0 | |
| 40 | 0.9748 | 3.0 | 2.3 | |
| 45 | 0.9964 | 2.7 | 2.3 | |
| CS | 25 | 0.9972 | 127.2 | 0.6 |
| 30 | 0.9946 | 112.2 | 0.6 | |
| 35 | 0.9983 | 115.5 | 0.5 | |
| 40 | 0.9937 | 102.1 | 0.5 | |
| 45 | 0.9550 | 116.9 | 0.6 | |
| CS-CUR | 25 | 0.8688 | 217.2 | 11.9 |
| 30 | 0.8482 | 229.7 | 16.9 | |
| 35 | 0.8469 | 231.5 | 18.0 | |
| 40 | 0.8461 | 246.0 | 20.4 | |
| 45 | 0.8093 | 263.4 | 19.6 |
| Sample | Zero Order | First Order | Peppas-Sahlin | |||||
|---|---|---|---|---|---|---|---|---|
| R2 | k0 (s−1) | R2 | k1 (s−1) | R2 | m | (s−m) | (10−1 s−2m) | |
| SA | 0.7950 | 0.03 | 0.9273 | 0.42 | 0.9569 | 0.40 | 0.40 | 0.45 |
| SA-CUR | 0.8288 | 0.01 | 0.9379 | 0.32 | 0.9446 | 0.45 | 0.13 | 0.12 |
| CS | 0.6813 | 0.04 | 0.9756 | 1.32 | 0.9455 | 0.33 | 0.71 | 1.31 |
| CS-CUR | 0.6950 | 0.02 | 0.9850 | 0.87 | 0.9083 | 0.42 | 0.27 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirillo, G.; Curcio, M.; Oliviero Rossi, C.; De Filpo, G.; Baratta, M.; De Luca, M.; Iemma, F.; Nicoletta, F.P. Curcumin–Sodium Alginate and Curcumin–Chitosan Conjugates as Drug Delivery Systems: An Interesting Rheological Behaviour. Molecules 2023, 28, 5893. https://doi.org/10.3390/molecules28155893
Cirillo G, Curcio M, Oliviero Rossi C, De Filpo G, Baratta M, De Luca M, Iemma F, Nicoletta FP. Curcumin–Sodium Alginate and Curcumin–Chitosan Conjugates as Drug Delivery Systems: An Interesting Rheological Behaviour. Molecules. 2023; 28(15):5893. https://doi.org/10.3390/molecules28155893
Chicago/Turabian StyleCirillo, Giuseppe, Manuela Curcio, Cesare Oliviero Rossi, Giovanni De Filpo, Mariafrancesca Baratta, Michele De Luca, Francesca Iemma, and Fiore Pasquale Nicoletta. 2023. "Curcumin–Sodium Alginate and Curcumin–Chitosan Conjugates as Drug Delivery Systems: An Interesting Rheological Behaviour" Molecules 28, no. 15: 5893. https://doi.org/10.3390/molecules28155893
APA StyleCirillo, G., Curcio, M., Oliviero Rossi, C., De Filpo, G., Baratta, M., De Luca, M., Iemma, F., & Nicoletta, F. P. (2023). Curcumin–Sodium Alginate and Curcumin–Chitosan Conjugates as Drug Delivery Systems: An Interesting Rheological Behaviour. Molecules, 28(15), 5893. https://doi.org/10.3390/molecules28155893









