Synthesis and Characterization of Methoxy-Exfoliated Montmorillonite Nanosheets as Potential Carriers of 5-Fluorouracil Drug with Enhanced Loading, Release, and Cytotoxicity Properties
Abstract
:1. Introduction
2. Experimental Work
2.1. Materials
2.2. Synthesis of Methoxy-Exfoliated Bentonite (Mth/EXBE)
2.3. Analytical Techniques
2.4. 5-Fu Loading Studies
2.5. The Release Studies
2.6. In Vitro Cytotoxicity
2.6.1. Cell Line
2.6.2. In Vitro Cytotoxicity
3. Results and Discussion
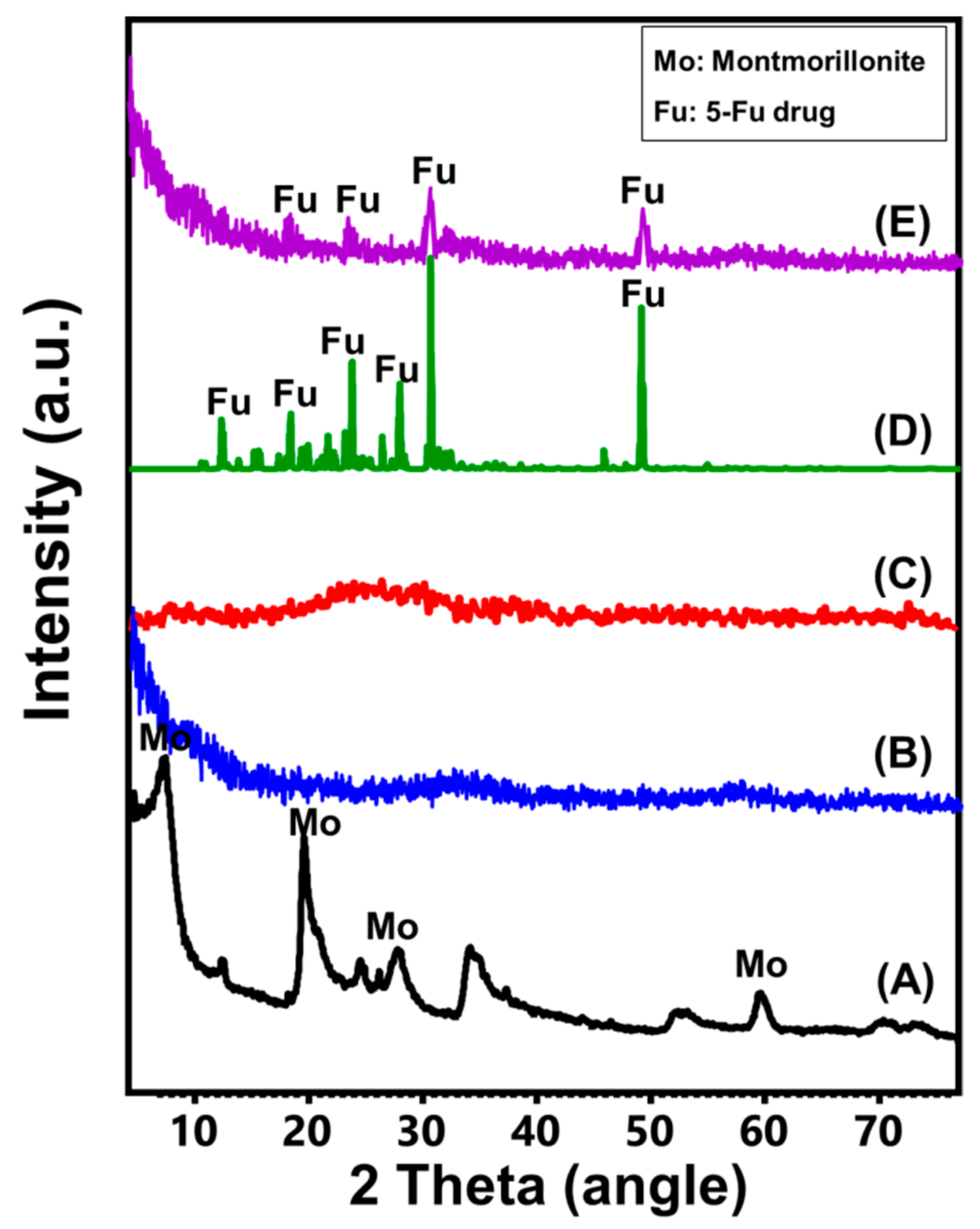
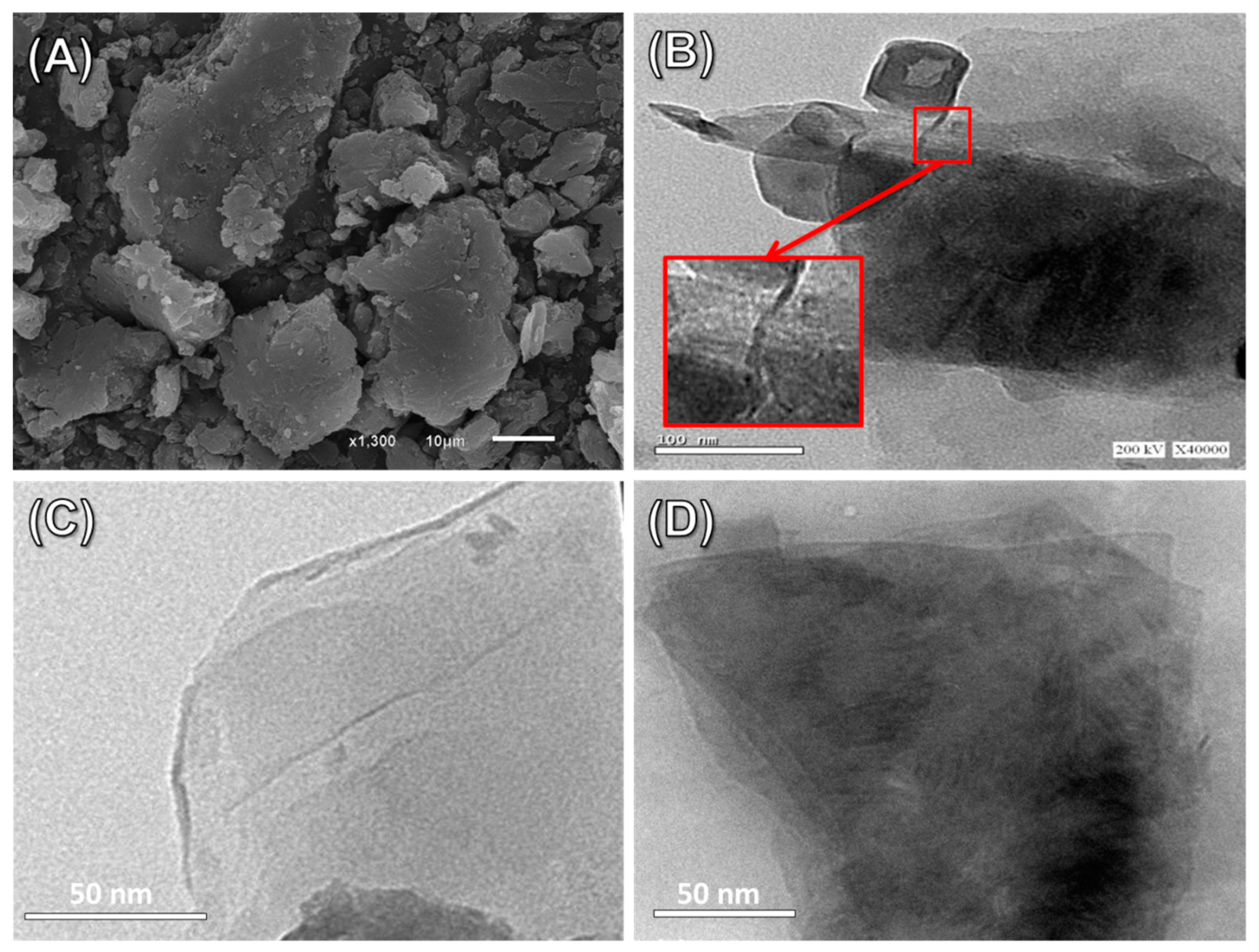
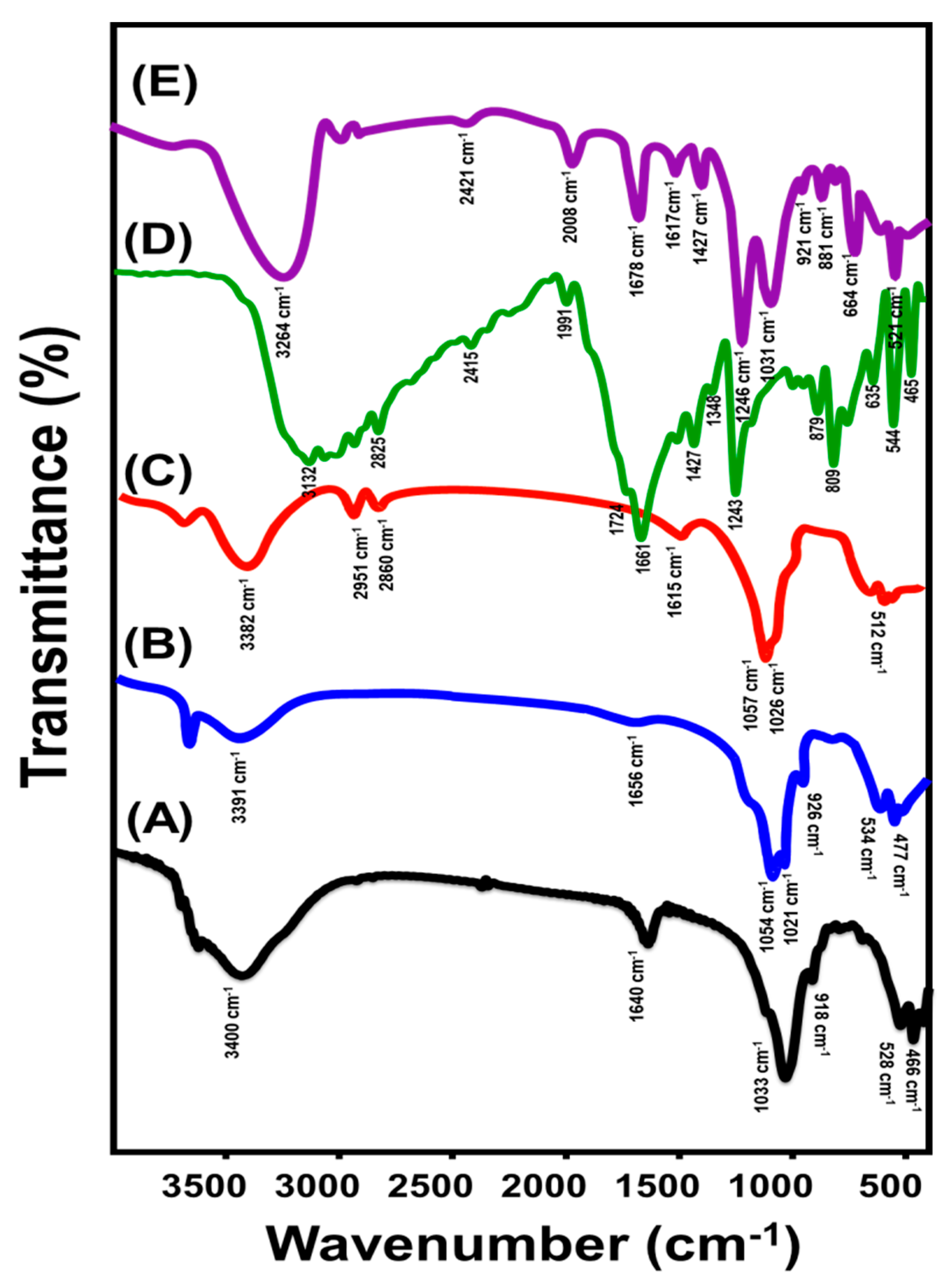
3.1. Characterization of the Carriers
3.2. Loading of 5-Fu Drug
3.2.1. Influence of the Encapsulation Parameters
Effect of pH
Loading Duration
5-Fu Concentration
3.2.2. Loading Mechanism
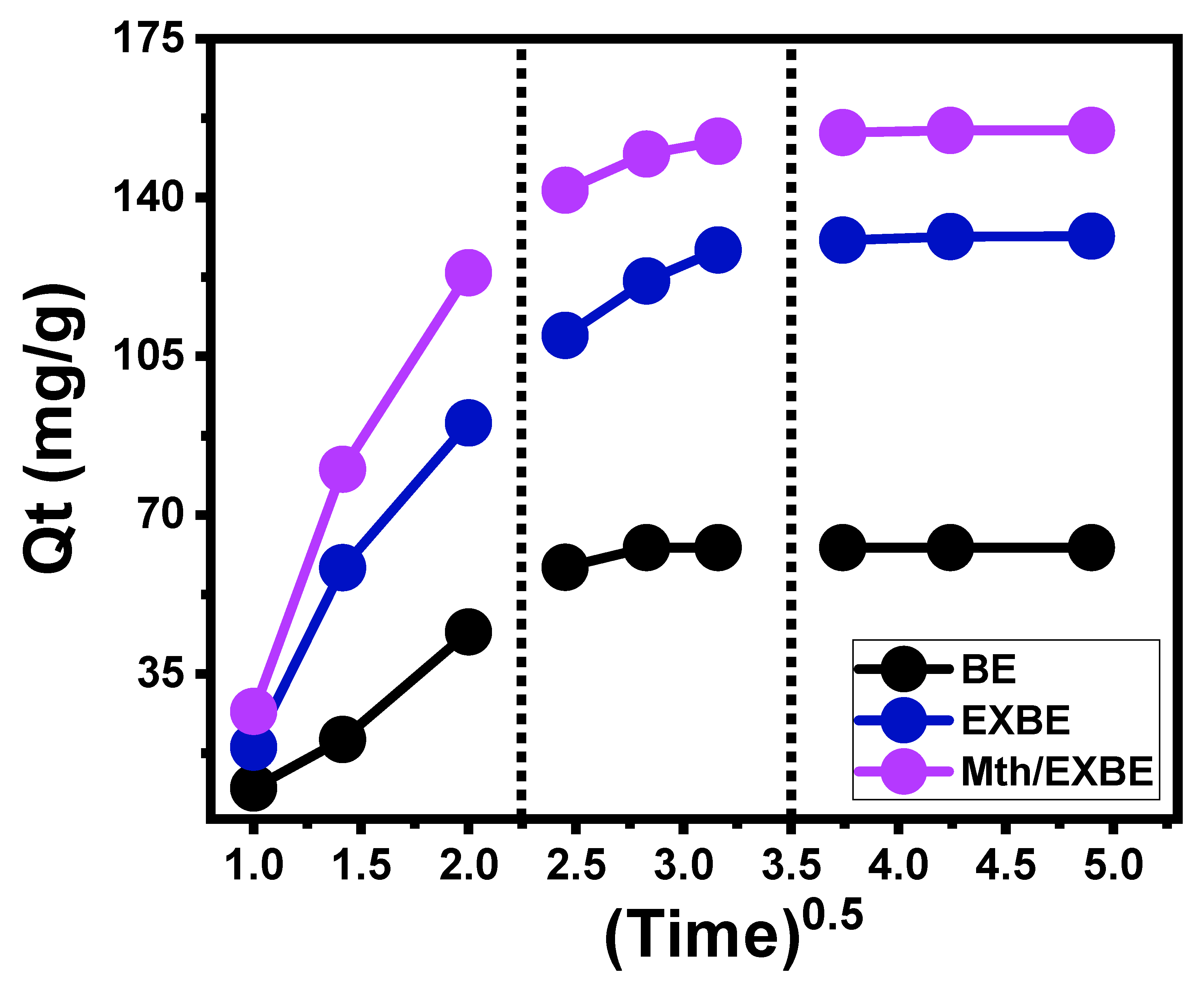
Kinetic Properties
- Intra-Particle Diffusion Behavior
- Kinetic Modeling
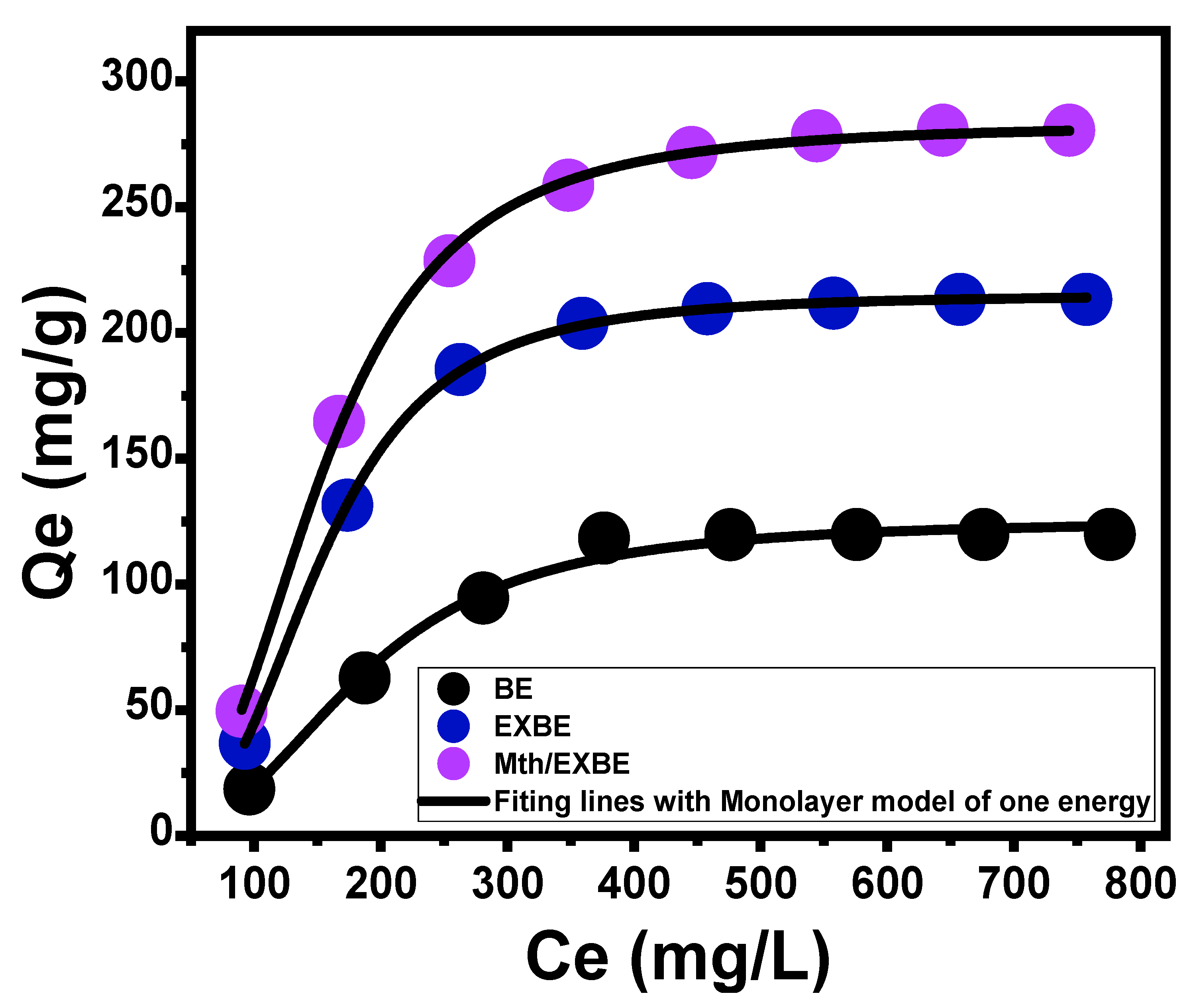
Isotherm Properties
- Classic Isotherm Models
- Advanced Isotherm Models
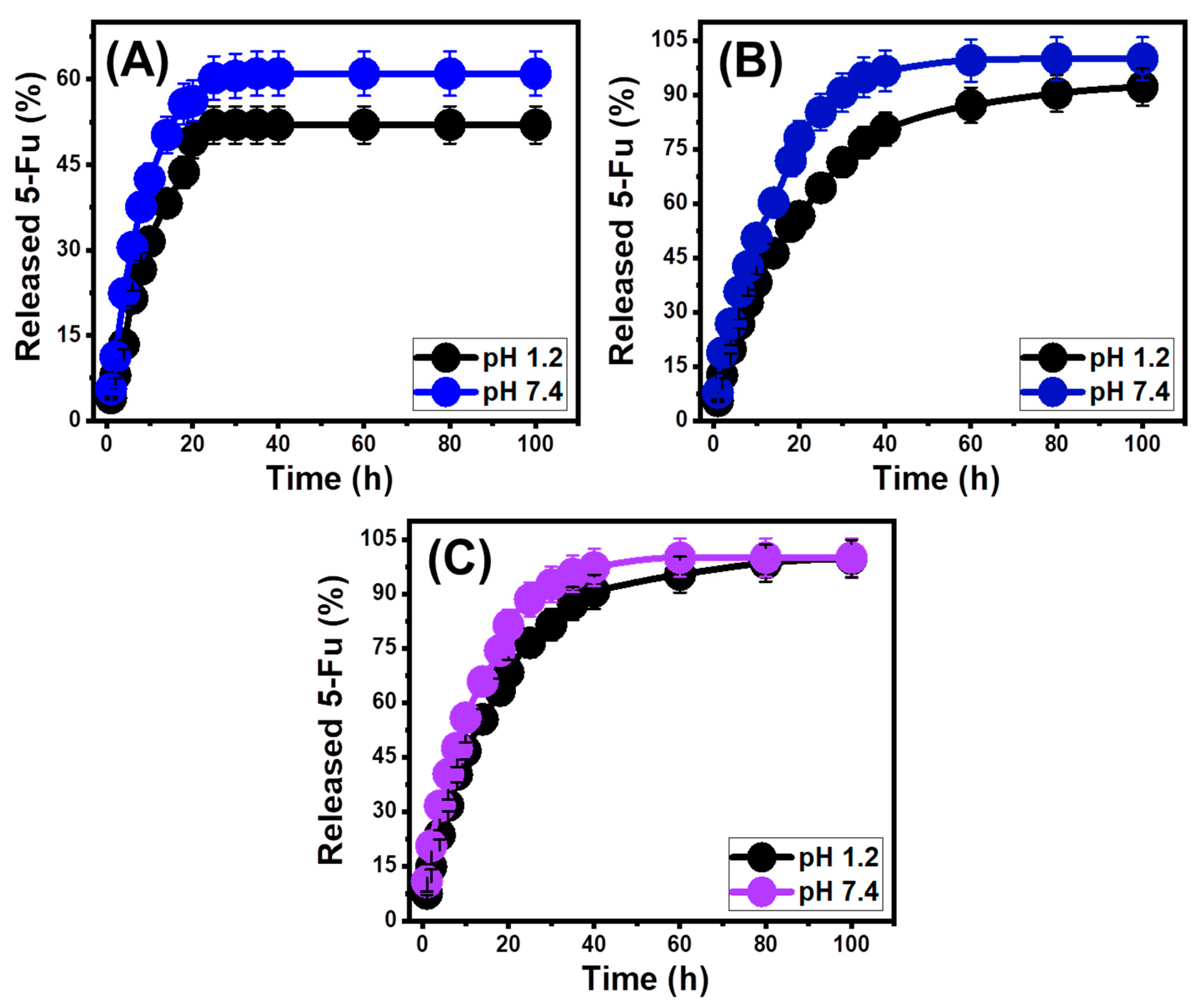
3.3. In Vitro Release Profiles
3.4. Release Kinetic Studies
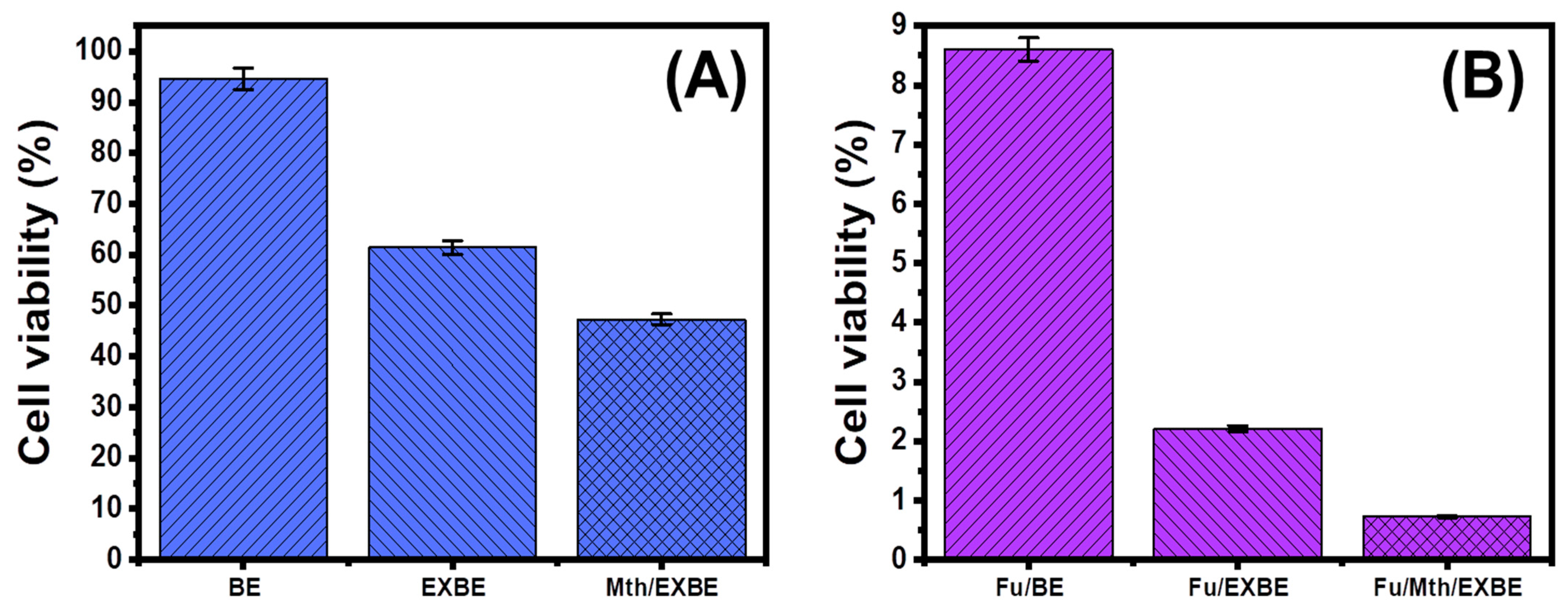
3.5. Cytotoxicity Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- El-Zeiny, H.M.; Abukhadra, M.R.; Sayed, O.M.; Osman, A.H.; Ahmed, S.A. Insight into novel β-cyclodextrin-grafted poly (Nvinylcaprolactam) nanogel structures as advanced carriers for 5-fluorouracil: Equilibrium behavior and pharmacokinetic modeling. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124197. [Google Scholar] [CrossRef]
- Cutrim, E.S.; Vale, A.A.; Manzani, D.; Barud, H.S.; Castellon, E.R.; Santos, A.P.; Alcântara, A.C. Preparation, characterization and in vitro anticancer performance of nanoconjugate based on carbon quantum dots and 5-Fluorouracil. Mater. Sci. Eng. C 2021, 120, 111781. [Google Scholar] [CrossRef] [PubMed]
- Abuzar, S.M.; Park, E.J.; Seo, Y.; Lee, J.; Baik, S.H.; Hwang, S.J. Preparation and evaluation of intraperitoneal long-acting oxaliplatin-loaded multi-vesicular liposomal depot for colorectal cancer treatment. Pharmaceutics 2020, 12, 736. [Google Scholar] [CrossRef]
- Othman, S.I.; Allam, A.A.; AlFassam, H.; Abu-Taweel, G.M.; Altoom, N.; Abukhadra, M.R. Sonoco green decoration of clinoptilolite with MgO nanoparticles as a potential carrier for 5-fluorouracil drug: Loading behavior, release profile, and cytotoxicity. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4608–4622. [Google Scholar] [CrossRef]
- Tian, L.; Abukhadra, M.R.; Mohamed, A.S.; Nadeem, A.; Ahmad, S.F.; Ibrahim, K.E. Insight into the loading and release properties of an exfoliated kaolinite/cellulose fiber (EXK/CF) composite as a carrier for oxaliplatin drug: Cytotoxicity and release kinetics. ACS Omega 2020, 5, 19165–19173. [Google Scholar] [CrossRef]
- Sundaramoorthy, P.; Ramasamy, T.; Mishra, S.K.; Jeong, K.Y.; Yong, C.S.; Kim, J.O.; Kim, H.M. Engineering of caveolae-specific self-micellizing anticancer lipid nanoparticles to enhance the chemotherapeutic efficacy of oxaliplatin in colorectal cancer cells. Acta Biomater. 2016, 42, 220–231. [Google Scholar] [CrossRef]
- Praphakar, R.A.; Jeyaraj, M.; Mehnath, S.; Higuchi, A.; Ponnamma, D.; Sadasivuni, K.K.; Rajan, M. A pH-sensitive guar gum-grafted-lysine-β-cyclodextrin drug carrier for the controlled release of 5-flourouracil into cancer cells. J. Mater. Chem. 2018, 6, 1519–1530. [Google Scholar] [CrossRef]
- Lee, J.E.; Abuzar, S.M.; Seo, Y.; Han, H.; Jeon, Y.; Park, E.J.; Baik, S.H.; Hwang, S.J. Oxaliplatin-loaded chemically cross-linked hydrogels for prevention of postoperative abdominal adhesion and colorectal cancer therapy. Int. J. Pharm. 2019, 565, 50–58. [Google Scholar] [CrossRef]
- Itoo, A.M.; Paul, M.; Ghosh, B.; Biswas, S. Oxaliplatin delivery via chitosan/vitamin E conjugate micelles for improved efficacy and MDR-reversal in breast cancer. Carbohydr. Polym. 2022, 282, 119108. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Cui, Y.; Zhang, H.; Zhang, S.; Wang, X.; Liu, S.; Gao, Q. Oxaliplatin derived monofunctional triazole-containing platinum (II) complex counteracts oxaliplatin-induced drug resistance in colorectal cancer. Bioorg. Chem. 2021, 107, 104636. [Google Scholar] [CrossRef]
- Sayed, M.A.; El-Zeiny, H.M.; Khim, J.S.; Ajarem, J.S.; Allam, A.A.; Abukhadra, M.R. Insight into the loading properties of Na+ green functionalized clinoptilolite as a potential carrier for the 5-fluorouracil drug, its release kinetics, and cytotoxicity. ACS Omega 2022, 7, 6991–7001. [Google Scholar] [CrossRef]
- Tan, D.; Yuan, P.; Dong, F.; He, H.; Sun, S.; Liu, Z. Selective loading of 5-fluorouracil in the interlayer space of methoxy-modified kaolinite for controlled release. Appl. Clay Sci. 2018, 159, 102–106. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Refay, N.M.; El-Sherbeeny, A.M.; Mostafa, A.M.; Elmeligy, M.A. Facile synthesis of bentonite/biopolymer composites as low-cost carriers for 5-fluorouracil drug; equilibrium studies and pharmacokinetic behavior. Int. J. Biol. Macromol. 2019, 141, 721–731. [Google Scholar] [CrossRef]
- Çiftçi, H.; Arpa, M.D.; Gülaçar, I.M.; Özcan, L.; Ersoy, B. Development and evaluation of mesoporous montmorillonite/magnetite nanocomposites loaded with 5-Fluorouracil. Microporous Mesoporous Mater. 2020, 303, 110253. [Google Scholar] [CrossRef]
- Elsayed, E.W.; El-Ashmawy, A.A.; El-Bassyouni, G.T.; Mousa, S.M.; El-Manawaty, M.; Emara, L.H. Formulation and evaluation of alginate-gelatin hydrogel scaffolds loaded with zinc-doped hydroxyapatite and 5-fluorouracil. Int. J. Biol. Macromol. 2023, 237, 124147. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, M.; Shariatinia, Z. AlN and AlP doped graphene quantum dots as novel drug delivery systems for 5-fluorouracil drug: Theoretical studies. J. Fluor. Chem. 2018, 211, 81–93. [Google Scholar] [CrossRef]
- Shad, P.M.; Karizi, S.Z.; Javan, R.S.; Mirzaie, A.; Noorbazargan, H.; Akbarzadeh, I.; Rezaie, H. Folate conjugated hyaluronic acid coated alginate nanogels encapsulated oxaliplatin enhance antitumor and apoptosis efficacy on colorectal cancer cells (HT29 cell line). Toxicol. Vitr. 2020, 65, 104756. [Google Scholar] [CrossRef]
- Luo, H.; Ji, D.; Li, C.; Zhu, Y.; Xiong, G.; Wan, Y. Layered nanohydroxyapatite as a novel nanocarrier for controlled delivery of 5-fluorouracil. Int. J. Pharm. 2016, 513, 17–25. [Google Scholar] [CrossRef]
- Khang, M.K.; Zhou, J.; Co, C.M.; Li, S.; Tang, L. A pretargeting nanoplatform for imaging and enhancing anti-inflammatory drug delivery. Bioact. Mater. 2020, 5, 1102–1112. [Google Scholar] [CrossRef]
- Rahbar, M.; Morsali, A.; Bozorgmehr, M.R.; Beyramabadi, S.A. Quantum chemical studies of chitosan nanoparticles as effective drug delivery systems for 5-fluorouracil anticancer drug. J. Mol. Liq. 2020, 302, 112495. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Refay, N.M.; El-Sherbeeny, A.M.; El-Meligy, M.A. Insight into the loading and release properties of MCM-48/biopolymer composites as carriers for 5-fluorouracil: Equilibrium modeling and pharmacokinetic studies. ACS Omega 2020, 5, 11745–11755. [Google Scholar] [CrossRef] [PubMed]
- Abukhadra, M.R.; Saad, I.; Othman, S.I.; Allam, A.A.; Fathallah, W. Synthesis of Co3O4@Organo/Polymeric Bentonite Structures as Environmental Photocatalysts and Antibacterial Agents for Enhanced Removal of Methyl Parathion and Pathogenic Bacteria. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2600–2614. [Google Scholar] [CrossRef]
- Dardir, F.M.; Mohamed, A.S.; Abukhadra, M.R.; Ahmed, E.A.; Soliman, M.F. Cosmetic and pharmaceutical qualifications of Egyptian bentonite and its suitability as drug carrier for Praziquantel drug. Eur. J. Pharm. Sci. 2018, 115, 320–329. [Google Scholar] [CrossRef]
- Momina, M.; Shahadat, M.; Ismail, S. Study of the adsorption/desorption of MB dye solution using bentonite adsorbent coating. J. Water Process Eng. 2020, 34, 101155. [Google Scholar] [CrossRef]
- Ain, Q.U.; Rasheed, U.; Yaseen, M.; Zhang, H.; He, R.; Tong, Z. Fabrication of magnetically separable 3-acrylamidopropyltrimethylammonium chloride intercalated bentonite composite for the efficient adsorption of cationic and anionic dyes. Appl. Surf. Sci. 2020, 514, 145929. [Google Scholar] [CrossRef]
- Almahri, A. The solid-state synthetic performance of bentonite stacked manganese ferrite nanoparticles: Adsorption and photo-fenton degradation of MB dye and antibacterial applications. J. Mater. Res. Technol. 2022, 17, 2935–2949. [Google Scholar] [CrossRef]
- Meng, B.; Guo, Q.; Men, X.; Ren, S.; Jin, W.; Shen, B. Modified bentonite by polyhedral oligomeric silsesquioxane and quaternary ammonium salt and adsorption characteristics for dye. J. Saudi Chem. Soc. 2020, 24, 334–344. [Google Scholar] [CrossRef]
- Gong, X.L.; Lu, H.Q.; Li, K.; Li, W. Effective adsorption of crystal violet dye on sugarcane bagasse–bentonite/sodium alginate composite aerogel: Characterisation, experiments, and advanced modelling. Sep. Purif. Technol. 2022, 286, 120478. [Google Scholar] [CrossRef]
- Ashiq, A.; Walpita, J.; Vithanage, M. Functionalizing non-smectic clay via methoxy-modification for enhanced removal and recovery of oxytetracycline from aqueous media. Chemosphere 2021, 276, 130079. [Google Scholar] [CrossRef]
- Li, X.; Cui, X.; Wang, S.; Wang, D.; Li, K.; Liu, Q.; Komarneni, S. Methoxy-grafted kaolinite preparation by intercalation of methanol: Mechanism of its structural variability. Appl. Clay Sci. 2017, 137, 241–248. [Google Scholar] [CrossRef]
- Tchoumene, R.; Dedzo, G.K.; Ngameni, E. Intercalation of 1, 2, 4-triazole in methanol modified-kaolinite: Application for copper corrosion inhibition in concentrated sodium chloride aqueous solution. J. Solid State Chem. 2022, 311, 123103. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Allah, A.F. Synthesis and characterization of kaolinite nanotubes (KNTs) as a novel carrier for 5-fluorouracil of high encapsulation properties and controlled release. Inorg. Chem. Commun. 2019, 103, 30–36. [Google Scholar] [CrossRef]
- Shawky, A.; El-Sheikh, S.M.; Rashed, M.N.; Abdo, S.M.; El-Dosoqy, T.I. Exfoliated kaolinite nanolayers as an alternative photocatalyst with superb activity. J. Environ. Chem. Eng. 2019, 7, 103174. [Google Scholar] [CrossRef]
- Salam, M.; Mokhtar, M.; Albukhari, S.M.; Baamer, D.F.; Palmisano, L.; Jaremko, M.; Abukhadra, M.R. Synthesis and Characterization of Green ZnO@polynaniline/Bentonite Tripartite Structure (G.Zn@PN/BE) as Adsorbent for As (V) Ions: Integration, Steric, and Energetic Properties. Polymers 2022, 14, 2329. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Abukhadra, M.R.; Refay, N.M.; Sharaf, M.F.; El-Meligy, M.A.; Awwad, E.M. Synthesis of chitosan/MCM-48 and β-cyclodextrin/MCM-48 composites as bio-adsorbents for environmental removal of Cd2+ ions; kinetic and equilibrium studies. React. Funct. Polym. 2020, 154, 104675. [Google Scholar] [CrossRef]
- Salam, M.A.; Abukhadra, M.R.; Mostafa, M. Effective decontamination of As (V), Hg (II), and U (VI) toxic ions from water using novel muscovite/zeolite aluminosilicate composite: Adsorption behavior and mechanism. Environ. Sci. Pollut. Res. 2020, 27, 13247–13260. [Google Scholar] [CrossRef]
- El Qada, E. Kinetic Behavior of the Adsorption of Malachite Green Using Jordanian Diatomite as Adsorbent. Jordanian J. Eng. Chem. Ind. (JJECI) 2020, 3, 1–10. [Google Scholar]
- Lin, X.; Xie, Y.; Lu, H.; Xin, Y.; Altaf, R.; Zhu, S.; Liu, D. Facile preparation of dual La-Zr modified magnetite adsorbents for efficient and selective phosphorus recovery. Chem. Eng. J. 2021, 413, 127530. [Google Scholar] [CrossRef]
- Albukhari, S.M.; Salam, M.A.; Abukhadra, M.R. Effective retention of inorganic Selenium ions (Se (VI) and Se (IV)) using novel sodalite structures from muscovite; characterization and mechanism. J. Taiwan Inst. Chem. Eng. 2021, 120, 116–126. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, X.; Guo, L.; Lan, J.; Zhang, L.; Cao, D. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018, 194, 462–469. [Google Scholar] [CrossRef]
- Sherlala, A.; Raman, A.; Bello, M.M.; Buthiyappan, A. Adsorption of arsenic using chitosan magnetic graphene oxide nanocomposite. J. Environ. Manag. 2019, 246, 547–556. [Google Scholar]
- Jasper, E.E.; Ajibola, V.O.; Onwuka, J.C. Nonlinear regression analysis of the sorption of crystal violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl. Water Sci. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Dawodu, F.; Akpomie, G.; Abuh, M. Equilibrium Isotherm Studies on the Batch Sorption of Copper (II) ions from Aqueous Solution unto Nsu Clay. Int. J. Sci. Eng. Res. 2012, 3, 1–7. [Google Scholar]
- Yang, X.; Wang, J.; El-Sherbeeny, A.M.; AlHammadi, A.A.; Park, W.H.; Abukhadra, M.R. Insight into the adsorption and oxidation activity of a ZnO/piezoelectric quartz core-shell for enhanced decontamination of ibuprofen: Steric, energetic, and oxidation studies. Chem. Eng. J. 2022, 431, 134312. [Google Scholar] [CrossRef]
- Sellaoui, L.; Guedidi, H.; Reinert, L.; Knani, S.; Duclaux, L.; Lamine, A.B. Experimental and theoretical studies of adsorption of ibuprofen on raw and two chemically modified activated carbons: New physicochemical interpretations. RSC Adv. 2016, 6, 12363–12373. [Google Scholar] [CrossRef]
- RAli, A.; Mobarak, M.; Badawy, A.M.; Lima, E.C.; Seliem, M.K.; Ramadan, H.S. New insights into the surface oxidation role in enhancing Congo red dye uptake by Egyptian ilmenite ore: Experiments and physicochemical interpretations. Surf. Interface 2021, 26, 101316. [Google Scholar]
- Ashraf, M.T.; AlHammadi, A.A.; El-Sherbeeny, A.M.; Alhammadi, S.; Al Zoubi, W.; Ko, Y.G.; Abukhadra, M.R. Synthesis of cellulose fibers/Zeolite-A nanocomposite as an environmental adsorbent for organic and inorganic selenium ions; Characterization and advanced equilibrium studies. J. Mol. Liq. 2022, 360, 119573. [Google Scholar] [CrossRef]
- AbouAitah, K.; Bil, M.; Pietrzykowska, E.; Szałaj, U.; Fudala, D.; Woźniak, B.; Nasiłowska, J.; Swiderska-Sroda, A.; Lojkowski, M.; Sokołowska, B.; et al. Drug-Releasing Antibacterial Coating Made from Nano-Hydroxyapatite Using the Sonocoating Method. Nanomaterials 2021, 11, 1690. [Google Scholar]
- Wang, J.; Cai, N.; Chan, V.; Zeng, H.; Shi, H.; Xue, Y.; Yu, F. Antimicrobial hydroxyapatite reinforced-polyelectrolyte complex nanofibers with long-term controlled release activity for potential wound dressing application. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126722. [Google Scholar] [CrossRef]
- Mostafa, M.; El-Meligy, M.A.; Sharaf, M.; Soliman, A.T.; AbuKhadra, M.R. Insight into chitosan/zeolite-A nanocomposite as an advanced carrier for levofloxacin and its anti-inflammatory properties; Loading, release, and anti-inflammatory studies. Int. J. Biol. Macromol. 2021, 179, 206–216. [Google Scholar]
- Elboraey, A.N.; Abo-Almaged, H.H.; El-Ashmawy, A.A.E.-R.; Abdou, A.R.; Moussa, A.R.; Emara, L.H.; El-Masry, H.M.; El Bassyouni, G.E.-T.; Ramzy, M.I. Biological and mechanical properties of denture base material as a vehicle for novel hydroxyapatite nanoparticles loaded with drug. Adv. Pharm. Bull. 2021, 11, 86–95. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Y.; Zhou, Y.; Guo, D.; Fan, Y.; Guo, F.; Zheng, Y.; Chen, W. Preparation of 5-fluorouracil-loaded chitosan nanoparticles and study of the sustained release in vitro and in vivo. Asian J. Pharm. Sci. 2017, 12, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.; Ahmed, K.; Rahim, A.; Muhammad, N.; Tariq, S.; Azhar, U.; Khan, A.J.; Sama, Z.; Volpe, P.L.; Airoldi, C. Organo-bridged silsesquioxane incorporated mesoporous silica as a carrier for the controlled delivery of ibuprofen and fluorouracil. J. Mol. Liq. 2018, 258, 319–326. [Google Scholar] [CrossRef]
- Ge, M.; Tang, W.; Du, M.; Liang, G.; Hu, G.; Alam, S.J. Research on 5-fluorouracil as a drug carrier materials with its in vitro release properties on organic modified magadiite. Eur. J. Pharm. Sci. 2019, 130, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.M.; Bin Jumah, M.N.; Othman, S.I.; Alruhaimi, R.S.; Al-Khalawi, N.; Salama, Y.F.; Allam, A.A.; Abukhadra, M.R. Synthesis of Chitosan/Diatomite Composite as an Advanced Delivery System for Ibuprofen Drug; Equilibrium Studies and the Release Profile. ACS Omega 2021, 6, 13406–13416. [Google Scholar] [CrossRef] [PubMed]
- El-Hamshary, H.; El-Newehy, M.H.; Abdulhameed, M.M.; ElFaham, A.; Elsherbiny, A.S. Evaluation of clay-ionene nanocomposite carriers for controlled drug delivery: Synthesis, in vitro drug release, and kinetics. Mater. Chem. Phys. 2019, 225, 122–132. [Google Scholar] [CrossRef]

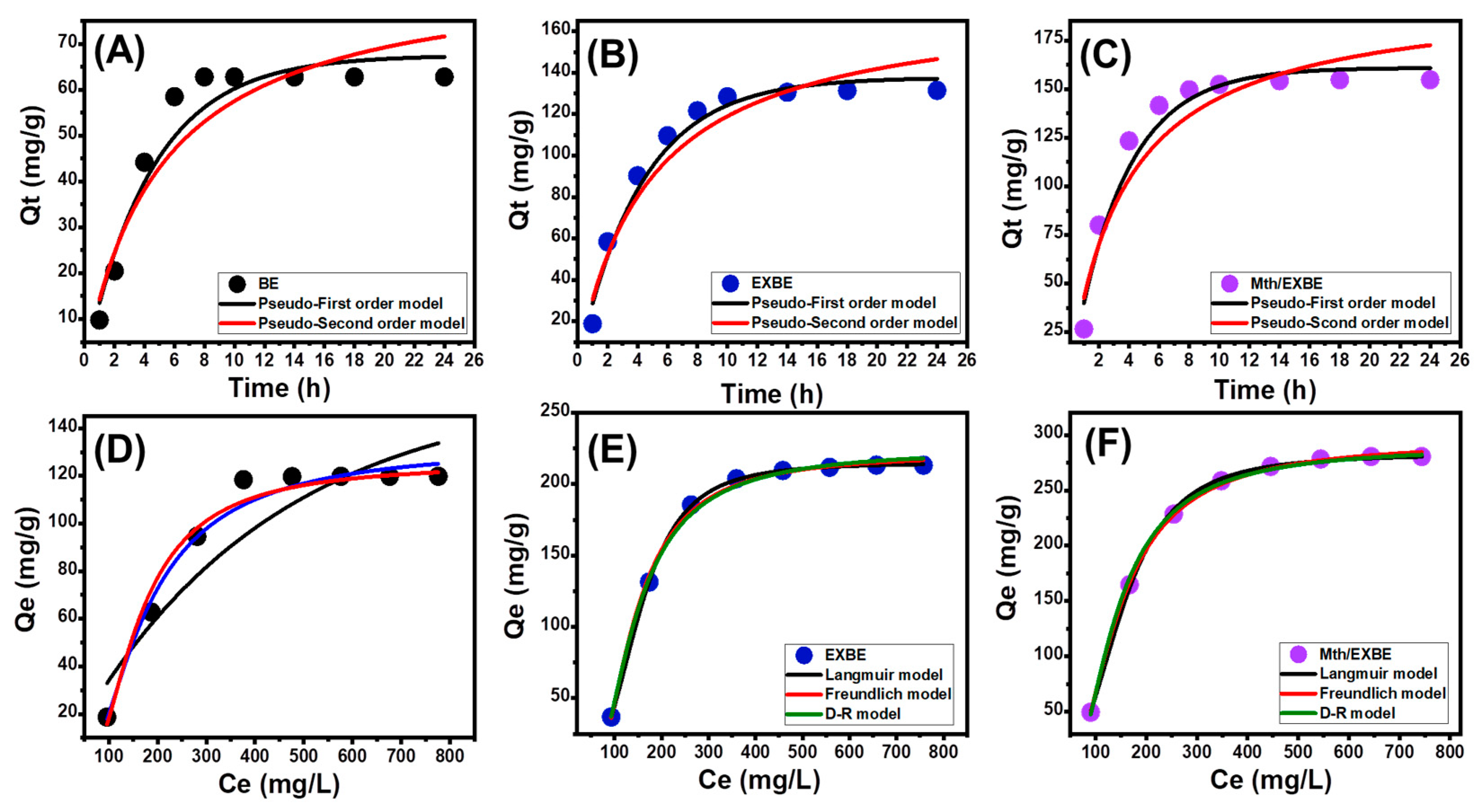

| Model | Parameters | BE | EXBE | Mth/EXBE | |||
|---|---|---|---|---|---|---|---|
| Kinetic models | |||||||
| Pseudo-first-order | K1 (min−1) | 0.223 ± 0.035 | 0.233 ± 0.031 | 0.285 ± 0.042 | |||
| Qe (Cal) (mg/g) | 67.5 ± 4.2 | 137.6 ± 7.19 | 160.9 ± 8.3 | ||||
| R2 | 0.95 | 0.97 | 0.96 | ||||
| X2 | 0.73 | 1.08 | 1.53 | ||||
| Pseudo-second-order | k2 (g mg−1 min−1) | 0.0022 ± 0.0001 | 0.0011 ± 0.0004 | 0.0013 ± 0.0005 | |||
| Qe (Cal) (mg/g) | 86.87 ± 6.8 | 175.7 ± 10.8 | 198.9 ± 12.87 | ||||
| R2 | 0.92 | 0.94 | 0.92 | ||||
| X2 | 1.35 | 2.04 | 2.92 | ||||
| Isotherm models | |||||||
| Langmuir | Qmax (mg/g) | 183.8 ± 4.65 | 215.1 ± 8.13 | 282.8 ± 10.52 | |||
| b (L/mg) | 2.8 × 10−3 ± 0.0011 | 8.35 × 10−8 ± 1.23 × 10−8 | 3.64 × 10−7 ± 1.11 × 10−8 | ||||
| R2 | 0.76 | 0.98 | 0.99 | ||||
| X2 | 5.47 | 0.12 | 0.076 | ||||
| Freundlich | 1/n | 0.702 ± 0.042 | 0.43 ± 0.051 | 0.48 ± 0.027 | |||
| kF (mg/g) | 1.215 ± 0.23 | 4.7 ± 1.16 | 5.34 ± 1.07 | ||||
| R2 | 0.89 | 0.98 | 0.99 | ||||
| X2 | 2.31 | 0.18 | 0.092 | ||||
| D-R model | β (mol2/kJ2) | 0.0042 ± 0.0011 | 0.0074 ± 0.0017 | 0.0081 ± 0.0022 | |||
| Qm (mg/g) | 125.5 ± 2.73 | 224.2 ± 4.49 | 289.7 ± 6.16 | ||||
| R2 | 0.98 | 0.99 | 0.99 | ||||
| X2 | 0.49 | 0.09 | 0.06 | ||||
| E (kJ/mol) | 10.2 ± 2.87 | 8.22 ± 1.64 | 7.85 ± 1.33 | ||||
| Monolayer model of one energy | n | 2.78 ± 0.196 | 3.24 ± 0.0195 | 2.95 ± 0.064 | |||
| Nm (mg/g) | 44.96 ± 3.97 | 66.2 ± 0.465 | 95.9 ± 2.51 | ||||
| Q(sat) (mg/g) | 124.9 ± 6.74 | 214.4 ± 6.39 | 282.6 ± 8.39 | ||||
| ∆E (kJ/mol) | 8.4 ± 1.82 | 9.2 ± 1.88 | 7.86 ± 1.17 | ||||
| Release kinetics | |||||||
| Models | Determination coefficient | ||||||
| BE | EXBE | Mth/EXBE | |||||
| Acetate buffer (pH 1.2) | Phosphate buffer (pH 7.4) | Acetate buffer (pH 1.2) | Phosphate buffer (pH 7.4) | Acetate buffer (pH 1.2) | Phosphate buffer (pH 7.4) | ||
| Zero-order | 0.39 | 0.36 | 0.73 | 0.59 | 0.67 | 0.56 | |
| First-order | 0.42 | 0.41 | 0.94 | 0.99 | 0.99 | 0.99 | |
| Higuchi | 0.60 | 0.56 | 0.91 | 0.83 | 0.88 | 0.81 | |
| Hixson–Crowell | 0.40 | 0.38 | 0.92 | 0.96 | 0.93 | 0.99 | |
| Korsmeyer–Peppas | 0.75 | 0.73 | 0.94 | 0.90 | 0.93 | 0.90 | |
| n | 0.62 | 0.55 | 0.59 | 0.54 | 0.55 | 0.48 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, M.D.; Bin Jumah, M.N.; Al-Hashimi, A.; Allam, A.A.; Abukhadra, M.R.; Bellucci, S. Synthesis and Characterization of Methoxy-Exfoliated Montmorillonite Nanosheets as Potential Carriers of 5-Fluorouracil Drug with Enhanced Loading, Release, and Cytotoxicity Properties. Molecules 2023, 28, 5895. https://doi.org/10.3390/molecules28155895
Alqahtani MD, Bin Jumah MN, Al-Hashimi A, Allam AA, Abukhadra MR, Bellucci S. Synthesis and Characterization of Methoxy-Exfoliated Montmorillonite Nanosheets as Potential Carriers of 5-Fluorouracil Drug with Enhanced Loading, Release, and Cytotoxicity Properties. Molecules. 2023; 28(15):5895. https://doi.org/10.3390/molecules28155895
Chicago/Turabian StyleAlqahtani, Mashael D., May N. Bin Jumah, Abdulrahman Al-Hashimi, Ahmed A. Allam, Mostafa R. Abukhadra, and Stefano Bellucci. 2023. "Synthesis and Characterization of Methoxy-Exfoliated Montmorillonite Nanosheets as Potential Carriers of 5-Fluorouracil Drug with Enhanced Loading, Release, and Cytotoxicity Properties" Molecules 28, no. 15: 5895. https://doi.org/10.3390/molecules28155895
APA StyleAlqahtani, M. D., Bin Jumah, M. N., Al-Hashimi, A., Allam, A. A., Abukhadra, M. R., & Bellucci, S. (2023). Synthesis and Characterization of Methoxy-Exfoliated Montmorillonite Nanosheets as Potential Carriers of 5-Fluorouracil Drug with Enhanced Loading, Release, and Cytotoxicity Properties. Molecules, 28(15), 5895. https://doi.org/10.3390/molecules28155895










