The Structural Characterisation and DFT-Aided Interpretation of Vibrational Spectra for Cyclo(l-Cys-d-Cys) Cyclic Dipeptide in a Solid State
Abstract
:1. Introduction
2. Results and Discussion
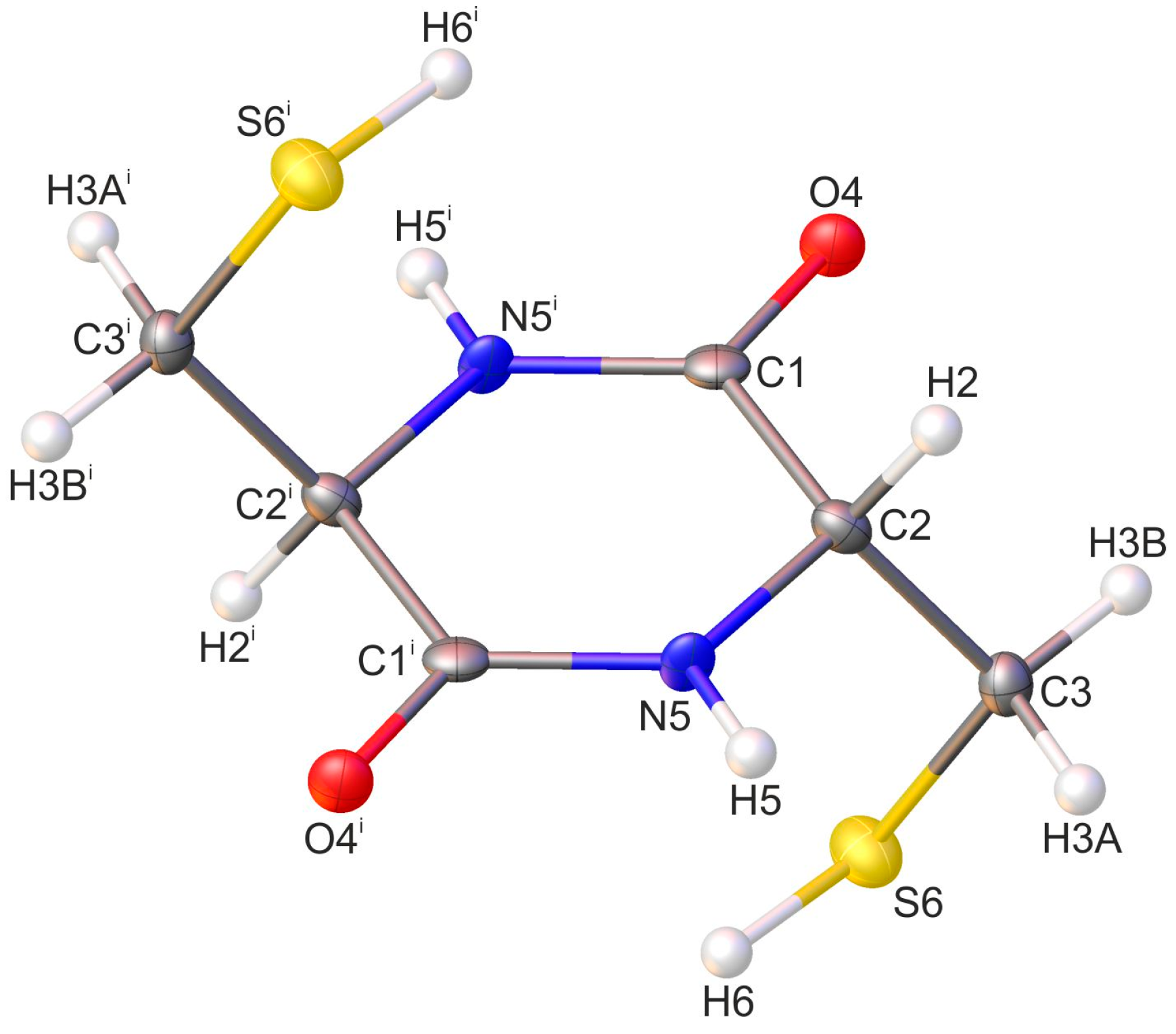
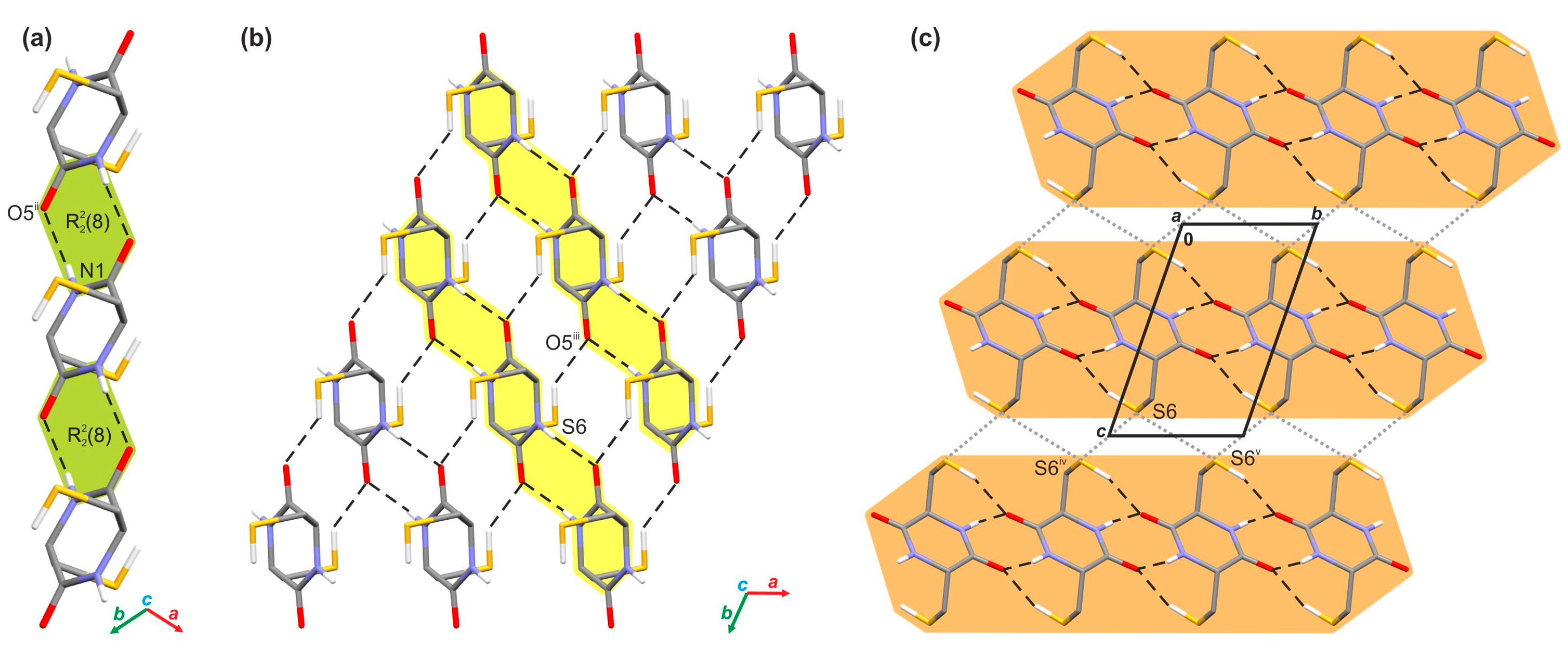
2.1. Crystallographic Analysis
2.2. DFT Studies of the Cyclo(l-Cys-d-Cys) Molecule
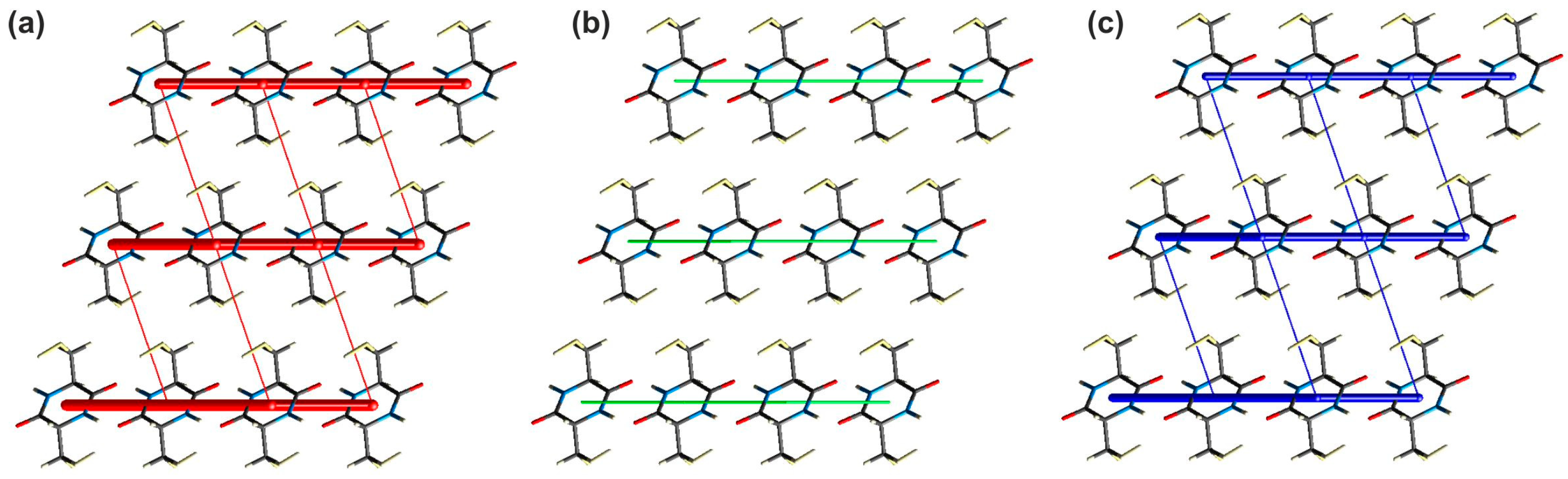
2.2.1. Investigation of the Interaction Energy in the Crystal
2.2.2. Optimising the Model of a Single Cyclo(l-Cys-d-Cys) Molecule
2.2.3. Structure of the Optimised Model
2.2.4. Conformational Analysis
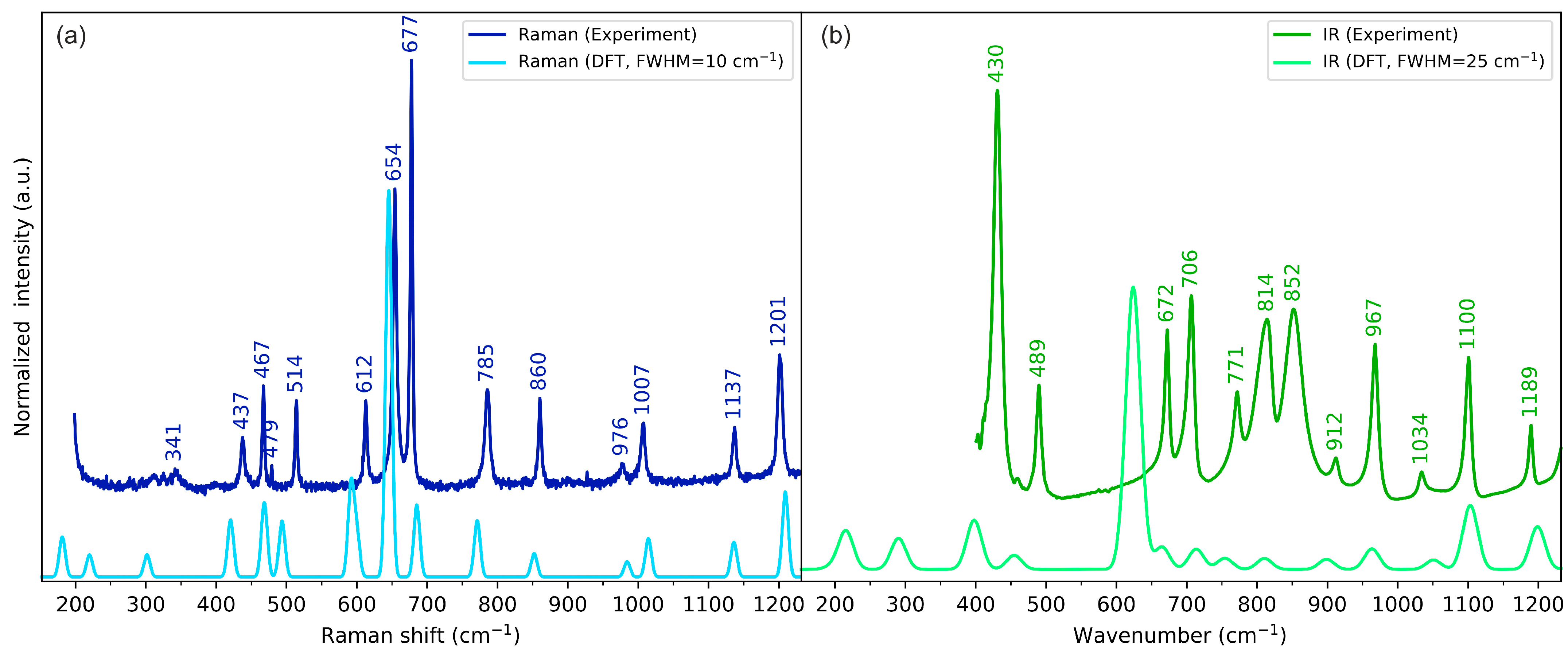
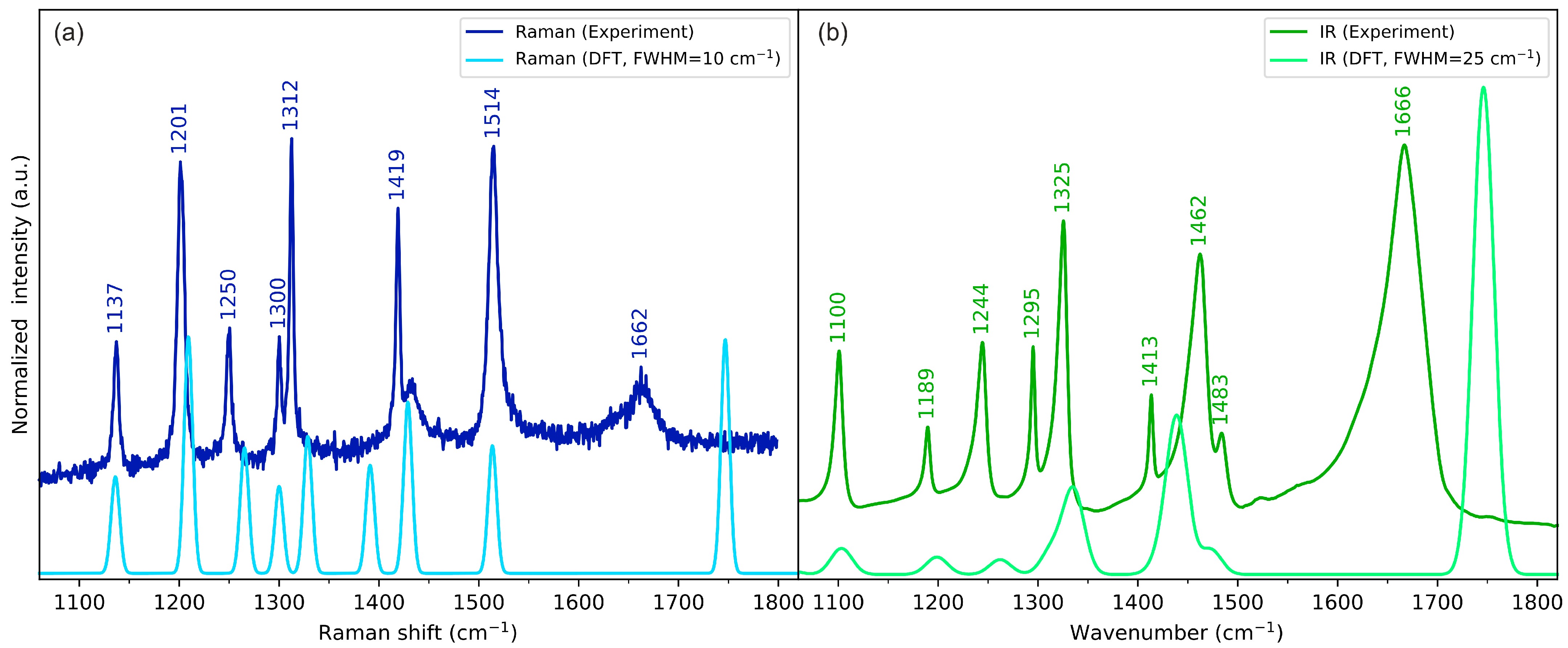
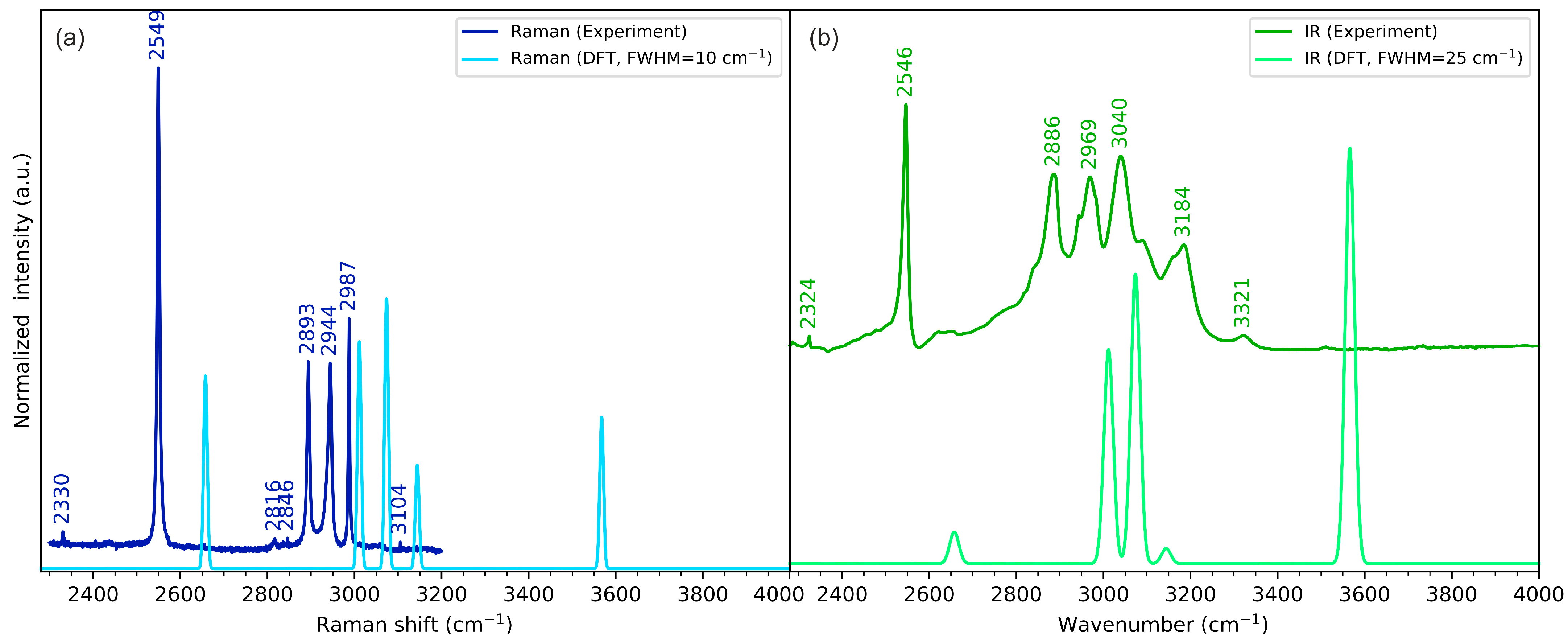
2.3. Vibrational Spectroscopy
2.3.1. N-H Stretching Vibrations
2.3.2. C=O Stretching Vibrations
2.3.3. S-H Stretching Vibrations
2.3.4. Skeletal Vibrations
3. Experimental Procedure
3.1. Materials and Methods
3.2. Raman Spectroscopy
3.3. IR Spectroscopy
3.4. Single-Crystal Growth
3.5. X-ray Diffraction Data Collection and Structural Refinement
3.6. Theoretical Calculations of Pairwise Intermolecular Interaction Energies
3.7. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, G.-Z.; Liu, J.; Wang, J.-M.; Fang, L.; Yu, S.-S. Secondary metabolites from the endophytic fungi Penicillium polonicum and Aspergillus fumigatus. J. Asian Nat. Prod. Res. 2013, 15, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C. Cyclo(His-Pro): Its distribution, origin and function in the human. Neurosci. Biobehav. Rev. 1988, 12, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Lautru, S.; Gondry, M.; Genet, R.; Pernodet, J.-L. The Albonoursin Gene Cluster of S. noursei: Biosynthesis of Diketopiperazine Metabolites Independent of Nonribosomal Peptide Synthetases. Chem. Biol. 2002, 9, 1355–1364. [Google Scholar] [CrossRef] [Green Version]
- Prasad, C.; Matsui, T.; Peterkofsky, A. Antagonism of ethanol narcosis by histidyl-proline diketopiperazine. Nature 1977, 268, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Conza, M.; Wennemers, H. Selective binding of two-armed diketopiperazine receptors to side-chain-protected peptides. J. Org. Chem. 2002, 67, 2696–2698. [Google Scholar] [CrossRef]
- Borthwick, A.D.; Liddle, J.; Davies, D.E.; Exall, A.M.; Hamlett, C.; Hickey, D.M.; Mason, A.M.; Smith, I.E.D.; Nerozzi, F.; Peace, S.; et al. Pyridyl-2,5-diketopiperazines as potent, selective, and orally bioavailable oxytocin antagonists: Synthesis, pharmacokinetics, and in vivo potency. J. Med. Chem. 2012, 55, 783–796. [Google Scholar] [CrossRef]
- Deigin, V.; Ksenofontova, O.; Yatskin, O.; Goryacheva, A.; Ignatova, A.; Feofanov, A.; Ivanov, V. Novel platform for the preparation of synthetic orally active peptidomimetics with hemoregulating activity. II. Hemosuppressor activity of 2,5-diketopiperazine-based cyclopeptides. Int. Immunopharmacol. 2020, 81, 106185. [Google Scholar] [CrossRef]
- Bojarska, J.; Mieczkowski, A.; Ziora, Z.M.; Skwarczynski, M.; Toth, I.; Shalash, A.O.; Parang, K.; El-Mowafi, S.A.; Mohammed, E.H.M.; Elnagdy, S.; et al. Cyclic Dipeptides: The Biological and Structural Landscape with Special Focus on the Anti-Cancer Proline-Based Scaffold. Biomolecules 2021, 11, 1515. [Google Scholar] [CrossRef]
- Fdhila, F.; Vázquez, V.; Sánchez, J.L.; Riguera, R. DD-diketopiperazines: Antibiotics active against Vibrio anguillarum isolated from marine bacteria associated with cultures of Pecten maximus. J. Nat. Prod. 2003, 66, 1299–1301. [Google Scholar] [CrossRef]
- Minelli, A.; Bellezza, I.; Grottelli, S.; Pinnen, F.; Brunetti, L.; Vacca, M. Phosphoproteomic analysis of the effect of cyclo-His-Pro dipeptide on PC12 cells. Peptides 2006, 27, 105–113. [Google Scholar] [CrossRef]
- Oku, J.-i.; Inoue, S. Asymmetric cyanohydrin synthesis catalysed by a synthetic cyclic dipeptide. J. Chem. Soc. Chem. Commun. 1981, 5, 229–230. [Google Scholar] [CrossRef]
- You, Y.; Xing, R.; Zou, Q.; Shi, F.; Yan, X. High-tolerance crystalline hydrogels formed from self-assembling cyclic dipeptide. Beilstein J. Nanotechnol. 2019, 10, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Akerlund, J.; Harmeier, S.; Pumphrey, J.; Timm, D.C.; Brand, J.I. Diketopiperazine-based polymers from common amino acids. J. Appl. Polym. Sci. 2000, 78, 2213–2218. [Google Scholar] [CrossRef]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef]
- Bellezza, I.; Peirce, M.J.; Minelli, A. Cyclic dipeptides: From bugs to brain. Trends Mol. Med. 2014, 20, 551–558. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhang, X.; Lai, D.; Zhou, L. Structural Diversity and Biological Activities of the Cyclodipeptides from Fungi. Molecules 2017, 22, 2026. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Xing, R.; Yan, X. Cyclic dipeptides: Biological activities and self-assembled materials. Pept. Sci. 2021, 113, 1713. [Google Scholar] [CrossRef]
- Martins, M.B.; Carvalho, I. Diketopiperazines: Biological activity and synthesis. Tetrahedron 2007, 63, 9923–9932. [Google Scholar] [CrossRef]
- Degeilh, R.; Marsh, R.E. A refinement of the crystal structure of diketopiperazine (2,5-piperazinedione). Acta Cryst. 1959, 12, 1007–1014. [Google Scholar] [CrossRef]
- Sletten, E. Conformation of cyclic dipeptides. The crystal and molecular structures of cyclo-d-alanyl-l-alanyl and cyclo-l-alanyl-l-alanyl (3,6-dimethylpiperazine-2,5-dione). J. Am. Chem. Soc. 1970, 92, 172–177. [Google Scholar] [CrossRef]
- Milne, P.J.; Oliver, D.W.; Roos, H.M. Cyclodipeptides: Structure and conformation of cyclo(tyrosyl-prolyl). J. Cryst. Spectr. Res. 1992, 22, 643–649. [Google Scholar] [CrossRef]
- BenNasr, F.; Pérez-Mellor, A.; Alata, I.; Lepere, V.; Jaïdane, N.-E.; Zehnacker, A. Stereochemistry-dependent hydrogen bonds stabilise stacked conformations in jet-cooled cyclic dipeptides: (LD) vs. (LL) cyclo tyrosine-tyrosine. Faraday Discuss. 2018, 212, 399–419. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.C.; Whitesides, G.M. Solid-State Structures of Hydrogen-Bonded Tapes Based on Cyclic Secondary Diamides. Chem. Rev. 1994, 94, 2383–2420. [Google Scholar] [CrossRef]
- Benedetti, E.; Bavoso, A.; Blasio, B.; Pavone, V.; Pedone, C.; Paolillo, L.; D’Alagni, M. Structural studies of cyclopeptides: Solid state and solution conformation of cyclo(l-histidyl-d-histidyl). Int. J. Pept. Protein Res. 1988, 31, 220–224. [Google Scholar] [CrossRef]
- Benedetti, E.; Corradini, P.; Pedone, C. Crystal and molecular structure of trans-3,6-dimethyl-2,5-piperazinedione (l-alanyl-d-alanine 2,5-diketopiperazine). J. Phys. Chem. 1969, 73, 2891–2895. [Google Scholar] [CrossRef]
- Avinash, M.B.; Raut, D.; Mishra, M.K.; Ramamurty, U.; Govindaraju, T. Bioinspired Reductionistic Peptide Engineering for Exceptional Mechanical Properties. Sci. Rep. 2015, 5, 16070. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, K.; Tanihara, M.; Imanishi, Y. Catalytic Hydrolysis of Charged Carboxylic Acid Active Esters with Cyclic Dipeptides Carrying Hydrophobic and Nucleophilic Groups. Polym. J. 1983, 15, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Gahan, L.R.; Cusack, R.M. Metal complexes of synthetic cyclic peptides. Polyhedron 2018, 153, 1–23. [Google Scholar] [CrossRef]
- Pandurangan, K.; Roy, B.; Rajasekhar, K.; Suseela, Y.V.; Nagendra, P.; Chaturvedi, A.; Satwik, U.R.; Murugan, N.A.; Ramamurty, U.; Govindaraju, T. Molecular Architectonics of Cyclic Dipeptide Amphiphiles and Their Application in Drug Delivery. ACS Appl. Bio Mater. 2020, 3, 3413–3422. [Google Scholar] [CrossRef]
- Feldstein, R.; Glass, J.; Steiner, S.S. Self Assembling Diketopiperazine Drug Delivery System. U.S. Patent US5352461A, 11 May 1992. [Google Scholar]
- Chibh, S.; Mishra, J.; Kour, A.; Chauhan, V.S.; Panda, J.J. Recent advances in the fabrication and bio-medical applications of self-assembled dipeptide nanostructures. Nanomedicine 2021, 16, 139–163. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lowe, A.B.; Bowman, C.N. Thiol-click chemistry: A multifaceted toolbox for small molecule and polymer synthesis. Chem. Soc. Rev. 2010, 39, 1355–1387. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Lu, Y.; Hou, M.; Xu, Z.; Li, B. A thiol-responsive and self-immolative podophyllotoxin prodrug for cancer therapy. Tetrahedron Lett. 2021, 71, 153044. [Google Scholar] [CrossRef]
- Nolan, M.D.; Scanlan, E.M. Applications of Thiol-Ene Chemistry for Peptide Science. Front. Chem. 2020, 8, 583272. [Google Scholar] [CrossRef] [PubMed]
- Vanslambrouck, S.; Riva, R.; Ucakar, B.; Préat, V.; Gagliardi, M.; Molin, D.G.M.; Lecomte, P.; Jérôme, C. Thiol-ene Reaction: An Efficient Tool to Design Lipophilic Polyphosphoesters for Drug Delivery Systems. Molecules 2021, 26, 1750. [Google Scholar] [CrossRef]
- Pace, N.J.; Weerapana, E. Zinc-binding cysteines: Diverse functions and structural motifs. Biomolecules 2014, 4, 419–434. [Google Scholar] [CrossRef] [Green Version]
- Li, N.C.; Manning, R.A. Some Metal Complexes of Sulfur-containing Amino Acids. J. Am. Chem. Soc. 1955, 77, 5225–5228. [Google Scholar] [CrossRef]
- Berthon, G. Critical evaluation of the stability constants of metal complexes of amino acids with polar side chains (Technical Report). Pure Appl. Chem. 1995, 67, 1117–1240. [Google Scholar] [CrossRef]
- Greenstein, J.P. Studies Of Multivalent Amino Acids and Peptides: IX. The Synthesis Of l-Cystinyl-I-Cystine. J. Biol. Chem. 1937, 121, 9–17. [Google Scholar] [CrossRef]
- Sakakibara, S.; Tani, H. Synthesis of Polycysteine. Bull. Chem. Soc. Japn. 1956, 29, 85–88. [Google Scholar] [CrossRef]
- Kamber, B. Cystinpeptide aus (S-Acetamidomethyl-cystein)-peptiden durch Oxydation mit Jod: Die Synthese von cyclo-l-Cystin. Helv. Chim. Acta 1971, 54, 927–930. [Google Scholar] [CrossRef]
- Weber, U.; Hartter, P. Synthese und Stabilität von cyclischen Disulfiden des Typs. Hoppe-Seyler Z. Physiol. Chem. 1974, 355, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Pastuszak, J.J.; Chimiak, A. tert-Butyl group as thiol protection in peptide synthesis. J. Org. Chem. 1981, 46, 1868–1873. [Google Scholar] [CrossRef]
- Iannotta, D.; Castellucci, N.; Monari, M.; Tomasini, C. A straightforward synthesis and partial hydrolysis of cysteine-derived 2,5-diketopiperazines. Tetrahedron Lett. 2010, 51, 4558–4559. [Google Scholar] [CrossRef]
- Mez, H.-C. Cyclo-L-cystine acetic acid. Cryst. Struct. Comm. 1974, 3, 657. [Google Scholar]
- Varughese, K.I.; Lu, C.T.; Kartha, G. Crystal structure and conformation of cyclo-L-cystine. Int. J. Pept. Protein Res. 1981, 18, 88–102. [Google Scholar] [CrossRef]
- Lu, C.T.; Kartha, G. The crystal and molecular structure of cyclo-L-cystine. In Proceedings of the Program and Abstracts Summer Meeting, Storrs, CT, USA, 17–22 June 1973; Yannoni, N.F., Frueh, A.J., Eds.; American Crystallographic Association: Buffalo, NY, USA, 1973; p. 188. [Google Scholar]
- van Wart, H.E.; Scheraga, H.A. Raman spectra of cystine-related disulfides. Effect of rotational isomerism about carbon-sulfur bonds on sulfur-sulfur stretching frequencies. J. Phys. Chem. 1976, 80, 1812–1823. [Google Scholar] [CrossRef]
- Schwyzer, R. Hormone-receptor interactions. Synthesis and conformational study of cyclo-L-cystathionine. Helv. Chim. Acta 1976, 59, 2187–2195. [Google Scholar] [CrossRef]
- Donzel, B.; Kamber, B.; Wüthrich, K.; Schwyzer, R. A Chiral Cystine Disulfide Group without Inherent Optical Activity in the Long-Wavelength Region. (1 H- and 13 C-NMR., UV., CD., and ORD. Studies with cyclo—L-cystine). Helv. Chim. Acta 1972, 55, 947–961. [Google Scholar] [CrossRef]
- Jung, G.; Ottnad, M. Temperature-induced change in helicity of the inherently dissymmetrical disulfide group in cyclocystine. Angew. Chem. Int. Ed. Engl. 1974, 13, 818–819. [Google Scholar] [CrossRef]
- Strickland, R.W.; Richardson, F.S. Chiroptical properties of cyclo-L-cystine. J. Chem. Soc. Perkin Trans. 2 1976, 15, 1818–1821. [Google Scholar] [CrossRef]
- Mitra, A.K.; Chandrasekaran, R. Conformational analysis of cyclo (L-cystine). Int. J. Pept. Protein Res. 1977, 10, 235–239. [Google Scholar] [CrossRef]
- Gockel, P.; Vogler, R.; Gelinsky, M.; Meißner, A.; Albrich, H.; Vahrenkamp, H. Zinc complexation of cyclic dipeptides containing cysteine and/or histidine. Inorg. Chim. Acta 2001, 323, 16–22. [Google Scholar] [CrossRef]
- Cook, J.M.; Baker, D.A.; Yin, W.; Johnson, E.M. Cysteine and Cystine Prodrugs to Treat Schizophrenia and Reduce Drug Cravings. U.S. Patent US8173809B2, 13 August 2009. [Google Scholar]
- Mendham, A.P.; Dines, T.J.; Snowden, M.J.; Withnall, R.; Chowdhry, B.Z. IR/Raman spectroscopy and DFT calculations of cyclic di-amino acid peptides. Part III: Comparison of solid state and solution structures of cyclo(L-Ser-L-Ser). J. Raman Spectrosc. 2009, 40, 1508–1520. [Google Scholar] [CrossRef]
- Mendham, A.P.; Dines, T.J.; Snowden, M.J.; Chowdhry, B.Z.; Withnall, R. Vibrational spectroscopy and DFT calculations of di-amino acid cyclic peptides. Part I: Cyclo(Gly-Gly), cyclo(L-Ala-L-Ala) and cyclo(L-Ala-Gly) in the solid state and in aqueous solution. J. Raman Spectrosc. 2009, 40, 1478–1497. [Google Scholar] [CrossRef]
- Mendham, A.P.; Dines, T.J.; Withnall, R.; Mitchell, J.C.; Chowdhry, B.Z. Vibrational spectroscopic studies of the structure of di-amino acid peptides. Part II: Cyclo(L-Asp-L-Asp) in the solid state and in aqueous solution. J. Raman Spectrosc. 2009, 40, 1498–1507. [Google Scholar] [CrossRef]
- Celik, S.; Ozel, A.E.; Akyuz, S.; Kecel, S.; Agaeva, G. Conformational preferences, experimental and theoretical vibrational spectra of cyclo(Gly–Val) dipeptide. J. Mol. Struct. 2011, 993, 341–348. [Google Scholar] [CrossRef]
- Celik, S.; Ozel, A.E.; Kecel, S.; Akyuz, S. Structural and IR and Raman spectral analysis of cyclo(His-Phe) dipeptide. Vib. Spectrosc. 2012, 61, 54–65. [Google Scholar] [CrossRef]
- Celik, S.; Ozel, A.E.; Akyuz, S. Comparative study of antitumor active cyclo(Gly-Leu) dipeptide: A computational and molecular modeling study. Vib. Spectrosc. 2016, 83, 57–69. [Google Scholar] [CrossRef]
- Celik, S.; Yilmaz, G.; Ozel, A.E.; Akyuz, S. Structural and spectral analysis of anticancer active cyclo(Ala-His) dipeptide. J. Biomol. Struct. Dyn. 2022, 40, 660–672. [Google Scholar] [CrossRef]
- Corey, R.B. The Crystal Structure of Diketopiperazine. J. Am. Chem. Soc. 1938, 60, 1598–1604. [Google Scholar] [CrossRef]
- Nonappa; Ahonen, K.; Lahtinen, M.; Kolehmainen, E. Cyclic dipeptides: Catalyst/promoter-free, rapid and environmentally benign cyclization of free amino acids. Green Chem. 2011, 13, 1203. [Google Scholar] [CrossRef]
- Corey, R.B. Interatomic Distances in Proteins and Related Substances. Chem. Rev. 1940, 26, 227–236. [Google Scholar] [CrossRef]
- Gasparri Fava, G.; Ferrari Belicchi, M.; Marchelli, R.; Dossena, A. Synthesis, crystal structure and conformation of the cyclic dipeptide cyclo (-L-seryl-L-seryl-). Acta Crystallogr. B Struct. Sci. 1981, 37, 625–629. [Google Scholar] [CrossRef]
- Mendham, A.P.; Spencer, J.; Chowdhry, B.Z.; Dines, T.J.; Mujahid, M.; Palmer, R.A.; Tizzard, G.J.; Coles, S.J. X-Ray Crystallographic Structure and Absolute Configuration of the Cyclic Di-amino Acid Peptide: Cyclo(l-HomoCySH-l-HomoCySH). J. Chem. Crystallogr. 2011, 41, 1328–1334. [Google Scholar] [CrossRef]
- Ramani, R.; Venkatesan, K.; Marsh, R.E. Crystal structure and conformation of the cyclic dipeptide cyclo-(L-histidyl-L-aspartyl) trihydrate. J. Am. Chem. Soc. 1978, 100, 949–953. [Google Scholar] [CrossRef]
- Bressan, M.; Ettore, R.; Marchiori, F.; Valle, G. Coordination chemistry of peptides: Part II. Crystal structure of cyclo-L-methionylglycine and studies of metal complexation. Int. J. Pept. Protein Res. 1982, 19, 402–407. [Google Scholar] [CrossRef]
- Cotrait, M.; Ptak, M. The crystal structure and conformation of the cyclic dipeptide cyclo (-L-seryl-L-histidyl-) monohydrate. Acta Crystallogr. B Struct. Sci. 1978, 34, 528–532. [Google Scholar] [CrossRef]
- Lin, C.-F.; Webb, L.E. Crystal structures and conformations of the cyclic dipeptides cyclo-(Glycyl-L-tyrosyl) and cyclo-(L-seryl-L-tyrosyl) Monohydrate. J. Am. Chem. Soc. 1973, 95, 6803–6811. [Google Scholar] [CrossRef]
- Sletten, J.; Grundnes, J.; Klæboe, P.; Tørneng, E.; Woldbæk, T.; Strand, T.G.; Sukhoverkhov, V.F. The Structure of a Second Crystallographic Modification of Cyclo-D-alanyl-L-alanyl. Acta Chem. Scand. 1980, 34a, 593–595. [Google Scholar] [CrossRef] [Green Version]
- Grant, G.D.; Hunt, A.L.; Milne, P.J.; Roos, H.M.; Joubert, J.A. The structure and conformation of the tryptophanyl diketopiperazines cyclo(Trp-Trp)·C2H6SO and cyclo(Trp-Pro). J. Chem. Crystallogr. 1999, 29, 435–447. [Google Scholar] [CrossRef]
- Görbitz, C.H. Crystal and Molecular Structure of cyclo-L-Aspartyl-L-alanyl (3,6-Dioxo-5-methyl-2-piperazineacetic acid). Acta Chem. Scand. 1987, 41b, 83–86. [Google Scholar] [CrossRef]
- Cotrait, M.; Ptak, M.; Busetta, B.; Heitz, A. Crystal structure and conformation of the cyclic dipeptide cyclo-(L-threonyl-L-histidyl) dihydrate. J. Am. Chem. Soc. 1976, 98, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, I.; Iwata, T.; Takahashi, N.; Ashida, T.; Tanihara, M. Cyclo- L -leucyl- L -histidyl monohydrate. Acta Crystallogr. B Struct. Sci. 1977, 33, 3902–3904. [Google Scholar] [CrossRef]
- Suguna, K.; Ramakumar, S.; Kopple, K.D. Structure of cyclo(-L-leucyl-L-tyrosyl-) monohydrate, C15H20N2O3·H2O. Acta Crystallogr. C 1984, 40, 2053–2056. [Google Scholar] [CrossRef]
- Budesinsky, M.; Cisarova, I.; Podlaha, J.; Borremans, F.; Martins, J.C.; Waroquier, M.; Pauwels, E. Structures of cyclic dipeptides: An X-ray and computational study of cis- and trans-cyclo(Pip-Phe), cyclo(Pro-Phe) and their N-methyl derivatives. Acta Crystallogr. B Struct. Sci. 2010, 66, 662–677. [Google Scholar] [CrossRef] [PubMed]
- Ramani, R.; Venkatesan, K.; Marsh, R.E.; Hu Kung, W.J. Crystal structure and conformation of the cyclic dipeptide cyclo-L-prolyl-D-phenylalanyl. Acta Crystallogr. B Struct. Sci. 1976, 32, 1051–1056. [Google Scholar] [CrossRef]
- Mendham, A.P.; Potter, B.S.; Palmer, R.A.; Dines, T.J.; Mitchell, J.C.; Withnall, R.; Chowdhry, B.Z. Vibrational spectra and crystal structure of the di-amino acid peptide cyclo(L-Met-L-Met): Comparison of experimental data and DFT calculations. J. Raman Spectrosc. 2010, 41, 148–159. [Google Scholar] [CrossRef]
- Mosetti, V.; Rosetti, B.; Pierri, G.; Bellotto, O.; Adorinni, S.; Bandiera, A.; Adami, G.; Tedesco, C.; Crosera, M.; Magnano, G.C.; et al. Cyclodipeptides: From Their Green Synthesis to Anti-Age Activity. Biomedicines 2022, 10, 2342. [Google Scholar] [CrossRef]
- Valle, G.; Guantieri, V.; Tamburro, A.M. On the molecular and crystal structure of cyclo(l-methionyl-l-methionyl). J. Mol. Struct. 1990, 220, 19–24. [Google Scholar] [CrossRef]
- Mendham, A.P.; Palmer, R.A.; Potter, B.S.; Dines, T.J.; Snowden, M.J.; Withnall, R.; Chowdhry, B.Z. Vibrational spectroscopy and crystal structure analysis of two polymorphs of the di-amino acid peptide cyclo(L-Glu-L-Glu). J. Raman Spectrosc. 2010, 41, 288–302. [Google Scholar] [CrossRef]
- Dreele, R.B. von. The crystal structure of cyclo- L -prolylglycyl: A refinement of high-angle diffraction data. Acta Crystallogr. B Struct. Sci. 1975, 31, 966–970. [Google Scholar] [CrossRef]
- Karle, I.L. Crystal structure and conformation of the cyclic dipeptide cyclo-L-prolyl-L-leucyl. J. Am. Chem. Soc. 1972, 94, 81–84. [Google Scholar] [CrossRef]
- Mazza, F.; Lucente, G.; Pinnen, F.; Zanotti, G. Cyclic dipeptides containing proline. Structure and conformation of cyclo-(-L-Phe-L-Pro-), C14H16N2O2. Acta Crystallogr. C 1984, 40, 1974–1976. [Google Scholar] [CrossRef] [Green Version]
- Palmer, R.A.; Potter, B.S.; Mendham, A.P.; Dines, T.J.; Chowdhry, B.Z. Refined Crystal Structure and Absolute Configuration of the Di-amino Acid Peptide Cyclo(l-Aspartyl-l-Aspartyl): Comparison with the DFT Calculated Structure. J. Chem. Crystallogr. 2010, 40, 608–615. [Google Scholar] [CrossRef]
- Tayhas, G.R.; Palmore; Luo, T.-J.M.; McBride-Wieser, M.T.; Picciotto, E.A.; Reynoso-Paz, C.M. Engineering Crystalline Architecture with Diketopiperazines: An Investigation of the Strength of Hydrogen-Bonded Tapes Based on the Cyclic Dipeptide of (S)-Aspartic Acid. Chem. Mater. 1999, 11, 3315–3328. [Google Scholar] [CrossRef]
- Benedetti, E.; Corradini, P.; Pedone, C. Crystal and molecular structure ofL-cis-3,6-dimethyl-2,5-piperazinedione (L-alanyl-L-alanyl-2,5-diketopiperazine). Biopolymers 1969, 7, 751–764. [Google Scholar] [CrossRef]
- Hong, Y.P.; Lee, S.-H.; Choi, J.-H.; Kashima, A.; Nakamura, G.; Suzuki, T. Crystal Structure and Spectroscopic Properties of Cyclic Dipeptide: A Racemic Mixture of cyclo(ᴅ-Prolyl-ʟ-Tyrosyl) and cyclo(ʟ-Prolyl-ᴅ-Tyrosyl). Bull. Kor. Chem. Soc. 2014, 35, 2299–2303. [Google Scholar] [CrossRef]
- Palacin, S.; Chin, D.N.; Simanek, E.E.; MacDonald, J.C.; Whitesides, G.M.; McBride, M.T.; Palmore, G.T.R. Hydrogen-Bonded Tapes Based on Symmetrically Substituted Diketopiperazines: A Robust Structural Motif for the Engineering of Molecular Solids. J. Am. Chem. Soc. 1997, 119, 11807–11816. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Cheam, T.C.; Krimm, S. Vibrational analysis of crystalline diketopiperazine—I. Raman and i.r. spectra. Spectrochim. Acta A 1984, 40, 481–501. [Google Scholar] [CrossRef] [Green Version]
- Cheam, T.C.; Krimm, S. Vibrational analysis of cyclo(d-Ala-l-Ala) in two crystalline forms. Effect of structure on peptide group and CH modes. Spectrochim. Acta A 1988, 44, 185–208. [Google Scholar] [CrossRef]
- Oxford Diffraction. CrysAlis CCD and CrysAlis RED; Oxford Diffraction Ltd.: Yarnton, UK, 2008. [Google Scholar]
- Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Crystallogr. A Found. Crystallogr. 1995, 51, 887–897. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer model energies and energy frameworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef] [Green Version]
- Gordon, M.S.; Binkley, J.S.; Pople, J.A.; Pietro, W.J.; Hehre, W.J. Self-consistent molecular-orbital methods. 22. Small split-valence basis sets for second-row elements. J. Am. Chem. Soc. 1982, 104, 2797–2803. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theoret. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Turner, M.J.; Thomas, S.P.; Shi, M.W.; Jayatilaka, D.; Spackman, M.A. Energy frameworks: Insights into interaction anisotropy and the mechanical properties of molecular crystals. Chem. Commun. 2015, 51, 3735–3738. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Pople, J.A.; Gill, P.M.W.; Johnson, B.G. Kohn—Sham density-functional theory within a finite basis set. Chem. Phys. Lett. 1992, 199, 557–560. [Google Scholar] [CrossRef]
- Su, T.-F.; Huang, R.; Su, Y.-Q.; Zhao, G.-Z.; Wu, D.-Y.; Wang, J.-A.; Gong, C.-R.; Xu, C.-L. Vibrational spectra of guaiacylglycerol-β-guaiacyl ether: Experiment and theory. Spectrochim. Acta A 2015, 139, 456–463. [Google Scholar] [CrossRef]
- Wrzosek, B.; Cukras, J.; Dobrowolski, M.A.; Bukowska, J. Real Chemical States of the 3-Sulfur Derivative of 1,2,4-Triazole in Different Conditions: Complex Experimental and Theoretical Studies. J. Phys. Chem. C 2017, 121, 9282–9295. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Wu, D.-Y.; Tian, Z.-Q. Theoretical Investigation on the Substituent Effect of Halogen Atoms at the C8 Position of Adenine: Relative Stability, Vibrational Frequencies, and Raman Spectra of Tautomers. J. Phys. Chem. A 2016, 120, 4049–4058. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef] [Green Version]
- Woon, D.E.; Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Papajak, E.; Zheng, J.; Xu, X.; Leverentz, H.R.; Truhlar, D.G. Perspectives on Basis Sets Beautiful: Seasonal Plantings of Diffuse Basis Functions. J. Chem. Theory Comput. 2011, 7, 3027–3034. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Avogadro: An Open-Source Molecular Builder and Visualization Tool. Version 1.2.0. Available online: http://avogadro.cc/ (accessed on 31 July 2023).
- Johnson, B.G.; Fisch, M.J. An implementation of analytic second derivatives of the gradient-corrected density functional energy. J. Chem. Phys. 1994, 100, 7429–7442. [Google Scholar] [CrossRef]
- Johnson, B.G.; Frisch, M.J. Analytic second derivatives of the gradient-corrected density functional energy. Effect of quadrature weight derivatives. Chem. Phys. Let. 1993, 216, 133–140. [Google Scholar] [CrossRef]
- Stratmann, R.E.; Burant, J.C.; Scuseria, G.E.; Frisch, M.J. Improving harmonic vibrational frequencies calculations in density functional theory. J. Chem. Phys. 1997, 106, 10175–10183. [Google Scholar] [CrossRef]
- Su, Y.-Q.; Liu, J.; Huang, R.; Yang, H.-T.; Li, M.-X.; Pang, R.; Zhang, M.; Yang, M.-H.; Su, H.-F.; Devasenathipathy, R.; et al. Plasmon-Mediated Photoelectrochemical Hot-Hole Oxidation Coupling Reactions of Adenine on Nanostructured Silver Electrodes. J. Phys. Chem. Lett. 2023, 14, 5163–5171. [Google Scholar] [CrossRef]
- Królikowska, A.; Cukras, J.; Witkowski, M.; Tymecka, D.; Hernik-Magoń, A.; Misicka, A.; Dzwolak, W. SERS and DFT Study of Noble-Metal-Anchored Cys-Trp/Trp-Cys Dipeptides: Influence of Main-Chain Direction and Terminal Modifications. J. Phys. Chem. C 2020, 124, 7097–7116. [Google Scholar] [CrossRef]
- Witkowski, M.; Królikowska, A.; Cukras, J.; Dzwolak, W. Hidden Dynamics of Noble-metal-bound Thiol Monolayers Revealed by SERS-monitored Entropy-driven Exchange of Cysteine Isotopologues. Appl. Surf. Sci. 2023, 623, 156985. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Jamróz, M.H. Vibrational Energy Distribution Analysis VEDA 4, Warsaw, 2004–2010. Available online: https://smmg.pl/software/veda (accessed on 20 November 2021).
- Jamróz, M.H.; Dobrowolski, J.C.; Brzozowski, R. Vibrational modes of 2,6-, 2,7-, and 2,3-diisopropylnaphthalene. A DFT study. J. Mol. Struct. 2006, 787, 172–183. [Google Scholar] [CrossRef]
- Suenaga, M. Facio 23.1.5. 2021. Available online: https://zzzfelis.sakura.ne.jp/ (accessed on 27 September 2021).
| Peptide | Ring Conformation (from the Reference in Parentheses) | Deviation from the Mean Plane (Å) |
|---|---|---|
| Cyclo(l-Cys-d-Cys) | Planar (this work) | 0.003 |
| Cyclo(Gly-Gly) | Planar [63] | 0.13 |
| Cyclo(Gly-Gly) | Planar [64,65] | Not stated |
| Cyclo(Gly-Gly) | Planar [19] | 0.009 |
| Cyclo(l-homoCys-l-homoCys) | Planar [67] | 0.0656–0.0668 |
| Cyclo(l-Ser-l-Ser) | Planar [66] | 0.020 |
| Cyclo(l-His-l-Asp) (trihydrate) | Planar [68] | <0.02 |
| Cyclo(l-Met-Gly) | Planar [69] | Not stated |
| Cyclo(l-Ser-l-His) (monohydrate) | Nearly planar [70] | 0.018 |
| Cyclo(l-Ser-l-Tyr) | Nearly planar [71] | Not stated |
| Cyclo(l-His-d-His) | Nearly planar (chair) [24] | Not stated |
| Cyclo(d-Ala-l-Ala) | Nearly planar (chair) [20] | Not stated |
| Cyclo(d-Ala-l-Ala) | Nearly planar [25] | 0.008 |
| Cyclo(d-Ala-l-Ala) | Nearly planar [72] | 0.016 |
| Cyclo(l-Trp-l-Trp) (cocrystal with DMSO) | Nearly planar [73] | Not stated |
| Cyclo(l-Ala-l-Asp) | Nearly planar (flagpole boat) [74] | Not stated |
| Cyclo(l-Thr-l-His) (dihyrate) | Flagpole boat [75] | 0.063 |
| Cyclo(Gly-l-Tyr) | Flagpole boat [71] | Not stated |
| Cyclo(l-Leu-l-His) | Flagpole boat [76] | Not stated |
| Cyclo(l-Leu-l-Tyr) (monohydrate) | Boat [77] | Not stated |
| Cyclo(l-Pro-l-Phe) | Boat [78] | Not stated |
| Cyclo(l-Pro-d-Phe) | Boat [79] | Not stated |
| Cyclo(l-Pro-d-Phe) | Boat [78] | Not stated |
| Cyclo(l-Met-l-Met) | Boat [80] | Not stated |
| Cyclo(l-Met-l-Met) | Boat [81] | Not stated |
| Cyclo(l-Met-l-Met) | Twist boat [82] | Not stated |
| Cyclo(l-Glu-l-Glu) | Boat [83] | Not stated |
| Cyclo(l-Pro-Gly) | Boat [84] | Not stated |
| Cyclo(l-Pro-l-Pro) | Boat [81] | Not stated |
| Cyclo(l-Pro-l-Leu) | Boat [85] | Not stated |
| Cyclo(l-Phe-l-Pro) | Boat [86] | Not stated |
| Cyclo(l-Asp-l-Asp) | Boat [87] | Not stated |
| Cyclo(l-Asp-l-Asp) | Boat [88] | Not stated |
| Cyclo(l-Trp-l-Pro) | Boat [73] | Not stated |
| Cyclo(l-Ala-l-Ala) | Bowsprit [89] | Not stated |
| Cyclo(l-Ala-l-Ala) | Twist boat [20] | Not stated |
| Cyclo(l-Tyr-l-Pro) | Flattened chair [21] | Not stated |
| rac-cyclo(d-Pro-l-Tyr/l-Pro-d-Tyr) | Twist boat [90] | Not stated |
| Cyclo-l-cystine | Twist boat [46] | Not stated |
| Vibration | Experimental Wavenumbers (cm−1) | |
|---|---|---|
| IR (Antisymmetric Vibrations) | Raman (Symmetric Vibrations) | |
| N-H stretching | 3185 | 3160 |
| C-H stretching (methylene) | 2970, 3040 | 2944, 2988 |
| C-H stretching (DKP ring) | 2886 | 2894 |
| S-H stretching | 2547 | 2549 |
| C=O stretching | 1667 | 1664 |
| Ring in-plane deformations + methylene group scissoring | 1462, 1484 | 1435, 1517 |
| Ring in-plane deformations + HCN bending | 1325 | 1300 |
| Hydrogen wagging | 1244, 1295 | 1250, 1300 |
| C-H twisting (methylene group) + HCN bending | 1190 | 1201 |
| N-C stretching | 1100 | 1137 |
| Cring-Cmethylene stretching | 1034 | 1007 |
| Methylene hydrogens rocking + CSH bending | 814, 967 | 785, 976 |
| Out-of-plane ring deformations | 707, 772, 912 | 679, 862 |
| Delocalised S-C and C-C stretching + out-of-plane N-H bending | 672 | 654 |
| OCN bending | 431 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witkowski, M.; Trzybiński, D.; Pawlędzio, S.; Woźniak, K.; Dzwolak, W.; Królikowska, A. The Structural Characterisation and DFT-Aided Interpretation of Vibrational Spectra for Cyclo(l-Cys-d-Cys) Cyclic Dipeptide in a Solid State. Molecules 2023, 28, 5902. https://doi.org/10.3390/molecules28155902
Witkowski M, Trzybiński D, Pawlędzio S, Woźniak K, Dzwolak W, Królikowska A. The Structural Characterisation and DFT-Aided Interpretation of Vibrational Spectra for Cyclo(l-Cys-d-Cys) Cyclic Dipeptide in a Solid State. Molecules. 2023; 28(15):5902. https://doi.org/10.3390/molecules28155902
Chicago/Turabian StyleWitkowski, Marcin, Damian Trzybiński, Sylwia Pawlędzio, Krzysztof Woźniak, Wojciech Dzwolak, and Agata Królikowska. 2023. "The Structural Characterisation and DFT-Aided Interpretation of Vibrational Spectra for Cyclo(l-Cys-d-Cys) Cyclic Dipeptide in a Solid State" Molecules 28, no. 15: 5902. https://doi.org/10.3390/molecules28155902






