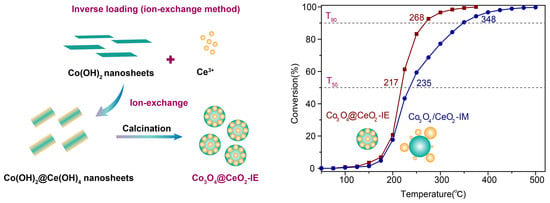
Enabling High Activity Catalyst Co3O4@CeO2 for Propane Catalytic Oxidation via Inverse Loading
Abstract
:1. Introduction
2. Results
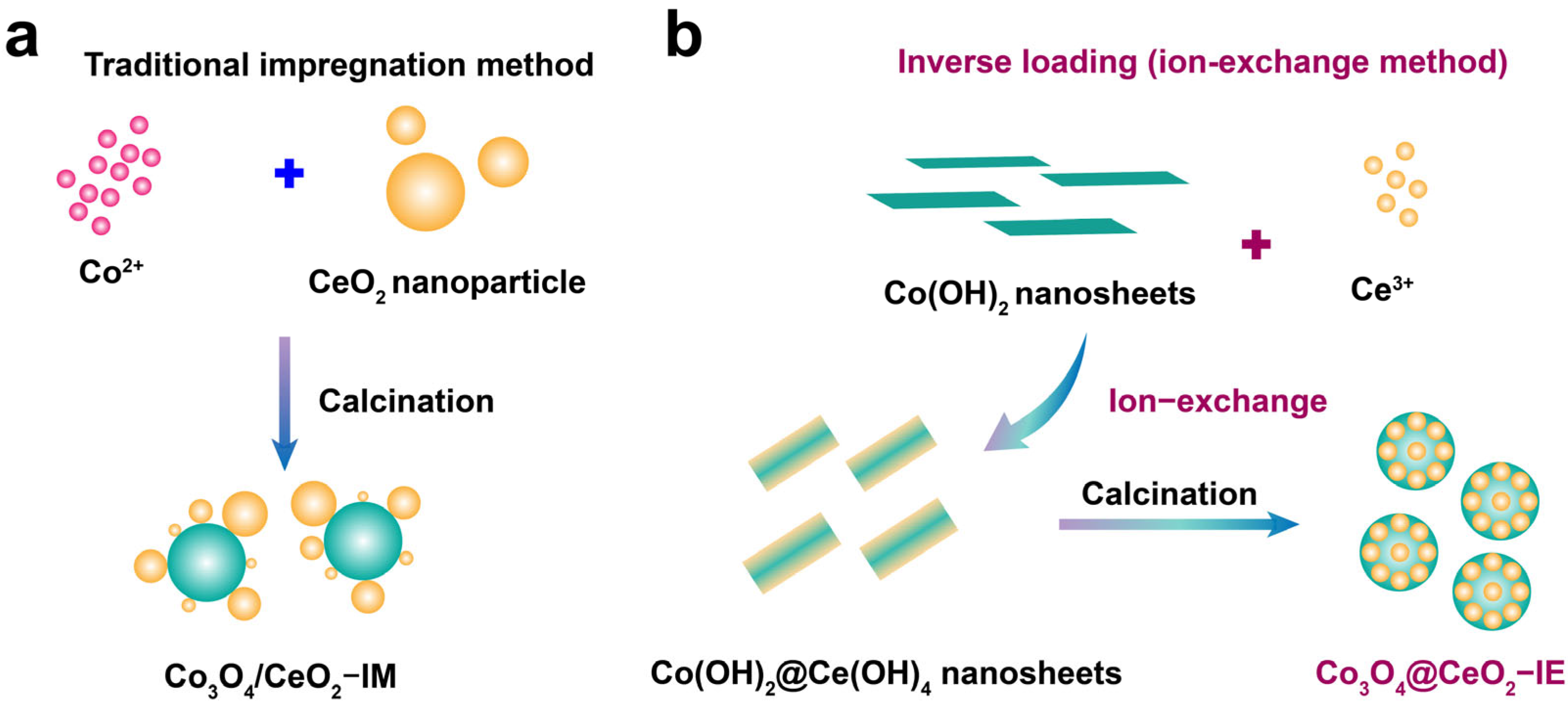
2.1. Structure and Morphology of the Obtained Samples
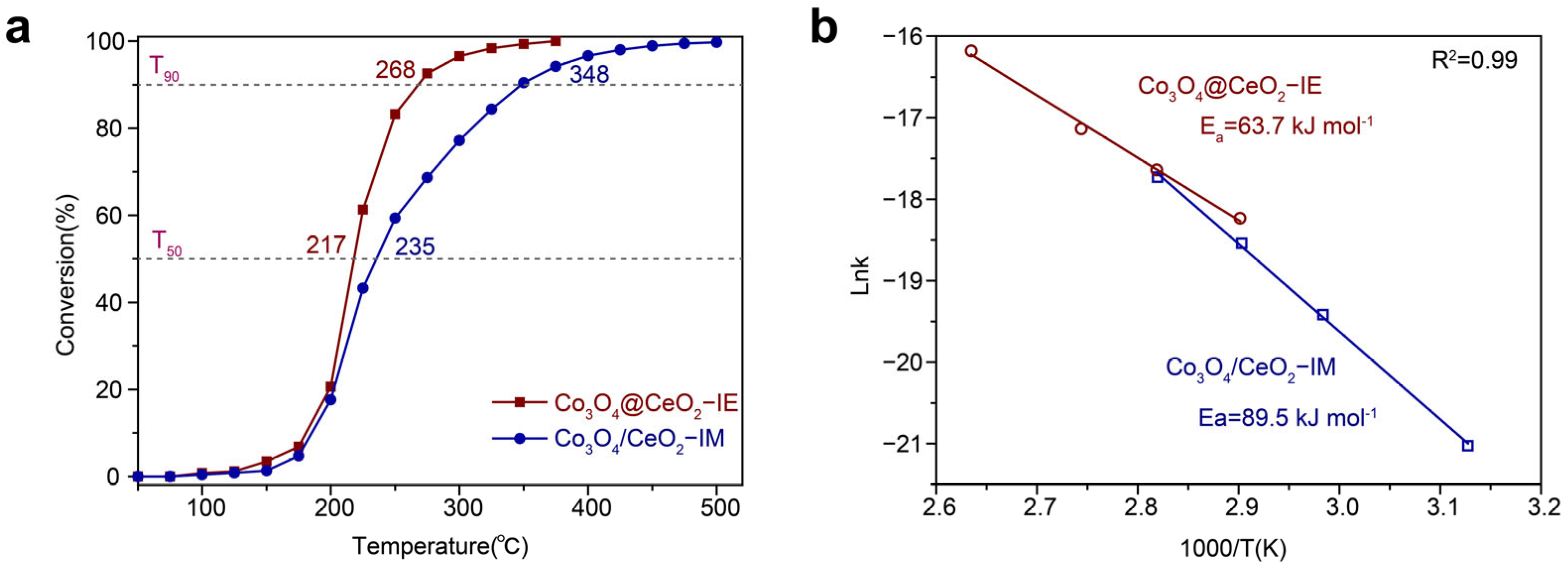
2.2. Catalytic Oxidation Performance
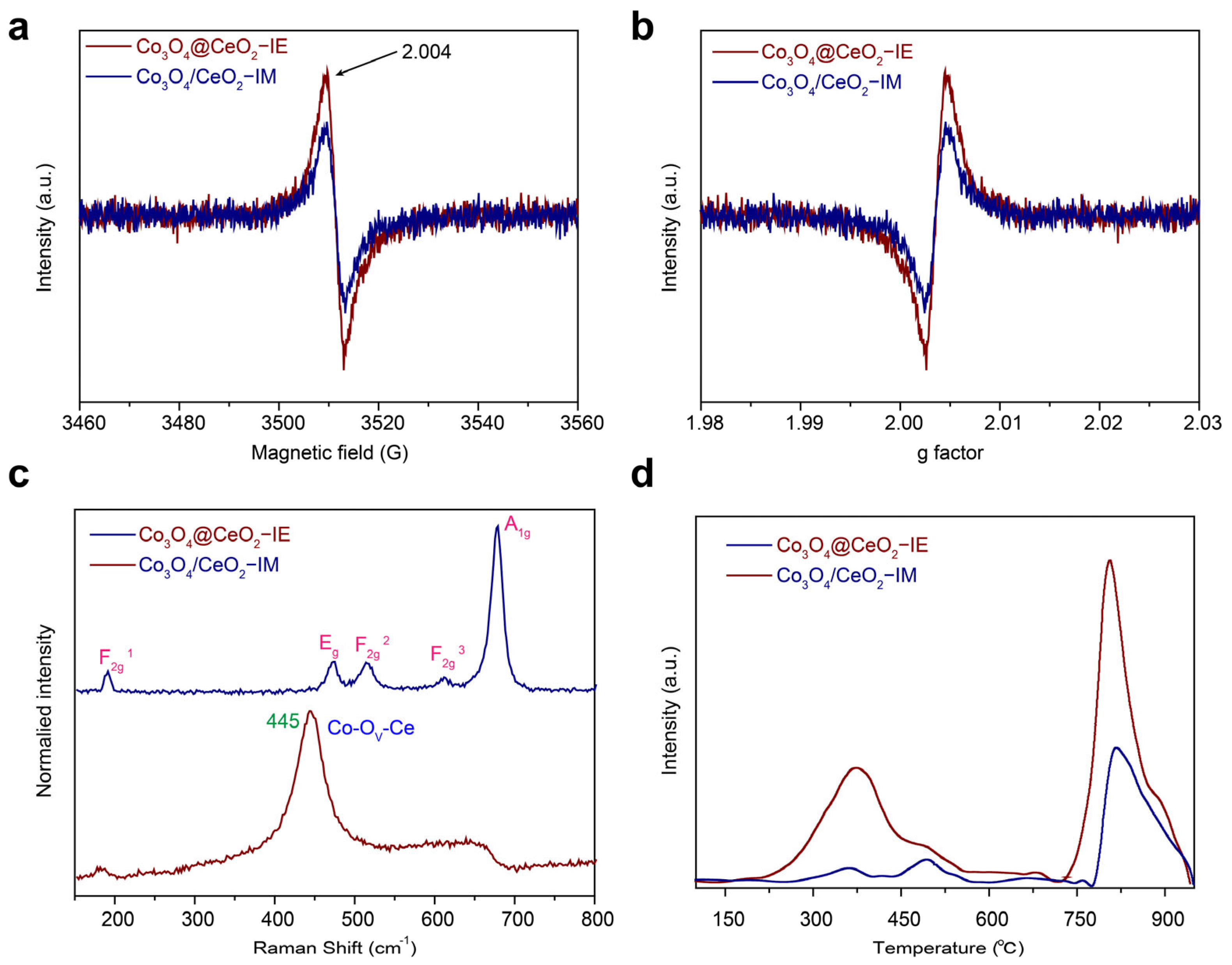
2.3. Surface Chemistry Analysis of the Catalysts
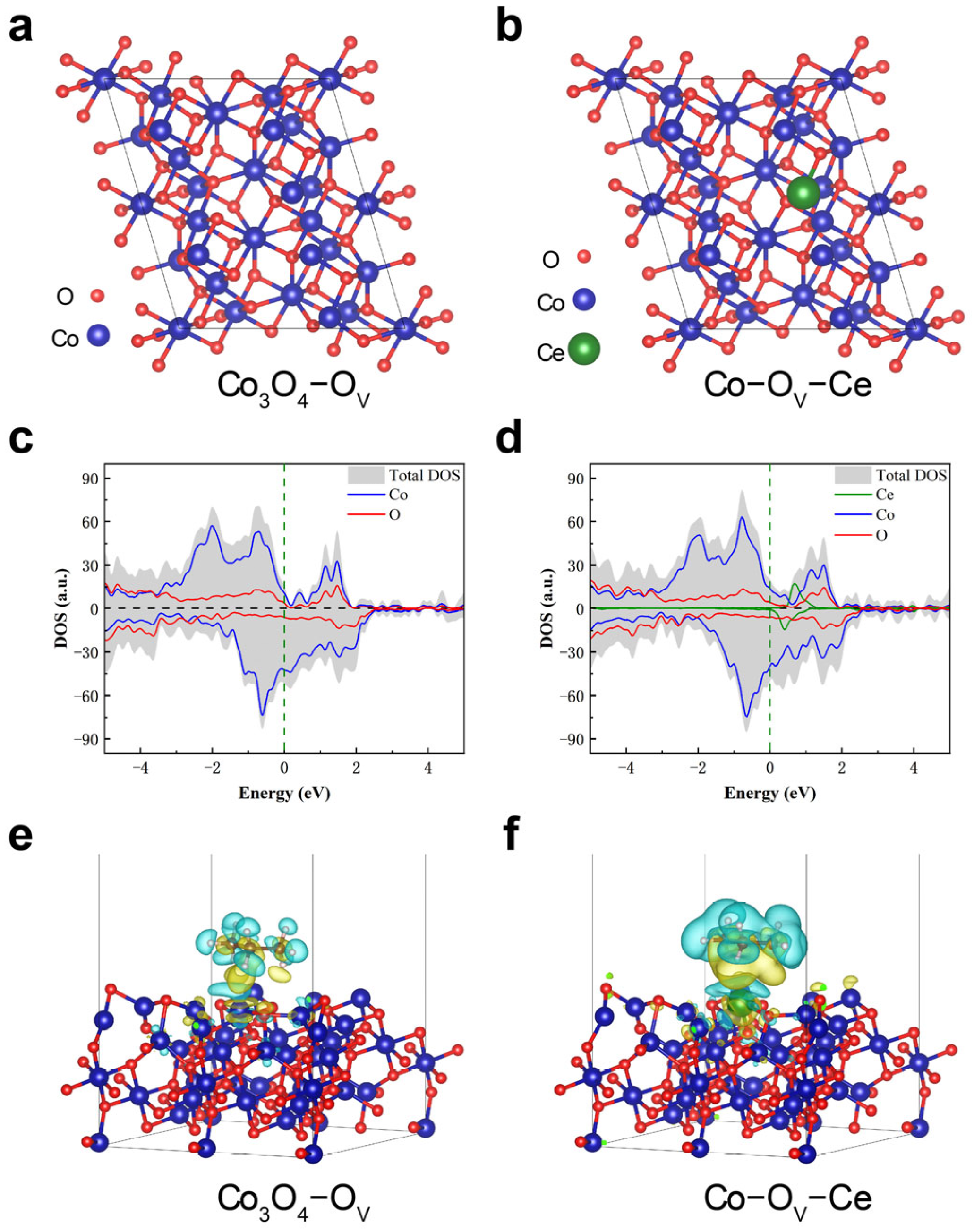
2.4. Catalytic Mechanism and Density−Functional Theory (DFT) Calculations
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.1.1. Preparation of Co(OH)2
4.1.2. Preparation of Co3O4@CeO2–IE Catalyst
4.1.3. Preparation of Co3O4/CeO2–IM Catalyst
4.2. Characterizations
4.3. Catalyst Evaluation
4.4. Computational Details
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mellouki, A.; Wallington, T.J.; Chen, J. Atmospheric chemistry of oxygenated volatile organic compounds: Impacts on air quality and climate. Chem. Rev. 2015, 115, 3984–4014. [Google Scholar] [CrossRef]
- Ravi Kumar, Y.; Deshmukh, K.; Kovářík, T.; Khadheer Pasha, S.K. A systematic review on 2D materials for volatile organic compound sensing. Coordin. Chem. Rev. 2022, 461, 214502. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Li, P.; Kim, S.; Jin, J.; Do, H.C.; Park, J.H. Efficient photodegradation of volatile organic compounds by iron-based metal-organic frameworks with high adsorption capacity. Appl. Catal. B Environ. 2020, 263, 118284. [Google Scholar] [CrossRef]
- Yin, F.; Yue, W.; Li, Y.; Gao, S.; Zhang, C.; Kan, H.; Niu, H.; Wang, W.; Guo, Y. Carbon–based nanomaterials for the detection of volatile organic compounds: A review. Carbon 2021, 180, 274–297. [Google Scholar] [CrossRef]
- Khatib, M.; Haick, H. Sensors for Volatile Organic Compounds. ACS Nano 2022, 16, 7080–7115. [Google Scholar] [CrossRef]
- Nadjafi, M.; Abdala, P.M.; Verel, R.; Hosseini, D.; Safonova, O.V.; Fedorov, A.; Müller, C.R. Reducibility and Dispersion Influence the Activity in Silica-Supported Vanadium-Based Catalysts for the Oxidative Dehydrogenation of Propane: The Case of Sodium Decavanadate. ACS Catal. 2020, 10, 2314–2321. [Google Scholar] [CrossRef]
- Zhang, M.; Sui, X.; Zhang, X.; Niu, M.; Li, C.; Wan, H.; Qiao, Z.-A.; Xie, H.; Li, X. Multi-defects engineering of NiCo2O4 for catalytic propane oxidation. Appl. Surf. Sci. 2022, 600, 154040. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Scirè, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B Environ. 2012, 125, 222–246. [Google Scholar] [CrossRef]
- Wang, B.; Wu, X.; Ran, R.; Si, Z.; Weng, D. IR characterization of propane oxidation on Pt/CeO2–ZrO2: The reaction mechanism and the role of Pt. J. Mol. Catal. A 2012, 356, 100–105. [Google Scholar] [CrossRef]
- Chen, J.; Lv, X.; Xu, W.; Li, X.; Chen, J.; Jia, H. Utilizing Cl coordination to facilitate Ru–Ag self–assembling into alloy and recover thermally-inactivated catalyst for propane combustion. Appl. Catal. B Environ. 2021, 290, 119989. [Google Scholar] [CrossRef]
- Chen, K.; Li, W.; Zhu, C.; Yuan, L. Construction of Hollow Cobalt Tetroxide Nanocages through the Metal Salt Bifunctional Etching Strategy for Catalytic Oxidation of Propane at Ultrahigh Space Velocity. ACS Appl. Nano Mater. 2022, 5, 6575–6584. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Gong, H. Catalytic Combustion of VOCs on Non-noble Metal Catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Feng, C.; Jiang, F.; Xiong, G.; Chen, C.; Wang, Z.; Pan, Y.; Fei, Z.; Lu, Y.; Li, X.; Zhang, R.; et al. Revelation of Mn4+–Osur–Mn3+ active site and combined Langmuir-Hinshelwood mechanism in propane total oxidation at low temperature over MnO2. Chem. Eng. J. 2023, 451, 138868. [Google Scholar] [CrossRef]
- Sun, L.; Liang, X.; Liu, H.; Cao, H.; Liu, X.; Jin, Y.; Li, X.; Chen, S.; Wu, X. Activation of Co–O bond in (110) facet exposed Co3O4 by Cu doping for the boost of propane catalytic oxidation. J. Hazard Mater. 2023, 452, 131319. [Google Scholar] [CrossRef]
- Chen, K.; Li, W.; Zhou, Z.; Huang, Q.; Liu, Y.; Duan, Q. Hydroxyl groups attached to Co2+ on the surface of Co3O4: A promising structure for propane catalytic oxidation. Catal. Sci. Technol. 2020, 10, 2573–2582. [Google Scholar] [CrossRef]
- Wu, S.; Liu, H.; Huang, Z.; Xu, H.; Shen, W. O-vacancy-rich porous MnO2 nanosheets as highly efficient catalysts for propane catalytic oxidation. Appl. Catal. B Environ. 2022, 312, 121387. [Google Scholar] [CrossRef]
- Xiao, Z.; Huang, Y.C.; Dong, C.L.; Xie, C.; Liu, Z.; Du, S.; Chen, W.; Yan, D.; Tao, L.; Shu, Z.; et al. Operando Identification of the Dynamic Behavior of Oxygen Vacancy-Rich Co3O4 for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2020, 142, 12087–12095. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Feng, Y.; Cheng, C.Q.; Qiu, W.K.; Dong, C.K.; Liu, H.; Du, X.W. Co3O4 Nanoparticles with Ultrasmall Size and Abundant Oxygen Vacancies for Boosting Oxygen Involved Reactions. Adv. Fun. Mater. 2019, 29, 1903444. [Google Scholar] [CrossRef]
- Hu, J.; Zeng, X.; Wang, G.; Qian, B.; Liu, Y.; Hu, X.; He, B.; Zhang, L.; Zhang, X. Modulating mesoporous Co3O4 hollow nanospheres with oxygen vacancies for highly efficient peroxymonosulfate activation. Chem. Eng. J. 2020, 400, 125869. [Google Scholar] [CrossRef]
- Iablokov, V.; Barbosa, R.; Pollefeyt, G.; Van Driessche, I.; Chenakin, S.; Kruse, N. Catalytic CO Oxidation over Well-Defined Cobalt Oxide Nanoparticles: Size-Reactivity Correlation. ACS Catal. 2015, 5, 5714–5718. [Google Scholar] [CrossRef]
- Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. Co3O4 nanocrystals with predominantly exposed facets: Synthesis, environmental and energy applications. J. Mater. Chem. A 2013, 1, 14427–14442. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Li, Y.; Liu, Z.Q.; Haruta, M.; Shen, W.-J. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, F.C. Superficial Chemistry and Solid Imperfections. Nature 1960, 186, 3–6. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Z.; Liu, P.; Wang, H.; Geng, J.; Lei, H.; Zhuo, O. Interfaces and Oxygen Vacancies-Enriched Catalysts Derived from Cu-Mn-Al Hydrotalcite towards High-Efficient Water–Gas Shift Reaction. Molecules 2023, 28, 1522. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Loh, H.; Low, B.Q.L.; Zhu, H.; Low, J.; Heng, J.Z.X.; Tang, K.Y.; Li, Z.; Loh, X.J.; Ye, E.; et al. Role of oxygen vacancy in metal oxides for photocatalytic CO2 reduction. Appl. Catal. B Environ. 2023, 321, 122079. [Google Scholar] [CrossRef]
- Zhu, K.; Shi, F.; Zhu, X.; Yang, W. The roles of oxygen vacancies in electrocatalytic oxygen evolution reaction. Nano Energy 2020, 73, 104761. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, Y.; Yang, Y.; Kong, T.; Song, Y.; Zhang, S.; Zheng, H. Elucidating the Role of Surface Ce4+ and Oxygen Vacancies of CeO2 in the Direct Synthesis of Dimethyl Carbonate from CO2 and Methanol. Molecules 2023, 28, 3785. [Google Scholar] [CrossRef]
- Su, Z.; Yang, W.; Wang, C.; Xiong, S.; Cao, X.; Peng, Y.; Si, W.; Weng, Y.; Xue, M.; Li, J. Roles of Oxygen Vacancies in the Bulk and Surface of CeO2 for Toluene Catalytic Combustion. Environ. Sci. Technol. 2020, 54, 12684–12692. [Google Scholar] [CrossRef]
- Yang, M.; Shen, G.; Wang, Q.; Deng, K.; Liu, M.; Chen, Y.; Gong, Y.; Wang, Z. Roles of Oxygen Vacancies of CeO2 and Mn-Doped CeO2 with the Same Morphology in Benzene Catalytic Oxidation. Molecules 2021, 26, 6363. [Google Scholar] [CrossRef]
- Lou, B.; Shakoor, N.; Adeel, M.; Zhang, P.; Huang, L.; Zhao, Y.; Zhao, W.; Jiang, Y.; Rui, Y. Catalytic oxidation of volatile organic compounds by non-noble metal catalyst: Current advancement and future prospectives. J. Clean. Prod. 2022, 363, 132523. [Google Scholar] [CrossRef]
- Singha, R.K.; Shukla, A.; Yadav, A.; Sivakumar Konathala, L.N.; Bal, R. Effect of Metal-Support Interaction on Activity and Stability of Ni-CeO2 Catalyst for Partial Oxidation of Methane. Appl. Catal. B Environ. 2017, 202, 473–488. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Pu, Y.; Liu, A.; Chen, C.; Zou, W.; Zheng, Y.; Huang, J.; Zhang, Y.; Yang, Y.; et al. Facile Ball-Milling Synthesis of CeO2/g-C3N4 Z-Scheme Heterojunction for Synergistic Adsorption and Photodegradation of Methylene Blue: Characteristics, Kinetics, Models, and Mechanisms. Chem. Eng. J. 2021, 420, 127719. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, S.; Liu, B.; Liu, J.; Wang, L.; Xiao, Y.; Xu, Y.; Liu, X. Insights into the Influence of CeO2 Crystal Facet on CO2 Hydrogenation to Methanol over Pd/CeO2 Catalysts. ACS Catal. 2020, 10, 11493–11509. [Google Scholar] [CrossRef]
- Chang, S.; Li, M.; Hua, Q.; Zhang, L.; Ma, Y.; Ye, B.; Huang, W. Shape-Dependent Interplay Between Oxygen Vacancies and Ag–CeO2 Interaction in Ag/CeO2 Catalysts and Their Influence on the Catalytic Activity. J. Catal. 2012, 293, 195–204. [Google Scholar] [CrossRef]
- Han, X.; Li, J.; Lu, J.; Luo, S.; Wan, J.; Li, B.; Hu, C.; Cheng, X. High mass-loading NiCoLDH nanosheet arrays grown on carbon cloth by electrodeposition for excellent electrochemical energy storage. Nano Energy 2021, 86, 106079. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, Q.; Li, S.; Ren, Q.; Zhang, M.; Xue, Y.; Peng, R.; Xiao, H.; Chen, Y.; Ye, D. Integrated Cobalt Oxide Based Nanoarray Catalysts with Hierarchical Architectures: In Situ Raman Spectroscopy Investigation on the Carbon Monoxide Reaction Mechanism. ChemCatChem 2018, 10, 3012–3026. [Google Scholar] [CrossRef]
- Spanier, J.E.; Robinson, R.D.; Zhang, F.; Chan, S.-W.; Herman, I.P. Size-Dependent Properties of CeO2−y Nanoparticles as Studied by Raman Scattering. Phys. Rev. B 2001, 64, 245407–245414. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Li, L.; Huang, Y.; Zhang, W.; Wang, X.; Wang, A.; Zhang, T. In Situ Calorimetric Study: Structural Effects on Adsorption and Catalytic Performances for CO Oxidation over Ir-in-CeO2 and Ir-on-CeO2 Catalysts. J. Phys. Chem. C 2011, 115, 16509–16517. [Google Scholar] [CrossRef]
- Chen, L.; Liu, F.; Li, X.; Tao, Q.; Huang, Z.; Zuo, Q.; Chen, Y.; Li, T.; Fu, M.; Ye, D. Surface adsorbed and lattice oxygen activated by the CeO2/Co3O4 interface for enhancive catalytic soot combustion: Experimental and theoretical investigations. J. Colloid Interface Sci. 2023, 638, 109–122. [Google Scholar] [CrossRef]
- Wu, S.; Liu, H.; Huang, Z.; Xu, H.; Shen, W. Mn1ZrxOy mixed oxides with abundant oxygen vacancies for propane catalytic oxidation: Insights into the contribution of Zr doping. Chem. Eng. J. 2023, 452, 139341. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, X.; Jin, J.; Di, X.; Liang, C.; Liu, Z. Insight into catalytic properties of Co3O4-CeO2 binary oxides for propane total oxidation. Chin. J. Catal. 2020, 41, 679–690. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.; Tchougreeff, A.; Dronskowski, R. LOBSTER: A Tool to Extract Chemical Bonding from Plane-Wave Based DFT. J. Comput. Chem. 2016, 37, 1030–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Zhan, Q.; Liu, F.; Wang, C.; Li, H.; Wang, X.; Guo, X.; Cheng, Y.; Sun, W.; Wang, L.; et al. Overturned Loading of Inert CeO2 to Active Co3O4 for Unusually Improved Catalytic Activity in Fenton-Like Reactions. Angew. Chem. Int. Ed. 2022, 61, e202200406. [Google Scholar] [CrossRef]
- Wang, X.; Song, H.; Ma, S.; Li, M.; He, G.; Xie, M.; Guo, X. Template ion-exchange synthesis of Co-Ni composite hydroxides nanosheets for supercapacitor with unprecedented rate capability. Chem. Eng. J. 2021, 432, 134319. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Buongiorno Nardelli, M.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Setten, M.J.; Giantomassi, M.; Bousquet, E.; Verstraete, M.J.; Hamann, D.R.; Gonze, X.; Rignanese, G.M. The PseudoDojo: Training and grading a 85 element optimized norm-conserving pseudopotential table. Comput. Phys. Commun. 2018, 226, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liang, W.; Lin, C.; Zhang, T.; Zhang, J.; Sheng, N.; Song, Z.; Jiang, J.; Sun, B.; Xu, W. Enabling High Activity Catalyst Co3O4@CeO2 for Propane Catalytic Oxidation via Inverse Loading. Molecules 2023, 28, 5930. https://doi.org/10.3390/molecules28155930
Wang X, Liang W, Lin C, Zhang T, Zhang J, Sheng N, Song Z, Jiang J, Sun B, Xu W. Enabling High Activity Catalyst Co3O4@CeO2 for Propane Catalytic Oxidation via Inverse Loading. Molecules. 2023; 28(15):5930. https://doi.org/10.3390/molecules28155930
Chicago/Turabian StyleWang, Xuan, Wei Liang, Changqing Lin, Tie Zhang, Jing Zhang, Nan Sheng, Zhaoning Song, Jie Jiang, Bing Sun, and Wei Xu. 2023. "Enabling High Activity Catalyst Co3O4@CeO2 for Propane Catalytic Oxidation via Inverse Loading" Molecules 28, no. 15: 5930. https://doi.org/10.3390/molecules28155930
APA StyleWang, X., Liang, W., Lin, C., Zhang, T., Zhang, J., Sheng, N., Song, Z., Jiang, J., Sun, B., & Xu, W. (2023). Enabling High Activity Catalyst Co3O4@CeO2 for Propane Catalytic Oxidation via Inverse Loading. Molecules, 28(15), 5930. https://doi.org/10.3390/molecules28155930