Enhancement of Electrocatalytic and Pseudocapacitive Properties as a Function of Structural Order in A2Fe2O5 (A = Sr, Ba)
Abstract
:1. Introduction
2. Results and Discussion
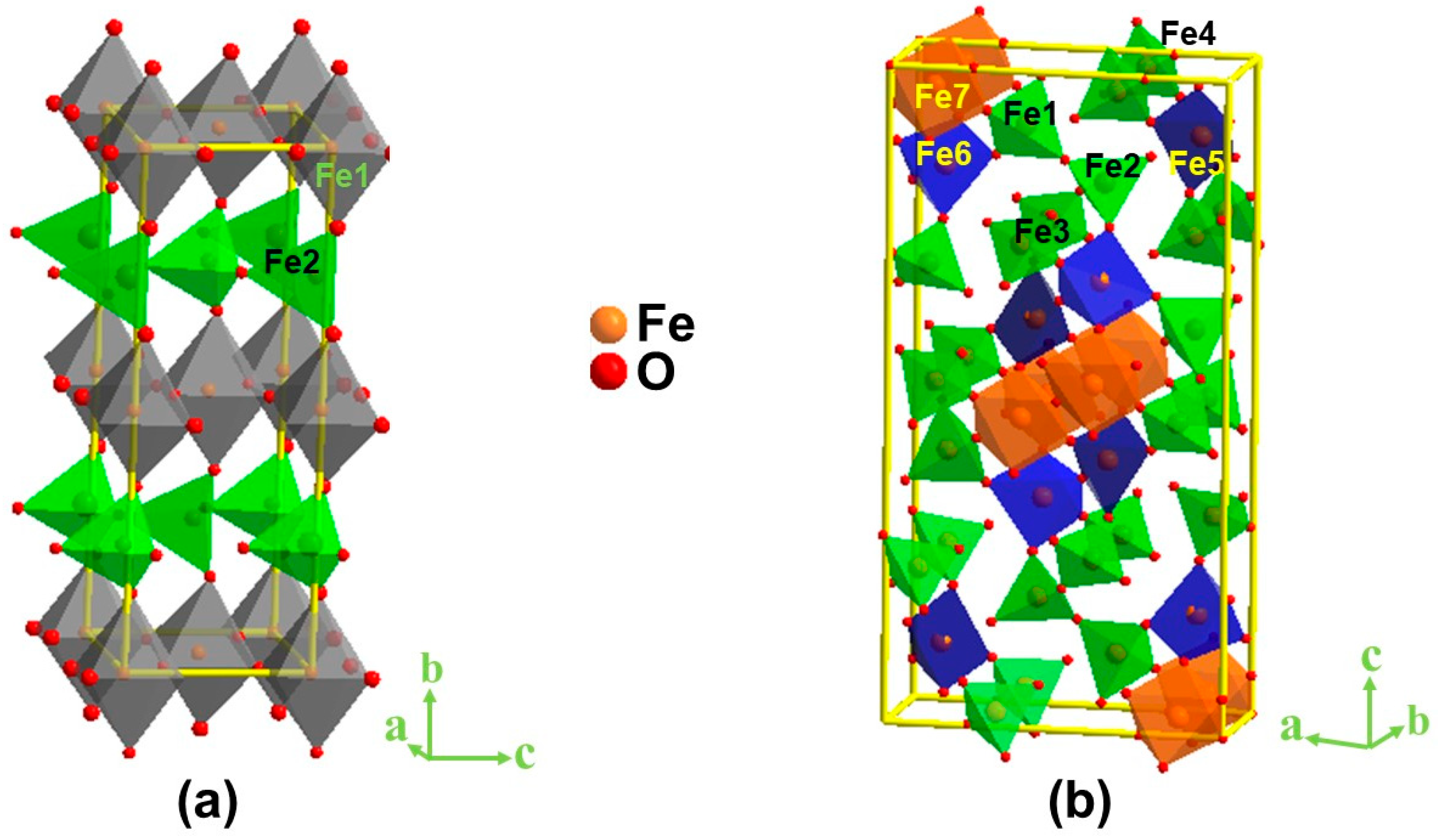
2.1. Crystal Structure
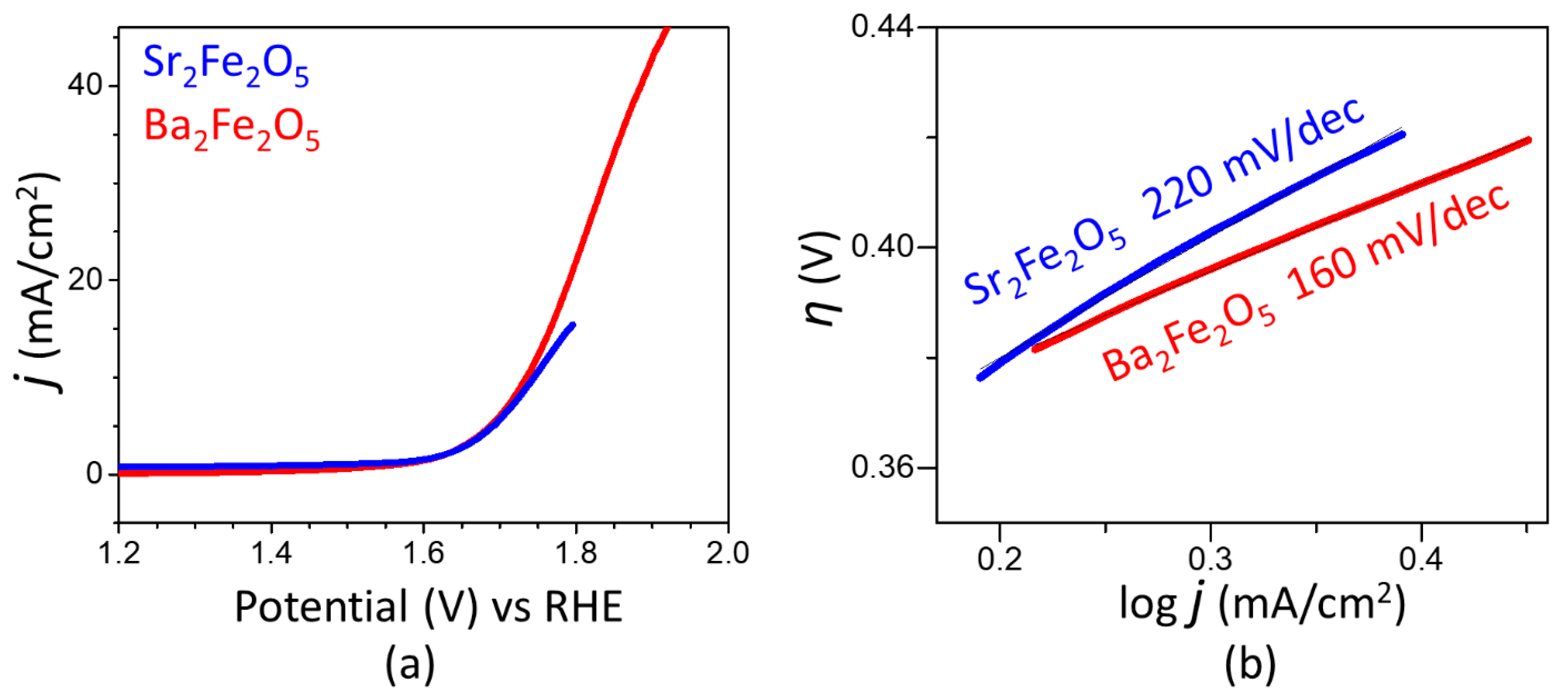
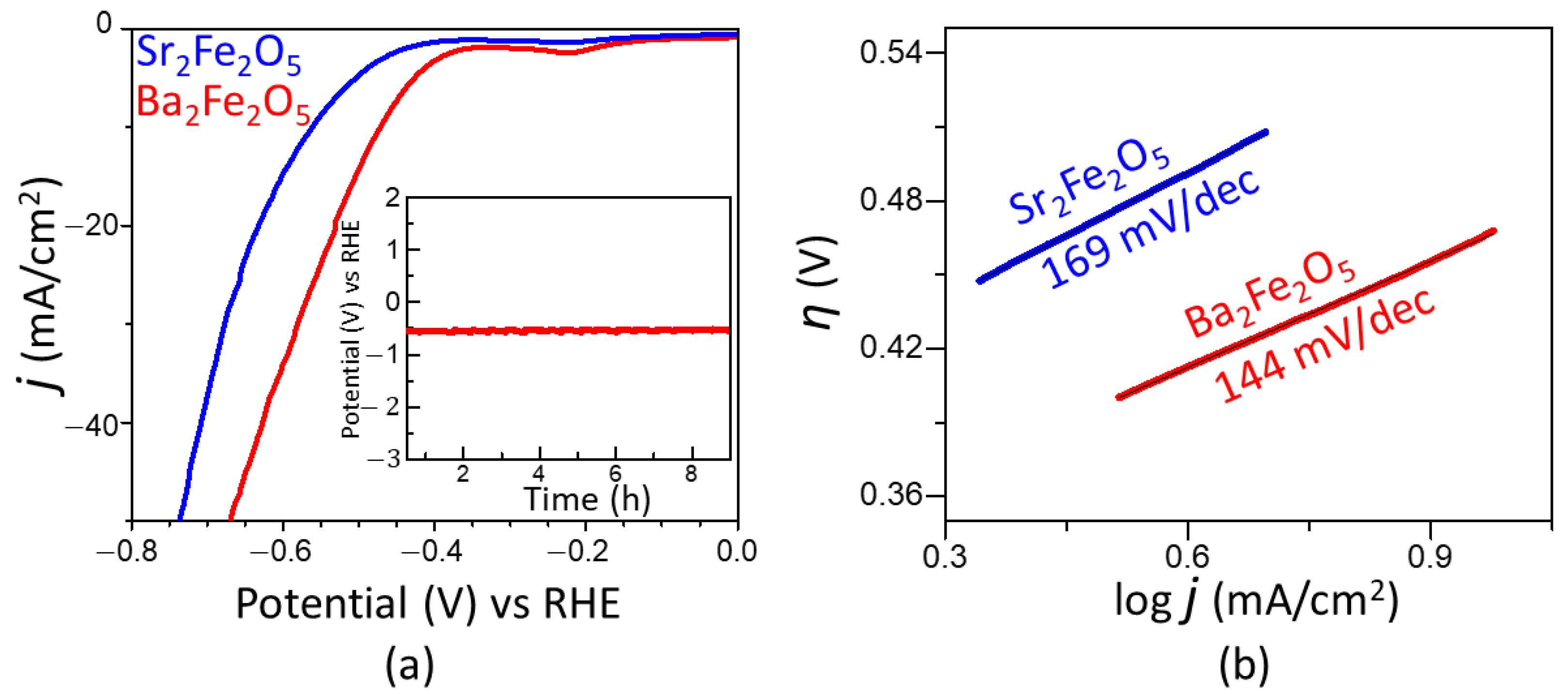
2.2. Electrocatalytic Properties for OER and HER
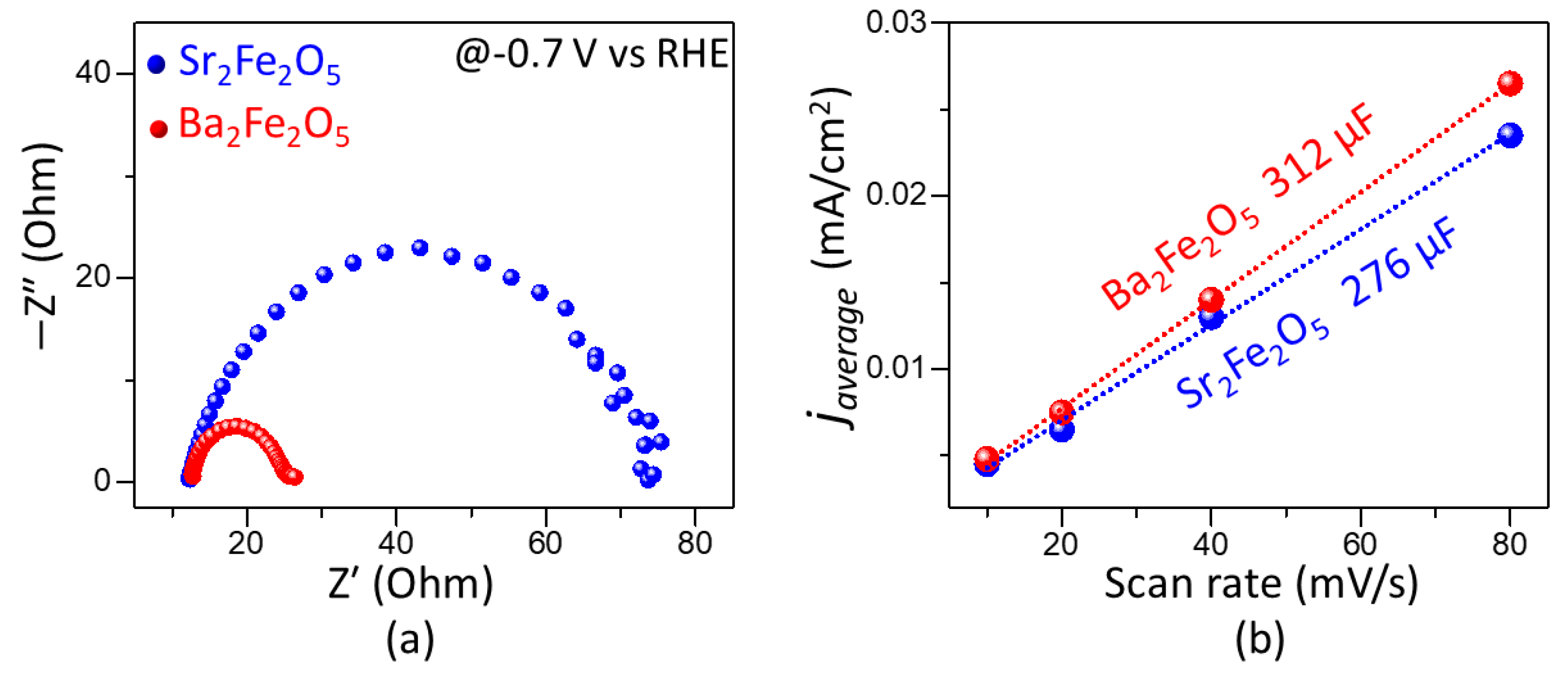
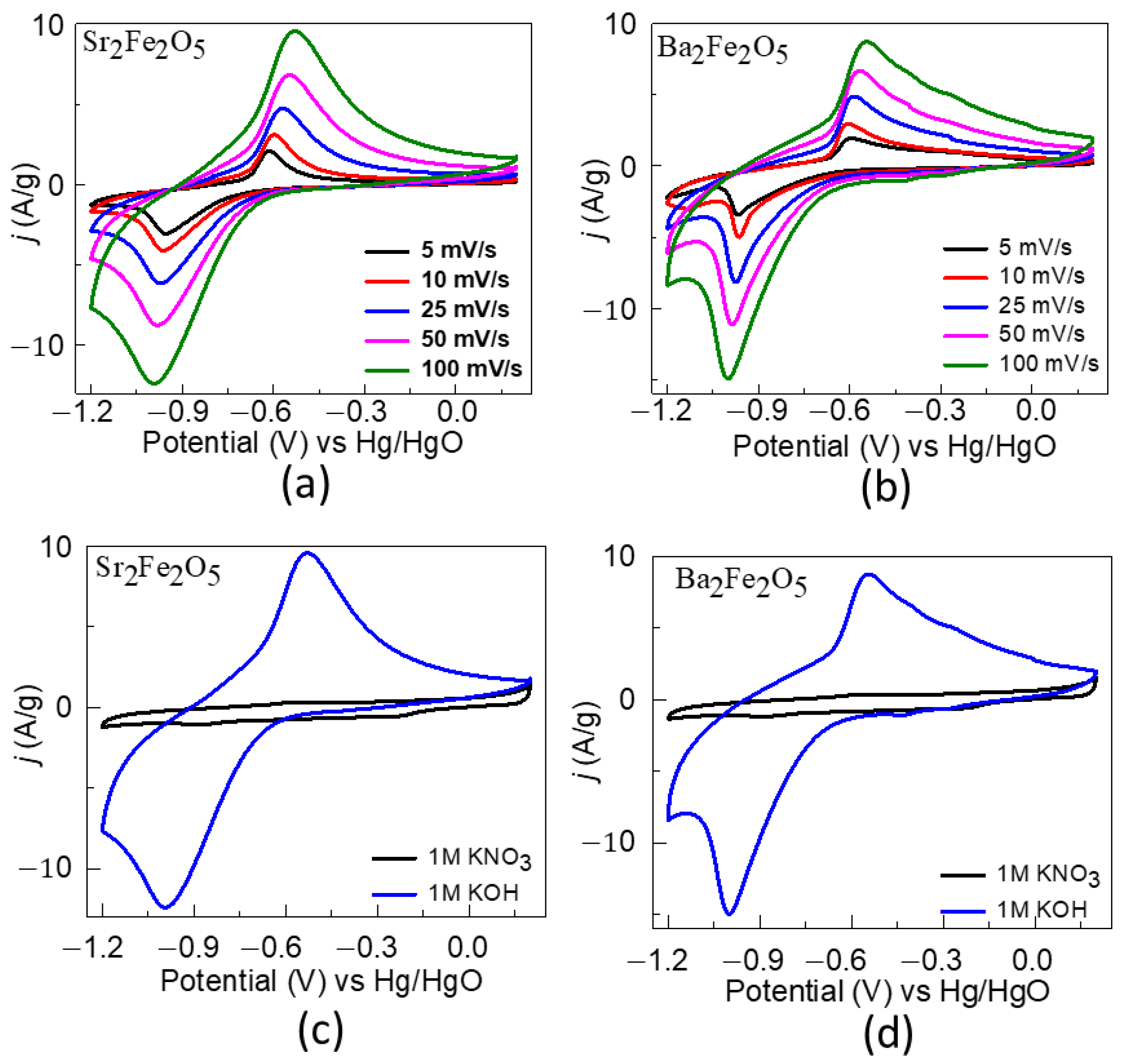
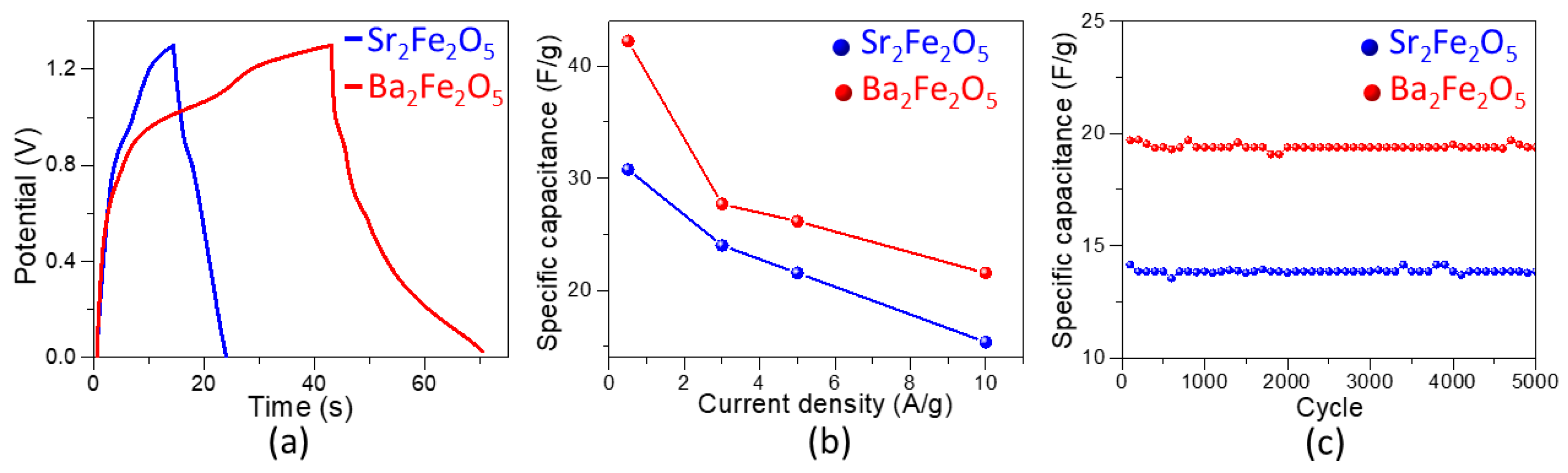
2.3. Pseudocapacitive Charge-Storage Properties
3. Experimental Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adler, S.B. Factors Governing Oxygen Reduction in Solid Oxide Fuel Cell Cathodes. Chem. Rev. 2004, 104, 4791–4844. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.S.; Guo, R.; Roy, R. The perovskite structure—A review of its role in ceramic science and technology. Mater. Res. Innov. 2000, 4, 3–26. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H.; Johnson, D.W.; Remeika, J.P.; Gallagher, P.K. Perovskite Oxides: Materials Science in Catalysis. Science 1977, 195, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Berenov, A.V.; Atkinson, A.; Kilner, J.A.; Bucher, E.; Sitte, W. Oxygen tracer diffusion and surface exchange kinetics in La0.6Sr0.4CoO3−δ. Solid State Ion. 2010, 181, 819–826. [Google Scholar] [CrossRef]
- Adler, S.B. Mechanism and kinetics of oxygen reduction on porous La1−xSrxCoO3−δ electrodes. Solid State Ion. 1998, 111, 125–134. [Google Scholar] [CrossRef]
- Ismail, M.; Liu, W.; Chan, M.S.C.; Dunstan, M.T.; Scott, S.A. Synthesis, Application, and Carbonation Behavior of Ca2Fe2O5 for Chemical Looping H2 Production. Energy Fuels 2016, 30, 6220–6232. [Google Scholar] [CrossRef] [Green Version]
- Saib, F.; Mekiri, M.; Bellal, B.; Chibane, M.; Trari, M. Photoelectrochemical properties of the brownmillerite Sr2Fe2O5: Application to electrochemical oxygen evolution. Russ. J. Phys. Chem. A 2017, 91, 1562–1570. [Google Scholar] [CrossRef]
- Fluri, A.; Gilardi, E.; Karlsson, M.; Roddatis, V.; Bettinelli, M.; Castelli, I.E.; Lippert, T.; Pergolesi, D. Anisotropic Proton and Oxygen Ion Conductivity in Epitaxial Ba2In2O5 Thin Films. J. Phys. Chem. C 2017, 121, 21797–21805. [Google Scholar] [CrossRef] [Green Version]
- Dhankhar, S.; Menon, S.S.; Gupta, B.; Baskar, K.; Singh, S. Electrochemical performance of brownmillerite calcium ferrite for application as supercapacitor. AIP Conf. Proc. 2017, 1832, 080050. [Google Scholar]
- Karki, S.B.; Hona, R.K.; Yu, M.; Ramezanipour, F. Enhancement of Electrocatalytic Activity as a Function of Structural Order in Perovskite Oxides. ACS Catal. 2022, 12, 10333–10337. [Google Scholar] [CrossRef]
- Wang, C.; Shang, H.; Xu, H.; Du, Y. Nanoboxes endow non-noble-metal-based electrocatalysts with high efficiency for overall water splitting. J. Mater. Chem. A 2021, 9, 857–874. [Google Scholar] [CrossRef]
- Tang, J.; Xu, X.; Tang, T.; Zhong, Y.; Shao, Z. Perovskite-Based Electrocatalysts for Cost-Effective Ultrahigh-Current-Density Water Splitting in Anion Exchange Membrane Electrolyzer Cell. Small Methods 2022, 6, 2201099. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Fang, S.; Zhu, X.; Liu, X.; Gu, J.; Liu, W.; Wang, D.; Zhang, W.; Lin, Y.; Lu, J.; Wei, S.; et al. Uncovering near-free platinum single-atom dynamics during electrochemical hydrogen evolution reaction. Nat. Commun. 2020, 11, 1029. [Google Scholar] [CrossRef] [Green Version]
- Das, D.; Das, A.; Reghunath, M.; Nanda, K.K. Phosphine-free avenue to Co2P nanoparticle encapsulated N,P co-doped CNTs: A novel non-enzymatic glucose sensor and an efficient electrocatalyst for oxygen evolution reaction. Green Chem. 2017, 19, 1327–1335. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhou, W.; Chen, Z.G.; Chen, Y.; Su, C.; Tadé, M.O.; Shao, Z. SrNb0.1Co0.7Fe0.2O3−δ perovskite as a next-generation electrocatalyst for oxygen evolution in alkaline solution. Angew. Chem. Int. Ed. 2015, 54, 3897–3901. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef]
- Karki, S.B.; Andriotis, A.N.; Menon, M.; Ramezanipour, F. Bifunctional Water-Splitting Electrocatalysis Achieved by Defect-Order in LaA2Fe3O8 (A = Ca, Sr). ACS Appl. Energy Mater. 2021, 4, 12063–12066. [Google Scholar] [CrossRef]
- Sankannavar, R.; Sandeep, K.C.; Kamath, S.; Suresh, A.K.; Sarkar, A. Impact of Strontium-Substitution on Oxygen Evolution Reaction of Lanthanum Nickelates in Alkaline Solution. J. Electrochem. Soc. 2018, 165, J3236–J3245. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Electrochemical Capacitors for Energy Management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Kluwer Academic/Plenum: New York, NY, USA, 1999. [Google Scholar]
- Mefford, J.T.; Hardin, W.G.; Dai, S.; Johnston, K.P.; Stevenson, K.J. Anion charge storage through oxygen intercalation in LaMnO3 perovskite pseudocapacitor electrodes. Nat. Mater. 2014, 13, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.B.; Ramezanipour, F. Pseudocapacitive Energy Storage and Electrocatalytic Hydrogen-Evolution Activity of Defect-Ordered Perovskites SrxCa3–xGaMn2O8 (x = 0 and 1). ACS Appl. Energy Mater. 2020, 3, 10983–10992. [Google Scholar] [CrossRef]
- Mondal, R.; Mishra, N.K.; Maiyalagan, T.; Gupta, A.; Singh, P. La1–xKxFeO3−δ: An Anion Intercalative Pseudocapacitive Electrode for Supercapacitor Application. ACS Omega 2021, 6, 30488–30498. [Google Scholar] [CrossRef]
- Mo, H.; Nan, H.; Lang, X.; Liu, S.; Qiao, L.; Hu, X.; Tian, H. Influence of calcium doping on performance of LaMnO3 supercapacitors. Ceram. Int. 2018, 44, 9733–9741. [Google Scholar] [CrossRef]
- Lang, X.; Mo, H.; Hu, X.; Tian, H. Supercapacitor performance of perovskite La1−xSrxMnO3. Dalton Trans. 2017, 46, 13720–13730. [Google Scholar] [CrossRef]
- Greaves, C.; Jacobson, A.J.; Tofield, B.C.; Fender, B.E.F. A powder neutron diffraction investigation of the nuclear and magnetic structure of Sr2Fe2O5. Acta Crystallogr. Sect. B 1975, 31, 641–646. [Google Scholar] [CrossRef]
- Hona, R.K.; Ramezanipour, F. Variation in electrical conductivity of A2Fe2O5 (A = Sr, Ba): The role of structural order. Mater. Res. Express 2018, 5, 076307. [Google Scholar] [CrossRef]
- Zou, X.D.; Hovmoller, S.; Parras, M.; Gonzalez-Calbet, J.M.; Vallet-Regi, M.; Grenier, J.C. The complex perovskite-related superstructure Ba2Fe2O5 solved by HREM and CIP. Acta Crystallogr. Sect. A 1993, 49, 27–35. [Google Scholar] [CrossRef]
- Harder, M.; Müller-Buschbaum, H. Darstellung und Untersuchung von Sr2Fe2O5-Einkristallen Ein Beitrag zur Kristallchemie von M2Fe2O5-Verbindungen. Z. Anorg. Allg. Chem. 1980, 464, 169–175. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Li, X.; He, L.; Zhong, X.; Zhang, J.; Luo, S.; Yi, W.; Zhang, L.; Hu, M.; Tang, J.; Zhou, X.; et al. Evaluation of A-Site Ba2+-Deficient Ba1-xCo0.4Fe0.4Zr0.1Y0.1O3-δ Oxides as Electrocatalysts for Efficient Hydrogen Evolution Reaction. Scanning 2018, 2018, 1341608. [Google Scholar] [CrossRef] [Green Version]
- Hona, R.K.; Karki, S.B.; Ramezanipour, F. Oxide Electrocatalysts Based on Earth-Abundant Metals for Both Hydrogen- and Oxygen-Evolution Reactions. ACS Sustain. Chem. Eng. 2020, 8, 11549–11557. [Google Scholar] [CrossRef]
- Alom, M.S.; Ramezanipour, F. Layered Oxides SrLaFe1-xCoxO4-δ (x=0–1) as Bifunctional Electrocatalysts for Water-Splitting. ChemCatChem 2021, 13, 3510–3516. [Google Scholar] [CrossRef]
- Allen, J.; Bard, L.R.F. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Hona, R.K.; Ramezanipour, F. Remarkable Oxygen-Evolution Activity of a Perovskite Oxide from the Ca2−xSrxFe2O6−δ Series. Angew. Chem. 2019, 58, 2060–2063. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Zhou, W.; Zhu, Z.; Su, C.; Liu, M.; Shao, Z. A Perovskite Electrocatalyst for Efficient Hydrogen Evolution Reaction. Adv. Mater. 2016, 28, 6442–6448. [Google Scholar] [CrossRef]
- Karki, S.B.; Hona, R.K.; Ramezanipour, F. Sr3Mn2O6 and Sr3FeMnO6 for oxygen and hydrogen evolution electrocatalysis. J. Solid State Electrochem. 2022, 26, 1303–1311. [Google Scholar] [CrossRef]
- Jung, S.; McCrory, C.C.L.; Ferrer, I.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking nanoparticulate metal oxide electrocatalysts for the alkaline water oxidation reaction. J. Mater. Chem. A 2016, 4, 3068–3076. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Cao, D.; Wang, P.; Wang, G.; Gao, Y. Oxygen evolution reaction on Ni-substituted Co3O4 nanowire array electrodes. Int. J. Hydrogen Energy 2011, 36, 72–78. [Google Scholar] [CrossRef]
- Alom, M.S.; Ramezanipour, F. Vacancy effect on the electrocatalytic activity of LaMn1/2Co1/2O3−δ for hydrogen and oxygen evolution reactions. Chem. Commun. 2023, 59, 5870–5873. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kim, H.; Kwon, Y.; Kim, M.; Cho, E.; Kwon, H. Porous Co–P foam as an efficient bifunctional electrocatalyst for hydrogen and oxygen evolution reactions. J. Mater.Chem. A 2016, 4, 18272–18277. [Google Scholar] [CrossRef]
- Hona, R.K.; Karki, S.B.; Cao, T.; Mishra, R.; Sterbinsky, G.E.; Ramezanipour, F. Sustainable Oxide Electrocatalyst for Hydrogen and Oxygen-Evolution Reactions. ACS Catal. 2021, 11, 14605–14614. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Sunarso, J.; Zhong, Y.; Shao, Z. Phosphorus-Doped Perovskite Oxide as Highly Efficient Water Oxidation Electrocatalyst in Alkaline Solution. Adv. Funct. Mater 2016, 26, 5862–5872. [Google Scholar] [CrossRef]
- Petrie, J.R.; Cooper, V.R.; Freeland, J.W.; Meyer, T.L.; Zhang, Z.; Lutterman, D.A.; Lee, H.N. Enhanced Bifunctional Oxygen Catalysis in Strained LaNiO3 Perovskites. J. Am. Chem. Soc. 2016, 138, 2488–2491. [Google Scholar] [CrossRef] [Green Version]
- Alom, M.S.; Ramezanipour, F. Pseudocapacitive charge storage in layered oxides SrLaFe1−xCoxO4−δ (x = 0–1). Mater. Lett. 2021, 295, 129859. [Google Scholar] [CrossRef]
- Che, W.; Wei, M.; Sang, Z.; Ou, Y.; Liu, Y.; Liu, J. Perovskite LaNiO3-δ oxide as an anion-intercalated pseudocapacitor electrode. J. Alloys Compd. 2018, 731, 381–388. [Google Scholar] [CrossRef]
- Kananke-Gamage, C.C.W.; Ramezanipour, F. Structure Effect on Pseudocapacitive Properties of A2LaMn2O7 (A = Ca, Sr). Energy Tech. 2023, 11, 2201249. [Google Scholar] [CrossRef]
- Alexander, C.T.; Mefford, J.T.; Saunders, J.; Forslund, R.P.; Johnston, K.P.; Stevenson, K.J. Anion-Based Pseudocapacitance of the Perovskite Library La1–xSrxBO3−δ (B = Fe, Mn, Co). ACS Appl. Mater. Interfaces 2019, 11, 5084–5094. [Google Scholar] [CrossRef]
- Alexander, C.T.; Forslund, R.P.; Johnston, K.P.; Stevenson, K.J. Tuning Redox Transitions via the Inductive Effect in LaNi1–xFexO3−δ Perovskites for High-Power Asymmetric and Symmetric Pseudocapacitors. ACS Appl. Energy Mater. 2019, 2, 6558–6568. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Su, C.; Zhou, W.; Liu, M.; Shao, Z. Perovskite SrCo0.9Nb0.1O3−δ as an Anion-Intercalated Electrode Material for Supercapacitors with Ultrahigh Volumetric Energy Density. Angew. Chem. Int. Ed. 2016, 55, 9576–9579. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Sun, W.; Ning, G.; Wei, T.; Zhang, Q.; Zhang, R.; Zhi, L.; Wei, F. Advanced Asymmetric Supercapacitors Based on Ni(OH)2/Graphene and Porous Graphene Electrodes with High Energy Density. Adv. Funct. Mater. 2012, 22, 2632–2641. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Shi, P.; Li, Y.; Huang, L.; Mai, W.; Tan, S.; Cai, X. Growth of nickel (111) plane: The key role in nickel for further improving the electrochemical property of hexagonal nickel hydroxide-nickel & reduced graphene oxide composite. J. Power Sources 2014, 267, 356–365. [Google Scholar]
- Guo, D.; Zhang, H.; Yu, X.; Zhang, M.; Zhang, P.; Li, Q.; Wang, T. Facile synthesis and excellent electrochemical properties of CoMoO4 nanoplate arrays as supercapacitors. J. Mater. Chem. A 2013, 1, 7247–7254. [Google Scholar] [CrossRef]
- Vellacheri, R.; Al-Haddad, A.; Zhao, H.; Wang, W.; Wang, C.; Lei, Y. High performance supercapacitor for efficient energy storage under extreme environmental temperatures. Nano Energy 2014, 8, 231–237. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Kshetri, T.; Tran, D.T.; Nguyen, D.C.; Kim, N.H.; Lau, K.-t.; Lee, J.H. Ternary graphene-carbon nanofibers-carbon nanotubes structure for hybrid supercapacitor. Chem. Eng. J. 2020, 380, 122543. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR; Los Alamos National Laboratory: Los Alamos, NM, USA, 1994; pp. 86–748.
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Karki, S.B.; Andriotis, A.N.; Menon, M.; Ramezanipour, F. Enhancement of Electrocatalytic Activity for both Hydrogen and Oxygen Evolution Reactions of a Perovskite Oxide. J. Phys. Chem. C 2022, 126, 20011–20019. [Google Scholar] [CrossRef]
- Tsuji, E.; Motohashi, T.; Noda, H.; Kowalski, D.; Aoki, Y.; Tanida, H.; Niikura, J.; Koyama, Y.; Mori, M.; Arai, H.; et al. Brownmillerite-type Ca2FeCoO5 as a Practicable Oxygen Evolution Reaction Catalyst. ChemSusChem 2017, 10, 2864–2868. [Google Scholar] [CrossRef] [Green Version]
- Niu, S.; Li, S.; Du, Y.; Han, X.; Xu, P. How to Reliably Report the Overpotential of an Electrocatalyst. ACS Energy Lett. 2020, 5, 1083–1087. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.G.; Hwang, J.; Hwang, H.J.; Jeon, O.S.; Jang, J.; Kwon, O.; Lee, Y.; Han, B.; Shul, Y.-G. A New Family of Perovskite Catalysts for Oxygen-Evolution Reaction in Alkaline Media: BaNiO3 and BaNi0.83O2.5. J. Am. Chem. Soc. 2016, 138, 3541–3547. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Chen, D.; Liu, J.; Zhang, Z.; Shao, Z.; Ciucci, F. Water Splitting with an Enhanced Bifunctional Double Perovskite. ACS Catal. 2018, 8, 364–371. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karki, S.B.; Ramezanipour, F. Enhancement of Electrocatalytic and Pseudocapacitive Properties as a Function of Structural Order in A2Fe2O5 (A = Sr, Ba). Molecules 2023, 28, 5947. https://doi.org/10.3390/molecules28165947
Karki SB, Ramezanipour F. Enhancement of Electrocatalytic and Pseudocapacitive Properties as a Function of Structural Order in A2Fe2O5 (A = Sr, Ba). Molecules. 2023; 28(16):5947. https://doi.org/10.3390/molecules28165947
Chicago/Turabian StyleKarki, Surendra B., and Farshid Ramezanipour. 2023. "Enhancement of Electrocatalytic and Pseudocapacitive Properties as a Function of Structural Order in A2Fe2O5 (A = Sr, Ba)" Molecules 28, no. 16: 5947. https://doi.org/10.3390/molecules28165947




