The Adsorption Behaviors of CO and H2 to FeO onto CaO Surfaces: A Density Functional Theory Study
Abstract
1. Introduction
2. Results and Discussion
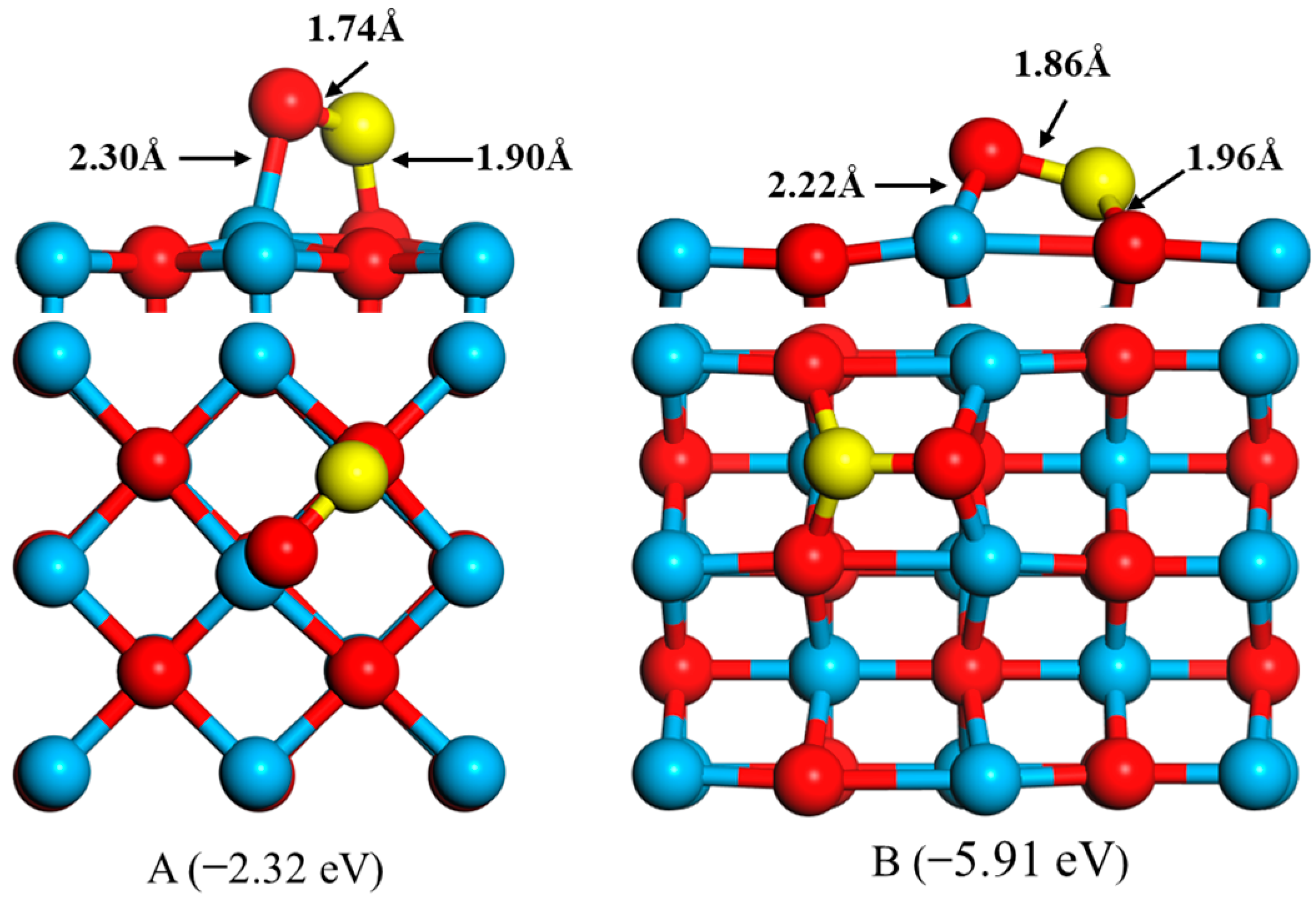
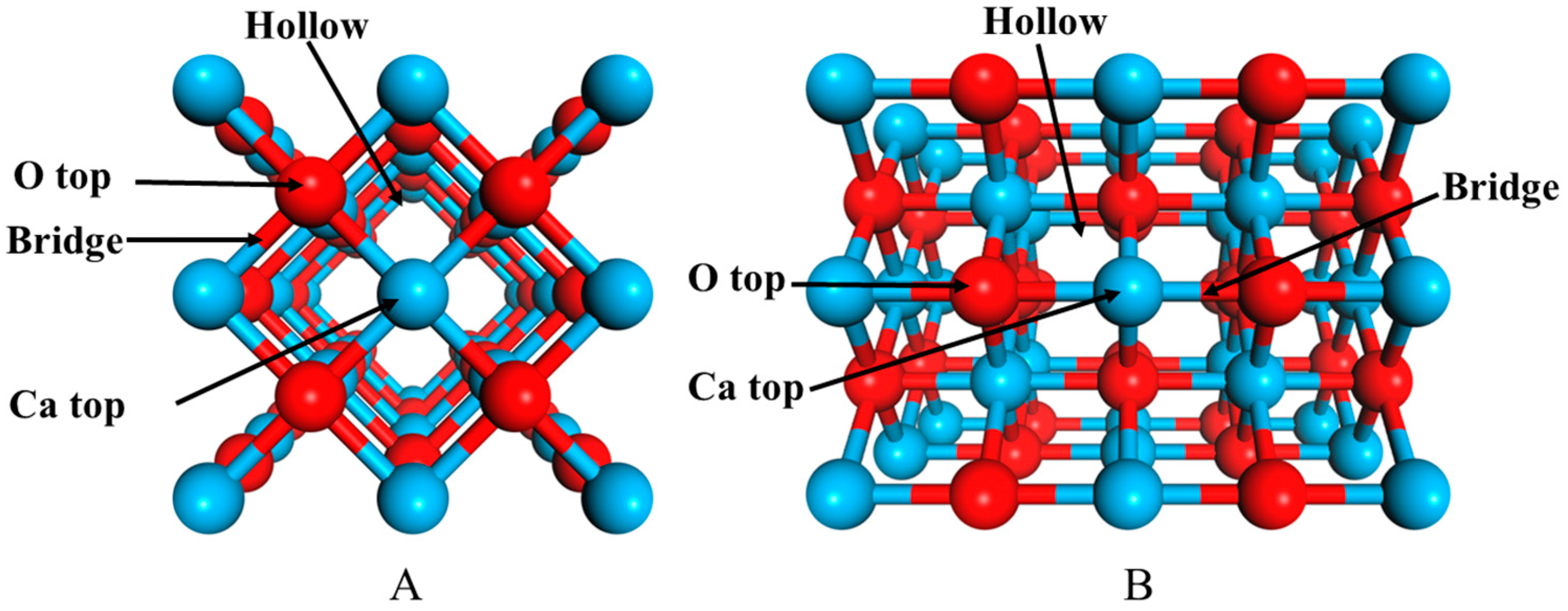
2.1. Adsorptions of FeO to CaO Surfaces
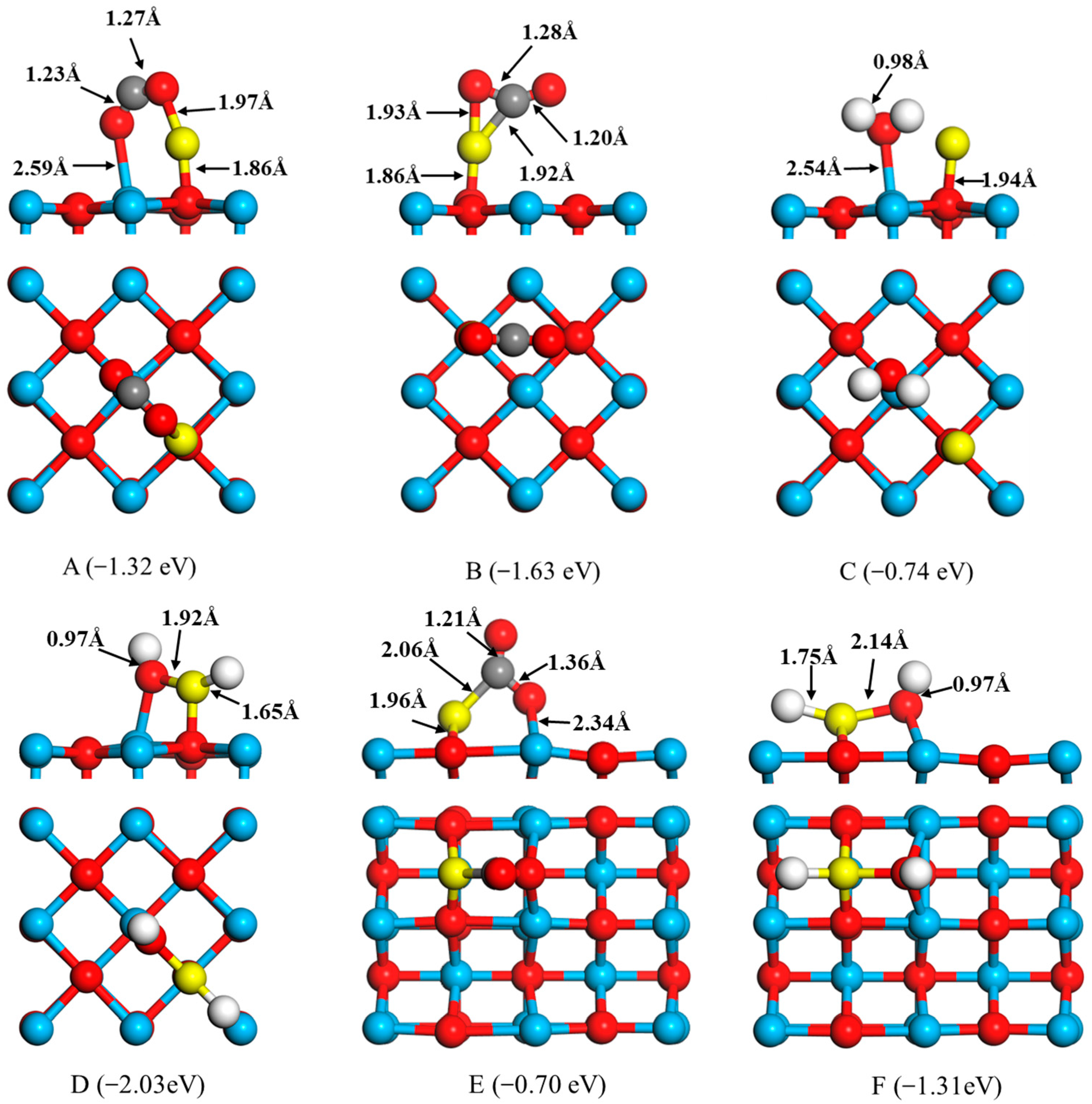
2.2. Adsorptions of CO and H2 to FeO onto the CaO Surfaces
2.3. Partial Density of States
2.4. Charge Transfer
2.5. Reaction Path of FeO by CO and H2 Molecule
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zieliński, J.; Zglinicka, I.; Znak, L.; Kaszkur, Z. Reduction of Fe2O3 with hydrogen. Appl. Catal. A Gen. 2010, 381, 191–196. [Google Scholar] [CrossRef]
- LMurr, E.; Foltz, J.V. Gaseous reduction of Fe2O3 compacts at 600 to 1050 °C. J. Mater. Sci. 1986, 21, 3889–3900. [Google Scholar]
- Saharuddin, T.S.T.; Samsuri, A.; Salleh, F.; Othaman, R.; Kassim, M.B.; Hisham, M.W.M.; Yarmo, M.A. Studies on reduction of chromium doped iron oxide catalyst using hydrogen and various concentration of carbon monoxide. Int. J. Hydrogen Energy 2017, 42, 9077–9086. [Google Scholar] [CrossRef]
- Sastri, M.V.C.; Viswanath, R.P.; Viswanathan, B. Studies on the reduction of iron oxide with hydrogen. Int. J. Hydrogen Energy 1982, 7, 951–955. [Google Scholar] [CrossRef]
- Yi, L.; Huang, Z.; Jiang, T. Sticking of iron ore pellets during reduction with hydrogen and carbon monoxide mixtures: Behavior and mechanism. Powder Technol. 2013, 235, 191–196. [Google Scholar] [CrossRef]
- Vinters, E.T.T.V. Gaseous reduction of iron oxides: Part I. Reduction of hematite in hydrogen. Metall. Mater. Trans. B 1971, 2, 3175–3188. [Google Scholar]
- Lin, H.Y.; Chen, Y.W.; Li, C. The mechanism of reduction of iron oxide by hydrogen. Thermochim. Acta 2003, 400, 61–67. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, H.; Li, H.; Zhu, Q. Study on Kinetics of Iron Oxide Reduction by Hydrogen. Chin. J. Chem. Eng. 2012, 20, 10–17. [Google Scholar] [CrossRef]
- Bahgat, M.; Khedr, M.H. Reduction kinetics, magnetic behavior and morphological changes during reduction of magnetite single crystal. Mater. Sci. Eng. B 2007, 138, 251–258. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, Z.; Chen, Z. Multistep reduction kinetics of fine iron ore with carbon monoxide in a micro fluidized bed reaction analyzer. Metall. Mater. Trans. B 2017, 48, 841–852. [Google Scholar] [CrossRef]
- Bonalde, A.; Henriquez, A.; Manrique, M. Kinetic analysis of the iron oxide reduction using hydrogen-carbon monoxide mixtures as reducing agent. ISIJ Int. 2005, 45, 1255–1260. [Google Scholar] [CrossRef]
- Kim, W.-H.; Lee, S.; Kim, S.-M.; Min, D.-J. The retardation kinetics of magnetite reduction using H2 and H2–H2O mixtures. Int. J. Hydrogen Energy 2013, 38, 4194–4200. [Google Scholar] [CrossRef]
- Jozwiak, W.; Kaczmarek, E.; Maniecki, T.; Ignaczak, W.; Maniukiewicz, W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl. Catal. A Gen. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, H.; Guo, Z. Micro behavior of the precipitation of metallic Fe in the reduction of Fe2O3 under CO Atmosphere. J. Iron Steel Res. 2012, 24, 26–28. [Google Scholar]
- Corbari, R.; Fruehan, R. Reduction of iron oxide fines to wustite with CO/CO2 gas of low reducing potential. Metall. Mater. Trans. B 2010, 41, 318–329. [Google Scholar] [CrossRef]
- Zhong, H.; Wen, L.; Li, J.; Xu, J.; Hu, M.; Yang, Z. The adsorption behaviors of CO and H2 on FeO surface: A density functional theory study. Powder Technol. 2016, 303, 100–108. [Google Scholar] [CrossRef]
- Umadevi, T.; Sah, R.; Mahapatra, P. Influence of sinter basicity (CaO/SiO2) on low and high alumina iron ore sinter quality. Miner. Process. Extr. Metall. 2014, 123, 75–85. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Z.; Guo, Z.; Tang, Q. Prevention of agglomeration/defluidization in fluidized bed reduction of Fe2O3 by CO: The role of magnesium and calcium oxide. Powder Technol. 2013, 241, 142–148. [Google Scholar] [CrossRef]
- Hayashi, S.; Iguchi, Y. Factors affecting the sticking of fine iron ores during fluidized bed reduction. ISIJ Int. 1992, 32, 962–971. [Google Scholar] [CrossRef]
- Komatina, M.; Gudenau, H.W. The sticking problem during direct reduction of fine iron ore in the fluidized bed. Metall. Mater. Eng. 2004, 10, 309–328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-L.; Tang, H.-Q.; Guo, Z.-C. Effects of CaO on precipitation morphology of metallic iron in reduction of iron oxides under CO atmosphere. J. Iron Steel Res. Int. 2013, 20, 16–24. [Google Scholar] [CrossRef]
- Geva, S.; Farren, M.; John, D.S.; Hayes, P. The effects of impurity elements on the reduction of wustite and magnetite to iron in CO/CO2 and H2/H2O gas mixtures. Metall. Trans. B 1990, 21, 743–751. [Google Scholar] [CrossRef]
- Zhong, H.; Er, D.; Wen, L. Theoretical study on influence of CaO and MgO on the reduction of FeO by CO. Appl. Surf. Sci. 2017, 399, 630–637. [Google Scholar] [CrossRef]
- Dong, C.; Sheng, S.; Qin, W.; Lu, Q.; Zhao, Y.; Wang, X.; Zhang, J. Density functional theory study on activity of α-Fe2O3 in chemical-looping combustion system. Appl. Surf. Sci. 2011, 257, 8647–8652. [Google Scholar] [CrossRef]
- Sun, X.; Kurahashi, M.; Pratt, A.; Yamauchi, Y. First-principles study of atomic hydrogen adsorption on Fe3O4 (100). Surf. Sci. 2011, 605, 1067–1073. [Google Scholar] [CrossRef]
- Qin, W.; Chen, Q.; Wang, Y.; Dong, C.; Zhang, J.; Li, W.; Yang, Y. Theoretical study of oxidation–reduction reaction of Fe2O3 supported on MgO during chemical looping combustion. Appl. Surf. Sci. 2013, 266, 350–354. [Google Scholar] [CrossRef]
- Lin, C.; Qin, W.; Dong, C. Reduction effect of ɑ-Fe2O3 on carbon deposition and CO oxidation during chemical-looping combustion. Chem. Eng. J. 2016, 301, 257–265. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Seriani, N.; Gebauer, R. Water adsorption and dissociation on α-Fe2O3 (0001): PBE+ U calculations. J. Chem. Phys. 2013, 138, 194709. [Google Scholar] [CrossRef] [PubMed]
- Cococcioni, M.; De Gironcoli, S. Linear response approach to the calculation of the effective interaction parameters in the LDA+ U method. Phys. Rev. B 2005, 71, 035105. [Google Scholar] [CrossRef]
- Rödl, C.; Fuchs, F.; Furthmüller, J.; Bechstedt, F. Quasiparticle band structures of the antiferromagnetic transition-metal oxides MnO, FeO, CoO, and NiO. Phys. Rev. B 2009, 79, 235114. [Google Scholar] [CrossRef]
- Eom, T.; Lim, H.-K.; Goddard, W.A.; Kim, H. First-principles study of iron oxide polytypes: Comparison of GGA+ U and hybrid functional method. J. Phys. Chem. C 2015, 119, 556–562. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, Y.; Dou, Y.; Li, K.; Yu, W.; Sheng, P. The Adsorption Behaviors of CO and H2 to FeO onto CaO Surfaces: A Density Functional Theory Study. Molecules 2023, 28, 5971. https://doi.org/10.3390/molecules28165971
Wang Z, Li Y, Dou Y, Li K, Yu W, Sheng P. The Adsorption Behaviors of CO and H2 to FeO onto CaO Surfaces: A Density Functional Theory Study. Molecules. 2023; 28(16):5971. https://doi.org/10.3390/molecules28165971
Chicago/Turabian StyleWang, Ziming, Yaqiang Li, Yaping Dou, Kejiang Li, Wanhai Yu, and Pengcheng Sheng. 2023. "The Adsorption Behaviors of CO and H2 to FeO onto CaO Surfaces: A Density Functional Theory Study" Molecules 28, no. 16: 5971. https://doi.org/10.3390/molecules28165971
APA StyleWang, Z., Li, Y., Dou, Y., Li, K., Yu, W., & Sheng, P. (2023). The Adsorption Behaviors of CO and H2 to FeO onto CaO Surfaces: A Density Functional Theory Study. Molecules, 28(16), 5971. https://doi.org/10.3390/molecules28165971






