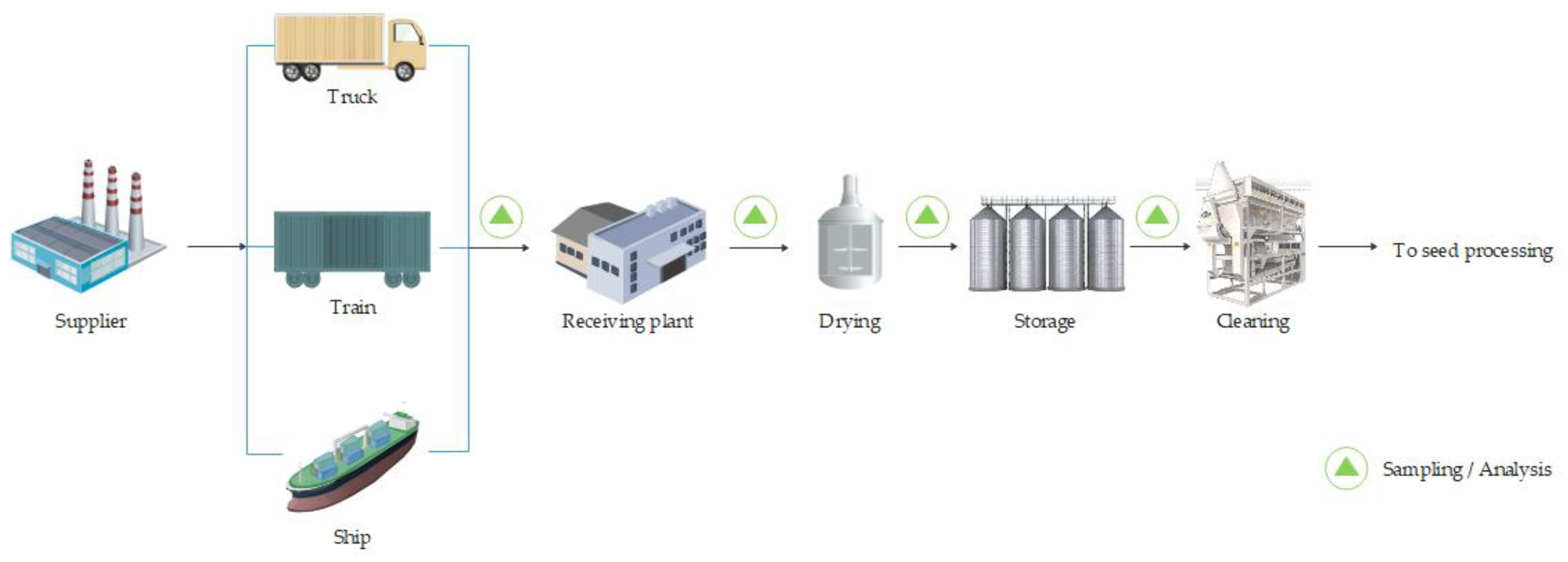
2. Oilseed Storage and Cleaning
The production of top-quality oils and meals requires the use of high-quality raw materials that are not damaged during transport, storage or processing. Seed storage is the step before the crushing and refining stages. Typical operations associated with storage include receiving, sampling, pre-cleaning, drying, storage and cleaning before processing [
5], as shown in
Figure 1. The main objective of storage is to keep the grain safe and deliver it to the processing plant at the right time and in the right condition.
Seeds arrive at the plant on an intermittent basis, by ship, train or truckload, in batches ranging from a few bags to loads of up to 60,000 tonnes. Each load of seed is controlled for weight and quality on receipt. Imported seed is usually also inspected on departure, while for locally harvested seed, the inspection at the plant may be the first it undergoes.
First, the received seed is weighed with weighbridges according to well-defined and fixed procedures to prevent fraud and ensure the absence of dead weights on entry and exit. The characteristics of the product, and consequently its economic value, can vary greatly depending on moisture, cracks, heat damage and other factors. When the product is received in storage plants, the quality of the raw material must be carefully and unequivocally controlled because it determines the type of processing and thus the costs, the type and the duration of storage. The moisture, protein content, oil content, presence of foreign matter and integrity of the seeds are periodically controlled, as deteriorated raw materials can cause serious capacity losses during refining. Crude oil obtained from damaged seeds (e.g., during harvest, transport or storage) may have a higher free fatty acid content, requiring further steps in the refining process, and exhibit increased oxidation resulting in a reduced stability [
6].
The foreign matter (up to 2%) consists of naturally occurring sticks and husks, metals and stones accumulated during processing, as well as weed seeds and other grains, and is generally removed twice, once before storage and then at the beginning of the continuous process. The residual metals must be removed first to avoid any damage to the equipment and are magnetically separated using plate magnets, drum magnets and magnets suspended from a conveyor belt. Sticks and pods are generally larger and lighter than seed materials. Therefore, they are removed by both course screening and aspiration. Fine screening removes weed seeds, sand and soil. Stones are heavier than ground nuts and must be removed by gravity separation in machines called destoners [
5].
The presence of impurities has a very negative impact on the sensory properties and chemical quality of oils, especially those obtained by mechanical pressing and not refined. Dimić et al. [
7] showed that the aroma (smell and taste), the colour and the oxidative stability of mechanically extracted oils are negatively affected by the presence of impurities. Usually the impurities differ so much from the seed in terms of density and flow resistance that their separation does not pose any difficulties [
8]. The contaminants removed by the magnet and scalper are normally disposed of as waste.
There are several factors to consider during storage, such as the humidity, oxygen, temperature and storage time. The temperature of the grain is monitored daily in order to immediately process the grain if heating occurs (temperature exceeds 40 °C). The rise in temperature is in fact seen as a precursor to qualitative damage to the seed due to the seed’s respiration processes triggering exothermic reactions. With a temperature of 70 °C, the seed is damaged within a few hours [
5].
Storage stability can be affected by microbial, physical and chemical changes in the grain. For example, microbial growth can prompt spoilage, characterised by the development of sour tastes and unpleasant aromas, as well as deterioration of the physical structure. In addition, the growth of pathogenic microorganisms makes the products obtained from the grain unsafe. Water activity (a
w) has become one of the most important intrinsic properties in predicting the survival of microorganisms in plant matrices and foods due to its direct influence on product quality and stability. In general, at a
w values below 0.61, bacterial proliferation is inhibited [
9]. In addition, many types of chemical reactions related to grain deterioration have been studied as a function of a
w (e.g., hydrolysis, oxidation, enzymatic activity), showing that a
w is a key factor in predicting grain storage stability. For safe long-term storage, the moisture content must be reduced from 20% in the freshly harvested grain to less than 13% [
5]. Moisture is localised in the non-fatty portion of the seed, where the critical moisture content reaches about 15%. The moisture content of the whole seed therefore depends on its oil content, e.g., the critical moisture content accounts for 9% for a seed with 40% oil and 8% for a seed with 47% oil [
3]. Soybeans can be safely stored at a moisture content of 11% or less for 1 year or longer, while rapeseed and sunflower seeds require 8% or less. In general, a high moisture content in the seed (above 14–15% moisture) and high a
w accelerate the lipolytic enzyme reactions and increase the content of free fatty acids in the oils [
10].
Grain drying can be performed using a vertical column system by direct fire, but today, air dryers are an essential part of modern storage facilities to preserve the quality of oilseeds [
10]. Steam and even solar energy are also used as heat sources in some systems. The higher the drying temperature, the faster the drying process, but excessively high temperatures can have negative effects on the grain. For example, temperatures above 63 °C lead to a darker colour of the flour and oil, denature the proteins and increase the content of non-hydratable phosphatides in the crude oil [
5]. Oilseeds are generally stored in vertical concrete or steel silos, which are usually galvanised, or in horizontal storage silos. Facilities that can hold seeds with high moisture content and store them for longer than 2 weeks should equip at least some silos with temperature-measuring devices and ventilation systems. However, ventilation can be more damaging than beneficial if it is not used properly. It is therefore important that fans are only operated when the air temperature is at least 3 °C cooler than the matrix and when the relative humidity is low [
3].
3. Oilseed Preparation
Seed preparation involves a series of steps that make the seed suitable for extraction. Good seed processing results in high extraction yields (low residual oil content after extraction) and high-quality products (crude oil and meal) while keeping production costs low. In fact, the efficiency of oil extraction is strongly influenced by seed processing.
The cell structure of oilseeds contains hundreds of very small oil bodies (e.g., 0.5–3.0 μm diameter in rapeseed) anchored to the inner surface of the cell wall and the outer surface of the protein bodies [
11]. Although oilseeds generally have a high oil content (20–50%
w/
w), the oil is firmly bound in the cell; thus, mechanical action is required to make it accessible for subsequent solvent extraction. However, the oilseed flakes, pellets or cake must be strong enough to withstand the impact of the solvent in percolation extractors but permeable enough for the solvent to penetrate their structure. The process presented here is a traditional method for oilseed preparation. Of course, each grain has its own characteristics, so different processes have been developed over the years. The main aims of the oilseed preparation include: weakening or breaking the walls of the oil-containing cells, increasing the oil extraction by pressing seeds with a high oil content before solvent extraction and shaping the material to facilitate solvent access to the oil [
3]. Typical steps in oilseed processing include scaling, cleaning, cracking, conditioning (or cooking) and flaking. Depending on the process and the oilseed to be processed, drying and dehulling can be used, as well as expanders and collet dryers/coolers. After the preparation process, the matrices are fed into the extraction system. The general process of oilseed processing is shown in
Figure 2.
Once the seed arrives at the processing plant, it is usually weighed and then sent to the cleaning process. These two processes are generally similar to those in the storage plants (described above). The traditional process continues with cracking; a series of two or three wavy high corrugated rollers rotate at relatively high speed, breaking the grain into several pieces. Rollers can have different configurations: pairs of horizontal corrugated rollers that rotate, rollers that rotate in a cylinder or pairs of rollers cavitated instead of toothed. The work capacity depends on the size of the machine but also varies from seed to seed. Modern cracking mills can process up to 1000 tonnes per day of oleaginous materials each [
5]. In addition, other technologies can be used for seed crushing, usually during the dehulling process. These include hammer mills and disk attrition mills, where the seed is fed through a hopper into the centre of vertical, corrugated disks. From here, the particles are thrown outwards, where they are collected. Another technique is based on pneumatic impact, where the seeds are blown against a wall, causing them to break [
8].
Many oilseeds are dehulled during preparation. Extraction without dehulling is possible, but the hull usually contains no fats and thus reduces the capacity of the plant. In addition, the hulls may contain components that should later be removed from the oil (e.g., waxes, pigments) so as not to reduce its quality [
7]. Even before screw pressing, it is better to remove the hulls because they absorb some of the oil released by the grains. In addition, the hulls are rich in fibres and are more abrasive, leading to a higher wear of the press and horsepower consumption [
12]. The proportion of the hull in the total weight of the seeds varies greatly, e.g., it is 7% for soybeans, 15% for rapeseed and 30% for sunflower seeds. Rapeseeds are usually not dehulled, while the hulls of sunflower and soybean seeds are usually removed. Although the proportion of hulls in soybeans is low, they are very often dehulled to obtain a protein-rich meal after extraction [
8]. The dehulling is essential when the final product is not an isolated protein but a concentrated one. In other words, when proteins are extracted for further purification (during isolated protein production), it is not essential to start from a previously dehulled material, as the hulls contain few soluble substances that can cause problems during purification [
13]. Various methods are used to separate the hulls from the kernels, including screening with vibrating sieves, air separation and electro-separation. Recent air separation technologies involve the use of cylindrical aspirators with multiple cascades. In these systems, the matrix is continuously fed to the upper part of the aspirator. In successive separation cascades, the material is repeatedly exposed to a transverse airflow that blows the light particles (hulls) to the centre, while the heavy particles (kernels) are directed to the product outlet.
Figure 3 shows the usual soybean dehulling process, in which the hulls are separated from the kernel with a stream of air and collected in a cyclone or bag philtre. Since some kernel particles are sucked in with the husks, it is customary to carry out a second separation of the hull’s fraction. In electro-separation, the mixture of hulls and kernels is passed from a vibrating chute onto a roller connected to a corona electrode. The electric field generated by the corona electrode causes the hulls and kernels to be deflected differently; the hulls are deflected more than the kernels and are therefore collected in different boxes [
8].
Hot dehulling (dehulling the seed while it is still hot) is widely used today, especially in the processing of soybeans. This results in energy savings and in a lower production of fine particles compared to the conventional system, using cold grain. Common hot dehulling systems consist of drying the grain from storage moisture to process moisture, dehulling the seed while it is still hot and delivering the conditioned cracks to the flakers without an intermediate cooling phase. In addition, these systems reduce residual oil content and refining losses.
Due to the difficulties associated with efficient dehulling before extraction, post-extraction dehulling methods have been developed. Solutions have been proposed for both rapeseed and sunflower. In some systems, hydrocyclones are used to separate a fibre and a protein fraction. In this way, Sosulski and Zadernowski [
14] obtained a rapeseed meal with a protein content of 45% after solvent extraction, recovering the 66% of the initial meal. McCurdy and March [
15], also using solvent-extracted rapeseed, washed the meal with water at pH 4.5 (9:1
w/
w water/meal, 22 °C). After drying, the meal was finely ground and sieved through a vibrating sieve (325 mm). In this way, they achieved an increase in the protein content of the meal from 40.4% to 46.7%, but the yield of the protein-rich fraction was relatively low (39% of the treated mass). To obtain meals with a higher protein concentration, Murru and Lera Calvo [
16] described a process combining milling, air classification and gravity separation. Starting from a dehulled industrial meal (36.5% protein), this system yielded a fraction with 43% protein, corresponding to 65% of the initial flow. Currently, this process is used in the industry for the production of meal with a high protein content [
13].
After cracking and/or dehulling, the seed is processed in a conditioner (or cooker) where gentle heat is applied to soften the seeds (heated to a temperature between 60 and 75 °C) and make them malleable for subsequent fine reduction and/or flaking. Apart from cold pressing, all oil extraction processes require heating and sometimes further drying of the oilseeds before extraction. There are different types of equipment for heating/drying oilseeds. The conditioning is important to achieve a high oil yield and to give the seed the right elasticity, preventing the flakes from finely crumbling at low humidity. The most common are a drum conditioner, stack conditioner, plugged flow conditioner and hot air conditioner [
5]. The stack conditioner (vertical), usually coupled with screw presses, and drum conditioner (horizontal) are the most common. These first ones consist of cylindrical vessels with a multitude of horizontal trays. The heat to raise the particle temperature and evaporate the moisture is conducted into the oleaginous materials from the upper surface of the trays filled with high-pressure steam.
The stack conditioner requires limited floor space but has the disadvantage that the residence time of the particles varies greatly. Drum conditioners consist of a series of horizontal drums (steam jacketed) that can be heated with steam up to 10 bar. The machine is slightly inclined, with the outlet end lower than the inlet end, so that the material advances each time it goes down. The rotation of the pipes raises the material continuously and it is discharged after about a third to half a revolution. They offer more consistent quality as the controlled seed flow provides more even heating and residence time [
8].
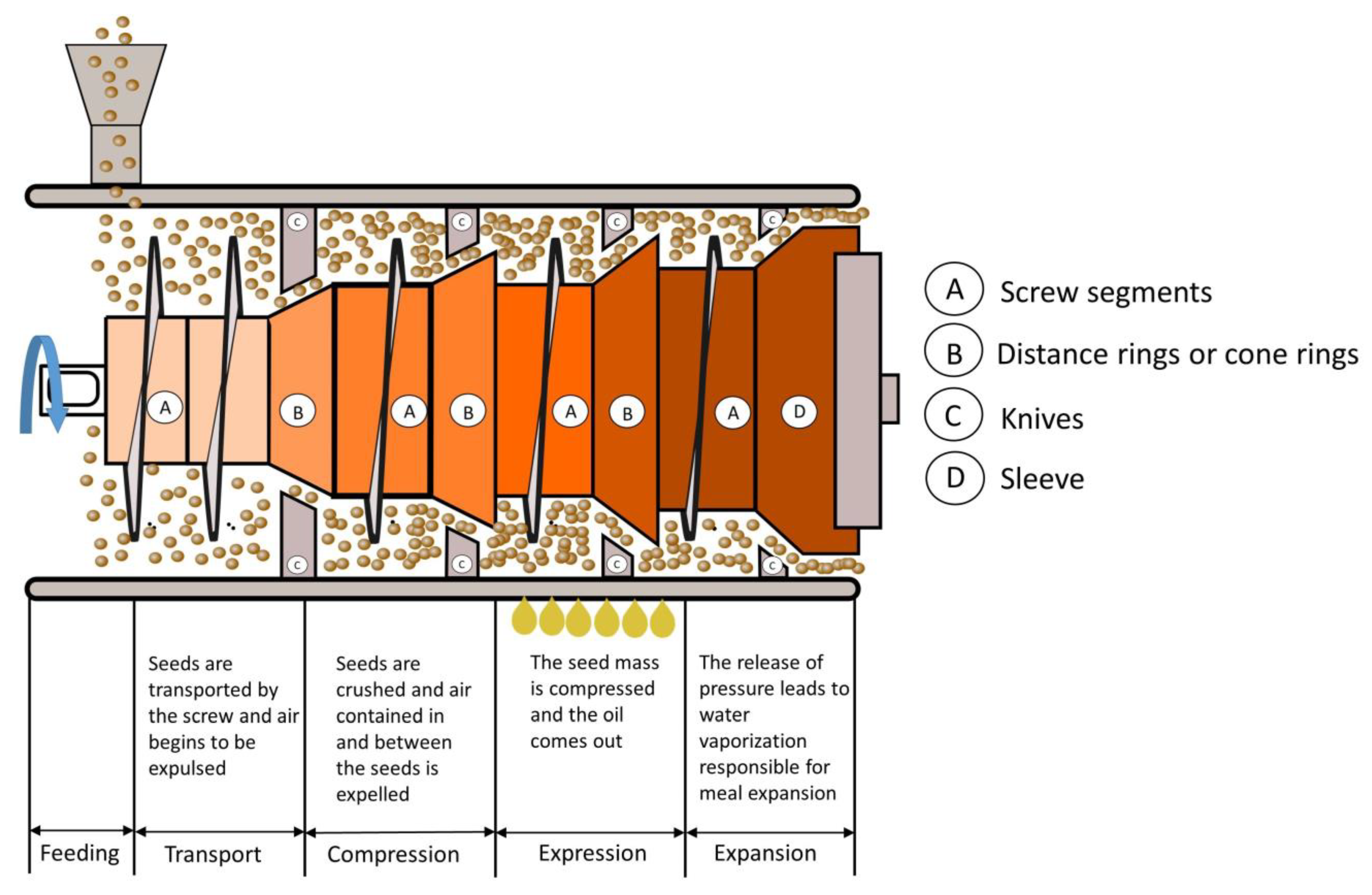
Generally, oilseeds are flaked before solvent extraction. A flaking mill has two large diameter rollers that rotate in opposite directions and are forced together by hydraulic cylinders. As the seeds are pulled through the flaking mill, they are stretched and flattened. The flaking process helps to ensure more uniform cooking prior to screw pressing and allows more efficient extraction of the oil in the solvent extraction plant [
12]. The shape of the flakes, with their large surface-to-volume ratio and the small distance between the oil cells and the flake surface, favours contact between the solvent and the solid and the migration of the oil into the solvent. However, flaking releases moisture that migrates to the flake surface and can hinder solvent penetration into the material. To overcome this problem, the surface moisture is removed by passing a strong stream of air through the discharge hoppers and conveyor belt of the flaker. Depending on the amount of seeds processed, there are different sized rollers (70–157 cm or larger) that press the seeds into flakes about 0.20–0.37 mm thick [
5]. The characteristics of the material for good flaking are a moisture content of about 10% and a temperature of about 70 °C.
Before mechanical screw pressing, the flakes can be cooked in systems similar to those used for conditioning. Oilseeds are cooked or tempered to denature proteins, release oil from cells and inactivate enzymes. Cooking is conducted at high humidity, usually 9–12%, and the flakes are then dried to bring the humidity down to 2–5%. This is performed in ventilated vessels, usually with a stream of air flowing through them, and with a heat source to raise the temperature of the flakes to 104–120 °C [
12].
Another process widely adopted by oilseed processing plants is the use of expanders. The expander consists of a short heat treatment at high vapour pressure followed by a rapid pressure drop to atmospheric pressure [
17]. Expanders are mainly used for soybeans and cottonseeds after conditioning and flaking of the matrix. After the expansion process, the density and porosity of the matrix increase significantly, and the seed proteins are denatured. These effects deconstruct the seed and increase the mass transfer during oil extraction. Extrusion and the expander differ mainly in the source of heating energy. While the expander heats the seed by saturated and superheated steam injection, the heating in extrusion derives from mechanical sharing between the screw and the container wall. The implementation of expanders in oilseed preparation systems increases the bulk density of the matrix entering the extractor, reduces the solvent retention of the meal in the desolventiser, improves percolation in the extractor, reduces the levels of non-hydratable phospholipids in the degummed oil and improves the qualitative–quantitative production of lecithin (increase in lecithin recovery by 50–100%) [
18]. The high pressure reduces the required residence time by a factor of sixty to a residence time of 30 s [
19]. The short residence time allows the use of a much smaller and less expensive vessel and leads to a better quality of the oils and meal. The system is, however, limited in its application to products with a low oil content. Seeds with a high oil content (>40%), such as rapeseed and sunflower seeds, do not usually allow sufficient pressure build-up in the units to achieve similar effects as seen with soybeans [
20].
Expanders work in the same way as mechanical screw presses but with a lower pressure. The system consists of a horizontal cylinder fed by a screw that transports the material. The cylinder can be heated and is endowed with nozzles for injecting water and live steam to increase the temperature, humidity and pressure inside the cylinder. In the first section of the system, the seed is brought to a water content of 10–15% and heated to 105–120 °C. Subsequently, the mechanical pressure in the extrusion phase leads to a further increase in temperature (about 160 °C). The material is forced through the outlet, which can be a plate with several openings or a hydraulic cone. When the product leaves the expander and is released to the ambient pressure, there is rapid evaporation of the water and expansion of the product, which takes on a sponge-like consistency. There are different types of expanders: closed-wall and slotted-wall expanders. Unlike closed-wall expanders, slotted-wall expanders allow the controlled release of excess oil through the slotted wall and produce collets with 20–30% oil. With this configuration, it is possible to process non-pre-pressed materials with a high oil content (e.g., full-fat sunflower), producing collets ready for solvent extraction [
12]. On leaving the expander, the hot, moist matrix is often cooled (to around 60 °C) and dried (2% moisture reduction) before extraction. Direct air cooling is the most common method for mass cooling after expander treatment. Dryers also allow the matrix to reach a more uniform humidity and temperature. During extraction, an uneven matrix can result in a miscella that is more prone to foaming, coating or clogging the evaporator tubes during second effect evaporation.
Alternative techniques for cooling the collets have been proposed to reduce energy consumption. These include the use of carbon dioxide (CO
2) snow, which is produced when liquid CO
2 is released at high pressure through a special spray nozzle at atmospheric pressure. The possibility of using CO
2 snow to cool the collets of soybean expansions was discovered about 25 years ago [
19]. However, this method proved to be not cost-effective, as a cryogenic reservoir of liquid carbon dioxide must be available on site and the CO
2 often has to be sourced from distant producers. In contrast, the use of water indirect heat exchangers proved more efficient, consuming up to 90% less energy than other technologies. These systems consist of one or more rows of vertical, hollow, stainless-steel plates in which the product flows slowly by gravity (residence times of 5–10 min). The cooling water flows through the plates in countercurrent to the flour tongs and cooling is based entirely on conduction. The system is usually connected at the bottom to an extraction screw press that generates a mass flow and regulates the flow rate. This process results in remarkably stable and uniform final product temperatures. As the air does not encounter the product, the risk of bacterial contamination and a change in the moisture content of the product is avoided. Finally, the water is used repeatedly in a closed loop system [
19].
For grains with low oil content, such as soybeans, the flakes are usually delivered directly to the solvent extraction plant. For oilseeds with higher oil content, such as sunflower or rapeseed, or for virgin oils (extracted mechanically only), the flakes are usually delivered to a mechanical pressing plant. For complete oil extraction, after mechanical pressure, cake is usually extracted with solvent.
3.1. New Sampling Techniques for Heat-Damaged Imported Seeds
Brazil, the United States and Argentina are among the main producing and exporting countries of oilseeds, which are often exported overseas. China is the world’s largest importer of soybean. Oilseeds are therefore often transported over long periods of time and can be damaged by high storage temperatures or poor ventilation during transport. Heat damage directly affects the quality of the product and significantly reduces the value of the seeds. In soybeans, heat damage negatively affects the properties of the protein isolate (protein denaturation) and the oil (reduced polyunsaturated fatty acid content and increased
n-hexaldehyde content). Assessing the quality of imported oilseeds is therefore essential. The current method for identifying heat-damaged oilseeds is manual sorting based on colour differences. However, this form of inspection is time-consuming, and the results are inevitably subjective. Furthermore, denatured proteins associated with heat damage do not always cause discolouration of the oilseeds. To improve the accuracy of identification, mechanised control has been introduced, as a fast, accurate, objective and reproducible method. However, this automated vision cannot provide detailed information on chemical composition because it is limited to the visible range. To overcome this problem, Near Infrared Spectroscopy (NIR), which has emerged as a non-destructive identification technique for studying the physical and chemical properties of materials, can be exploited. Indeed, changes in oilseeds affect light scattering and energy absorption patterns, resulting in differences in NIR absorption between heat-damaged and healthy oilseeds [
21,
22]. In addition to NIR spectroscopy, the emerging hyperspectral imaging (HSI) technology offers the advantages of rapid and non-destructive analysis. HSI also integrates spectral information with spatial information from imaging techniques. Furthermore, HSI requires no sample preparation and scans many samples simultaneously. A recent study investigated the possibility of identifying heat-damaged soybeans using HSI. The results show that the HSI technology is an accurate, effective and non-destructive technique for classifying sound- and heat-damaged soybeans [
23].
3.2. Microwave, Ultrasound and Pulsed Electric Fields Pre-Treatments
Recently, Koubaa et al. (2016) described the use of new technologies such as ultrasound (US) and MW to improve the yield and quality of oil extraction from oil crops. In the extraction of plant matrices, a partially damaged cell wall significantly improves the accessibility of the oil for extraction. In all the reported articles, US was used during extraction with various solvents (e.g., hexane, isopropanol, supercritical CO
2) and not as a pre-treatment of the matrix [
24]. US produces acoustic cavitation, which consists of the formation, growth and decay of gaseous bubbles in a liquid. The cavitation effect on the matrix improves the extraction yield through the mechanisms of erosion, fragmentation, sonoporation and the so-called ultrasonic capillary effect [
25]. Zdanowska et al. [
26] evaluated the effects of pre-treatment with ultrasound on the process of the continuous oil pressing of rapeseed. Pre-treatment with US did not significantly influence the oil yield, but the temperature during pressing was much lower and the flow rate of the oil was higher over time. In summary, the energy efficiency of the press was about 25% higher when the seeds were pressed after pre-treatment with US. However, some unfavourable changes in the oxidation stability of the oil were observed after US pre-treatment, and the need for additional drying of the seeds before pressing could be economically disadvantageous. As far as the authors are aware, the industrial use of US to pre-treat the matrix before oil extraction has only been used in the production of extra-virgin olive oil (Clodoveo et al. [
27]). The use of a continuous flow sono-heat exchanger, which combines the mechanical energy of US with the ability to modulate the heat exchange of the olive paste, eliminated malaxation, increased the extraction yield and improved the polyphenol content of the oil.
Microwave (MW) irradiation is an energy-efficient alternative to conventional heating treatments. Among the various new methods available, MW-assisted pre-treatment of oilseeds has proven an efficient method for producing high quality vegetable oil with high nutritional aspects. Furthermore, it can increase oil extraction yields compared to conventional extraction under the same conditions. MW pre-treatment of oilseeds increases mass transfer as it favours the rupture of the cell membrane. In addition, the formation of pores in the cell walls makes them more permeable to the passage of oil during extraction. An example of a continuous industrial-scale plant for the pre-treatment of oilseeds with microwaves was proposed by Koubaa et al. [
24]. In such a plant, the seeds are transported by a screw conveyor along horizontal pipes coupled with MW horns, into which steam is injected (
Figure 4). In addition to the higher yield and high oil quality, MW treatment allowed lower energy consumption, faster processing times and lower solvent consumption compared to conventional methods. However, high MW power or excessively long pre-treatment times can have a negative effect on oil quality, e.g., by reducing the amount of polyunsaturated fatty acids [
28].
Azadmard-Damirchi et al. [
29] showed that a short MW pre-treatment of rapeseed (2–4 min) resulted in an increase by 10% in the oil yield extracted with the press. In addition, the pre-treated oil was richer in phytosterols (15% increase), tocopherols (55% increase), canolol and phenolic compounds, resulting in higher oxidative stability compared to the oil extracted without pre-treatment. Zhou et al. [
30] investigated the influence of MW pre-treatment on the flavour properties of rapeseed oil extracted by cold pressing. The profile of the volatile components of rapeseed oil was positively affected by MW pre-treatment. A treatment at 800 W for 6 min was indeed sufficient to improve the flavour of rapeseed oils by releasing the pyrazine compounds that impart a pleasant roasted flavour to the oil. The authors concluded that the use of this technology as a pre-treatment process would allow the production of pressed oils with a special flavour [
30]. Despite the advantages presented, MW pre-treatment of oilseeds in large-scale plants has not yet been reported. There is also a lack of information on the economic costs of this process in large-scale production.
Due to the electroporation of the cell membrane, pulsed electric fields (PEF) are used as an innovative pre-treatment to facilitate the recovery of intracellular material, such as oil, in the subsequent extraction step. Pre-treatment of rapeseed and sunflower seeds with PEF has proved to increase oil yield in both mechanical and solvent pressing extraction. A continuous flow pre-treatment and/or extraction system under combined US and PEF conditions is shown in
Figure 5 [
25]. Guderjan et al. [
31] showed that pre-treatment with PEF increases the rapeseed oil extraction yield and the concentrations of total antioxidants, tocopherols, polyphenols and phytosterols in the oil. Moradi et al. [
32] evaluated the effects of pre-treatment with US and PEF both individually and in combination on the extraction of sunflower oil with hexane. The results showed the superiority of PEF treatment over other methods in terms of oil yield. Scanning electron microscopy showed that the surfaces of the PEF-treated samples were more porous, and thus the solvent diffusion rate may be much higher. However, PEFs require an aqueous medium to be effective, so the matrix need to be soaked or directly immersed in water before treatment. This requires an additional, energy-consuming drying step of seeds after pre-treatment. In addition, both studies described lab-scale systems, so further investigations are needed on the application of this technology on a pilot scale.
3.3. Instant Controlled Pressure Drop Technology
Instantaneous controlled pressure drop (DIC) technology is a thermomechanical process that uses a short-term treatment at high temperature and pressure. In DIC, biological matrices are subjected to a saturated vapour pressure of 100 to 900 kPa for a few seconds, followed by an abrupt controlled pressure drop at a rate of more than 500 kPa per second, leading to an absolute ultimate vacuum of 10 to 5 kPa. The instantaneous pressure drop leads to instantaneous autovaporisation of the water, rapid cooling of the biological products and expansion and texturization of the matrix. DIC is widely used in the food industry, e.g., for microbial decontamination, deodorisation, swell drying and texturization [
33].
DIC has also been investigated as a possible processing technique for oilseeds prior to oil extraction. The porous structure achieved by DIC improves mass transfer and increases both the effective diffusivity and accessibility of the matrix, thereby improving the overall kinetics of oil extraction [
34]. DIC has a very high texturing capacity, which can lead to rupturing of the cell walls. The intensity of texturization usually depends on the amount of vapour produced by autovaporisation, which is directly related to the temperature drop. Oilseed treatment with expanders, which is usually carried out at an absolute vapour pressure of 0.6 MPa and a treatment temperature of about 160 °C, results in a temperature drop of 60 °C when decompressed to atmospheric pressure. Under similar treatment conditions, DIC flash decompression at a vacuum of 4 kPa (equilibrium end temperature of about 30 °C) results in a temperature drop of 130 °C. The texturing effect of DIC is therefore greater [
17]. A comparison between DIC and expander treatments is shown in
Figure 6.
Pech-Almeida et al. [
33] provided an overview of the use of DIC in the food industry, including its use in the extraction of oilseeds. Several studies investigated the effects of DIC on the extraction of oilseeds (such as rapeseed, soybean and sunflower) by mechanical and solvent extraction. In the most recent paper, Jablaoui et al. [
17] compared the effects of expanders and DIC on the extraction of soybean oil with
n-hexane. The use of DIC resulted in higher extraction yields with faster extraction kinetics. To achieve the same yield as that obtained in 160 min for cracked or cracked/flaked soybeans, the expander required 120 min, compared to 35 min for DIC. Due to the brevity of the heat treatment and immediate cooling, DIC maximally preserved the quality of the soybean oil, with fatty acid concentrations almost identical to those of the untreated seeds. The authors concluded that pre-treatment with DIC can substitute expanders by reducing the extraction time and increasing yields, while maintaining oil quality. However, the large quantities of treated seeds in the food industry lead to questions regarding the feasibility of this technology. A flow-through concept for DIC is still a long way off today and further research in this direction is needed.
An alternative pre-treatment method to intensify the extraction process is to bring the natural material into contact with CO
2 under high pressure and then decompress it under atmospheric conditions. This pre-treatment showed a positive effect on the extraction kinetics, reduced solvent consumption and, in some cases, improved the extraction yield. The efficiency of pre-treatment with CO
2 depends on the exposure time to the dense gas atmosphere, the pre- and post-expansion pressure, and the decompression rate [
35]. Meyer et al. [
35] studied the effect of rapid CO
2 decompression pre-treatment on rapeseed and sunflower seeds. A significant positive effect on extraction directly related to the pre-treatment was evident for sunflower seeds but not for rapeseed. The authors concluded that the mechanism controlling the process is the macroscopic destruction of the material. Although it cannot completely replace conventional mechanical treatments, rapid CO
2 decompression could be applied in addition to those to possibly avoid the frictional heat stress of extensive mechanical pre-treatment methods.
5. Green Solvents’ Extraction
5.1. Alcohols
Ethanol has been most studied in the literature as a possible alternative to hexane for oil extraction. Not only is it safe for humans, but it can also be obtained from biological resources without producing toxic waste. Among the solvents listed in Directive 2009/32/EC, ethanol has a high acceptance and is the only solvent except for water and CO2 that is included in the list of solvents allowed in organic animal feed production.
The main problems related to oil extraction with ethanol are the low oil solubility (especially at low temperatures and in the presence of water) and the high latent heat of vaporisation (846 kJ/kg compared to 333 kJ/kg for hexane), which requires more energy for distillation. The low solubility of the oil in ethanol can be exploited to separate the oil after extraction by simply cooling the solvent. In this way, the oil can be recovered without the need to evaporate the entire solvent, thus reducing the costs associated with the higher energy required to regenerate the solvent [
13]. Johnson and Lusas [
57] proposed that the energy requirement of the entire process can be reduced by about 25% compared to the corresponding process with hexane, although it is not clear whether the drying of the oilseeds was taken into account. The problem with cold separation is the formation of a third emulsified phase containing gums at the interface between the solvent and oil phases. Hron and Koltun [
58] proposed a method to improve the decanting of miscella from ethanol extraction. The heterogeneous solution is processed through a phase separator where free and emulsified oil and gum are separated from oil-lean miscella. The proposed method involves heating of the interface to 38 °C, a temperature at which the gums lose some of their entrapped mixture, agglomerate and sink to the bottom of the oil phase. Then, the oil and gum phases are treated with caustic soda and centrifuged to produce semi-refined oil [
58]. Oliveira et al. [
59] investigated the composition of the two oil- and alcohol-rich phases formed during the cooling of the miscella as it leaves the extractor. The authors predicted an ethanol recovery of up to 98% when the mixture is cooled from 80 °C to 25 °C.
Another technique for solvent separation involves the use of ethanol-compatible membranes. The miscella can be significantly concentrated using nanofiltration membranes [
60]. The concentrated miscella could significantly reduce the energy required for distillation. Theoretically, then, it can be assumed that the energy cost for ethanol distillation is not an obstacle, as this step can be bypassed.
The composition of the extract obtained with ethanol differs from that of the oil obtained with hexane. The extraction of polar lipids (e.g., phospholipids) is more effective with ethanol due to its polar nature [
49]. Similarly, more polar lipids can be extracted when isopropanol is used, while more triacylglycerols are extracted when hexane is used. The solubility of oils in ethanol has been studied for most commercial oils;
Figure 13 shows the solubility of oil in absolute ethanol and isopropanol and azeotropic mixtures as well as of sunflower oil in various ethanol/water mixtures.
At the azeotropic concentration (95.6%), ethanol is saturated with about 1% oil at 40 °C and 8% at 80 °C, slightly above its boiling point at atmospheric pressure. In addition, the presence of free fatty acids has a significant influence on the solubility of oil in alcohols, so that these saturation values may vary. In general, at the temperatures achievable in industrial extraction plants, the oil concentration in the miscella is unlikely to exceed 8–10%. More solvent is therefore needed to extract the same amount of oil as with hexane (S/L ratio of 3–4 versus about 0.8–1 with hexane), resulting in higher process costs.
The most recent studies on the extraction of the major vegetable oils with alcohols are listed in
Table 4. Sawada et al. [
62] showed that an increase in water content in the ethanol strongly suppressed soybean oil extraction, while an increase in temperature favoured it. The opposite behaviour was observed for proteins: an increase in the water content of the solvent enhanced the extraction of these compounds, while increasing the temperature decreased the protein content in the meal. The fatty acid profile of the ethanol-extracted oils showed a composition typical of soybean oil, regardless of the extraction conditions. Toda et al. [
63] described the extraction kinetics of soybean oil and free fatty acids using ethanol with different degrees of hydration (0 and 5.98% mass of water) at temperatures of 40, 50 and 60 °C. Increasing the degree of ethanol hydration suppresses the extraction of soybean oil but increases that of FFA, while temperature favours the solubility of both fatty compounds. Bessa et al. [
64] successfully used a multi-batch solid–liquid extraction system to simulate continuous countercurrent ethanol extraction of rice bran oil. The theoretical number of equilibrium steps required for the extraction of rice bran oil was higher for ethanol extraction than for hexane extraction. Ethanol extraction required working with a higher solvent to solid mass ratio. However, ethanol can extract the solid matrix completely in five steps (residual oil less than 0.5% in the meal). The fatty acid composition of the crude oil shows that the oil extracted with ethanol has the typical composition of rice bran oil. Capellini et al. [
49] showed how the water content in the solvent and the temperature of the process strongly affected the properties of the protein fraction of the meal. The nitrogen solubility index decreased from about 40% in anhydrous solvents to 17% and 15% in aqueous ethanol and isopropanol, respectively. Minor nutraceutical compounds (e.g., γ-oryzanol, tocopherols and tocotrienols) were recovered more efficiently in oil extracted with aqueous ethanol and isopropanol than with hexane.
However, several obstacles make the ethanol non-competitive with hexane. The first is the need to dry the oilseeds (water content below 2–3%) to avoid water entering the system, which is dissolved by the ethanol, reducing its affinity to the oil. Conventional drying is associated with high energy costs. Secondly, the affinity of the matrix for ethanol is much higher than for hexane, which means that a higher quantity of solvent is retained and must be evaporated from the meal. Ethanol extraction also results in the denaturation of proteins, which lose their solubility on prolonged contact with this solvent. The displacement of structural water results in a conformational change due to the merging of domains with opposite electrostatic charges and the formation of new bonds [
13]. However, this loss of solubility is reversible after some hydration treatments, unlike that occurring when the proteins are exposed to heat. Thirdly, there is a significant risk of protein degradation in the meal due to the mass of solvent to be evaporated and the higher boiling point of ethanol. In addition, the use of direct steam should be avoided in order not to rectify the solvent at each extraction cycle. Desolventisation technologies other than those used in the existing plants should be used. These include the use of larger heating surfaces to work only with indirect steam or vacuum desolventiser. Both solutions are associated with higher capital investments. Fourth, the affinity of the solvent to the matrix, the difficulty of using direct steam and the need to maintain temperatures compatible with protein stability result in a much higher solvent content in the meal than in the case of hexane. This can lead to increased flammability of the meals.
Carré [
13] proposed a process in which the dehulled seeds are first cold pressed to mechanically extract a high-quality oil and then extracted with azeotropic ethanol to complete the defatting. Subsequently, a washing step with aqueous ethanol removes soluble carbohydrates, phenolic compounds and most glucosinolates. The meal is pressed to mechanically remove as much of the retained solvent before a gentle desolventisation. This model is more expensive than the current one, but the different products obtained (pressed oil, solvent-extracted oil, meal and an ethanol extract of lecithin, phenolic compounds, etc.) could make it economically attractive. Carré et al. [
69] proposed a cost estimation to evaluate the feasibility of a large-scale hot ethanol extraction process. The simulated extractor involved extraction at a temperature above the boiling point (90–95 °C) in a module consisting of hydrocyclones operating in partial countercurrent. The total cost of processing was estimated at EUR 47.4 per tonne of seed processed, which was higher than the cost of a conventional hexane process (EUR 30/tonne). The additional profit margin after processing costs (EUR 14.64 more than the hexane process for 1 tonne of seed) is mainly due to a better valuation of the meal and oil and a lower phospholipid and pigment content. In addition, in contrast to the conventional process with hexane, extraction with hot ethanol produces molasses, whose value was estimated at 70% of the meal price. However, the greatest uncertainty of the simulation relied on the performance of the process under real countercurrent extraction and solvent regeneration conditions.
Potrich et al. [
70] simulated the replacement of hexane by ethanol (hydro or anhydrous) as a solvent for the extraction of soybean oil. Different solvent recovery methods (simple distillation and extractive distillation with glycerol or monoethylene glycol) were evaluated for ethanol. An economic analysis and life cycle assessment of the process were carried out. The economic analysis showed that hexane possessed a net present value (NPV) about 10.2% higher than that of the best-case ethanol process (hydrous). However, replacing hexane with ethanol resulted in a lower global warming potential (GWP) by avoiding the emission of about 10,600 tonnes of CO
2 equivalent per year in an industry that crushes 125 tonnes of soybean per hour.
Citeau et al. [
68] evaluated the extraction of rapeseed oil with aqueous ethanol (92% and 96% by weight, respectively) and isopropanol (84% and 88% by weight, respectively) and compared them with hexane as a reference. The alcoholic solvents, together with the oil, extracted 11–15% non-lipid substances, increased the protein concentration to 42–43% and reduced the glucosinolates concentration to 7–19% in the meal, depending on the type of alcohol. In comparison, protein and glucosinolates concentrations after extraction with hexane were 38% and 25% in the meal, respectively. Alcohol extraction increased the protein content of the meal by 13% compared to hexane extraction but greatly reduced the solubility of the protein. The increased water content improved the extractability of the glucosinolates. Isopropanol with the highest water content reduced the glucosinolates concentration by 49–73% compared to meal extracted with other alcohols.
Isopropanol is another alcohol used in the extraction of vegetable oils. It is generally obtained by chemical synthesis, while production by fermentation is limited. However, isopropanol is more expensive than other alcoholic solvents, such as ethanol. Its main advantage is that oils are more soluble in this solvent than in ethanol, even in the presence of water (
Figure 13), and it would be possible to obtain miscella with 20% oil concentration. Compared to ethanol, isopropanol has a higher boiling point of 4 °C, but its latent heat of vaporisation of 666 kJ/kg is only 78% of that of ethanol, and its dielectric constant is lower (18.6 vs. 26.5) [
13]. On the other hand, the azeotropic water contents are different with 12.3% (isopropanol) and 4.4% (ethanol) by mass. Compared to ethanol, this solvent can represent a relatively easy substitute for hexane as it remains effective in the presence of water. However, it incurs additional costs compared to the hexane process. Furthermore, the literature lacks data on the effects of this solvent on rapeseed and sunflower seeds.
Comerlatto et al. [
71] suggested that for ethanol-rich mixtures, mass transfer is limited by convection at the solid–liquid interface, while for isopropanol-rich mixtures, internal diffusion limits the extraction process. An economic analysis considering solvent costs at different extraction yields evidenced that isopropanol is more suitable for low-yield extractions (less than 70%) and ethanol for high-yield extractions.
According to the work by Li et al. [
65], the extraction of oil from coarsely ground rapeseed was more promising using isopropanol (83.1%) and butanol (78.3%) than ethanol (22.8%) (analytical grade solvents). The oil extracted in isopropanol was 94.7% TAG. Perrier et al. [
67] obtained a higher extraction yield with isopropanol than with aqueous ethanol (96%), even with the use of ultrasound. Compared to the diffusivity of oil in hexane, the diffusivity in isopropanol and ethanol was slower. In another study by Sicaire et al. [
66], the extraction of rapeseed with ethanol showed a similar yield to that obtained with isopropanol (technical grade solvents). However, the extract in isopropanol contained large amounts of polar lipids (only 80.19% of TAG). Citeau et al. [
68] reported a lower rapeseed oil extraction yield with isopropanol at 87.8% (89.3% of yield), compared to hexane (93.5%) and ethanol at 95.6% (92.7%). A small increase in water content significantly reduced the extraction yield of alcohol solvents. Due to the variability in extraction conditions (extraction technique, temperature, liquid/solid ratio, agitation, etc.), sampling conditions (moisture content, drying efficiency, oilseeds’ pre-treatment) and oilseed composition, it is difficult to compare the different authors’ experimental work.
5.2. 2-Methyloxolane
2-Methyltetrahydrofuran, also known as 2-methyloxolane (2-MeOx), is a biodegradable solvent derived from levulinic acid or furfural obtained from lignocellulosic biomass conversion (e.g., corncobs and sugarcane bagasse). 2-MeOx shows interesting properties that are technically comparable to those of hexane and could easily be transported on an industrial scale [
72]. 2-MeOx is predominantly lipophilic (log
p = 1.85) and can therefore dissolve both fatty molecules, such as hexane (log
p = 4.00), and more polar molecules, due to the presence of an oxygen atom (dipole moment = 1.38 D). Its boiling point (80 °C) is high enough to allow a good extraction temperature but low enough to be easily removed from the final products and recycled. In addition, its density (0.855) and viscosity (0.6 cP) are close to those of hexane and within an acceptable range for efficient diffusion through solid particles [
73]. Moreover, 2-MeOx has a much safer toxicological profile than hexane, and the use of 2-MeOx in industrial production could lead to a 97% reduction in CO
2 emissions compared to petroleum-based solvents [
72].
2-MeOx has already been approved for the extraction of organic and natural cosmetic ingredients (COSMOS label) and pharmaceutical products. On 20 March 2022, EFSA declared 2-MeOx a safe solvent for food applications, based on a comprehensive review of scientific studies. Methyloxolane (EcoXtract
®) was added to the list of permitted solvents for food and feed production in Europe on 26 January 2023 (Directive 2009/32/EC) [
74,
75]. The main studies on the extraction of vegetable oils with 2-MeOx are listed in
Table 5.
The partial miscibility of 2-MeOx with water can lead to better diffusion in cases where the solids to be extracted contain moisture. 2-MeOx forms an azeotrope with water (10.6% water and 89.4% 2-MeOx) and a second distillation is required to obtain the dry solvent. In the recovery of 2-MeOx in industrial extraction plants, the recovered organic phase is saturated with water after liquid/liquid separation. The 2-MeOx/H
2O mixture (95.5/4.5% at 55 °C) must be fed to another distillation step to recover the dry 2-MeOx. However, studies have shown that 2-MeOx 95.5% can be used directly for oil extraction, showing a similar efficiency to the dry solvent [
77,
78]. Using COSMO-RS and Hansen solubility predictions, Sicaire et al. [
66] showed that 2-MeOx can be considered the best alternative to
n-hexane among all solvents tested, as it can efficiently dissolve the desired compounds. The extraction yield with 2-MeOx was comparable to that with hexane on a laboratory scale (45.96 and 46.34 g/100 g DM, respectively).
Using the 6-litre pilot percolation extractor, the percentage of residual oil in the rapeseed meal was 1.8% for hexane and 0.8% for 2-MeOx [
76]. In addition, the extraction with 2-MeOx was faster, as three washing cycles allowed the extraction of almost 95% of the total oil, while five washes were required to extract 96% of the total oil for hexane. The energy evaluation of the industrial extraction process showed that the total amount of energy was slightly higher with 2-MeOx (365 MJ/t seed) than with hexane (284 MJ/t seed) [
76]. Sicaire et al. [
66] obtained a rapeseed oil yield of 47.19% with 2-MeOx and 46.7% with hexane, but the extract with 2-MeOx had a lower percentage of TAGs (92.83% and 99.12%, respectively) due to the extraction of more polar lipids [
66]. Claux et al. [
77] investigated the use of dry 2-MeOx and 2-MeOx 95.5% as alternatives for the extraction of soybean oil. Due to their higher polarity, dry 2-MeOx (23.5%) and 2-MeOx 95.5% (23.7%) gave higher oil yields than hexane (18.8%). The yield differences were ascribed to the co-extraction of more polar additional compounds such as phospholipids and isoflavones. However, the protein dispersibility index (PDI) and KOH protein solubility were slightly lower for meal extracted with dry 2-MeOx and 2-MeOx 95.5%. The concentrations of antinutritional factors in the meals were the same after extraction with hexane and 2-MeOx.
Annual production of 2-MeOx is estimated at about 4500 tonnes, which is far below the amount needed for its continuous large-scale use. 2-MeOx trades at EUR 8.0/kg for purchases over 100 tonnes from Pennakem (Memphis, Tennessee), and hexane at EUR 0.9/kg [
79]. A price reduction would increase the competitiveness of this solvent. The second economic disadvantage is the higher energy consumption for evaporation of the solvent from the miscella and desolventisation of the meal compared to extraction with hexane. However, the yield with 2-MeOx is higher, and with further optimisation of recycling and the reduction in losses, the estimated total cost of the process increases by only EUR 0.47 per tonne of extracted seed. This difference is minimal and could easily be compensated by a slight increase in the retail price of 2-MeOx-extracted oils: one cent per kilo, or 1 per cent of the premium, could actually bring more profit than hexane [
72].
Among the various solvents here presented, 2-MeOx, ethanol and SFE-CO
2 are the ones at the most advanced stage of study. Rapinel et al. [
72] reported industrial-scale studies that demonstrated the scalability of the process. The extraction of 46 tonnes of canola press cake (22.8% oil) at 340 kg/h in an industrial immersion extractor allowed a very good extraction performance (0.3% residual oil). The replacement of hexane by 2-MeOx does not require any significant plant changes. The only adjustments encompass the replacement of incompatible polymeric materials, the replacement of the wastewater boiler with a distillation column to maximise the recovery of the solvent present in the aqueous phase and the installation of a thermostatically controlled settling tank to reduce the amount of 2-MeOx in the aqueous phase. An advantage is also the possible conversion of the mineral oil absorption system replaced by cold water.
Further studies on the extraction of secondary metabolites, antinutrients and protein quality with 2-MeOx are needed. The refining process of crude oils extracted with 2-MeOx also requires further research.
5.3. Supercritical and Subcritical Fluids
Supercritical fluids are an alternative to conventional organic solvents. A fluid reaches a critical state when it is simultaneously heated and pressurised above its critical pressure. The main advantages of supercritical fluid extraction (SFE) include the absence of explosion hazards, higher selectivity in the extraction of neutral lipids, shorter extraction times, easy solvent recovery that preserves the oil and meal from thermal and oxidative degradation, and the avoidance of halogenated organic solvents. On the other hand, due to the high initial cost of equipment installation, and the energy and capital costs associated with regular maintenance, SFE is more commonly used in industrial processes that aim to obtain a product with a high economic value, resulting in a high-quality lipid extract.
Carbon dioxide (CO
2) is the most widely used solvent for SFE, being inert, non-toxic, non-flammable, inexpensive, abundant and endowed with moderate critical properties (Tc = 31.1 °C, Pc = 7.38 MPa). CO
2 is a generally recognised safe solvent (GRAS), so products containing extracts made from food-grade CO
2 are safe for human health. The use of supercritical CO
2 extraction techniques allows the efficient extraction of lipophilic compounds at generally mild temperatures (30–70 °C), without leaving traces in the extract. Since supercritical CO
2 is a non-polar solvent, its solubilising capacity is known to be between that of pentane and toluene [
47]. The solubility of oil in CO
2 is strongly dependent on pressure and temperature. At about 300 bar, it is between 0.3 and 0.7%, with temperature having a negative effect (raising the temperature from 40 to 80 °C leads to lower oil solubility) [
80]. Only above 350 bar is there a positive relationship between solubility and temperature. As a result, in today’s systems (which are generally limited to 3–400 bar), a large amount of solvent must be used to extract the oil. Oil separation from the solvent should also be carried out under supercritical conditions to save energy. The solubility of triglycerides in CO
2 is so low under 160 bar that excellent separation is possible. Another advantage of this system is that there is no need for a condenser to liquefy the CO
2 ahead of the pump. Cavitation in the plunger pump due to the phase change of the CO
2 is also avoided. Specific mass flows between 10 and 50 kg CO
2/kg·h at surface velocities of the solvent between 1 and 5 mm/sec have been used for SFE of oilseeds [
81].
SFE plants comprise four main components: (1) a pump to ensure the volumetric flow of the fluid; optionally, it can be preceded by a cooler to transport the gaseous components in a liquid state, (2) a heat exchanger, (3) an extractor, where static and dynamic extractions take place by modulating the pressure regulated by a valve, and (4) a separator [
82].
To improve the solubility of polar compounds, a polar co-solvent such as methanol or ethanol can be added. Moreover, systems have been developed that integrate ultrasonic treatments sequentially or simultaneously with SFE. Duarte et al. [
83] described a system with an ultrasound probe internally coupled into the supercritical extraction cell. To avoid the use of CO
2 at high pressures, binary gas mixtures were investigated. A mixture of CO
2 and propane allows complete miscibility of triglycerides below 300 bar and at temperatures up to 70 °C [
81]. Ultra-high-pressure SFE (pressures greater than 70 MPa) severely limits the amount of solvent to be used and the extraction time. Recent studies have shown that extracts obtained under high pressure are enriched with important ingredients that can only be obtained under these conditions. Although the investment costs are higher, the operating costs are lower at higher pressure. This is due to the shorter extraction times resulting from the higher oil solubility and the extraction of high-value compounds. Depending on the application, ultra-high-pressure SFE could be competitive [
79].
The use of liquid CO
2 has emerged as an innovative extraction technique that offers many of the same benefits as supercritical CO
2, but at lower pressures (≤15 MPa) and temperatures (about 25 °C), therefore reducing energy costs. In addition, due to its low polarity compared to most organic solvents, liquid CO
2 might have higher selectivity for neutral lipids, while affinity for non-neutral lipids is limited. The overall extraction yield is usually lower than that of organic solvents due to the lower polarity. The use of co-solvents such as methanol is also compatible with this technique [
84].
Liquified gas extraction (LGE) is a promising alternative for the extraction of lipids and other compounds. LGE involves extraction with gaseous organic solvents under pressure, which allows the solvent to be in a condensed state. Unlike SCF, LGE requires moderate pressures (1–10 bar), which results in lower energy consumption and facilitates industrial-scale application. Depending on the pressure and temperature conditions, the dissolving power can be adjusted by changing the solvent density, which enables selective extractions. The most common liquid gases are propane, butane and dimethyl ether (the first two are listed in the Directive 2009/32/EC). The main advantages of LGE are the following: (1) they can be used at low temperatures: due to their low boiling point, liquefied gases can be vaporised at moderate temperatures, preserving oil and meal and leaving no traces of solvent residues; (2) they can be used at moderate pressures; (3) under “normal” solid–liquid extraction conditions, most liquefied gases are chemically inert [
47].
In LGE, the temperature increase has a positive effect on the extraction yield up to a certain limit (e.g., 55 °C at 0.5 MPa for
n-butane). Above these temperatures, the solubility of the oil decreases as the gasification rate of the solvent increases [
2]. The main studies on the extraction of vegetable oils with supercritical and subcritical fluids are listed in
Table 6. Some studies have compared SFE and LGE in the extraction of oilseeds. For example, Pederssetti et al. [
85] analysed the extraction of canola seeds using SFE-CO
2 and LGE-propane. For SFE, increasing pressure had a positive effect, while increasing temperature had a negative effect on the extraction yield. For LGE extractions, the effect of temperature was more pronounced compared to pressure due to the low variation in density with changing pressure over the experimental range examined. LGE with propane shows faster extraction kinetics than SFE since propane solubilises triacylglycerols better than CO
2. The oxidative stability and fatty acid profile of the extracted oil were similar for the two solvents. However, the oil yields obtained with SFE and LGE were only 52.7% and 64.3% of those obtained with hexane Soxhlet extraction. Due to the lower oil yield, the protein content of the meal was also lower with SFE (29.9%) and LGE (29.9%) than with hexane extraction (36.7%). Sun et al. [
86] compared canola meal extracted with SFE-CO
2 with pressed cake and meal extracted with hexane. Both the hexane-extracted and SFE-CO
2-extracted meals had a higher protein content than the pressed cake. The glucosinolates content was lower in the meal extracted with SFE-CO
2, while the phosphorus content was higher than in the meal extracted with hexane. The phenolic acid contents of the meal extracted with hexane and SFE-CO
2 were similar. The addition of ethanol as a co-solvent during SFE-CO
2 reduced the phosphorus and phenolic acid content in the meal. It is well known that the addition of ethanol enhances phospholipid extraction with SFE-CO
2. Both meals extracted with hexane and SFE-CO
2 showed a similar protein solubility, high water-holding capacity, high oil absorption and high emulsifying capacity. Boutin and Badens [
87] investigated the influence of different parameters on the oil extraction yield for rapeseed and sunflower seeds using SFE-CO
2. The authors found that pressure and extraction duration are the most influencing parameters together with temperature in the case of rapeseed. Under optimal conditions, SFE-CO
2 yielded 89.9% and 78.5% of oil from rapeseed and sunflower, respectively. SFE-CO
2 proved to be a selective process as no traces of phospholipids were detected in the extracted oil. This would make it possible to avoid degumming during oil refining. Nimet et al. [
88] showed that LGE-propane afforded higher yields of sunflower oil than SFE-CO
2, working at a lower pressure and with a shorter extraction time. The fatty acid profile of the sunflower oil and the protein content of the meal were not significantly affected by the solvents or operating conditions. The values for the tocopherol content and oxidative stability of the oil obtained during extraction with LGE-propane and SFE-CO
2 were higher than those obtained with conventional extraction with hexane. In another study by Rapinel et al. [
89], LGE of sunflower oil with
n-butane at low pressure (0.2 to 0.4 MPa) resulted in a lower extraction yield compared to hexane extraction (36.9% vs. 53.4%).
In general, oils extracted with LGE have a higher quality (lower acid value), a higher content of phytosterols and carotenoids (antioxidants), a better shelf life (lower iodine value) and a better oxidation stability than oils extracted with conventional techniques. The fatty acid profiles of the oil extracted with LGE or SFE do not differ from those of the conventional methods [
2]. The separation of oil from the miscella after LGE can also be performed by membrane filtration. This technique can lead to energy savings (reduced use of steam, full or partial replacement of traditional degumming, refining and bleaching steps). An ideal membrane for solvent recovery must combine specific properties such as high oil retention and permeate fluxes suitable for industrial scales, as well as heat resistance and mechanical and chemical compatibility with the process [
93].
Currently, the largest SFE-CO
2 plant is in Korea and is dedicated to the extraction of sesame oil for food purposes (capacity of 2 × 2600 L/550 bar). A techno-economic analysis of the SFE-CO
2 performance for the extraction of pequi pulp oil showed that the production costs in pilot (100 L) and industrial (500 L) plants were attractive in eight scenarios tested. The most promising one showed a production cost of about EUR 33/kg pequi oil, with the purchase of pequi and other feedstocks accounting for about 85% of the cost. Moreover, the payback period was estimated as less than one year, which is attractive as the initial investment can be recovered quickly [
79].
Fiori [
94] developed a model to predict the economic feasibility of an industrial scale SFE-CO
2 plant (three extractors in series operating in countercurrent) designed to extract 3000 tonnes of grape seeds per year. The authors suggested that switching from a single mode to a cascade of two extractors increases the extraction efficiency from 83% to 86%. The break-even point that makes the process economically viable is a value of 5.9 EUR/kg for grape seed oil extracted with SFE-CO
2. According to these results, SFE-CO
2 proves to be a cost-effective alternative for the extraction of high-value-added specialty oils.
However, the high pressures required to reach the supercritical state limit its use to high-value-added products, as the investment costs for an industrial plant are correspondingly high. A clear disadvantage of SFE-CO
2 is also the need to extract large quantities of material in a semi-continuous process by filling and emptying the extraction vessels at atmospheric pressure. In conclusion, the use of SFE-CO
2 in the extraction of lipids for the large-scale production of biofuels and commodity vegetable oils is an uneconomic alternative, as the high costs of installation and maintenance, combined with energy costs, would significantly increase the price of the final product [
79].
For liquefied gases’ pilot-scale extraction, the company CELSIUS Sarl (Villette de Vienne, France) developed an isobaric LGE process (NECTACEL extractor) where the system always stays at liquid/vapor equilibrium. The liquefied gas is evaporated in the boiler at the same pressure (isobaric mode) and the vapours rise naturally to the condenser for solvent regeneration. No solvent pump or compressor is required to run the cycle, including solvent injection or recycling. The absence of mechanical equipment means lower energy consumption and maintenance costs. The extraction and separation steps are carried out at room temperature, which benefits heat-sensitive or thermo-oxidisable molecules. Solvation properties can be improved by using a co-solvent with complementary ionic character. The most used solvents are butane, fluorocarbons (HFO1234ze) and dimethyl ether. Extraction and separation take place at a relatively low pressure (less than 10 bar), with no technological capacity limit for the equipment. CELSIUS built several prototypes with 1 L, 200 L and 500 L capacities. The main applications of this system concern the extraction of molecules for pharmaceutical, cosmetic (flavours and perfumes) and nutraceutical purposes [
95].
5.4. Terpenes
Terpenes (also called isoprenoids or terpenoids) are acyclic, bicyclic or monocyclic hydrocarbons formed biosynthetically from isoprene units (C
5H
8). Terpenes are a large and extensive family of natural products (over 30,000) with relatively different physical properties. They are generally considered biodegradable solvents with low environmental impact and low toxicity, and they are mainly extracted from tree leaves and agricultural sources. In addition, microbial production of terpenes has enormous potential as it avoids dependence on natural resources. For example, limonene, which is generally obtained from the by-products of citrus juice production by steam distillation and condensation, can be produced by microbial bioconversion of glucose by
E. coli or
S. cerevisiae [
82].
D-limonene, α-pinene and
p-limonene have been investigated as alternative solvents for the extraction of vegetable oils.
Terpenes have molecular weights and structures suitable to substitute for
n-hexane. The solubility parameters of the solvents were investigated using Hansen’s parameters and COSMO-RS (Conductor-like Screening Model for Realistic Solvents) simulations. They gave similar results for terpenes and
n-hexane for dissolving TAGs as well as sterols and tocopherols. However, when their dielectric constants are considered, terpenes are slightly more polar and show a higher dissociation force than
n-hexane. In terms of safety, they have a higher flash point and are therefore less flammable and dangerous. Although the results in the laboratory scale are encouraging, the scalability of the terpene extraction process is limited. The main disadvantage of using terpenes is their high viscosity and density, and the higher energy consumption in solvent recovery by evaporation due to their higher boiling points (155–176 °C) and higher enthalpies of evaporation (37–39 kJ/mol) compared to
n-hexane (Bp = 69 °C, ΔHvap = 29.74 kJ/mol) [
66,
76,
96].
Heteroazeotropic distillation of the binary terpene–water mixture (about 50%
v/
v) leads to evaporation of the terpene and separation of the terpene–water distillation product by phase decantation. With this system, the solvent can be recovered at a lower temperature (about 97–98 °C at atmospheric pressure), and a high recovery rate of terpenes can be achieved (about 90% with 98% purity for α-pinene) [
97]. The main studies on the extraction of vegetable oils with terpenes are listed in
Table 7.
Bertouche et al. [
97] showed a higher yield with α-pinene compared to hexane when extracting soybean oil (21.1 vs. 19.1 g/100 g DM) and sunflower oil (67.2 vs. 52.6 g/100 g DM). The fatty acids extracted from both solvents were equivalent in terms of identified compounds and relative proportions. The higher boiling point of α-pinene reduces the viscosity of the oil, which leads to better diffusion through the matrix. Li et al. [
65] evaluated the use of different terpenes (
p-cymene, d-limonene and α-pinene) to extract rapeseed oil. The three solvents afforded higher oil extraction yields than
n-hexane (88.9%, 80.8% and 65.5%, respectively, compared to 58.2% for
n-hexane). The composition of the oil extracted with
p-cymene contained more FFA and DAG than
n-hexane-extracted oil, and the tocopherol and tocotrienol content was lower. Sicaire et al. [
66] reported lower yields with
p-cymene (39.71%) and d-limonene (36.94%) compared to hexane (46.71%) for rapeseed oil extraction. The percentage of TAG in the oil extracted with
p-cymene (82.03%) and d-limonene (51.31%) was significantly lower than with hexane (99.12%).
D-limonene and other unsaturated terpenes are readily oxidised in air and the resulting oxidation products are labelled as allergens.
p-menthane (stable saturated derivative of d-limonene) and cis/trans-pinane: 7:3 (stable saturated derivative of α-pinene and β-pinene) were investigated as new saturated terpene solvents for oil extraction. Both solvents showed promising results with comparable oil yields to
n-hexane (40.5 vs. 39.5 g/100 g DM for
p-menthane and
n-hexane, respectively [
99], and 42.5 vs. 43.2 g/100 g DM for pinane and
n-hexane, respectively [
98]). The encouraging lab-scale results still need further research for the development of a pilot-scale terpene extraction process.
5.5. Alternative Hydrocarbon Solvents
Among the alternative hydrocarbon solvents, isohexane, cyclohexane and
n-heptane have been proposed to replace hexane. These solvents also originate from the petrochemical industry, and although they are considered less harmful to health than hexane, comprehensive studies are lacking, especially on their chronic toxicity [
100,
101]. In contrast to
n-hexane, the C6 isomers without
n-hexane have not shown neurotoxic effects in animal experiments. This is attributed to the fact that no neurotoxic 1,4-diketones are formed during the metabolism of these hexane isomers. However, further studies on the chronic effects of exposure to these substances are needed (e.g., lack of information on teratogenicity and genotoxicity) [
102].
Wan et al. (1995) compared five hydrocarbon solvents (heptane, isohexane, neohexane, cyclohexane and cyclopentane) with hexane for the extraction of cottonseed oil. Extraction with the six hydrocarbon solvents at their respective boiling points showed that normal paraffins (hexane and heptane) were more efficient and significantly better than cyclohexane or branched paraffins in extracting oil from cottonseed flakes. Cyclohexane extracted 95.4% of the oil, with respect to normal paraffins, while cyclopentane, isohexane and neohexane extracted 91.6%, 88.8% and 88.8%, respectively. The laboratory-scale extraction study (S/L ratio of 1 to 5.5
w/
w, temperature 10–45 °C below the boiling point of the solvent) showed that hexane removed 100% of the oil from the flakes at 55 °C after a one-step extraction; heptane extracted 100% at 75 °C and 95.9% at 55 °C; and isohexane extracted 93.1% at 45 °C. The extraction efficiencies of cyclopentane, cyclohexane and neohexane were lower. Based on these results, heptane and isohexane are the most suitable alternative hydrocarbon solvents to replace hexane [
103].
These two solvents were therefore investigated as potential substitutes for hexane in a cottonseed processing plant with a capacity of 300 tonnes/day. The extraction efficiencies of isohexane and heptane, as measured by extraction time and residual oil in the meal, colour of refined and bleached oil and solvent loss, were comparable to that of hexane. However, isohexane appears the best solution to replace hexane with minimal equipment adjustment. The higher boiling point of heptane increased the energy consumption of the desolventiser/toaster (D/T) system, reducing the daily production by 20–30%. Heptane would require more D/T capacity to achieve the same tonne production as hexane. In addition, the solvent loss of heptane was 12% higher than the average annual loss of hexane. However, due to the high boiling point of heptane, it can be used in a wide temperature range from ambient to 80 °C.
For isohexane, the D/T processing rate was 10–20% higher than for hexane. Isohexane boils at 55 °C and therefore has a fairly narrow operating temperature range. This lower extraction temperature may also affect the extraction efficiency of isohexane, as shown by the slightly higher residual oil content in the meal compared to hexane. However, with isohexane, daily production increased by more than 20% and gas consumption decreased by more than 40% [
104]. Isohexane is more expensive than hexane because of the additional isomerisation process to produce it. The improved production and lower energy requirements for isohexane should offset the solvent cost difference. Isohexane was shown to be a viable choice to replace hexane in oilseed extraction, but the current need for solvents that are safe and not derived from petroleum makes this solvent out of step with the new frontiers defined by green extraction.
5.6. Other Organic Solvents
Acetone, ethyl acetate, cyclopentyl methyl ether (CPME) and dimethyl carbonate (DMC) have also been tested as alternative solvents to hexane for the extraction of lipids from oilseeds. Of these solvents, only acetone and ethyl acetate are included in Directive 2009/32/EC.
Acetone has been used mainly for the extraction of cotton oil, but its use in the extraction of oilseeds is limited. Acetone ranks between hexane and ethanol in terms of selectivity towards lipids. This solvent is completely miscible with water and its volatility is higher than that of hexane due to its lower boiling point, which is not an advantage from a safety point of view. Acetone has a relatively low latent heat of vaporisation (534 kJ/kg) and does not form an azeotrope with water, which allows easy recovery by distillation. As with ethanol and isopropyl alcohol, the oil extracted with acetone can be recovered by simply cooling the miscella. On the other hand, phospholipids are not soluble in acetone, which facilitates refining but does not allow recovery of the lecithin. The presence of water in acetone significantly reduces its ability to extract the oil. In addition, acetone can reduce the amount of the gossypol and aflatoxins in the meal. One of the main problems with acetone extraction is that it usually imparts a very unpleasant odour (cat’s urine smell) to the meal. This smell is due to the reaction of mesityl oxide (which is obtained from acetone by condensation reactions) with hydrogen sulphide (coming from the breakdown of one or more of the sulphur-containing amino acids). Further drying of the flakes and solvent before extraction can reduce these unpleasant odours but significantly increases production costs [
105].
Ethyl acetate is a colourless ester of acetic acid and ethanol, characterised by low toxicity and a pleasant odour. Ethyl acetate is one of the solvents approved for food production (Directive 2009/32/EU) and is safer and cheaper (33% less) than hexane. It has a similar boiling temperature and latent heat of vaporisation, but the main disadvantages of using ethyl acetate are its higher viscosity, density and dielectric point than hexane [
106]. This solvent is partially miscible with water (8.5% at 20 °C) and forms with it an azeotrope with a boiling point lower than that of the pure compound (70 °C). Ethyl acetate seems to have been relatively little studied compared to other solvents. The researchers who have made comparisons seem to prefer isopropanol. However, as the economic environment has changed, the impact of energy costs may tip the balance in favour of a solvent that is easier to distil. Lohani et al. (2015) used an accelerated pressure extractor (at 1500 psi) to extract seed oils (rapeseed, flaxseed) using ethyl acetate and hexane for comparison. Ethyl acetate shows a lower viscosity and polarity under subcritical conditions, affording an oil yield (120 °C, 90 min) close to that of Soxhlet extraction with hexane, reaching 40.38% for rapeseed and 33.33% for flaxseed, compared to 42.96% and 35.62% with hexane, respectively. These results evidenced that the quality (heating value, density, viscosity, fatty acid profile and unsaponifiable matter) of the oils extracted from different oilseeds with ethyl acetate is almost equivalent to that of the oils extracted with hexane [
106]. The use of solvent mixtures such as water/ethanol/ethyl acetate was investigated for the extraction of rapeseed oil, obtaining the maximum rate of oil yield (32.35 mg/mm) with the mixture containing 9.17%, 6.61% and 84.17% of water, ethanol and ethyl acetate, respectively [
107].
Dimethyl carbonate (DMC) is a non-polar aprotic solvent with good miscibility with water (139 g/L), and it is readily biodegradable in the atmosphere and non-toxic. Currently, the most common commercial route to produce DMC is the oxidative carbonylation of methanol using O
2; however, new alternative processes have been developed to produce DMC from CO
2. DMC showed a good extraction performance for triglycerides and could also be used as a mild solvent for biocatalyst-assisted biodiesel conversion [
108].
Although cyclopentyl methyl ether (CPME) is currently synthesized by petrochemical-based routes (using cyclopentene and methanol to obtain CPME with excellent atom economy), some bio-based alternatives paved the way for a future biogenic source of this solvent. In particular, several biomass-based processes focused on the production of chemical precursors that can be used for CPME production, such as cyclopentanone or cyclopentanol [
109]. CPME is endowed with interesting properties, such as low solubility in water (1.1 g CPME/100 g), the formation of a positive azeotrope and low enthalpy of vaporisation, as well as stability in strongly acidic or basic conditions. CPME also shows low toxicity, a negligible peroxide formation rate and a narrow explosion range. However, it has a high flash point (compared to hexane) and a higher boiling temperature (106 °C). The main studies on the extraction of vegetable oils with other organic solvents are listed in
Table 8.
Sicaire et al. [
66] investigated the use of ethyl acetate, CPME and DMC as alternatives to hexane for the extraction of rapeseed oil by conventional Soxhlet extraction. All these three solvents gave similar oil yields (42.83%, 42.77% and 41.53% for ethyl acetate, DMC and CPME, respectively), but these values were about 10% lower than those of hexane (46.71%). Furthermore, HPTLC analysis showed that the oils extracted with these solvents had different compositions. Only 88% of the extracted components were triglycerides (TAGs), compared to 99% of the oil extracted with hexane.
5.7. Enzymatic and Surfactant-Assisted Aqueous Extraction
The aqueous extraction of oil from oilseeds involves the diffusion of lipids in water and the subsequent formation of emulsions. The emulsified oil can be separated by demulsification, e.g., through temperature change or using enzymes. Aqueous oil extraction is an environmentally friendly and safe process that allows the simultaneous production of edible oil and protein isolates or concentrates in the same process. This process also brings economic advantages as it avoids the use of organic solvents, effectively removes antinutritive factors and toxins from the meal, and avoids the need for solvent evaporation and for degumming of crude oils [
110]. The main limitations are the lower efficiency of the aqueous oil extraction, the necessary demulsification of the extract to recover the oil, the wastewater treatment, and the lowest protein content and quality of the meal. To improve the yield of the aqueous processes, enzymes have been used to facilitate the oil release. These mainly hydrolyse the structural polysaccharides (cellulose, hemicellulose and pectin) that form the cell wall, or the proteins of the cytoplasm or of the lipid bodies’ membrane.
Selected enzymes were tested on different types of oilseeds and achieved much higher extraction yields than the original aqueous process (in some cases over 90%) [
111]. Several factors influence the efficiency of aqueous enzyme extraction. Pre-treatment to reduce the size of oily materials by grinding or flaking increases the extraction yield due to there being better accessibility for the enzymes. The type of enzyme is a key factor in the whole process. The use of enzymes alone or in combination can be determined based on the structure of the oilseed, the types of enzymes and the experimental conditions. Due to the high protein content of soybean, the use of proteases led to more efficient oil extraction. In the extraction of rapeseed, whose cell wall consists mainly of pectin, treatment with pectinase resulted in an 85.9% increase in oil yield. The use of enzyme mixtures in some cases showed better performance than single enzymes, probably due to a synergistic effect. Since for each enzyme there is a certain optimal pH value, special attention must be paid to optimising this parameter. In general, higher extraction efficiency is obtained at pH values far from the isoelectric point. For example, low oil yields were achieved in soybeans, rapeseed and sunflower seed extractions at the isoelectric point due to low protein solubility.
Temperature also plays an important role as enzyme activities are generally effective at 45 °C or less and an increase in temperature can lead to the denaturation of proteins, reducing the release of oil from the oilseed. In general, increasing the concentration of the enzyme (up to about 1% enzyme by weight of raw material) until the active sites of the substrate are saturated results in greater degradation of the desired product and greater oil recovery. Further increases in enzyme concentration are not only not cost-effective, but can also induce off flavours, bitterness and caramelisation of sugars that can interfere with the oil extraction process. Adequate agitation allows partial breakdown of the mechanical barriers (cell wall) and uniform mixing of the entire contents of the reaction mixture. However, too intensive stirring is not only energetically costly but can also form stable emulsions that are difficult to separate. In general, oils obtained by aqueous enzyme extraction contained low levels of phosphatides and peroxides and similar levels of free fatty acids compared to oils extracted by conventional methods [
112].
The optimisation of the entire process must not only focus on achieving the highest possible oil yield but must also consider the ease of splitting the resulting emulsion. In particular, some parameters such as oil droplet size and viscosity of the bulk emulsion are very useful in evaluating emulsion stability [
111]. In addition, some proteins show an emulsifying capacity that generally increases during enzymatic proteolysis until a certain degree of hydrolysis is reached, and then decreases. Therefore, the extent of the enzymatic proteolytic action must be optimised to obtain both a higher oil yield and a less stable oil in water emulsion. The main studies on the extraction of vegetable oils with aqueous enzymatic extraction are listed in
Table 9.
Cheng et al. [
121] assessed the environmental impacts of soybean oil production through an extruding–expelling process, hexane extraction and aqueous extraction. Hexane extraction showed the highest environmental impact due to the use of an organic solvent. Extrusion led to the highest greenhouse gas and criteria pollutant emissions due to the high energy required for heat-pressing processes. Finally, enzyme-assisted aqueous oil extraction had similar environmental impacts as the extrusion process but also reduced greenhouse gas and criteria pollutant emissions.
Enzyme-assisted oil extraction was also studied in combination with other green technologies such as microwaves and ultrasound. Enzyme-assisted extraction combined with microwaves, in addition to the general extractive benefits of microwaves, accelerates the enzyme reactions and delays the denaturalisation of the enzymes compared to conventional heating. Similarly, the use of ultrasound in combination with enzymatic extraction can increase oil yield and improve its quality and stability over time [
4].
The necessary drying of the wet meal to be stored and the limited recycling of the enzymes represent additional costs in this process [
4]. Immobilisation of the enzyme on a solid support is a possible strategy that allows many cycles of oil extraction and reduces costs [
110]. Aqueous enzymatic extraction has good potential for simultaneous oil and protein recovery from oilseeds. However, the application of this technology is still hampered by the high cost for the production of enzymes, the lower extraction yields compared to solvent extraction, the long incubation time and the additional de-emulsification process.
Surfactant aqueous oil extraction (SAOE) is another aqueous extraction technique that seems to be a promising method on a laboratory scale and allows an oil yield of slightly more than 90%. The basic concept of SAEP is to use surfactants to reduce the interfacial tension (IFT) between oil and water (about 19–24 mN/m), making aqueous oil extraction possible [
122,
123]. Surfactants reduce the IFT due to their amphiphilic structure, which gives them the property to adsorb at the oil–water interface. Compared to conventional surfactants, extended surfactants contain polar intermediate groups, such as polypropylene oxide and/or polyethylene oxide, inserted between the hydrophilic head and the lipophilic tail. This molecular structure gives surfactants the ability to achieve a very low IFT (≤10
−2 mN/m). Lowering the IFT to such low values allows the formation of so-called microemulsions, which are ideal systems for the co-solubilisation of oil and water. In addition, salts (e.g., NaCl, CaCl
2 and KCl) and alcohols (from ethanol to medium-chain alcohols such as octanol and decanol) are often added to surfactant solutions to improve their interfacial properties and thus reduce both the critical micelle concentration and the surface or interfacial tension. SAOE involves two main steps: extraction and separation. In the first step, the oil is extracted by immersing the matrix in the surfactant solution, usually with stirring. The oil and water form a turbid mixture that must be separated and purified to obtain a free oil phase. First, the solid phase is separated from the liquid phase by centrifugation. Then, in the liquid phase of interest, three layers are generally observed: (i) a layer of free oil, (ii) a microemulsion of oil in aqueous solution and (iii) the surfactant solution. In order to recover the oil from this liquid fraction, the system must be destabilised, whereby the oil–water–surfactant system is relatively stable. Gagnon et al. [
122] described in detail the properties of extended surfactants, the state of the art of SAOE and the limitations that need to be overcome for the industrial application of this extraction method. The authors concluded that although initial results have shown this technology to be effective on a laboratory scale, it is still far from industrial application. From a technical point of view, the fractionation process of the oil–water–surfactant mixture, the recycling of the surfactants, the removal of salts and dehydration of the residual cake after extraction are the main critical points. In addition, the energy costs of the process should be taken into account and more information on the quality of the oil and solid fractions should be collected and compared with those obtained in the conventional way. Finally, the identification of new extended-like, bio-based and environmentally friendly surfactants would be desirable.
5.8. Extraction Optimization Methods
Oil extraction represents one of the most critical steps in oilseed processing, as it determines the quality and quantity of the oil obtained. By carefully optimising the extraction conditions for each extraction method, yield and quality can be improved. In addition, a proper optimisation process can save time and energy, thus reducing the cost of the entire oil extraction process. While conventional extraction methods are highly optimised today, especially at the industrial scale, new extraction technologies require extensive optimisation. Nde and Fonchaet [
1] gave an overview of techniques for optimising the extraction of oil from plant material.
Most of these methods involve the use of statistical software to optimise control variables (e.g., extraction time, solids/solvent ratio, type of solvent, seed pre-treatment, etc.) to reduce costs and increase performance while avoiding degradation of product quality as much as possible. Two main methods for process optimisation can be identified in the literature: the single parameter optimisation method and the response surface methodology (RSM).
In single parameter optimisation, the best conditions for oil extraction are determined for a single factor at a time, holding the other factors constant. In this case, the ideal level of a factor that gives the best response is defined. However, this laborious procedure does not allow the interactions between factors to be considered. The RSM adopts several mathematical and statistical techniques to jointly model and analyse the effects of two or more process factors that have an impact on the desired response. A major step in optimization is screening the factors studied to have the significant effects of the analytical method. RSM reduces the number of experimental trials but maintains the expected accuracy and determines the responses for the interactive effect of multiple factors. However, RSM is limited by fitting the data to a second order polynomial, but not all systems with curvatures are compatible with this model. RSM is based on the relationship between product properties and regression equations that describe the correlations between input parameters and product properties. In many extraction procedures, the regression coefficient R
2 and/or the absolute/standard error of deviation are used to validate the models used. A model is considered good if R
2 > 0.70 and/or error < 10%. Among the many RSM software available, Design experts, Sigma Plot version 12.5, MATLAB version 5.0, IBM SPSS and Minitab version 15.5 have been commonly used to optimise oilseed extraction [
1].