Machine Learning and Quantum Calculation for Predicting Yield in Cu-Catalyzed P–H Reactions
Abstract
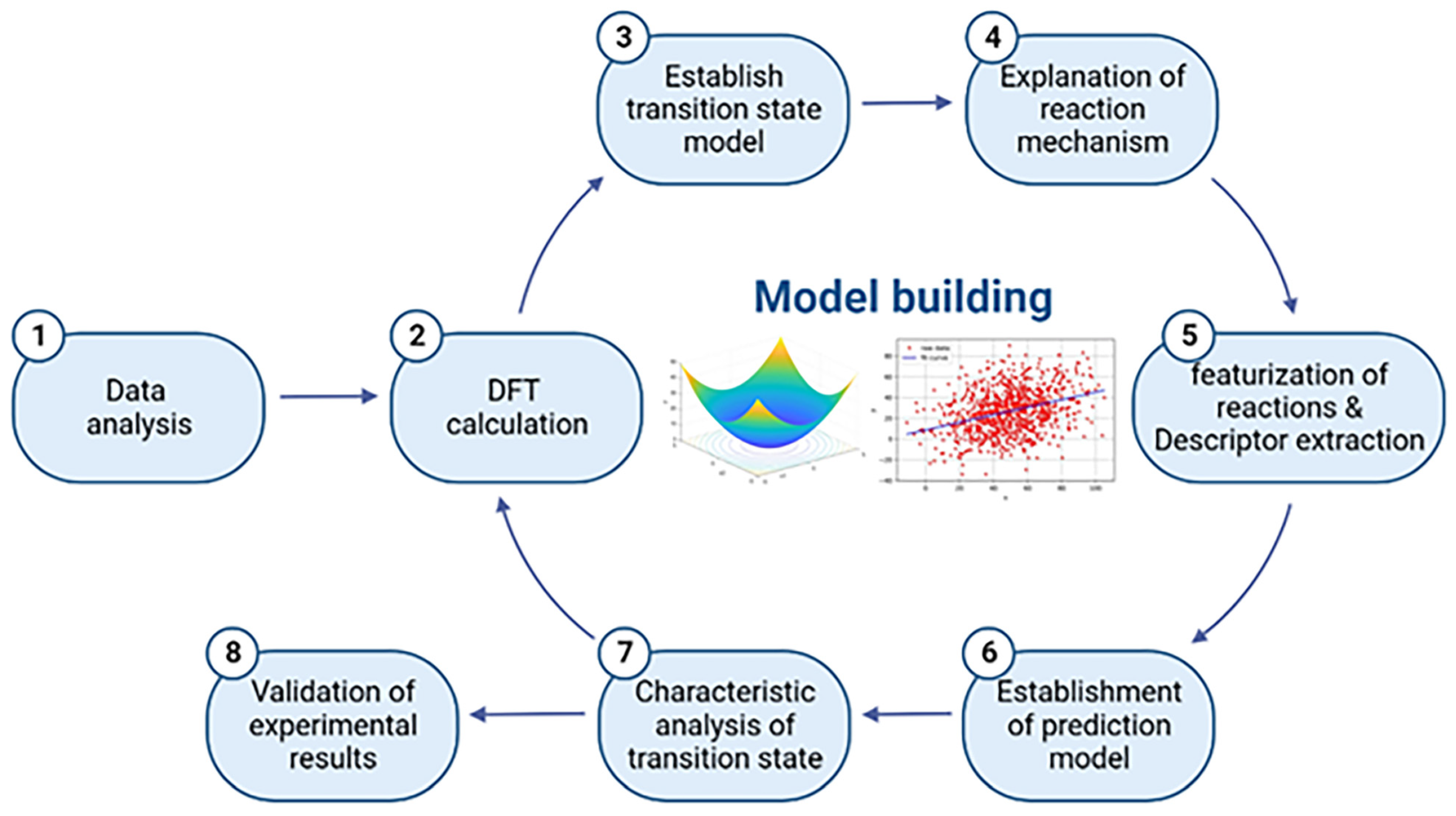
1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Data Source
3.2. Quantum Chemistry Calculations and Descriptor Acquisition
3.3. Machine Learning Models
3.3.1. Partial Least Squares Regression (PLSR)
3.3.2. Multiple Linear Regression (MLR)
3.3.3. Stepwise Multiple Linear Regression (SMLR)
3.3.4. Artificial Neural Networks (ANN)
3.3.5. Support Vector Machine (SVM)
3.4. Synthesis
3.5. Structural Validation of Synthesized Product
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Monai, M.; Weckhuysen, B.M. Crowded catalyst, better catalyst. Natl. Sci. Rev. 2021, 8, nwab141. [Google Scholar] [CrossRef]
- Lim, X. The new breed of cutting-edge catalysts. Nature 2016, 537, 156–158. [Google Scholar] [CrossRef]
- Dai, L. Metal-Free Carbon Electrocatalysts: Recent Advances and Challenges Ahead. Adv. Mater. 2019, 31, e1900973. [Google Scholar] [CrossRef]
- Zhu, S.F.; Zhou, Q.L. Transition-Metal-Cataiyzed Enantioselective Heteroatom-Hydrogen Bond InsertionReactions. Acc. Chem. Res. 2012, 45, 1365–1377. [Google Scholar] [CrossRef]
- Bergstrom, B.D.; Nickerson, L.A.; Shaw, J.T.; Souza, L.W. Transition Metal Catalyzed Insertion Reactions with Donor/Donor Carbenes. Angew. Chem. 2020, 133, 6940–6954. [Google Scholar] [CrossRef]
- Batista, V.F.; GA Pinto, D.C.; Silva, A.M. Iron: A Worthy Contender in Metal Carbene Chemistry. ACS Catal. 2020, 10, 10096–10116. [Google Scholar] [CrossRef]
- Candeias, N.R.; Paterna, R.; Gois, P. Homologation Reaction of Ketones with Diazo Compounds. Chem. Rev. 2016, 116, 2937–2981. [Google Scholar] [CrossRef]
- Wang, Y.F.; Wang, C.J.; Feng, Q.Z.; Zhai, J.J.; Qi, S.S.; Zhong, A.G.; Chu, M.M.; Xu, D.Q. Copper-catalyzed asymmetric 1,6-conjugate addition of in situ generated para-quinone methides with β-ketoesters. Chem. Commun. 2022, 58, 6653–6656. [Google Scholar] [CrossRef]
- Doyle, M.P.; McKervey, M.A.; Ye, T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds (From Cyclopropanes to Ylides); Wiley-Interscience: New York, NY, USA, 1998; 652p, ISBN 0-47113556-9. [Google Scholar]
- Hosseinian, A.; Farshbaf, S.; Fekri, L.Z.; Nikpassand, M.; Vessally, E. Cross-Dehydrogenative Coupling Reactions Between P(O)-H and X-H (X = S, N, O, P) Bonds. Top. Curr. Chem. 2018, 376, 23. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Liang, C.; Jiang, J. Copper-catalyzed P-H insertion reactions of sulfoxonium ylides. Org. Biomol. Chem. 2021, 19, 5767–5771. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, K.; Ge, X.; Ma, P.; Xu, Z.; Lu, H.; Li, G. Redox-Neutral P(O)-N Coupling between P(O)-H Compounds and Azides via Dual Copper and Photoredox Catalysis. Org. Lett. 2020, 22, 6143–6149. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, S.; Gao, Y.; Zhao, Y.; Goto, M.; Han, L.B. Selective P-P and P-O-P bond formations through copper-catalyzed aerobic oxidative dehydrogenative couplings of H-phosphonates. Angew. Chem. Int. Ed. Engl. 2010, 49, 6852–6855. [Google Scholar] [CrossRef] [PubMed]
- Ess, D.; Gagliardi, L.; Hammes-Schiffer, S. Introduction: Computational Design of Catalysts from Molecules to Materials. Chem. Rev. 2019, 119, 6507–6508. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Su, J.; Huang, F.; Jiao, L.; Liang, Y.; Yang, D.; Zhang, S.; Wender, P.A.; Yu, Z.-X. A Computationally Designed Rh(I)-Catalyzed Two-Component [5+2+1] Cycloaddition of Ene-vinylcyclopropanes and CO for the Synthesis of Cyclooctenones. J. Am. Chem. Soc. 2007, 129, 10060–10061. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, P.J.; Helquist, P.; Norrby, P.-O.; Wiest, O. Prediction of Enantioselectivity in Rhodium Catalyzed Hydrogenations. J. Am. Chem. Soc. 2009, 131, 410–411. [Google Scholar] [CrossRef]
- Rowley, C.N.; Woo, T.K. Computational design of ruthenium hydride olefin-hydrogenation catalysts containing hemilabile ligands. Can. J. Chem. 2009, 87, 1030–1038. [Google Scholar] [CrossRef]
- Baik, M.-H.; Mazumder, S.; Ricci, P.; Sawyer, J.R.; Song, Y.-G.; Wang, H.; Evans, P.A. Computationally Designed and Experimentally Confirmed Diastereoselective Rhodium-Catalyzed Pauson−Khand Reaction at Room Temperature. J. Am. Chem. Soc. 2011, 133, 7621–7623. [Google Scholar] [CrossRef]
- Fernandez, L.E.; Horvath, S.; Hammes-Schiffer, S. Theoretical Design of Molecular Electrocatalysts with Flexible Pendant Amines for Hydrogen Production and Oxidation. J. Phys. Chem. Lett. 2013, 4, 542–546. [Google Scholar] [CrossRef]
- Nielsen, M.C.; Bonney, K.J.; Schoenebeck, F. Computational Ligand Design for the Reductive Elimination of ArCF3 from a Small Bite Angle PdII Complex: Remarkable Effect of a Perfluoroalkyl Phosphine. Angew. Chem. Int. Ed. 2014, 53, 5903–5906. [Google Scholar] [CrossRef]
- Bernales, V.; League, A.B.; Li, Z.; Schweitzer, N.M.; Peters, A.W.; Carlson, R.K.; Hupp, J.T.; Cramer, C.J.; Farha, O.K.; Gagliardi, L. Computationally Guided Discovery of a Catalytic Cobalt-Decorated Metal–Organic Framework for Ethylene Dimerization. J. Phys. Chem. C 2016, 120, 23576–23583. [Google Scholar] [CrossRef]
- Boddeda, A.; Hossain, M.M.; Mirzaei, M.S.; Lindeman, S.V.; Mirzaei, S.; Rathore, R. Angular ladder-type meta-phenylenes: Synthesis and electronic structural analysis. Org. Chem. Front. 2020, 7, 3215–3222. [Google Scholar] [CrossRef]
- Cao, X.; Rong, C.; Zhong, A.; Lu, T.; Liu, S. Molecular acidity: An accurate description with information-theoretic approach in density functional reactivity theory. J. Comput. Chem. 2018, 39, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Schumann, J.; Medford, A.J.; Yoo, J.S.; Zhao, Z.J.; Norskov, J.K. Selectivity of Synthesis Gas Conversion to C2+ Oxygenates on fcc(111) Transition-Metal Surfaces. Acs Catal. 2018, 8, 3447–3453. [Google Scholar] [CrossRef]
- Ulissi, Z.W.; Medford, A.J.; Bligaard, T.; Nrskov, J.K. To address surface reaction network complexity using scaling relations machine learning and DFT calculations. Nat. Commun. 2017, 8, 14621. [Google Scholar] [CrossRef]
- Ahneman, D.T.; Estrada, J.G.; Lin, S.; Dreher, S.D.; Doyle, A.G. Predicting reaction performance in C–N cross-coupling using machine learning. Science 2018, 360, eaar5169. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, N.; Jonayat, A.; Janik, M.J.; Senftle, T.P. Interaction trends between single metal atoms and oxide supports identified with density functional theory and statistical learning. Nat. Catal. 2018, 1, 531–539. [Google Scholar] [CrossRef]
- Sun, M.; Wu, T.; Xue, Y.; Dougherty, A.W.; Yan, C.H. Mapping of atomic catalyst on graphdiyne. Nano Energy 2019, 62, 754–763. [Google Scholar] [CrossRef]
- Wang, X.; Ye, S.; Hu, W.; Sharman, E.; Liu, R.; Liu, Y.; Luo, Y.; Jiang, J. Electric Dipole Descriptor for Machine Learning Prediction of Catalyst Surface–Molecular Adsorbate Interactions. J. Am. Chem. Soc. 2020, 142, 7737–7743. [Google Scholar] [CrossRef]
- Tran, K.; Ulissi, Z.W. Active learning across intermetallics to guide discovery of electrocatalysts for CO2 reduction and H2 evolution. Nat. Catal. 2018, 1, 696–703. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Cheng, T.; Wang, L.-W.; Goddard, W.A., III. Identification of the Selective Sites for Electrochemical Reduction of CO to C2+ Products on Copper Nanoparticles by Combining Reactive Force Fields, Density Functional Theory, and Machine Learning. ACS Energy Lett. 2018, 3, 2983–2988. [Google Scholar] [CrossRef]
- Sun, M.; Dougherty, A.W.; Huang, B.; Li, Y.; Yan, C.-H. Accelerating Atomic Catalyst Discovery by Theoretical Calculations-Machine Learning Strategy. Adv. Energy Mater. 2020, 10, 1903949. [Google Scholar] [CrossRef]
- Artrith, N.; Lin, Z.; Chen, J.G. Predicting the Activity and Selectivity of Bimetallic Metal Catalysts for Ethanol Reforming using Machine Learning. ACS Catal. 2020, 10, 9438–9444. [Google Scholar] [CrossRef]
- Meyer, B.; Sawatlon, B.; Heinen, S.; Lilienfeld, O.; Corminboeuf, C. Machine learning meets volcano plots: Computational discovery of cross-coupling catalysts. Chem. Sci. 2018, 9, 7069–7077. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wencong, L.; Chunrong, P.; Qiang, S.; Jin, G. Two semi-empirical approaches for the prediction of oxide ionic conductivities in ABO3 perovskites. Comput. Mater. Sci. 2009, 46, 860–868. [Google Scholar] [CrossRef]
- Chen, N.; Lu, W.; Yang, J.; Li, G. Support Vector Machine in Chemistry; World Scientific: Singapore, 2004. [Google Scholar]
- Lvarez, M.; Besora, M.; Molina, F.; Maseras, F.; Pérez, P. Two Copper-Carbenes from One Diazo Compound. J. Am. Chem. Soc. 2021, 143, 4837–4843. [Google Scholar] [CrossRef] [PubMed]
- Balhara, R.; Chatterjee, R.; Jindal, G. A computational approach to understand the role of metals and axial ligands in artificial heme enzyme catalyzed C–H insertion. Phys. Chem. Chem. Phys. 2021, 23, 9500–9511. [Google Scholar] [CrossRef]
- Lübcke, M.; Szabó, K. Diazocarbonyl Compounds in Organofluorine Chemistry. Synlett 2020, 32, 1060–1071. [Google Scholar]
- Wu, Y.; Cao, S.; Douair, I.; Maron, L.; Bi, X. Computational Insights into Different Mechanisms for Ag-, Cu-, and Pd-Catalyzed Cyclopropanation of Alkenes and Sulfonyl Hydrazones. Chem. Eur. J. 2021, 27, 5999–6006. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Zhao, J.; Xue, Y. Mechanism and Diastereoselectivity of [3+3] Cycloaddition between Enol Diazoacetate and Azomethine Imine Catalyzed by Dirhodium Tetracarboxylate: A Theoretical Study. Eur. J. Org. Chem. 2018, 2018, 3086–3094. [Google Scholar] [CrossRef]
- Varnek, A.; Baskin, I. Machine learning methods for property prediction in chemoinformatics: Quo Vadis? J. Chem. Inf. Model. 2012, 52, 1413–1437. [Google Scholar] [CrossRef]
- Vogt, M.; Bajorath, J. Chemoinformatics: A view of the field and current trends in method development. Bioorg. Med. Chem. 2012, 20, 5317–5323. [Google Scholar] [CrossRef]
- Burbidge, R.; Trotter, M.; Buxton, B.; Holden, S. Drug design by machine learning: Support vector machines for pharmaceutical data analysis. Comput. Chem. 2001, 26, 5–14. [Google Scholar] [CrossRef]
- Geppert, H.; Vogt, M.; Bajorath, J. Current trends in ligand-based virtual screening: Molecular representations, data mining methods, new application areas, and performance evaluation. J. Chem. Inf. Model. 2010, 50, 205–216. [Google Scholar] [CrossRef]
- Heikamp, K.; Bajorath, J. Support vector machines for drug discovery. Expert Opin. Drug Discov. 2014, 9, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Shlens, J. A Tutorial on Principal Component Analysis. Int. J. Remote Sens. 2014, 51. [Google Scholar] [CrossRef]
- Shen, L.B. Copper(II) Acetate-Catalyzed Synthesis of Phosphorylated Pyridines via Denitrogenative C-P Coupling between Pyridotriazoles and P(O)H Compounds. Adv. Synth. Catal. 2018, 360, 4252–4258. [Google Scholar] [CrossRef]
- Qian, C.; Xinxing, Y.; Chunxiao, W.; Jiekun, Z.; Yulin, H.; Xingguo, L.; Kun, Z. Copper-Catalyzed Addition of H-P(O) Bonds to Arynes. J. Org. Chem. 2016, 81, 9476–9482. [Google Scholar]
- Lu, L.Q.; Wang, B.C.; Wang, Y.N.; Zhang, M.M.; Xiao, W.J. Copper-catalyzed decarboxylative cyclization via tandem C–P and C–N bond formation: Access to 2-phosphorylmethyl indoles. Chem. Commun. 2018, 54, 3154–3157. [Google Scholar]
- Liu, X.-Y.; Zou, Y.-X.; Ni, H.-L.; Zhang, J.; Dong, H.-B.; Chen, L. Copper-catalyzed tandem phosphorylative allenylation/cyclization of 1-(o-aminophenyl)prop-2-ynols with the P(O)–H species: Access to C2-phosphorylmethylindoles. Org. Chem. Front. 2020, 7, 980–986. [Google Scholar] [CrossRef]
- Shen, R.; Yang, J.; Luo, B.; Zhang, L.; Han, L.B. Copper-Catalyzed Allenylation-Isomerization Sequence of Penta-1,4-diyn-3-yl Acetates with P(O)H Compounds: Facile Synthesis of 1-Phosphonyl 2,4-Diynes. Adv. Synth. Catal. 2016, 358, 3897–3906. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09, Revision B.01. 2010. Available online: https://www.scienceopen.com/document?vid=45b5a7ba-f6ee-40ce-b346-7407f99a540d (accessed on 30 June 2023).
- Aleku, G.A.; Saaret, A.; Bradshaw-Allen, R.T.; Derrington, S.R.; Titchiner, G.R.; Gostimskaya, I.; Gahloth, D.; Parker, D.A.; Hay, S.; Leys, D. Enzymatic C–H activation of aromatic compounds through CO2 fixation fixation. Nat. Chem. Biol. 2020, 16, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Raghavachari, K. Perspective on “Density functional thermochemistry. III. The role of exact exchange”. Theor. Chem. Acc. 2000, 103, 361–363. [Google Scholar] [CrossRef]
- Deng, Y.; Yu, D.; Cao, X.; Liu, L.; Rong, C.; Lu, T.; Liu, S. Structure, aromaticity and reactivity of corannulene and its analogues: A conceptual density functional theory and density functional reactivity theory study. Mol. Phys. 2017, 116, 956–968. [Google Scholar] [CrossRef]
- Qi, D.; Zhang, L.; Wan, L.; Zhang, Y.; Bian, Y.; Jiang, J. Conformational effects, molecular orbitals, and reaction activities of bis(phthalocyaninato) lanthanum double-deckers: Density functional theory calculations. Phys. Chem. Chem. Phys. 2011, 13, 13277–13286. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 247–257. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Roy, L.E.; Hay, P.J.; Martin, R.L. Revised Basis Sets for the LANL Effective Core Potentials. J. Chem. Theory Comput. 2008, 4, 1029. [Google Scholar] [CrossRef]
- Hariharan, P.C.P.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Fukui, K. A Formulation of Reaction Coordinate. J. Phys. Chem. 1970, 74, 4161–4163. [Google Scholar] [CrossRef]
- Fukui, K. The Path of Chemical Reactions—The IRC Approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Uddin, K.M.; Poirier, R.A. Computational Study of the Deamination of 8-Oxoguanine. J. Phys. Chem. B 2011, 115, 9151–9159. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self consistent molecular orbital methods. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar]
- Mclean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 119, 525. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Xu, L.; Wencong, L.; Shengli, J.; Yawei, L.; Nianyi, C. Support Vector Regression Applied to Materials Optimization of SiAlON Ceramics. Chemom. Intell. Lab. Syst. 2006, 82, 8–14. [Google Scholar] [CrossRef]
- Lorber, A.; Wangen, L.E.; Kowalski, B.R. A Theoretical Foundation for the PLS Algorithm. J. Chemom. 1987, 1, 19–31. [Google Scholar] [CrossRef]
- Bin, L. Multiple linear regression analysis and its application. China Sci. Technol. Inf. 2010, 3, 1–25. [Google Scholar]
- Xia, Z.; Yu, B.; Yuan, X. ON 13 C NMR SPECTROSCOPY: Approach to Chemical Shift Sum (CSS) in Alkanes by Stepwise Multiple Linear Regression (SMR) with Molecular Path Index Vector (VPM). Chin. J. Magn. Reson. 1999, 16, 243–254. [Google Scholar]
- Mao, J.; Jain, A.K.; Jain, A. Artificial neural networks for feature-extraction and multivariate data projection. IEEE Trans. Neural Netw. 1995, 6, 296–317. [Google Scholar]
- Vapnik, V. Statistical Learning Theory; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Sheng, G.; Huang, K.; Chi, Z.; Ding, H.; Xing, Y.; Lu, P.; Wang, Y. Preparation of 3-diazoindolin-2-imines via cascade reaction between indoles and sulfonylazides and their extensions to 2,3-diaminoindoles and imidazo[4,5-b]indoles. Org. Lett. 2015, 46, 5096. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zhang, X.; Zhu, L.; Feng, X.; Kowah, J.A.H.; Jiang, J.; Wang, L.; Jiang, L.; Liu, X. Machine Learning and Quantum Calculation for Predicting Yield in Cu-Catalyzed P–H Reactions. Molecules 2023, 28, 5995. https://doi.org/10.3390/molecules28165995
Ma Y, Zhang X, Zhu L, Feng X, Kowah JAH, Jiang J, Wang L, Jiang L, Liu X. Machine Learning and Quantum Calculation for Predicting Yield in Cu-Catalyzed P–H Reactions. Molecules. 2023; 28(16):5995. https://doi.org/10.3390/molecules28165995
Chicago/Turabian StyleMa, Youfu, Xianwei Zhang, Lin Zhu, Xiaowei Feng, Jamal A. H. Kowah, Jun Jiang, Lisheng Wang, Lihe Jiang, and Xu Liu. 2023. "Machine Learning and Quantum Calculation for Predicting Yield in Cu-Catalyzed P–H Reactions" Molecules 28, no. 16: 5995. https://doi.org/10.3390/molecules28165995
APA StyleMa, Y., Zhang, X., Zhu, L., Feng, X., Kowah, J. A. H., Jiang, J., Wang, L., Jiang, L., & Liu, X. (2023). Machine Learning and Quantum Calculation for Predicting Yield in Cu-Catalyzed P–H Reactions. Molecules, 28(16), 5995. https://doi.org/10.3390/molecules28165995





