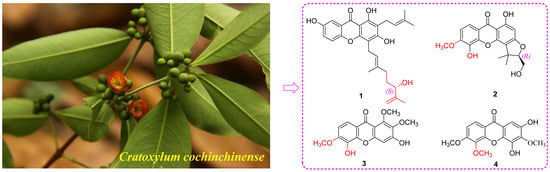
Xanthones with Potential Anti-Inflammatory and Anti-HIV Effects from the Stems and Leaves of Cratoxylum cochinchinense
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Investigation
2.2. Anti-Inflammatory Activity
2.3. Anti-HIV-1 Activity
3. Experimental Section
3.1. General Experiment Procedure
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Anti-Inflammatory Bioassays
3.5. Anti-HIV-1 Activity Bioassays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Editorial Committee of Flora of China. Chinese Flora; Science Press: Beijing, China, 1990; Volume 50, pp. 75–79. [Google Scholar]
- Bennett, G.J.; Harrison, L.J.; Sia, G.L.; Sim, K.Y. Triterpenoids, tocotrienols and xanthones from the bark of Cratoxylum cochinchinense. Phytochemistry 1993, 32, 1245–1251. [Google Scholar] [CrossRef]
- Seo, E.K.; Kim, N.C.; Wani, M.C.; Wall, M.E.; Navarro, H.A.; Burgess, J.P.; Kawanishi, K.; Kardono, L.B.; Riswan, S.; Rose, W.C.; et al. Cytotoxic prenylated xanthones and the unusual compounds anthraquinobenzophenones from Cratoxylum sumatranum. J. Nat. Prod. 2002, 65, 299–305. [Google Scholar] [CrossRef]
- Reutrakul, V.; Chanakul, W.; Pohmakotr, M.; Jaipetch, T.; Yoosook, C.; Kasisit, J.; Napaswat, C.; Santisuk, T.; Prabpai, S.; Kongsaeree, P.; et al. Anti-HIV-1 constituents from leaves and twigs of Cratoxylum arborescens. Planta Med. 2006, 72, 1433–1435. [Google Scholar] [CrossRef]
- Boonnak, N.; Karalai, C.; Chantrapromma, S.; Ponglimanont, C.; Fun, H.K.; Kanjana-Opas, A.; Laphookhieo, S. Bioactive prenylated xanthones and anthraquinones from Cratoxylum formosum ssp. pruniflorum. Tetrahedron 2006, 62, 8850–8859. [Google Scholar] [CrossRef]
- Laphookhieo, S.; Syers, J.K.; Kiattansakul, R.; Chantrapromma, K. Cytotoxic and antimalarial prenylated xanthones from Cratoxylum cochinchinense. Chem. Pharm. Bull. 2006, 54, 745–747. [Google Scholar] [CrossRef] [Green Version]
- Kukongviriyapan, U.; Luangaram, S.; Leekhaosoong, K.; Kukongviriyapan, V.; Preeprame, S. Antioxidant and vascular protective activities of Cratoxylum formosum, Syzygium gratum and Limnophila aromatica. Biol. Pharm. Bull. 2007, 30, 661–666. [Google Scholar] [CrossRef] [Green Version]
- Mahabusarakam, W.; Rattanaburi, S.; Phongpaichit, S.; Kanjana-Opas, A. Antibacterial and cytotoxic xanthones from Cratoxylum cochinchinense. Phytochem. Lett. 2008, 1, 211–214. [Google Scholar] [CrossRef]
- Laphookhieo, S.; Maneerat, W.; Koysomboon, S. Antimalarial and cytotoxic phenolic compounds from Cratoxylum maingayi and Cratoxylum cochinchinense. Molecules 2009, 14, 1389–1395. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.L.; Matthew, S.; Lantvit, D.D.; Ninh, T.N.; Chai, H.; Fuchs, J.R.; Soejarto, D.D.; de Blanco, E.J.C.; Swanson, S.M.; Kinghorn, A.D. Cytotoxic and NF-κB inhibitory constituents of the stems of Cratoxylum cochinchinense and their semisynthetic analogues. J. Nat. Prod. 2011, 74, 1117–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Y.H.; Dai, Y.; Wang, G.H.; Chen, L.Y.; Chen, H.F.; Zeng, D.Q.; Li, Y.L.; Yao, X.S. Bioactive prenylated xanthones from the stems of Cratoxylum cochinchinense. J. Asian Nat. Prod. Res. 2015, 17, 519–531. [Google Scholar] [CrossRef]
- Tantapakul, C.; Maneerat, W.; Sripisut, T.; Ritthiwigrom, T.; Andersen, R.J.; Cheng, P.; Cheenpracha, S.; Raksat, A.; Laphookhieo, S. New benzophenones and xanthones from Cratoxylum sumatranum ssp. neriifolium and their antibacterial and antioxidant activities. J. Agric. Food Chem. 2016, 64, 8755–8762. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.P.; Lee, H.H.; Uddin, Z.; Song, Y.H.; Park, K.H. Caged xanthones displaying protein tyrosine phosphatase 1B (PTP1B) inhibition from Cratoxylum cochinchinense. Bioorg. Chem. 2018, 78, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kaewpiboon, C.; Boonnak, N.; Yawut, N.; Kaowinn, S.; Chung, Y.H. Caged-xanthone from Cratoxylum formosum ssp. pruniflorum inhibits malignant cancer phenotypes in multidrug-resistant human A549 lung cancer cells through down-regulation of NF-κB. Bioorg. Med. Chem. 2019, 27, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Raksat, A.; Phukhatmuen, P.; Yang, J.; Maneerat, W.; Charoensup, R.; Andersen, R.J.; Wang, Y.A.; Pyne, S.G.; Laphookhieo, S. Phloroglucinol benzophenones and xanthones from the leaves of Garcinia cowa and their nitric cxide production and α-glucosidase inhibitory activities. J. Nat. Prod. 2020, 83, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.H.; Liu, Y.P.; Huang, Z.H.; Wang, T.T.; Feng, X.Y.; Yue, H.; Guo, W.; Fu, Y.H. Cytotoxic dihydrobenzofuran neolignans from Mappianthus iodoies. Bioorg. Chem. 2017, 7, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Hu, S.; Wen, Q.; Ma, Y.L.; Jiang, Z.H.; Tang, J.Y.; Fu, Y.H. Novel γ-lactone derivatives from Trigonostemon heterophyllus with their potential antiproliferative activities. Bioorg. Chem. 2018, 79, 107–110. [Google Scholar] [CrossRef]
- Ma, Y.L.; Liu, Y.P.; Zhang, C.; Zhao, W.H.; Shi, S.; He, D.N.; Zhang, P.; Liu, X.H.; Han, T.T.; Fu, Y.H. Carbazole alkaloids from Clausena hainanensis with their potential antiproliferative activities. Bioorg. Chem. 2018, 76, 359–364. [Google Scholar] [CrossRef]
- Liu, Y.P.; Guo, J.M.; Liu, Y.Y.; Hu, S.; Yan, G.; Qiang, L.; Fu, Y.H. Carbazole alkaloids with potential neuroprotective activities from the fruits of Clausena lansium. J. Agric. Food Chem. 2019, 67, 5764–5771. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, Y.P.; Wang, X.P.; Qiao, Z.H.; Yu, X.M.; Zhu, Y.Z.; Xie, L.; Qiang, L.; Fu, Y.H. Bioactive daphnane diterpenes from Wikstroemia chuii with their potential anti-inflammatory effects and anti-HIV activities. Bioorg. Chem. 2020, 105, 104388. [Google Scholar] [CrossRef]
- Fu, Y.H.; Xie, Y.T.; Guo, J.M.; Wang, X.P.; Jiang, B.; Zhang, W.; Qiang, L.; Kong, L.Y.; Liu, Y.P. Limonoids from the fresh young leaves and buds of Toona sinensis and their potential neuroprotective effects. J. Agric. Food Chem. 2020, 68, 12326–12335. [Google Scholar] [CrossRef]
- Liu, Y.P.; Guo, J.M.; Xie, Z.; Suo, X.Y.; Liu, Z.Y.; Qiao, Z.H.; Guan, R.Q.; Bian, Y.; Qiang, L.; Fu, Y.H. Clausanisumine, a prenylated bicarbazole alkaloid from the fruits of Clausena anisum-olens and its potential anti-HIV activity. J. Org. Chem. 2021, 86, 17722–17726. [Google Scholar] [CrossRef]
- Liu, Y.P.; Wang, T.W.; Xie, Z.; Bian, Y.; Liu, Y.Y.; Guan, R.Q.; Liu, Z.Y.; Qiang, L.; Chen, G.Y.; Fu, Y.H. Artapilosines A and B, unusual phenanthrene derivatives related to aporphine alkaloids from Artabotrys pilosus. J. Nat. Prod. 2021, 84, 3117–3121. [Google Scholar] [CrossRef]
- Liu, Y.P.; Xie, Z.; Guan, R.Q.; Du, M.R.; Qiao, Z.H.; Suo, X.Y.; Liu, Z.Y.; Bian, Y.; Qiang, L.; Fu, Y.H. Syzysamalactone, an unusual 11-carbon δ-lactone derivative from the fresh ripe fruits of Syzygium samarangense (wax apple). J. Nat. Prod. 2022, 85, 2100–2103. [Google Scholar] [CrossRef]
- Mahabusarakam, W.; Nuangnaowarat, W.; Taylor, W.C. Xanthone derivatives from Cratoxylum cochinchinense roots. Phytochemistry 2006, 67, 470–474. [Google Scholar] [CrossRef]
- Klaiklay, S.; Sukpondma, Y.; Rukachaisirikul, V.; Phongpaichit, S. Friedolanostanes and xanthones from the twigs of Garcinia hombroniana. Phytochemistry 2013, 85, 161–166. [Google Scholar] [CrossRef]
- Boonnak, N.; Khamthip, A.; Karalai, C.; Chantrapromma, S.; Ponglimanont, C.; Kanjana-Opas, A.; Tewtrakul, S.; Chantrapromma, K.; Fun, H.K.; Kato, S. Nitric oxide inhibitory activity of xanthones from the green fruits of Cratoxylum formosum ssp. pruniflorum. Aust. J. Chem. 2010, 63, 1550–1556. [Google Scholar] [CrossRef]
- Fun, H.K.; Chantrapromma, S.; Boonnak, N.; Karalai, C.; Chantrapromma, K. Redetermination and absolute configuration of pruniflorone M monohydrate. Acta Crystallogr. E 2011, 67, o1916–o1917. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Jin, D.Z.; Chen, W.L.; Wang, J.; Liu, Q.F.; Zhu, X.Z.; Zhao, W.M. Selective cyclooxygenase-2 inhibitors from Calophyllum membranaceum. J. Nat. Prod. 2005, 68, 1514–1518. [Google Scholar] [CrossRef]
- Luz Cardona, M.; Fernandez, I.; Pedro, J.R.; Serrano, A. Xanthones from Hypericum reflexum. Phytochemistry 1990, 29, 3003–3006. [Google Scholar] [CrossRef]
- Fouotsa, H.; Lannang, A.M.; Dzoyem, J.P.; Tatsimo, S.J.N.; Neumann, B.; Mbazoa, C.D.; Razakarivony, A.A.; Nkengfack, A.E.; Eloff, J.N.; Sewald, N. Antibacterial and antioxidant xanthones and benzophenone from Garcinia smeathmannii. Planta Med. 2015, 81, 594–599. [Google Scholar] [CrossRef]
- Kainz, K.; Zehl, M.; Bleier, J.; Merkinger, B.; Pemmer, T.; Schmidt, N.; Winkler, J.; Kahlig, H.; Krenn, L. New compounds from the tree fern Metaxya rostrata C. Presl. Rec. Nat. Prod. 2014, 8, 348–353. [Google Scholar]
- Elya, B.; He, H.P.; Kosela, S.; Hanafi, M.; Hao, X.J. Two new xanthons from Garcinia rigida leaves. Nat. Prod. Res. 2006, 20, 788–791. [Google Scholar] [CrossRef]
- Zhang, L.J.; Yang, X.D.; Xu, L.Z.; Yang, S.L. Three new xanthones from the roots of Securidaca inappendiculata. Heterocycles 2005, 65, 1685–1690. [Google Scholar] [CrossRef]
- Xue, Q.C.; Li, C.J.; Zuo, L.; Yang, J.Z.; Zhang, D.M. Three new xanthones from the roots of Polygala japonica Houtt. J. Asian Nat. Prod. Res. 2009, 11, 465–469. [Google Scholar] [CrossRef]
- Hua, Y.; Chen, C.X.; Liu, Y.Q.; Chen, S.K.; Zhou, J. Three new xanthones from Polygala crotalarioides. Chinese Chem. Lett. 2006, 17, 773–775. [Google Scholar]
- Liu, Y.P.; Liu, Q.L.; Zhang, X.L.; Niu, H.Y.; Guan, C.Y.; Sun, F.K.; Xu, W.; Fu, Y.H. Bioactive monoterpene indole alkaloids from Nauclea officinalis. Bioorg. Chem. 2019, 83, 1–5. [Google Scholar] [CrossRef]
- Fu, Y.H.; Guo, J.M.; Xie, Y.T.; Hua, J.; Dai, Y.Y.; Zhang, W.; Lin, T.C.; Liu, Y.P. Structural characterization, antiproliferative and anti-inflammatory activities of alkaloids from the roots of Zanthoxylum austrosinense. Bioorg. Chem. 2020, 102, 104101. [Google Scholar] [CrossRef]
- Liu, Y.P.; Guo, J.M.; Yan, G.; Zhang, M.M.; Zhang, W.H.; Qiang, L.; Fu, Y.H. Anti-inflammatory and antiproliferative prenylated isoflavone derivatives from the fruits of Ficus carica. J. Agric. Food Chem. 2019, 67, 4817–4823. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zhao, W.H.; Feng, X.Y.; Zhang, Z.J.; Zong, K.; Sun, Z.G.; Zheng, Y.T.; Fu, Y.H. Novel tetrahydrofuran derivatives from Trigonostemon howii with their potential anti-HIV-1 activities. Bioorg. Chem. 2018, 79, 111–114. [Google Scholar] [CrossRef]
- Fu, Y.H.; Guo, J.M.; Xie, Y.T.; Yu, X.M.; Su, Q.T.; Qiang, L.; Kong, L.Y.; Liu, Y.P. Prenylated chromones from the fruits of Artocarpus heterophyllus and their potential anti-HIV-1 activities. J. Agric. Food Chem. 2020, 68, 2024–2030. [Google Scholar] [CrossRef]
- Liu, Y.P.; Yan, G.; Guo, J.M.; Liu, Y.Y.; Li, Y.J.; Zhao, Y.Y.; Qiang, L.; Fu, Y.H. Prenylated coumarins from the fruits of Manilkara zapota with potential anti-inflammatory effects and anti-HIV activities. J. Agric. Food Chem. 2019, 67, 11942–11947. [Google Scholar] [CrossRef] [PubMed]


| Position | Cratocochinone A (1) | Cratocochinone B (2) | ||
|---|---|---|---|---|
| δH a | δC b | δH c | δC d | |
| 1 | 158.1 s | 163.4 s | ||
| 2 | 108.8 s | 6.22 (1H, s) | 93.0 d | |
| 3 | 160.9 s | 165.1 s | ||
| 4 | 105.1 s | 112.6 s | ||
| 5 | 7.26 (1H, d, J = 8.0 Hz) | 118.8 d | 150.4 s | |
| 6 | 7.18 (1H, dd, J = 8.0, 1.8 Hz) | 124.1 d | 134.6 s | |
| 7 | 150.2 s | 6.91 (1H, d, J = 8.8 Hz) | 115.3 d | |
| 8 | 7.48 (1H, d, J = 1.8 Hz) | 108.6 d | 7.69 (1H, d, J = 8.8 Hz) | 121.0 d |
| 9 | 180.8 s | 179.1 s | ||
| 4a | 153.0 s | 152.0 s | ||
| 8a | 120.4 s | 111.1 s | ||
| 9a | 103.1 s | 102.2 s | ||
| 10a | 152.5 s | 160.3 s | ||
| 1′ | 3.42 (2H, d, J = 6.8 Hz) | 21.5 t | 42.6 s | |
| 2′ | 5.28 (1H, d, J = 6.8 Hz) | 121.5 d | 4.44 (1H, dd, J = 6.8, 4.6 Hz) | 94.2 d |
| 3′ | 135.5 s | 3.79 (1H, dd, J = 12.1, 4.6 Hz) | 59.6 t | |
| 3.74 (1H, dd, J = 12.1, 6.8 Hz) | ||||
| 4′ | 1.77 (3H, s) | 25.8 q | 1.62 (3H, s) | 26.3 q |
| 5′ | 1.84 (3H, s) | 17.6 q | 1.34 (3H, s) | 20.9 q |
| 1″ | 3.51 (2H, d, J = 6.8 Hz) | 21.7 t | ||
| 2″ | 5.37 (1H, d, J = 6.8 Hz) | 122.5 d | ||
| 3″ | 136.7 s | |||
| 4″ | 2.11 (2H, m) | 35.9 t | ||
| 5″ | 1.70 (2H, m) | 32.8 t | ||
| 6″ | 4.08 (1H, d, J = 6.8 Hz) | 75.9 d | ||
| 7″ | 147.1 s | |||
| 8″ | 4.90 (1H, s) | 111.2 t | ||
| 4.81 (1H, s) | ||||
| 9″ | 1.89 (3H, s) | 16.2 q | ||
| 10″ | 1.70 (3H, s) | 17.9 q | ||
| 1-OH | 13.02 (1H, s) | 13.52 (1H, s) | ||
| 6-OCH3 | 3.86 (3H, s) | 60.5 q | ||
| Position | Cratocochinone C (3) | Cratocochinone D (4) | ||
|---|---|---|---|---|
| δH a | δC b | δH a | δC b | |
| 1 | 152.0 s | 6.99 (1H, s) | 94.0 d | |
| 2 | 140.8 s | 149.8 s | ||
| 3 | 166.4 s | 141.9 s | ||
| 4 | 6.23 (1H, s) | 99.5 d | 140.1 s | |
| 5 | 148.5 s | 144.9 s | ||
| 6 | 145.2 s | 149.7 s | ||
| 7 | 7.16 (1H, d, J = 8.8 Hz) | 121.4 d | 7.37 (1H, d, J = 9.1 Hz) | 123.5 d |
| 8 | 6.98 (1H, d, J = 8.8 Hz) | 112.1 d | 7.24 (1H, d, J = 9.1 Hz) | 113.6 d |
| 9 | 173.1 s | 174.8 s | ||
| 4a | 154.4 s | 141.8 s | ||
| 8a | 117.1 s | 115.7 s | ||
| 9a | 104.8 s | 117.1 s | ||
| 10a | 146.3 s | 146.6 s | ||
| 1-OCH3 | 3.75 (3H, s) | 61.1 q | ||
| 2-OH | 10.89 (1H, s) | |||
| 2-OCH3 | 3.62 (3H, s) | 60.0 q | ||
| 3-OCH3 | 3.82 (3H, s) | 60.3 q | ||
| 4-OH | 9.47 (1H, s) | |||
| 5-OCH3 | 3.79 (3H, s) | 60.9 q | ||
| 6-OCH3 | 3.72 (3H, s) | 60.9q | 3.85 (3H, s) | 55.7 q |
| Compound | IC50 (μM) a | Compound | IC50 (μM) a |
|---|---|---|---|
| 1 | 1.29 ± 0.06 | 7 | 12.58 ± 0.12 |
| 2 | 2.17 ± 0.09 | 8 | 4.16 ± 0.08 |
| 3 | 0.86 ± 0.05 | 9 | 18.36 ± 0.21 |
| 4 | 9.42 ± 0.13 | 10 | 7.47 ± 0.11 |
| 5 | 6.47 ± 0.10 | 11 | 3.16 ± 0.09 |
| 6 | 8.35 ± 0.15 | 12 | 6.21 ± 0.08 |
| Hydrocortisone b | 4.08 ± 0.08 |
| Compound | CC50 (µM) a | EC50 (µM) b | TI c |
|---|---|---|---|
| 1 | >200.00 | 0.22 | >909.09 |
| 2 | >200.00 | 0.68 | >294.12 |
| 3 | >200.00 | 1.89 | >105.82 |
| 4 | >200.00 | 4.07 | >49.14 |
| 5 | >200.00 | 2.53 | >79.05 |
| 6 | >200.00 | 3.08 | >64.94 |
| 7 | >200.00 | 4.16 | >48.08 |
| 8 | >200.00 | 8.91 | >22.45 |
| 9 | >200.00 | 5.32 | >37.59 |
| 10 | >200.00 | 2.67 | >74.91 |
| 11 | >200.00 | 11.23 | >17.81 |
| 12 | >200.00 | 6.35 | >31.50 |
| AZT d | 4018.26 | 0.02078 | 193,371.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Guo, J.-M.; Zhang, M.-M.; Wang, R.; Liang, C.-H.; Zhao, Y.-M.; Deng, Y.-Y.; Liu, Y.-P.; Fu, Y.-H. Xanthones with Potential Anti-Inflammatory and Anti-HIV Effects from the Stems and Leaves of Cratoxylum cochinchinense. Molecules 2023, 28, 6050. https://doi.org/10.3390/molecules28166050
Zhang Y, Guo J-M, Zhang M-M, Wang R, Liang C-H, Zhao Y-M, Deng Y-Y, Liu Y-P, Fu Y-H. Xanthones with Potential Anti-Inflammatory and Anti-HIV Effects from the Stems and Leaves of Cratoxylum cochinchinense. Molecules. 2023; 28(16):6050. https://doi.org/10.3390/molecules28166050
Chicago/Turabian StyleZhang, Yong, Jia-Ming Guo, Ming-Ming Zhang, Ran Wang, Chai-Huan Liang, Yi-Meng Zhao, Ya-Yuan Deng, Yan-Ping Liu, and Yan-Hui Fu. 2023. "Xanthones with Potential Anti-Inflammatory and Anti-HIV Effects from the Stems and Leaves of Cratoxylum cochinchinense" Molecules 28, no. 16: 6050. https://doi.org/10.3390/molecules28166050
APA StyleZhang, Y., Guo, J.-M., Zhang, M.-M., Wang, R., Liang, C.-H., Zhao, Y.-M., Deng, Y.-Y., Liu, Y.-P., & Fu, Y.-H. (2023). Xanthones with Potential Anti-Inflammatory and Anti-HIV Effects from the Stems and Leaves of Cratoxylum cochinchinense. Molecules, 28(16), 6050. https://doi.org/10.3390/molecules28166050