Mairá-Potato (Casimirella sp.): Botanical, Food, Pharmacological, and Phytochemical Aspects
Abstract
1. Introduction
2. Results and Discussion
2.1. Origin and Distribution of the Mairá-Potato
2.2. Taxonomic Classification and Botanical Characterisation of the Mairá-Potato
2.3. Food Use of the Mairá-Potato
2.4. Pharmacological Properties of the Mairá-Potato
Anti-Parasitic and Anti-Fungal Potential of Mairá-Potato Compounds
2.5. Phytochemicals Present in Parts of the Mairá-Potato Plant
Toxicity of Mairá-Potato and Indications for Evaluation to Ensure Application as a New Product or Ingredient
2.6. What Advances Can Contribute to the Development of New Technologies on the Basis of What Has Been Learned?
2.6.1. Application of Starch for the Development of Biodegradable Films
2.6.2. Application of Bioactive Ingredients Contained in the Mairá-Potato
2.6.3. Material for Printing 3D
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO Food and Agriculture Organization of the United Nations. International Fund for Agricultural Development. World Food Programme. In The State of Food Insecurity in the World. Strengthening the Enabling Environment for Food Security and Nutrition; FAO: Rome, Italy, 2014; ISBN 9789251085424. [Google Scholar]
- FAO. Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable. In The State of Food Security and Nutrition in the World 2022; FAO: Rome, Italy, 2022; ISBN 9789251364994. [Google Scholar]
- Saath, K.C.d.O.; Fachinello, A.L. Crescimento Da Demanda Mundial de Alimentos e Restrições Do Fator Terra No Brasil. Rev. Econ. e Sociol. Rural 2018, 56, 195–212. [Google Scholar] [CrossRef]
- Lebot, V. Tropical Root and Tuber Crops: Cassava, Sweet Potato, Yams and Aroids. In Crop Production Science in Horticulture; CAB International: Wallingford, UK, 2009; ISBN 1845936213. [Google Scholar]
- Pradeepika, C.; Selvakumar, R.; Nabi, S.U.; Sajeev, M.S.; Giri, N.A. Ethnopharmacology and Toxicology of Threatened Tuberous Plant Genus Ceropegia sp. L.: A Review. Pharma Innov. 2018, 7, 192–196. [Google Scholar]
- Naczk, M.; Shahidi, F. Phenolics in Cereals, Fruits and Vegetables: Occurrence, Extraction and Analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Kumar, T.J. Roots and Tuber Crops as Functional Foods: A Review on Phytochemical Constituents and Their Potential Health Benefits. Int. J. Food Sci. 2016, 2016, 3631647. [Google Scholar] [CrossRef]
- Ribeiro, R.G. Estudo Etnobotânico e Físico-Químico Da Batata-Mairá (Casimirella spp.—Icacinaceae). Master’s Thesis, INPA, Manaus, Brazil, 2018; p. 118. [Google Scholar]
- Kinupp, V.F.; Lorenzi, H. Plantas Alimentícias Não Convencionais (PANC) No Brasil: Guia de Identificação, Aspectos Nutricionais e Receitas Ilustradas, 2nd ed.; Instituto Plantarum de Estudos da Flora: São Paulo, Brazil, 2014. [Google Scholar]
- Paschoal, V.; Souza, N.S. Plantas Alimentícias Não Convencionais (PANC). In Nutrição Clínica Funcional: Compostos Bioativos Dos Alimentos; Chaves, D.F.S., Ed.; VP Editora: São Paulo, Brazil, 2015; Chapter 13; pp. 302–323. [Google Scholar]
- Rizzini, C.T.; Mors, W.B. Botânica Econômica Brasileira; Editora da Universidade de São Paulo: São Paulo, Brazil, 1976. [Google Scholar]
- Aparício, M. Os Suruwaha e Sua Rede de Relações. Uma Hipótese Sobre Localidades e Coletivos Arawa. In Paisagens Ameríndias: Lugares, Circuitos e Modos de Vida Na Amazônia; Amoroso, M., Mendes Dos Santos, G., Eds.; Editora EDUA: Manaus, Brazil, 2013; pp. 247–273. ISBN 978-85-7816-126-2. [Google Scholar]
- Zoghbi, M.D.G.B.; Roque, N.F.; Gottlieb, H.E. Humirianthenolides, New Degraded Diterpenoids from Humirianthera Rupestris. Phytochemistry 1981, 20, 1669–1673. [Google Scholar] [CrossRef]
- Zoghbi, M.D.G.B.; Roque, Ν.F.; Cabral, J.A.S. Estudo Químico de Humirianthera Ampla (Miers) Baehni (Icacinaceae). Notas & Comunicações. Acta Amaz. 1983, 13, 215–216. [Google Scholar]
- Zoghbi, M.d.G.B.; Varejão, M.d.J.C.; Ribeiro, M.N.d.S. A Presença de Substâncias Inorgânicas Tóxicas No Gênero Humirianthera (Icacinaceae). Acta Amaz. 1988, 18, 61–66. [Google Scholar] [CrossRef][Green Version]
- Varejão, M.J.C.; Ribeiro, M.N.S.; Zoghbi, M.G.B. Variação Sazonal Dos Macro e Microelementos No Gênero Humirianthera (Icacinaceae), Em Função Da Idade. Acta Amaz. 1992, 22, 381–390. [Google Scholar]
- Varejão, M.D.J.C.; Zoghbi, M.d.G.B.; Diniz, R.E.O.; Ribeiro, M.N.d.S. Variação Sazonal de Sulfato, Enxofre Total e Matéria Orgânica No Gênero Humirianthera, Em Função Da Idade. Acta Amaz. 1992, 22, 391–397. [Google Scholar] [CrossRef]
- Howard, R.A. A Revision of Casimirella, Including Humirianthera (Icacinaceae). Brittonia 1992, 44, 166–172. [Google Scholar] [CrossRef]
- Graebner, I.B.; Mostardeiro, M.A.; Ethur, E.M.; Burrow, R.A.; Dessoy, E.C.S.; Morel, A.F. Diterpenoids from Humirianthera Ampla. Phytochemistry 2000, 53, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Graebner, I.B.; Morel, A.F.; Burrow, R.A.; Mostardeiro, M.A.; Ethur, E.M.; Dessoy, E.C.M.; Scher, A. Diterpenos Isolados de Humirianthera Ampla. Miers. Rev. Bras. Farmacogn. 2002, 12, 80–81. [Google Scholar] [CrossRef]
- Monks, N.R.; Bordignon, S.A.L.; Ferraz, A.; Machado, K.R.; Faria, D.H.; Lopes, R.M.; Mondin, C.A.; De Souza, I.C.C.; Lima, M.F.S.; Da Rocha, A.B.; et al. Anti-Tumour Screening of Brazilian Plants. Pharm. Biol. 2002, 40, 603–616. [Google Scholar] [CrossRef]
- Burrow, R.A.; Morel, A.F.; Graebner, I.B.; Farrar, D.H.; Lough, A.J. The Acetyl Derivative of Humirianthol. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, 347–349. [Google Scholar] [CrossRef]
- Burrow, R.A.; Morel, A.F.; Graebner, I.B.; Lough, A.J.; Farrar, D.H. Absolute Configuration of Diacetylated Acrenol as Its Chloroform Solvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, 350–352. [Google Scholar] [CrossRef]
- Graebner, I.B. Estudo Dos Constituintes Químicos Isolados de Plantas Medicinais Da Região Do Vale Do Purus No Acre (Amazônia) 133 f. Tese (Doutorado); Universidade Federal de Santa Maria: Santa Maria, Brazil, 2003. [Google Scholar]
- Adou, E.; Williams, R.B.; Schilling, J.K.; Malone, S.; Meyer, J.; Wisse, J.H.; Frederik, D.; Koese, D.; Werkhoven, M.C.M.; Snipes, C.E.; et al. Cytotoxic Diterpenoids from Two Lianas from the Suriname Rainforest. Bioorg. Med. Chem. 2005, 13, 6009–6014. [Google Scholar] [CrossRef]
- Marques, R.D.E.A. Estudo Fitoquímico e Biológico de Humirianthera Ampla Miers (ICACINACEAE). Master’s Thesis, Universidade Federal do Ceará, Fortaleza-Ceará, Brazil, 2007; p. 132. [Google Scholar]
- Luiz, A.P.; Moura, J.D.Á.; Meotti, F.C.; Guginski, G.; Guimarães, C.L.S.; Azevedo, M.S.; Rodrigues, A.L.S.; Santos, A.R.S. Antinociceptive Action of Ethanolic Extract Obtained from Roots of Humirianthera Ampla Miers. J. Ethnopharmacol. 2007, 114, 355–363. [Google Scholar] [CrossRef]
- Strauch, M.A.; Tomaz, M.A.; Monteiro-Machado, M.; Ricardo, H.D.; Cons, B.L.; Fernandes, F.F.A.; El-Kik, C.Z.; Azevedo, M.S.; Melo, P.A. Antiophidic Activity of the Extract of the Amazon Plant Humirianthera Ampla and Constituents. J. Ethnopharmacol. 2013, 145, 50–58. [Google Scholar] [CrossRef]
- Marques, R.A.; Gomes, A.O.C.V.; de Brito, M.V.; dos Santos, A.L.P.; da Silva, G.S.; de Lima, L.B.; Nunes, F.M.; de Mattos, M.C.; de Oliveira, F.C.E.; do Ó Pessoa, C.; et al. Annonalide and Derivatives: Semisynthesis, Cytotoxic Activities and Studies on Interaction of Annonalide with DNA. J. Photochem. Photobiol. B Biol. 2018, 179, 156–166. [Google Scholar] [CrossRef]
- Amorim, B.S.; Cardozo, N.D.; Albuquerque, P.M.; Cabral, F.N. Flora of the Ducke Reserve, Amazonas, Brazil: Icacinaceae. Rodriguésia 2020, 71, e00712018. [Google Scholar]
- Amorim, B.S. Casimirella. In Flora Do Brasil 2020 Em Construção. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB8022 (accessed on 6 June 2020).
- Albuquerque, M.; Pinheiro, E. Tuberosas Feculentas. Instituto de Pesquisas e Experimentação Agropecuárias Do Norte—IPEAN. Série Fitotec. 1970, 1, 1970. [Google Scholar]
- Baehni, C. Revision Des Genres Neoleretia, Mappia et Humirianthera. Candollea 1936, 7, 167–181. [Google Scholar]
- GBIF. G.B.I.F.—Acesso Livre e Aberto a Dados de Biodiversidade—Taxonomia. Available online: https://www.gbif.org/occurrence/search?dataset_key=7aeb3ded-7443-4512-95f7-2ec7ab0f308candtaxon_key=3596825 (accessed on 15 June 2020).
- Amorim, B.S.; Stefano, R.D. Icacinaceae. In: Flora Do Brasil 2020 Em Construção. Available online: http://reflora.jbrj.gov.br/reflora/floradobrasil/FB84155 (accessed on 15 June 2020).
- Reflora-Herbário Virtual—Humirianthera Rupestris. Available online: http://floradobrasil.jbrj.gov.br/reflora/herbarioVirtual/ConsultaPublicoHVUC/ConsultaPublicoHVUC.do?idTestemunho=414989 (accessed on 15 January 2023).
- Reflora-Herbário Virtual—Humirianthera Ampla. Available online: http://floradobrasil.jbrj.gov.br/reflora/herbarioVirtual/ConsultaPublicoHVUC/ConsultaPublicoHVUC.do?idTestemunho=5472396 (accessed on 15 January 2023).
- Spruce, R. Journal of a Voyage up the Amazon and Rio Negro. In Hooker’s Journal of Botany and Kew Garden Miscellany; Hooker, J., Ed.; Lovell Reeve: London, UK, 1851; Volume 3, pp. 210–212. [Google Scholar]
- Balestra, A.A. Tempos Mansos: História, Socialidade e Transformação No Juruá-Purus Indígena. Master’s Thesis, Em Antropologia Social da Universidade de Brasília, Brasília, Brazil, 2013; p. 233. [Google Scholar]
- Hegnauer, R. Icacinaceae. In Chemotaxonomie Der Pflanzen. Lehrbücher Und Monographien Aus Dem Gebiete Der Exakten Wissenschaften (Chemische Reihe); Birkhäuser: Basel, Switzerland, 1966; Volume 19, pp. 275–277. [Google Scholar]
- Leitão-Barboza, M.S.; Kawa, N.C.; Junqueira, A.B.; Oyuela-Caycedo, A. Open Air Laboratories: Amazonian Home Gardens as Sites of Experimentation, Collaboration, and Negotiation across Time. J. Anthropol. Archaeol. 2021, 62, 101302. [Google Scholar] [CrossRef]
- dos Santos, G.M.; Cangussu, D.; Furquim, L.P.; Watling, J.; Neves, E.G. Indigenous Bread and Vegetable Pulp: Bonds between Past and Present in Indigenous Amazon. Bol. Do Mus. Para. Emilio GoeldiCiencias Humanas 2021, 16, 1–20. [Google Scholar] [CrossRef]
- Watling, J.; Shock, M.P.; Almeida, G.Z.M.F.O.; Kater, T.; De Oliveira, P.E.; Neves, E.G. Direct Archaeological Evidence for Southwestern Amazonia as an Early Plant Domestication and Food Production Centre. PLoS ONE 2018, 13, e0199868. [Google Scholar] [CrossRef]
- Mostafa, M.; Nahar, N.; Mosihuzzaman, M.; Sokeng, S.D.; Fatima, N.; Rahman, A.U.; Choudhary, M.I. Phosphodiesterase-I Inhibitor Quinovic Acid Glycosides from Bridelia Ndellensis. Nat. Prod. Res. 2006, 20, 686–692. [Google Scholar] [CrossRef]
- Hernandez-Perez, M.; Rabanal, R.M.; De La Torre, M.C.; Rodriguez, B. Analgesic, Anti-Inflammatory, Antipyretic and Haematological Effects of Aethiopinone, an o-Naphthoquinone Diterpenoid from Salvia Aethiopis Roots and Two Hemisynthetic Derivatives. Planta Med. 1995, 61, 505–509. [Google Scholar] [CrossRef]
- Liu, C.F.; Lin, N. Progress in Research on Mechanisms of Anti-Rheumatoid Arthritis of Triptolide. Zhongguo Zhong Yao Za Zhi 2006, 31, 1575–1579. [Google Scholar]
- Laszczyk, M.N. Pentacyclic Triterpenes of the Lupane, Oleanane and Ursane Group as Tools in Cancer Therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef]
- Sharma, V.; Kaur, M.; Sandhu, K.S.; Godara, S.K. Effect of Cross-Linking on Physico-Chemical, Thermal, Pasting, in Vitro Digestibility and Film Forming Properties of Faba Bean (Vicia faba L.) Starch. Int. J. Biol. Macromol. 2020, 159, 243–249. [Google Scholar] [CrossRef]
- Siddique, H.R.; Saleem, M. Beneficial Health Effects of Lupeol Triterpene: A Review of Preclinical Studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Lehtonen, M.; Suuronen, T.; Kaarniranta, K.; Huuskonen, J. Terpenoids: Natural Inhibitors of NF-ΚB Signaling with Anti-Inflammatory and Anticancer Potential. Cell. Mol. Life Sci. 2008, 65, 2979–2999. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M. Lupeol, a Novel Anti-Inflammatory and Anti-Cancer Dietary Triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Shahlaei, M.; Ghanadian, S.M.; Ayatollahi, A.M.; Mesaik, M.A.; Abdalla, O.M.; Afsharypour, S.; Rabbani, M. Molecular Modeling, Structure Activity Relationship and Immunomodulatory Properties of Some Lupeol Derivatives. Med. Chem. Res. 2013, 22, 1795–1803. [Google Scholar] [CrossRef]
- Saleem, M.; Maddodi, N.; Zaid, M.A.; Khan, N.; Bin Hafeez, B.; Asim, M.; Suh, Y.; Yun, J.M.; Setaluri, V.; Mukhtar, H. Lupeol Inhibits Growth of Highly Aggressive Human Metastatic Melanoma Cells in Vitro and in Vivo by Inducing Apoptosis. Clin. Cancer Res. 2008, 14, 2119–2127. [Google Scholar] [CrossRef]
- Gallo, M.B.C.; Sarachine, M.J. Biological Activities of Lupeol. Syst. Rev. Pharm. 2009, 3, 46–66. [Google Scholar] [CrossRef]
- Fotie, J.; Bohle, D.S.; Leimanis, M.L.; Georges, E.; Rukunga, G.; Nkengfack, A.E. Lupeol Long-Chain Fatty Acid Esters with Antimalarial Activity from Holarrhena Floribunda. J. Nat. Prod. 2006, 69, 62–67. [Google Scholar] [CrossRef]
- Boakye, Y.D.; Groyer, L.; Heiss, E.H. An Increased Autophagic Flux Contributes to the Anti-Inflammatory Potential of Urolithin A in Macrophages. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 61–70. [Google Scholar] [CrossRef]
- Saha, S.; Profumo, E.; Togna, A.R.; Riganò, R.; Saso, L.; Buttari, B. Lupeol Counteracts the Proinflammatory Signalling Triggered in Macrophages by 7-Keto-Cholesterol: New Perspectives in the Therapy of Atherosclerosis. Oxid. Med. Cell. Longev. 2020, 2020, 1232816. [Google Scholar] [CrossRef]
- Sharma, N.; Palia, P.; Chaudhary, A.S.; Verma, K.K. A Review on Pharmacological Activities of Lupeol and its Triterpene Derivatives. J. Drug Deliv. Ther. 2020, 10, 325–332. [Google Scholar] [CrossRef]
- Colquhoun, A.J.; Venier, N.A.; Vandersluis, A.D.; Besla, R.; Sugar, L.M.; Kiss, A.; Fleshner, N.E.; Pollak, M.; Klotz, L.H.; Venkateswaran, V. Metformin Enhances the Antiproliferative and Apoptotic Effect of Bicalutamide in Prostate Cancer. Prostate Cancer Prostatic Dis. 2012, 15, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Zachos, G.; Spandidos, D.A. Expression of Ras Proto-Oncogenes: Regulation and Implications in the Development of Human Tumors. Crit. Rev. Oncol. Hematol. 1997, 26, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Santos-Hidalgo, A.B.; Saura-Calixto, F. Common Sources and Estimated Intake of Plant Sterols in the Spanish Diet. J. Agric. Food Chem. 2006, 54, 3462–3471. [Google Scholar] [CrossRef]
- Nair, P.P.; Turjman, N.; Kesie, G.; Calkins, B.; Goodman, G.T.; Davidovitz, H.; Ninmagadda, G. Diet, Nutrition Intake, and Metabolism in Populations at High and Low Risk for Colon Cancer. Metabolism of Bile Acids. Am. J. Clin. Nutr. 1984, 40, 937–941. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, A.K.; Dobhal, M.P.; Sharma, M.C.; Gupta, R.S. Antidiabetic and Antioxidant Potential of β-Sitosterol in Streptozotocin-Induced Experimental Hyperglycemia. J. Diabetes 2011, 3, 29–37. [Google Scholar] [CrossRef]
- Shi, C.; Wu, F.; Xu, J. Incorporation of β-Sitosterol into Mitochondrial Membrane Enhances Mitochondrial Function by Promoting Inner Mitochondrial Membrane Fluidity. J. Bioenerg. Biomembr. 2013, 45, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Junaid, M.; Ullah, F.; Subhan, F.; Sadiq, A.; Ali, G.; Ovais, M.; Shahid, M.; Ahmad, A.; Wadood, A.; et al. Anti-Alzheimer’s Studies on ß-Sitosterol Isolated from Polygonum hydropiper L. Front. Pharmacol. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Cheng, J.; Guo, B.; Duan, J.; Che, C.T. Momilactone and Related Diterpenoids as Potential Agricultural Chemicals. J. Agric. Food Chem. 2018, 66, 7859–7872. [Google Scholar] [CrossRef]
- Ramos, D.V.B.; Neto, H.G.; Azevedo, M.S.; Jardim, I.S.; Souza, A.C.F.; Cunha, E.M.F.; Marques, E.; Cunha, F. Estudo Fitoquímico e Atividade Leishmanicida in Vitro de Extrato e Substâncias Isoladas de Humirianthera Ampla (MIERS). In Proceedings of the Amazônia Ciência e Cultura. 61a Reunião Anual da SBPC, Manaus, Brazil, 12–17 July 2009; Anais da Sociedade Brasileira do Progresso e da Ciência: Rio de Janeiro, Brazil, 2009; p. 5541. [Google Scholar]
- Gonçalves, H.P.; Gonçalves-Neto, H.; Aprígio, C.J.L.; Azevedo, M.S.; Silva-Jardim, I. QT54—Chemical study and evaluation of leishmanicidal activity from the ethanolic extract, eluate and isolated substance from Humirianthera ampla Miers. Quimioterapia- Chemotherapy. In Proceedings of the SBPZ. Sociedade Brasileira de Protozoologia, Caxambu, Brazil, 9–11 November 2015; Available online: https://sbpz.org.br/wp-content/uploads/2015/12/QT.pdf (accessed on 12 October 2020).
- Huggett, A.C.; Verschuren, P.M. The Safety Assurance of Functional Foods. Nutr. Rev. 1996, 54, S132–S140. [Google Scholar] [CrossRef]
- OECD Organization for Economic Co-Operation and Development. OECD Test Guidelines for Chemicals. Available online: https://www.oecd.org/chemicalsafety/testing/oecdguidelinesforthetestingofchemicals.htm (accessed on 22 November 2021).
- Barbosa, J.R.; de Carvalho Junior, R.N. Occurrence and Possible Roles of Polysaccharides in Fungi and Their Influence on the Development of New Technologies. Carbohydr. Polym. 2020, 246, 116613. [Google Scholar] [CrossRef]
- Romero-Bastida, C.A.; Bello-Pérez, L.A.; García, M.A.; Martino, M.N.; Solorza-Feria, J.; Zaritzky, N.E. Physicochemical and Microstructural Characterization of Films Prepared by Thermal and Cold Gelatinization from Non-Conventional Sources of Starches. Carbohydr. Polym. 2005, 60, 235–244. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent Advances on Chitosan-Based Films for Sustainable Food Packaging Applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Valencia-Sullca, C.; Vargas, M.; Atarés, L.; Chiralt, A. Thermoplastic Cassava Starch-Chitosan Bilayer Films Containing Essential Oils. Food Hydrocoll. 2018, 75, 107–115. [Google Scholar] [CrossRef]
- Requena, R.; Vargas, M.; Chiralt, A. Obtaining Antimicrobial Bilayer Starch and Polyester-Blend Films with Carvacrol. Food Hydrocoll. 2018, 83, 118–133. [Google Scholar] [CrossRef]
- Sudheesh, C.; Sunooj, K.V.; Sasidharan, A.; Sabu, S.; Basheer, A.; Navaf, M.; Raghavender, C.; Sinha, S.K.; George, J. Energetic Neutral N2 Atoms Treatment on the Kithul (Caryota Urens) Starch Biodegradable Film: Physico-Chemical Characterization. Food Hydrocoll. 2020, 103, 105650. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-Based Biodegradable Materials: Challenges and Opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Pascoal, A.M.; Di-Medeiros, M.C.B.; Batista, K.A.; Leles, M.I.G.; Lião, L.M.; Fernandes, K.F. Extraction and Chemical Characterization of Starch from S. Lycocarpum Fruits. Carbohydr. Polym. 2013, 98, 1304–1310. [Google Scholar] [CrossRef]
- Tao, K.; Yu, W.; Prakash, S.; Gilbert, R.G. High-Amylose Rice: Starch Molecular Structural Features Controlling Cooked Rice Texture and Preference. Carbohydr. Polym. 2019, 219, 251–260. [Google Scholar] [CrossRef]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and Physical Modifications of Starch for Renewable Polymeric Materials. Mater. Today Sustain. 2020, 7–8, 100028. [Google Scholar] [CrossRef]
- Silva, D.A.; Aires, G.C.M.; Pena, R.d.S. Gums—Characteristics and Applications in the Food Industry. In Innovation in the Food Sector Through the Valorization of Food and Agro-Food By-Products; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Menzel, C.; González-Martínez, C.; Vilaplana, F.; Diretto, G.; Chiralt, A. Incorporation of Natural Antioxidants from Rice Straw into Renewable Starch Films. Int. J. Biol. Macromol. 2020, 146, 976–986. [Google Scholar] [CrossRef]
- Tapia, M.S.; Pérez, E.; Rodríguez, P.E.; Guzmán, R.; Ducamp-Collin, M.N.; Tran, T.; Rolland-Sabaté, A. Some Properties of Starch and Starch Edible Films from Under-Utilized Roots and Tubers from the Venezuelan Amazons. J. Cell. Plast. 2012, 48, 526–544. [Google Scholar] [CrossRef]
- Galindez, A.; Daza, L.D.; Homez-Jara, A.; Eim, V.S.; Váquiro, H.A. Characterization of Ulluco Starch and Its Potential for Use in Edible Films Prepared at Low Drying Temperature. Carbohydr. Polym. 2019, 215, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.C.M.; Miki, K.S.L.; Ramos, A.d.S.; Teixeira-Costa, B.E. Development of Biodegradable Films Based on Purple Yam Starch/Chitosan for Food Application. Heliyon 2020, 6, e03718. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Thory, R.; Sinhmar, A.; Pathera, A.K.; Nain, V. Development and Characterization of Nano Starch-Based Composite Films from Mung Bean (Vigna Radiata). Int. J. Biol. Macromol. 2020, 144, 242–251. [Google Scholar] [CrossRef]
- Liporacci, J.S.N.; Mali, S.; Grossmann, M.V.E. Efeito Do Método de Extração Na Composição Química e Nas Propriedades Funcionais Do Amido de Inhame (Dioscorea Alata). Semin. Ciências Agrárias 2005, 26, 345. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Hagenmaier, R.; Bai, J. Edible Coatings and Films to Improve Food Quality, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Nakamura, Y. Starch Metabolism and Structure; Springer: Berlin, Germany, 2015. [Google Scholar]
- Zhu, F. Structure, Properties, and Applications of Aroid Starch. Food Hydrocoll. 2016, 52, 378–392. [Google Scholar] [CrossRef]
- Yao Désiré, A.; Charlemagne, N.; Degbeu Claver, K.; Fabrice Achille, T.; Marianne, S. Starch-Based Edible Films of Improved Cassava Varieties Yavo and TMS Reinforced with Microcrystalline Cellulose. Heliyon 2021, 7, e06804. [Google Scholar] [CrossRef]
- Niu, X.; Ma, Q.; Li, S.; Wang, W.; Ma, Y.; Zhao, H.; Sun, J.; Wang, J. Preparation and Characterization of Biodegradable Composited Films Based on Potato Starch/Glycerol/Gelatin. J. Food Qual. 2021, 2021, 6633711. [Google Scholar] [CrossRef]
- Ye, C.; Chi, H. A Review of Recent Progress in Drug and Protein Encapsulation: Approaches, Applications and Challenges. Mater. Sci. Eng. C 2018, 83, 233–246. [Google Scholar] [CrossRef]
- Ahmad, S.U.; Li, B.; Sun, J.; Arbab, S.; Dong, Z.; Cheng, F.; Zhou, X.; Mahfuz, S.; Zhang, J. Recent Advances in Microencapsulation of Drugs for Veterinary Applications. J. Vet. Pharmacol. Ther. 2021, 44, 298–312. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ma, J.; Khoo, T.S.; Abdullah, N.; Nik Md Noordin Kahar, N.N.F.; Abdul Hamid, Z.A.; Mustapha, M. Polysaccharide-Based Hydrogels for Microencapsulation of Stem Cells in Regenerative Medicine. Front. Bioeng. Biotechnol. 2021, 9, 735090. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, J.D. Phytochemistry and Medicinal Plants. Phytochemistry 2001, 56, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Functionality of Food Components and Emerging Technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Noore, S.; Rastogi, N.K.; O’Donnell, C.; Tiwari, B. Novel Bioactive Extraction and Nano-Encapsulation. Encyclopedia 2021, 1, 632–664. [Google Scholar] [CrossRef]
- Alemzadeh, I.; Hajiabbas, M.; Pakzad, H.; Sajadi Dehkordi, S.; Vossoughi, A. Encapsulation of Food Components and Bioactive Ingredients and Targeted Release. Int. J. Eng. Trans. A Basics 2020, 33, 1–11. [Google Scholar] [CrossRef]
- Chaudhary, V.; Thakur, N.; Kajla, P.; Thakur, S.; Punia, S. Application of Encapsulation Technology in Edible Films: Carrier of Bioactive Compounds. Front. Sustain. Food Syst. 2021, 5, 734921. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Boostani, S.; Katouzian, I.; Rezaei, A.; Assadpour, E.; Jafari, S.M. Design and Formulation of Nano/Micro-Encapsulated Natural Bioactive Compounds for Food Applications. In Application of Nano/Microencapsulated Ingredients in Food Products; Academic Press: Cambridge, MA, USA, 2020; pp. 1–41. ISBN 978-0-12-815726-8. [Google Scholar]
- Favaro-Trindade, C.S.; de Matos Junior, F.E.; Okuro, P.K.; Dias-Ferreira, J.; Cano, A.; Severino, P.; Zielińska, A.; Souto, E.B. Encapsulation of Active Pharmaceutical Ingredients in Lipid Micro/Nanoparticles for Oral Administration by Spray-Cooling. Pharmaceutics 2021, 13, 1186. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Pandya, J.K.; McClements, D.J.; Lu, J.; Kinchla, A.J. Advancements in 3D Food Printing: A Comprehensive Overview of Properties and Opportunities. Crit. Rev. Food Sci. Nutr. 2021, 62, 4752–4768. [Google Scholar] [CrossRef]
- Bird, D.T.; Ravindra, N.M. Additive Manufacturing of Sensors for Military Monitoring Applications. Polymers 2021, 13, 1455. [Google Scholar] [CrossRef]
- Mahmood, M.A. 3D Printing in Drug Delivery and Biomedical Applications: A State-of-the-Art Review. Compounds 2021, 1, 94–115. [Google Scholar] [CrossRef]
- Agrawaal, H.; Thompson, J.E. Additive Manufacturing (3D Printing) for Analytical Chemistry. Talanta Open 2021, 3, 100036. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, L.; Zou, Y.; Tong, Z.; Han, S.; Wang, S. 3D Food Printing: Main Components Selection by Considering Rheological Properties. Crit. Rev. Food Sci. Nutr. 2019, 59, 2335–2347. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, D.; Zang, Z.; Sun, X.; Tan, H.; Si, X.; Tian, J.; Teng, W.; Wang, J.; Liang, Q.; et al. 3D Food Printing: Applications of Plant-Based Materials in Extrusion-Based Food Printing. Crit. Rev. Food Sci. Nutr. 2021, 62, 7184–7198. [Google Scholar] [CrossRef]
- Singhal, S.; Rasane, P.; Kaur, S.; Garba, U.; Bankar, A.; Singh, J.; Gupta, N. 3D Food Printing: Paving Way towards Novel Foods. An. Acad. Bras. Cienc. 2020, 92, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Shen, F.; Chua, C.K.; Zhou, K. Polymeric Composites for Powder-Based Additive Manufacturing: Materials and Applications. Prog. Polym. Sci. 2019, 91, 141–168. [Google Scholar] [CrossRef]
- Pérez, B.; Nykvist, H.; Brøgger, A.F.; Larsen, M.B.; Falkeborg, M.F. Impact of Macronutrients Printability and 3D-Printer Parameters on 3D-Food Printing: A Review. Food Chem. 2019, 287, 249–257. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, M.; Bhandari, B. Materials Properties of Printable Edible Inks and Printing Parameters Optimization during 3D Printing: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3074–3081. [Google Scholar] [CrossRef]
- Dankar, I.; Pujolà, M.; El Omar, F.; Sepulcre, F.; Haddarah, A. Impact of Mechanical and Microstructural Properties of Potato Puree-Food Additive Complexes on Extrusion-Based 3D Printing. Food Bioprocess Technol. 2018, 11, 2021–2031. [Google Scholar] [CrossRef]
- Sun, J.; Peng, Z.; Zhou, W.; Fuh, J.Y.H.; Hong, G.S.; Chiu, A. A Review on 3D Printing for Customized Food Fabrication. Procedia Manuf. 2015, 1, 308–319. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Huang, D.; Fuh, J.Y.H.; Hong, G.S. An Overview of 3D Printing Technologies for Food Fabrication. Food Bioprocess Technol. 2015, 8, 1605–1615. [Google Scholar] [CrossRef]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the Printability of Hydrogels in 3D Bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]
- Ma, Y.; Schutyser, M.A.I.; Boom, R.M.; Zhang, L. Predicting the Extrudability of Complex Food Materials during 3D Printing Based on Image Analysis and Gray-Box Data-Driven Modelling. Innov. Food Sci. Emerg. Technol. 2021, 73, 102764. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D Printing: Printing Precision and Application in Food Sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef]
- Liu, Z.; Bhandari, B.; Prakash, S.; Mantihal, S.; Zhang, M. Linking Rheology and Printability of a Multicomponent Gel System of Carrageenan-Xanthan-Starch in Extrusion Based Additive Manufacturing. Food Hydrocoll. 2019, 87, 413–424. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]


| No | Title | Principal Findings | Publication | Ref. |
|---|---|---|---|---|
| 01 | Humirianthenolides, new degraded diterpenoids from Humirianthera rupestris | The tuber of Humirianthera rupestris (Icacinaceae) contains the degraded diterpenoids 3fi,20-epoxy-3uhydroxy-14-oxo-9βpodocarpan-19,6β-olide (humirianthenolide A), 3β,20-epoxy-3α,14u-dihydroxy-9β-podocarpan19,6β-olide (humirianthenolide B), 3β,20; 16,14-diepoxy-3cl-hydroxy-17-nor-15-oxo-9β-abiet-l3-en-l9,6β-olide (humirianthenolide C), 3β,20-epoxy-3a,14-dihydroxy-13-oxo-9β-podocarp-8(14)-en-l9,6β-olide (humirianthenolide D), 3β,20-epoxy-3a-hidroxy-14-oxo-8α,9β-podocarpan-l9,6β-olide (humirianthenolide E), and 3β,20-epoxy-3α,14β-dihydroxy-8α,9 β podocarpan-19,6β-olide (humirianthenolide F). ‘H NMR and ‘3C NMR spectroscopy were effective for the determination of the humirianthenolide structures. | Phytochemistry | [13] |
| 02 | Chemical study of Humirianthera ampla. (Miers) Baehni (Icacinaceae) | From the tubers of H. ampla (Miers) Baehni (Icacinaceae), the humiriantenolides A, C, and D were isolated in addition to sitosterol. | Acta Amazonica | [14] |
| 03 | The presence of toxic inorganic substances in the genus Humirianthera (Icacinaceae) | The isolation of thiocyanate crystals, sodium and potassium nitrate, and nitrite in the tuber and stem of Humirianthera ampla has been reported. The nitrate and nitrite contents in the leaves of H. ampla and H. rupestris at young and adult ages were also determined. The entire contents of N-NO3 observed for H. ampla and H. rupestris were 8.83 mg % and 6.42 mg %. | Acta Amazonica | [15] |
| 04 | Seasonal variation of macro- and microelements in the genus Humirianthera (Icacinaceae) as a function of age | Twenty-six specimens of H. ampla and nine of H. rupestris at young and adult ages collected during the dry and rainy seasons were analyzed for the total content of N, F, K, Ca, Mg, Mo, Cu, Zn, Mn, Fe, B, Al, Co, Cr, Na, Pb, Si, and Sr in the different vegetative parts of the plants. The concentration of macro-elements in the leaves, stems, and tubers of H. ampla and H. rupestris obeys the relationship N > Ca > Mg > F > K. The average concentration of these elements indicates no significant variability in the organs analyzed nor in relation to the ages, except for N, which presents a higher content in the leaves and in relation to the other elements. The micro-elements are uniformly distributed in the various organs, with Fe, Al, and Na having the highest concentration. | Acta Amazonica | [16] |
| 05 | Seasonal variation of sulfate, total sulfur and organic matter in the genus Humirianthera as a function of age | Twenty-eight (28) H. ampla and nine (09) H. rupestris adult and young specimens were collected to determine the levels of S04β and total-S in the leaf, stem, tuber, and soil where they grew. In H. ampla, the level of S04β varied from 0.22 to 0.78% and in H. rupestris from 0.22 to 138%. The level of S in H. ampla varied from 0.74 to 0.96% and in H. rupestris from 0.75 to 1.02%. The level of S04β IH H. ampla followed the patterned leaf > tuber > stem independent of time of year and physiological age, while in H. rupestris, the pattern was tuber > leaf > stem. The 5 showed a different behavior, maintaining the pattern tuber > stem > leaf for H. ampla and tuber > leaf > stem for H. rupestris. | Acta Amazonica | [17] |
| 06 | A revision of Casimirella, including Humirianthera (Icacinaceae) | Casimirella Hassler (1913) is accepted and Humirianthera Huber (1914) is considered a synonym. Casimirella diversifolia and C. lanata from Brazil are described as new species. Casimirella ampla (Miers) based on Leretia ampla Miers, C. crispula (Howard), based on Humirianthera crispula Howard, and C. rupestris (Ducke), based on Humirianthera rupestris Ducke, are new combinations. | Brittonia | [18] |
| 07 | Diterpenoids from Humirianthera ampla | Two diterpenoids (humirianthol and acrenol) and the known annonalide were isolated from Humirianthera ampla. Humirianthol and acrenol were determined by 1D and 2D NMR spectroscopic techniques to br: 3 beta,20:14 beta,16-diepoxy-3 alpha,15 alpha-dihydroxy-7-pimaren-19,6 beta-olide, and 3 beta,20-epoxy-3 alpha,15,16-trihydroxy-7-pimaren-19,6 beta-olide, respectively. | Phytochemistry | [19] |
| 08 | Diterpenes isolated from Humirianthera ampla. Miers | From Humirianthera ampla, Icacinaceae have isolated a phthalate, lupeol, ß-sitosterol, glycosyl–sitosterol, one known annonalide diterpene, and two new diterpenes named humirianthol and acrenol. Humirianthol and acrenol were determined by 1D and 2D NMR spectroscopic techniques to be 3 ß, 20:14 ß, 16-diepoxy-3 a, 15 a-dihydroxy-7-primary-19, 6 ß-olide, and 3 ß, 20-epoxy-3 a, 15, 16-trihydroxy-7-primary-19, 6 ß-olide, respectively. Acrenol has anti-microbial activity. | Revista Brasileira de Farmacognosia | [20] |
| 09 | Antitumour screening of Brazilian plants | Organic and aqueous extracts of 145 Brazilian plants (538) from 34 families were evaluated for antitumor activity against the human tumor cell lines HT29 and NCIH460. Of the extracts tested, 117 (22%) demonstrated cytotoxicity against one or both cell lines at a 100 mg/mL concentration. These results also confirm the continuing importance of natural product screening models, alongside targeted drug development, in discovering new anti-neoplastic pharmacophores. | Pharmaceutical Biology | [21] |
| 10 | The acetyl derivative of humirianthol | The title compound, 15alpha-acetate-3beta,20:14beta,16-diepoxy-3alpha-hydroxy-9-epi-7-pimaren-19,6beta-olide, C22H28O7, formed from the acetylation of humirianthol, isolated from the tubers of Humirianthera ampla, crystallized in the chiral space group P2(1)2(1)2(1). The structure comprised a pimarane ring system with a methylene ether bridge over ring A, a double bond in ring B, and two five-membered furanyl rings, one fused to rings A and B and the other to ring C. The absolute configuration was set using the absolute configuration of C15, as determined by the Horeau method. | Acta Crystallographica Section E structure reports online | [22] |
| 11 | Absolute configuration of diacetylated acrenol as its chloroform solvate | The title compound, (15S)-15,16-diacetate-3beta,20-epoxy-3beta-hydroxy-9-epi-7-pimaren-19,6beta-olide chloroform solvate, C24H32O8.CHCl3, formed from diacetylated acrenol, isolated from the tubers of Humirianthera ampla, crystallized as a chloroform solvate. The structure was based on a pimarane skeleton and was identical to the previously determined structures of icancinol and the acetylated derivative of humirianthol. The anomalous dispersion of the Cl atoms allowed the absolute configuration to be determined. | Acta Crystallographica Section E structure reports online | [23] |
| 12 | Study of chemical constituents isolated from medicinal plants of the Purus valley region in Acre (Amazonia) | From the tubers of the species Humirianthera ampla, the diterpenes humirianthol (3,18: 14,16 diepoxy 3,15-dihydroxy 7-pimarene-17,6β-olide) (18) and acrenol (15,16 diol-3β,20 epoxy-3α-hydroxy 9 epi-7 -pimarene-19,6β-olide) (19) and the constituents β-amyrin (37), glycosylated β-sitosterol (45), glycosylated plumeride (32), and glycoplumeric acid (1β-O-β-Dglycopyranosylplumeric) (33) were isolated and identified. | Thesis | [24] |
| 13 | Cytotoxic diterpenoids from two lianas from the Suriname rainforest | Bioassay-guided fractionation of the MeOH and EtOAc fractions of extracts of two lianas collected in Suriname has led to the isolation of five new diterpenoids, humirianthone 1, 1-hydroxy-humirianthone 2, 15R-humirianthol 3, patagonol 4, and patagonal 5, and the five known diterpenoids, humirianthol 7, annonalide 8, acrenol 9, icacinol 10, and the oxidized annonalide 11. All 10 diterpenoids showed cytotoxic activity against the A2780 human ovarian cancer cell line; compounds 1, 3, 8, and 9 also showed activity against phytopathogenic fungi. | Bioorganical & Medicinal Chemistry | [25] |
| 14 | Phytochemical and Biological Study of Humirianthera ampla Miers (Icacinaceae). | The phytochemical investigation of the roots of Humiranthera ampla (Icacinaceae) resulted in the isolation and identification of a mixture of beta-sitosterol and stigmasterol, annonalide, lupeol, and the 3-beta-O-beta-D-glucopyranosyl sitosterol. The structures of these compounds were established by spectrometric analysis (IR, MS, NMR 1H, and 13C), including bidimensional NRM techniques (COSY, HMQC, HMBC, and NOESY) and for comparison with data described in the literature. All extracts were tested using the Ellman assay. Only ethyl acetate extracts and their fractions showed acetylcholinesterase inhibition. The ethanolic extract was the most active. The ethyl acetate extract showed xanthine oxidase inhibition. | Dissertation | [26] |
| 15 | Anti-nociceptive action of ethanolic extract obtained from roots of Humirianthera ampla Miers | The anti-nociceptive actions of ethanolic extract (EE) of roots of Humirianthera ampla in chemical and thermal models of pain in mice were investigated. Oral treatment with ethanolic extract inhibited, in a dose-dependent manner, glutamate-, capsaicin- and formalin-induced licking. However, it did not prevent nociception caused by radiant heat in the tail jerking test. The ethanolic extract (30 mg/kg) caused marked inhibition of the nociceptive bite response induced by glutamate, (±) -1-aminocyclopentane- trans -1,3-dicarboxylic acid (trans-ACPD), N- methyl- d- aspartate (NMDA), and substance P. The anti-nociception caused by the ethanolic extract was significantly attenuated by naloxone, L- arginine, WAY100635, ondansetron, or ketanserin, but not by caffeine or naloxone methiodide. The ethanolic extract of Humirianthera ampla roots produced anti-nociception against neurogenic and inflammatory models of nociception. | Journal of ethnopharmacology | [27] |
| 16 | Anti-ophidic activity of the extract of the Amazon plant Humirianthera ampla and constituents | Although serotherapy against snakebites was discovered more than one hundred years ago, anti-venom is not available all over Brazil. The use of plants from folk medicine is common mainly in the Brazilian Amazon area. One of these plants is named Humirianthera ampla (HA). | Journal of ethnopharmacology | [28] |
| 17 | Annonalide and derivatives: semi-synthesis, cytotoxic activities, and studies on interaction of annonalide with DNA | The cytotoxic activity of the pimarane diterpene annonalide (1) and nine of its semisynthetic derivatives (2–10) was investigated against the human tumor cell lines HL-60 (leukemia), PC-3 (prostate adenocarcinoma), HepG2 (hepatocellular carcinoma), SF-295 (glioblastoma), and HCT-116 (colon cancer) and normal mouse fibroblast (L929) cells. The preparation of 2–10 involved derivatization of the side chain of 1 at C-13. Except for 2, all derivatives were reported for the first time. Most of the tested compounds presented IC50s below 4.0 μM, being considered potential anti-tumor agents. The interaction of annonalide (1) with ctDNA was evaluated using spectroscopic techniques; the formation of a supramolecular complex with the macromolecule was confirmed. Competition assays with fluorescent probes (Hoechst and ethidium bromide) and theoretical studies confirmed that 1 interacted preferentially via DNA intercalation with stoichiometric ratio of 1:1 (1:ctDNA). | Journal of Photochemistry and Photobiology B: Biology | [29] |
| 18 | Ethnobotanical and physicochemical study of the Mairá-potato (Casimirella spp.-Icacinaceae) | An ethnobotanical survey and physical–chemical characterization of the Mairá-potato was carried out. The form of starch extraction was very similar among the different ethnic groups. Only the Apurinã reported the existence of cultivation (vegetative propagation) and management, where the liana was kept alive in the fields. The physical–chemical study indicated that the tuberous root was a source of starch (68.23% on a dry basis). The yield obtained from starch extraction was up to 15.4% and the mineral contents of calcium, copper, iron, manganese, and zinc were higher than those of manioc and potato starch. The toxicity test revealed that from the fifth wash of the starch, the material extracted from the supernatant was non-toxic to Artemia salina. The functional properties of the starch revealed granules that possessed stability to thermal and mechanical action and that were relatively large, with an average size of 24.48 μm, and possessed high amylose content (38%). | Dissertation | [8] |
| 19 | Flora of the Ducke Reserve, Amazonas, Brazil: Icacinaceae | Three species belonging to two genera were recorded: Casimirella rupestris, Pleurisanthes emarginata, and P. parviflora. Casimirella rupestris is easily differentiated from Pleurisanthes species by presenting branches covered by stellate trichomes and paniculate inflorescence (vs. glabrous or puberulent branches in Pleurisanthes). | Rodriguésia | [30] |
| Kingdom | Plantae |
|---|---|
| Phylum | Tracheophyta |
| Class | Magnoliopsida |
| Order | Icacinales |
| Family | Icacinaceae (Benth.) Miers |
| Genus | Humirianthera Huber (synonym Casimirella Hassler) |
| Species | Humirianthera ampla (Miers) Baehni (synonym Casimirella ampla (Miers) R.A.Howard) and Humirianthera rupestris (synonym Casimirella rupestris (Ducke) R.A.Howard) [35] |
| Common name | Mairá-potato |
| Species | Casimirella ampla | Casimirella rupestris |
|---|---|---|
| Plant | Rhizomatous shrub or vine with branches to 30 m climbing on trees, young branches somewhat angular, glabrous; leaves with petioles 8–10 mm long, glabrate, blade broadly lanceolate to elliptic, 8–20 × 3–10 cm, apex obtuse to acuminate, base nearly acute or rounded, central vein and veins prominent below | Scandent shrub, caudex tuber large and starchy; stems angular, stellate densely red–brown, pubescent |
| Leaf | Mature leaves glabrous or nearly so; pubescence of the inflorescence single-haired. Leaves 8–20 × 3–10 cm with petioles 8–10 mm long | Leaves with petioles 6–9 mm long, stellate pubescent; blade rhomboid to ovate, 10–15 × 6–8 cm, apex acuminate, base rounded, stellate pubescent above in central vein and veins. |
| Inflorescences | Inflorescence axillary or terminal, strigose | Inflorescence axis moderately star-shaped or tomentosa |
| Flowers | Flowers with calyx patelliform, lobes 1.3–1.6 mm long, lanceolate, densely hairy, petals oval–lanceolate oval, 3.5–4.3 × 1.4–2.0 mm, nearly equal, strigose outside, villous or tomentose or rarely crispate inside, apex inflexed, glabrous; filaments 2–3 mm, anther sacs globose, connective tapering to an apex extension, 0.6–0.8 mm; ovary glabrous, diam. 1 mm; hirsute; style 0.7 mm long, glabrous, slightly curved | Flowers with calyx 4 mm in diameter, lobes triangular–acute, 1.3 mm long, hirsute pubescent on the outside; petals oval–oblong, 4.1–4.3 × 1.6–1.9 mm, hirsute, villous inside, with a glabrate base, apex flexed; filaments glabrous, 2.5–2.6 mm long; ovary globose, diam. 1.2 mm hirsute. |
| Fruit | Globose to oblong, 7.5–8 × 3.8–4.0 cm, strigose inside the endocarp | Ovoid to globose drupe, 5 cm long, 4 cm in diameter, densely stellate pubescent, endocarp woody, smooth, to 0.7 mm thick, pubescent inside |
| Name of the Isolated/Identified Compound | Species | Technique of Analysis | Structural Formula of Compound | Ref. |
|---|---|---|---|---|
| Humiriantenolides A | C. rupestris | Thin layer chromatography (TLC) and nuclear magnetic resonance spectroscopy (NMR) | 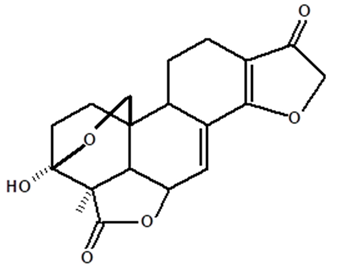 | [13] |
| C. ampla | Liquid chromatography | [14] | ||
| Humiriantenolides B | C. rupestris | Thin layer chromatography and nuclear magnetic resonance spectroscopy | 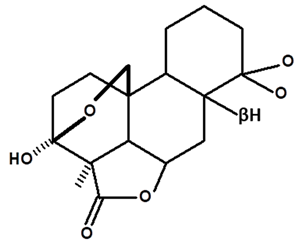 | [13] |
| Humiriantenolides C | C. rupestris | Thin layer chromatography and nuclear magnetic resonance spectroscopy | 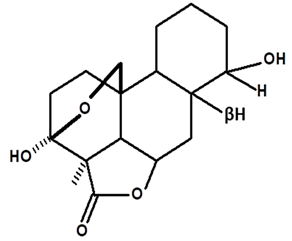 | [13] |
| C. ampla | Liquid chromatography | [14] | ||
| Humiriantenolides D | C. rupestris | Thin layer chromatography and nuclear magnetic resonance spectroscopy | 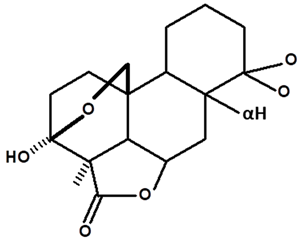 | [13] |
| C. ampla | Liquid chromatography | [14] | ||
| Humiriantenolides E | C. rupestris | Thin layer chromatography and nuclear magnetic resonance spectroscopy | 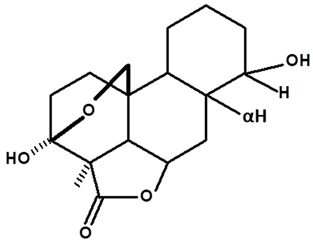 | [13] |
| Humiriantenolides F | C. rupestris | Thin layer chromatography and nuclear magnetic resonance spectroscopy | 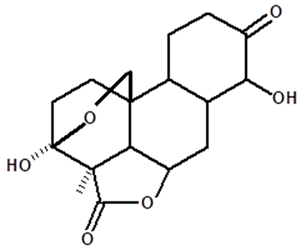 | [13] |
| β-sitosterol | C. rupestris | Thin layer chromatography and nuclear magnetic resonance spectroscopy |  | [13] |
| C. ampla | Liquid chromatography | [14] | ||
| C. ampla | Column chromatography and nuclear magnetic resonance spectroscopy | [20] | ||
| C. ampla | Thin layer chromatography and nuclear magnetic resonance spectroscopy | [26] | ||
| Sodium thiocyanate | C. ampla | Liquid chromatography and atomic absorption spectroscopy | 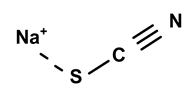 | [15] |
| Potassium thiocyanate | C. ampla | Liquid chromatography and atomic absorption spectroscopy | 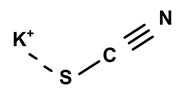 | [15] |
| Sodium nitrite | C. ampla | Liquid chromatography and atomic absorption spectroscopy | 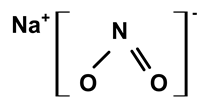 | [15] |
| Potassium nitrite | C. ampla | Liquid chromatography and atomic absorption spectroscopy | 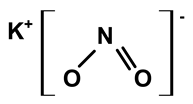 | [15] |
| Sodium nitrate | C. ampla | Liquid chromatography and atomic absorption spectroscopy | 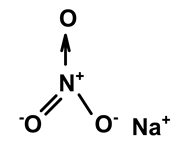 | [15] |
| Potassium nitrate | C. ampla | Liquid chromatography and atomic absorption spectroscopy | 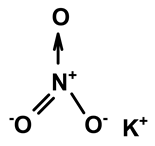 | [15] |
| Lupeol | C. ampla | Gas chromatography coupled with a flame ionization detector (GC-FID) | 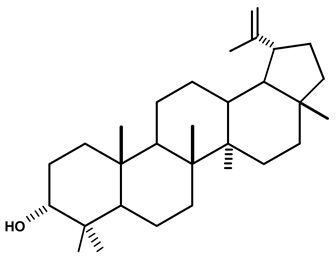 | [19] |
| C. ampla | Column chromatography and nuclear magnetic resonance spectroscopy | [20] | ||
| C. ampla | Thin layer chromatography and nuclear magnetic resonance spectroscopy | [26] | ||
| β-sitosterol glycosylate | C. ampla | Gas chromatography coupled with a flame ionization detector | 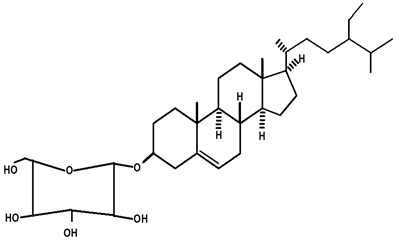 | [19] |
| C. ampla | Column chromatography and nuclear magnetic resonance spectroscopy | [20] | ||
| Annonalide | C. ampla | Gas chromatography coupled with a flame ionization detector | 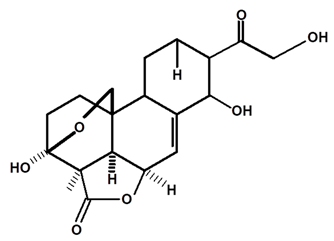 | [19] |
| C. ampla | Thin layer chromatography, nuclear magnetic resonance spectroscopy and infrared spectroscopy | [24] | ||
| C. ampla | Column chromatography and nuclear magnetic resonance spectroscopy | [20] | ||
| C. ampla | High efficiency liquid chromatography, nuclear magnetic resonance spectroscopy, and infrared spectroscopy | [25] | ||
| C. ampla | Thin layer chromatography and nuclear magnetic resonance spectroscopy | [26] | ||
| Acrenol | C. ampla | Gas chromatography coupled with a flame ionization detector | 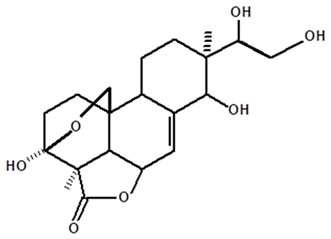 | [19] |
| C. ampla | Column chromatography and nuclear magnetic resonance spectroscopy | [20] | ||
| C. ampla | Thin layer chromatography, nuclear magnetic resonance spectroscopy and infrared spectroscopy | [24] | ||
| C. ampla | High efficiency liquid chromatography, nuclear magnetic resonance spectroscopy, and infrared spectroscopy | [25] | ||
| Humirianthol | C. ampla | Gas chromatography coupled with a flame ionization detector | 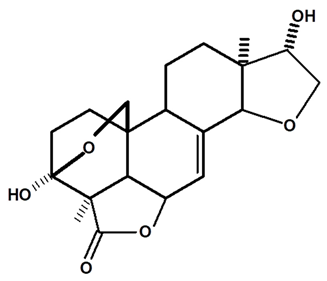 | [19] |
| C. ampla | Column chromatography and nuclear magnetic resonance spectroscopy | [20] | ||
| C. ampla | Thin layer chromatography, nuclear magnetic resonance spectroscopy and infrared spectroscopy | [24] | ||
| C. ampla | High efficiency liquid chromatography, nuclear magnetic resonance spectroscopy, and infrared spectroscopy | [25] | ||
| Phthalate | C. ampla | Column chromatography and nuclear magnetic resonance spectroscopy | 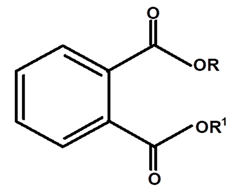 | [20] |
| β-amyrin | C. ampla | Thin layer chromatography, nuclear magnetic resonance spectroscopy, and infrared spectroscopy | 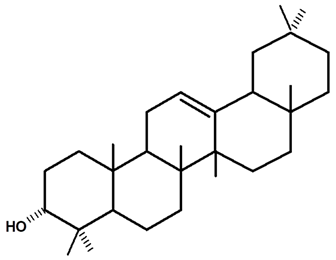 | [24] |
| Glycosylated Plumerideum | C. ampla | Thin layer chromatography, nuclear magnetic resonance spectroscopy, and infrared spectroscopy | 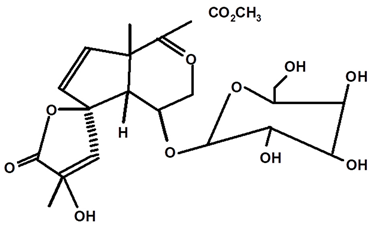 | [24] |
| 1βOβ-D glycopyranosyl plumeric | C. ampla | Thin layer chromatography, nuclear magnetic resonance spectroscopy, and infrared spectroscopy | 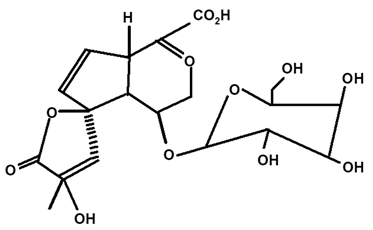 | [24] |
| Acetylated Humirianthol | C. ampla | Gas chromatography coupled with a flame ionization detector and solvation with chloroform |  | [22] |
| Acrenol diacetylate | C. ampla | Gas chromatography coupled with a flame ionization detector and thin layer chromatography | 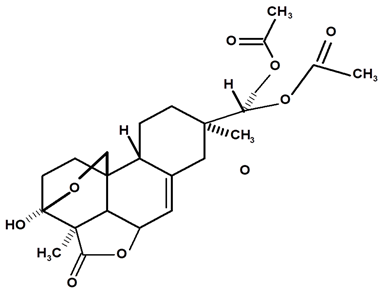 | [23] |
| Icacinol | C. ampla | Gas chromatography coupled with a flame ionization detector and solvation with chloroform | 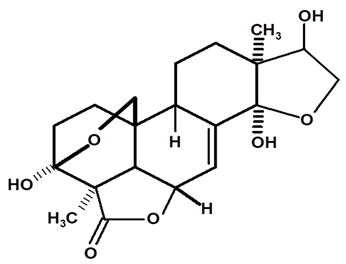 | [22] |
| C. ampla | High efficiency liquid chromatography, nuclear magnetic resonance spectroscopy, and infrared spectroscopy | [25] | ||
| Humirianthone | C. ampla | High efficiency liquid chromatography, nuclear magnetic resonance spectroscopy, and infrared spectroscopy | 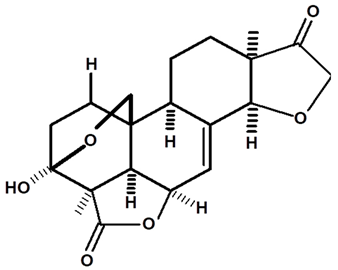 | [25] |
| 1-hydroxy-humirianthone | C. ampla | High efficiency liquid chromatography, nuclear magnetic resonance spectroscopy, and infrared spectroscopy | 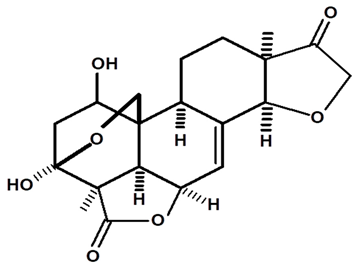 | [25] |
| 15R-humirianthol | C. ampla | High efficiency liquid chromatography, nuclear magnetic resonance spectroscopy, and infrared spectroscopy | 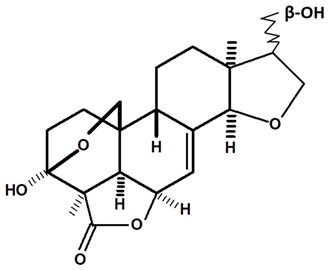 | [25] |
| Patogonol | C. ampla | High efficiency liquid chromatography, nuclear magnetic resonance spectroscopy, and infrared spectroscopy | 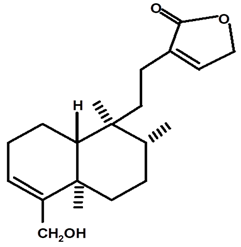 | [25] |
| Patogonal | C. ampla | High efficiency liquid chromatography, nuclear magnetic resonance spectroscopy, and infrared spectroscopy | 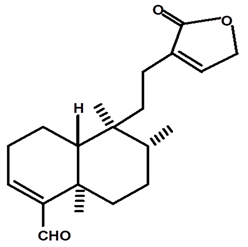 | [25] |
| Stigmasterol | C. ampla | Thin layer chromatography and nuclear magnetic resonance spectroscopy | 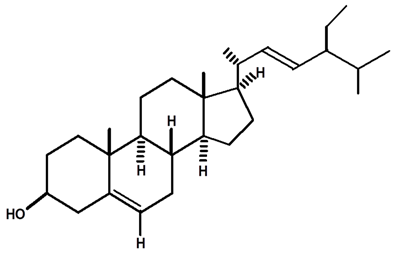 | [26] |
| 3-βOβ-D glycopyranosyl sitosterol | C. ampla | Thin layer chromatography and nuclear magnetic resonance spectroscopy | 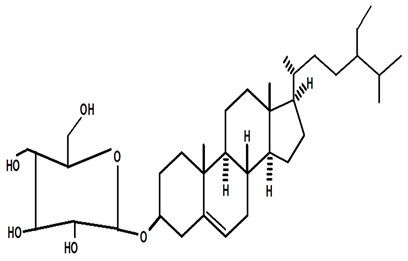 | [26] |
| Database | Descriptor Terms and Boolean Operators Used in the Search |
|---|---|
| Google Scholar | Humirianthera ampla AND Humirianthera rupestris Humirianthera ampla OR Humirianthera rupestris |
| Scielo | Humirianthera ampla AND Humirianthera rupestris Humirianthera ampla OR Humirianthera rupestris |
| Science Direct | Humirianthera ampla AND Humirianthera rupestris Humirianthera ampla OR Humirianthera rupestris |
| Scopus | ALL FIELDS = (Humirianthera ampla AND Humirianthera rupestris) ALL FIELDS = (Humirianthera ampla OR Humirianthera rupestris) |
| PubMed | Humirianthera |
| Web of Science | ALL = (Humirianthera ampla AND Humirianthera rupestris) ALL = (Humirianthera ampla OR Humirianthera rupestris) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, D.S.d.; Santos, L.N.d.; Ferreira, N.R.; Takeuchi, K.P.; Lopes, A.S. Mairá-Potato (Casimirella sp.): Botanical, Food, Pharmacological, and Phytochemical Aspects. Molecules 2023, 28, 6069. https://doi.org/10.3390/molecules28166069
Costa DSd, Santos LNd, Ferreira NR, Takeuchi KP, Lopes AS. Mairá-Potato (Casimirella sp.): Botanical, Food, Pharmacological, and Phytochemical Aspects. Molecules. 2023; 28(16):6069. https://doi.org/10.3390/molecules28166069
Chicago/Turabian StyleCosta, Danusa Silva da, Lucely Nogueira dos Santos, Nelson Rosa Ferreira, Katiuchia Pereira Takeuchi, and Alessandra Santos Lopes. 2023. "Mairá-Potato (Casimirella sp.): Botanical, Food, Pharmacological, and Phytochemical Aspects" Molecules 28, no. 16: 6069. https://doi.org/10.3390/molecules28166069
APA StyleCosta, D. S. d., Santos, L. N. d., Ferreira, N. R., Takeuchi, K. P., & Lopes, A. S. (2023). Mairá-Potato (Casimirella sp.): Botanical, Food, Pharmacological, and Phytochemical Aspects. Molecules, 28(16), 6069. https://doi.org/10.3390/molecules28166069







