Few Layer Ti3C2 MXene-Based Label-Free Aptasensor for Ultrasensitive Determination of Chloramphenicol in Milk
Abstract
:1. Introduction
2. Results and Discussion
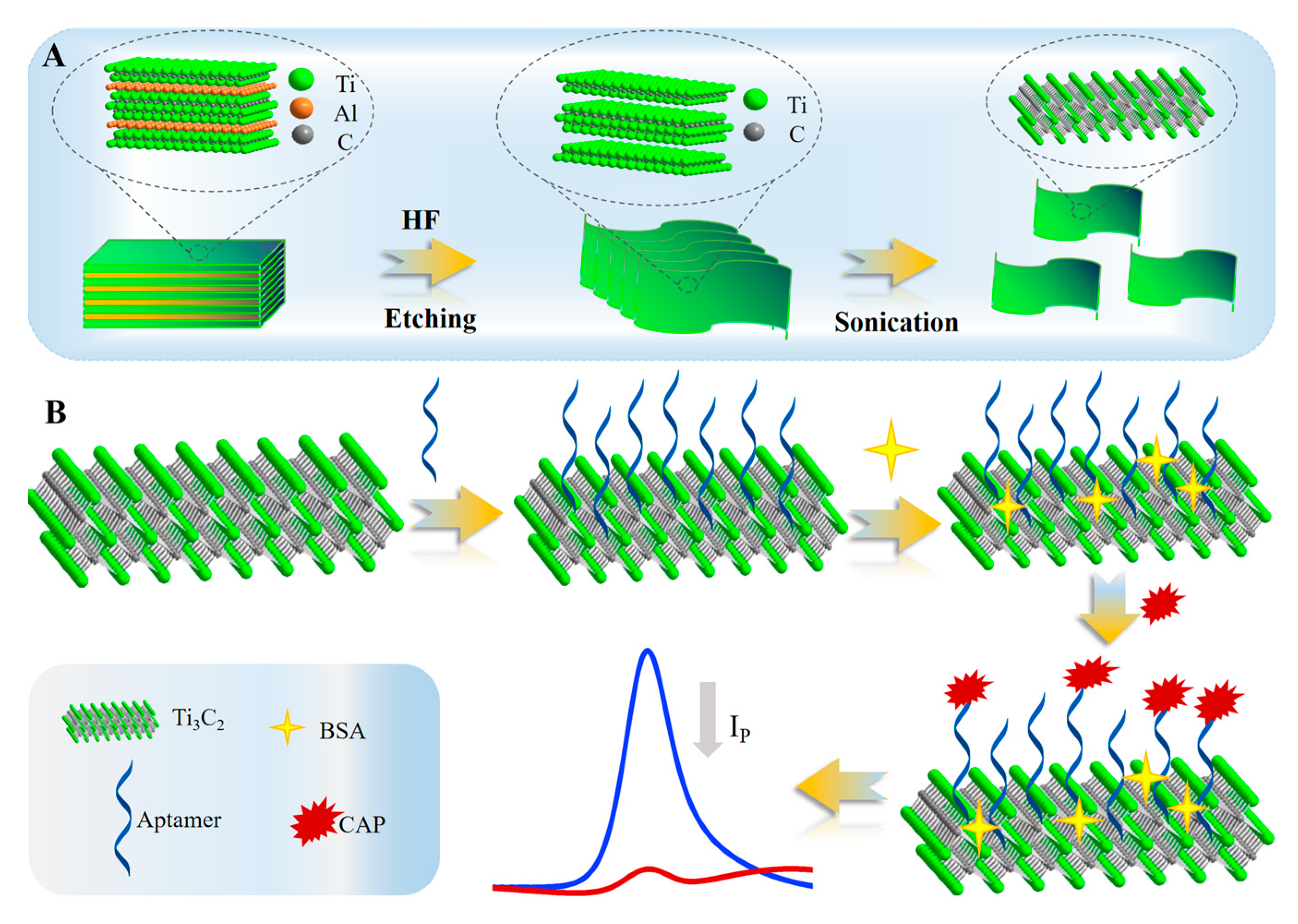
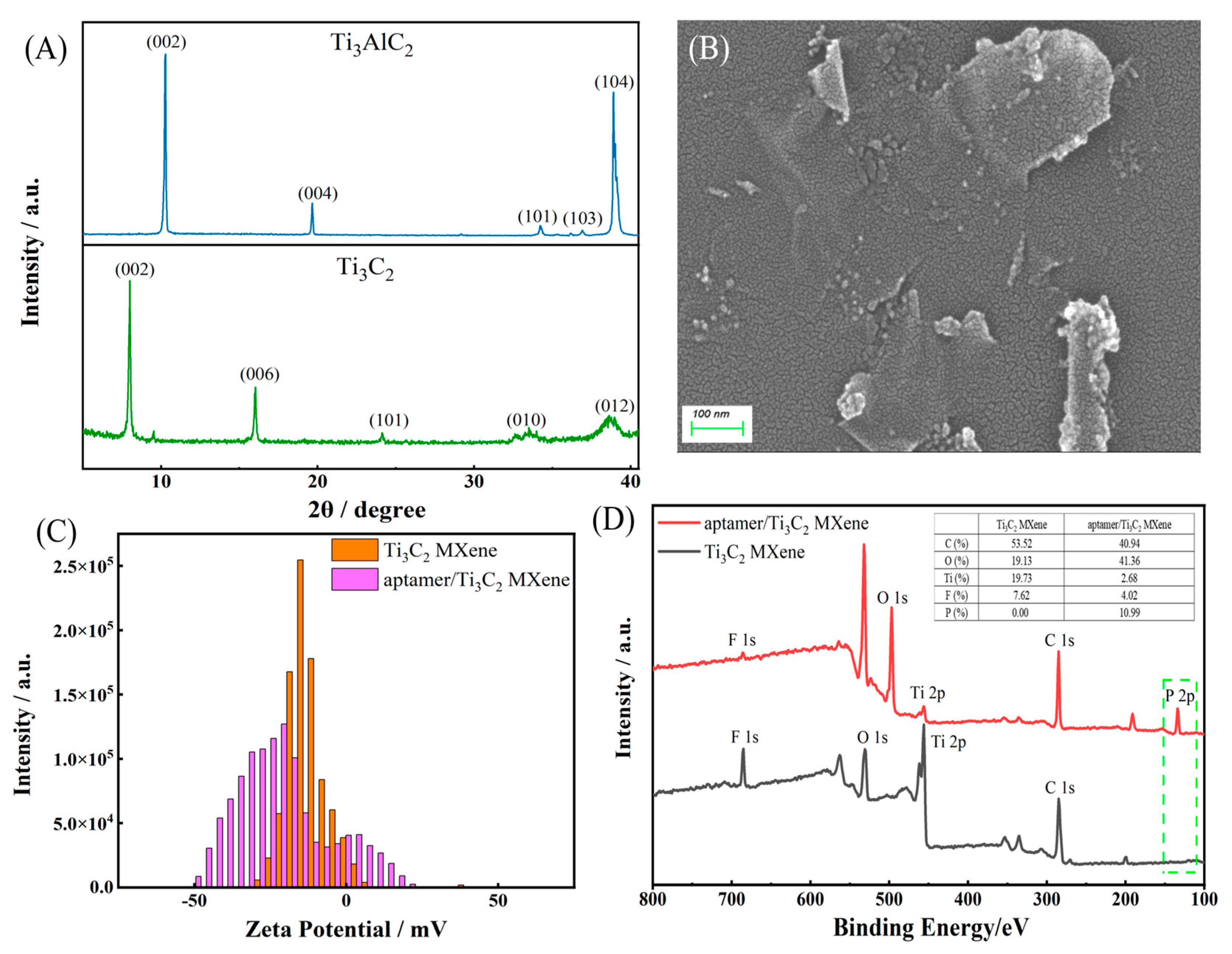
2.1. Characterization of Ti3C2 MXene
2.2. Electrochemical Characterization of the Aptasensor Assembly Process
2.3. Optimization of Experimental Conditions
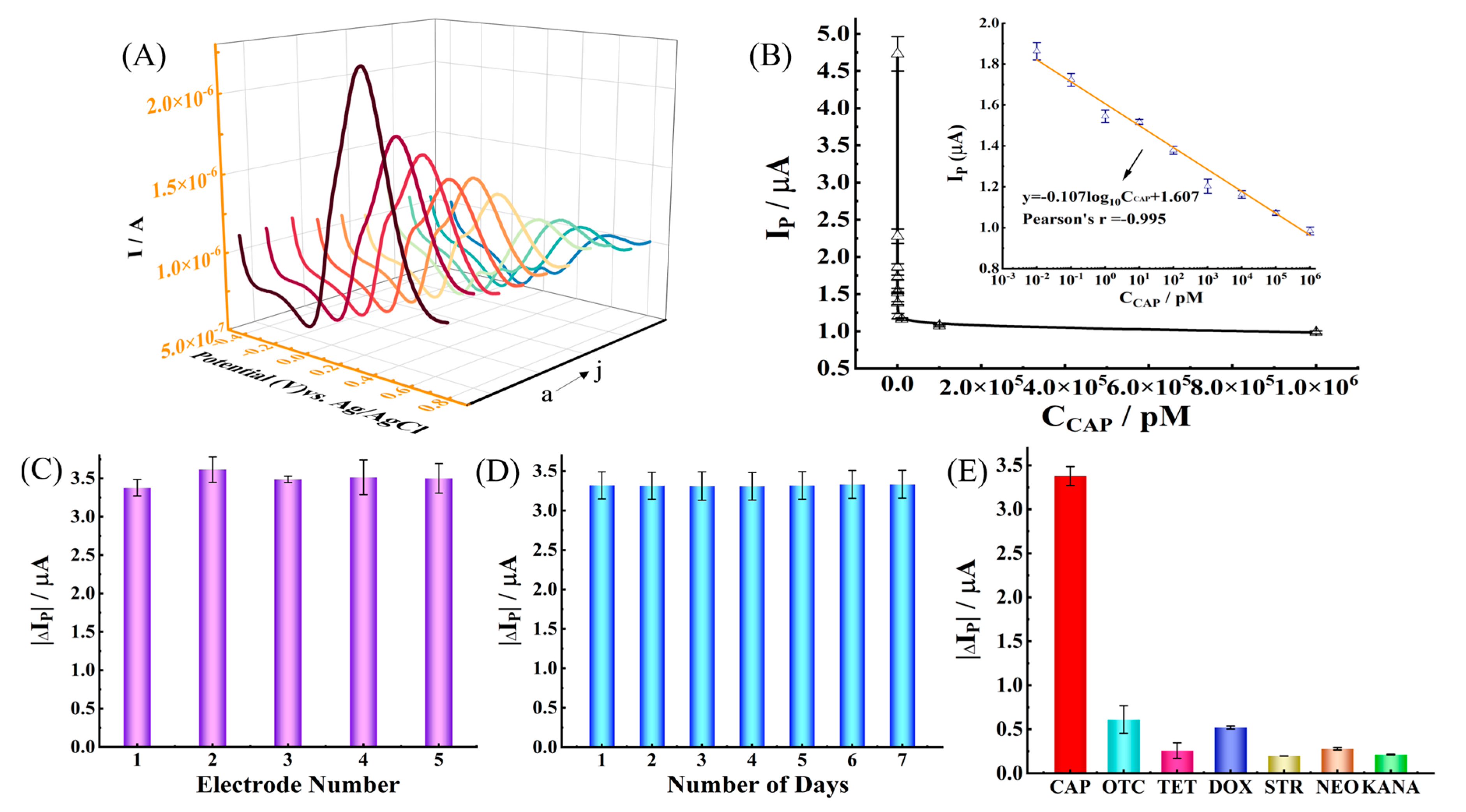
2.4. Analytical Performance of the Electrochemical Aptasensors
2.5. Detection of Chloramphenicol in Real Samples
3. Materials and Methods
3.1. Materials
3.2. Apparatus
3.3. Synthesis of Ti3C2 MXene
3.4. Construction of the Aptasensor
3.5. Milk Sample Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mosher, R.H.; Camp, D.J.; Yang, K.; Brown, M.P.; Shaw, W.V.; Vining, L.C. Inactivation of chloramphenicol by O-phosphorylation. A novel resistance mechanism in Streptomyces venezuelae ISP5230, a chloramphenicol producer. J. Biol. Chem. 1995, 270, 27000–27006. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Chen, Y.; Li, W.; Qu, J.; Chen, T.; Wang, Y.; Geng, N. Deciphering the microbial community tolerance mechanism and alteration of antibiotic resistance genes during chloramphenicol wastewater treatment. Int. Biodeter. Biodegr. 2023, 178, 105546. [Google Scholar] [CrossRef]
- Aran, G.C.; Bayraç, C. Simultaneous Dual-Sensing platform based on Aptamer-Functionalized DNA hydrogels for visual and fluorescence detection of chloramphenicol and aflatoxin m1. Bioconjugate Chem. 2023, 34, 922–933. [Google Scholar] [CrossRef]
- Mehrabi, F.; Ghaedi, M. Magnetic nanofluid based on green deep eutectic solvent for enrichment and determination of chloramphenicol in milk and chicken samples by high-performance liquid chromatography-ultraviolet: Optimization of microextraction. J. Chromatogr. A 2023, 1689, 463705. [Google Scholar] [CrossRef]
- Chen, D.; Delmas, J.; Hurtaud-Pessel, D.; Verdon, E. Development of a multi-class method to determine nitroimidazoles, nitrofurans, pharmacologically active dyes and chloramphenicol in aquaculture products by liquid chromatography-tandem mass spectrometry. Food Chem. 2020, 311, 125924. [Google Scholar] [CrossRef]
- Shen, J.; Xia, X.; Jiang, H.; Li, C.; Li, J.; Li, X.; Ding, S. Determination of chloramphenicol, thiamphenicol, florfenicol, and florfenicol amine in poultry and porcine muscle and liver by gas chromatography-negative chemical ionization mass spectrometry. J. Chromatogr. B 2009, 877, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Jian, D.; Zhang, Y.; Shan, Y.; Wang, S.; Liu, F. Paper-based antibiotic sensor (PAS) relying on colorimetric indirect competitive enzyme-linked immunosorbent assay for quantitative tetracycline and chloramphenicol detection. Sens. Actuators B Chem. 2021, 329, 129173. [Google Scholar] [CrossRef]
- Shakila, R.J.; Saravanakumar, R.; Vyla, S.A.P.; Jeyasekaran, G. An improved microbial assay for the detection of chloramphenicol residues in shrimp tissues. Innov. Food Sci. Emerg. 2007, 8, 515–518. [Google Scholar] [CrossRef]
- Xia, Y.; Su, R.; Huang, R.; Ding, L.; Wang, L.; Qi, W.; He, Z. Design of elution strategy for simultaneous detection of chloramphenicol and gentamicin in complex samples using surface plasmon resonance. Biosens. Bioelectron. 2017, 92, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Kotelnikova, P.A.; Iureva, A.M.; Nikitin, M.P.; Zvyagin, A.V.; Deyev, S.M.; Shipunova, V.O. Peroxidase-like activity of silver nanowires and its application for colorimetric detection of the antibiotic chloramphenicol. Talanta Open 2022, 6, 100164. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, Z.; Yao, Y.; Shi, W.; Liu, Y.; Zhang, S. A monoclonal antibody-based time-resolved fluoroimmunoassay for chloramphenicol in shrimp and chicken muscle. Anal. Chim. Acta 2006, 575, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.J.; He, X.; Cui, P.L.; Liu, J.X.; Wang, J.P. Detection of chloramphenicol in meat with a chemiluminescence resonance energy transfer platform based on molecularly imprinted graphene. Anal. Chim. Acta 2019, 1063, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ouyang, H.; Li, W.; Long, Y. An effective electrochemical platform for chloramphenicol detection based on Carbon-Doped boron nitride nanosheets. Biosensors 2023, 13, 116. [Google Scholar] [CrossRef]
- Cao, X.; Chen, C.; Zhu, Q. Biosensors based on functional nucleic acids and isothermal amplification techniques. Talanta 2023, 253, 123977. [Google Scholar] [CrossRef]
- Mohamad, N.; Azizan, N.I.; Mokhtar, N.F.K.; Mustafa, S.; Mohd Desa, M.N.; Hashim, A.M. Future perspectives on aptamer for application in food authentication. Anal. Biochem. 2022, 656, 114861. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Li, H.; Zhao, L.; Zhang, Y.; Liu, Z. Oligonucleotide aptamers: Recent advances in their screening, molecular conformation and therapeutic applications. Biomed. Pharmacother. 2021, 143, 112232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yin, M.; Wei, X.; Sun, Y.; Chen, Y.; Qi, S.; Tian, X.; Qiu, J.; Xu, D. Synthesis of rGO@PDA@AuNPs for an effective electrochemical chloramphenicol sensor. Diam. Relat. Mater. 2022, 128, 109311. [Google Scholar] [CrossRef]
- Macchia, E.; Torricelli, F.; Bollella, P.; Sarcina, L.; Tricase, A.; Di Franco, C.; Österbacka, R.; Kovács-Vajna, Z.M.; Scamarcio, G.; Torsi, L. Large-Area interfaces for Single-Molecule label-free bioelectronic detection. Chem. Rev. 2022, 122, 4636–4699. [Google Scholar] [CrossRef]
- Krečmarová, M.; Gulka, M.; Vandenryt, T.; Hrubý, J.; Fekete, L.; Hubík, P.; Taylor, A.; Mortet, V.; Thoelen, R.; Bourgeois, E.; et al. A Label-Free diamond microfluidic DNA sensor based on active Nitrogen-Vacancy center charge state control. ACS Appl. Mater. Inter. 2021, 13, 18500–18510. [Google Scholar] [CrossRef]
- Chen, Q.; Du, M.; Xu, X. A label-free and selective electrochemical aptasensor for ultrasensitive detection of Di(2-ethylhexyl) phthalate based on self-assembled DNA nanostructure amplification. J. Electroanal. Chem. 2022, 914, 116300. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, D.; Li, Y.; Chen, T.; You, T. Label-free ratiometric homogeneous electrochemical aptasensor based on hybridization chain reaction for facile and rapid detection of aflatoxin B1 in cereal crops. Food Chem. 2022, 373, 131443. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Chen, Q.; Xu, X. A novel and label-free electrochemical aptasensor based on exonuclease III and G-quadruplex DNAzyme for sensitive and selective detection of metronidazole. Microchem. J. 2022, 179, 107577. [Google Scholar] [CrossRef]
- Mahar, I.; Memon, F.H.; Lee, J.; Kim, K.H.; Ahmed, R.; Soomro, F.; Rehman, F.; Memon, A.A.; Thebo, K.H.; Choi, K.H. Two-Dimensional transition metal carbides and nitrides (MXenes) for water purification and antibacterial applications. Membranes 2021, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Giménez, R.; Serrano, B.; San-Miguel, V.; Cabanelas, J.C. Recent advances in MXene/Epoxy composites: Trends and prospects. Polymers 2022, 14, 1170. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Yang, L.; Fu, X.; Li, G.; Xing, S.; Wang, X. Ti3C2 MXene-based aptasensor for sensitive and simultaneous detection of two diabetes biomarkers. Surf. Interfaces 2023, 41, 103196. [Google Scholar] [CrossRef]
- Wang, H.; Sun, J.; Lu, L.; Yang, X.; Xia, J.; Zhang, F.; Wang, Z. Competitive electrochemical aptasensor based on a cDNA-ferrocene/MXene probe for detection of breast cancer marker Mucin1. Anal. Chim. Acta 2020, 1094, 18–25. [Google Scholar] [CrossRef]
- Yue, F.; Liu, M.; Bai, M.; Hu, M.; Li, F.; Guo, Y.; Vrublevsky, I.; Sun, X. Novel electrochemical aptasensor based on ordered mesoporous Carbon/2D Ti3C2 MXene as nanocarrier for simultaneous detection of aminoglycoside antibiotics in milk. Biosensors 2022, 12, 626. [Google Scholar] [CrossRef]
- Manzanares-Palenzuela, C.L.; Pourrahimi, A.M.; Gonzalez-Julian, J.; Sofer, Z.; Pykal, M.; Otyepka, M.; Pumera, M. Interaction of single- and double-stranded DNA with multilayer MXene by fluorescence spectroscopy and molecular dynamics simulations. Chem. Sci. 2019, 10, 10010–10017. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.Y.; Lin, H.; Barui, A.K.; Gomez, A.M.U.; Wendt, M.K.; Stanciu, L.A. DNA-Functionalized ti3c2tx MXenes for selective and rapid detection of SARS-CoV-2 nucleocapsid gene. ACS Appl. Nano Mater. 2022, 5, 1902–1910. [Google Scholar] [CrossRef]
- Yadav, P.; Cao, Z.; Barati Farimani, A. DNA detection with Single-Layer TI3C2 MXene nanopore. ACS Nano 2021, 15, 4861–4869. [Google Scholar] [CrossRef]
- Du, X.; Sun, J.; Li, Y.; Du, W.; Jiang, D. Self-accelerated electrochemiluminescence luminophor of Ag3PO4-Ti3C2 for trace lincomycin aptasensing. Microchem. J. 2022, 179, 107578. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Zhang, Y.; Ge, H.; Zhang, J.; Yang, P.; Wu, Z. Facile fabrication of 2D MXene loading Co-doped Prussian blue nanoparticles for ultrasensitive electrochemical assay of trace lead ions. J. Electroanal. Chem. 2023, 935, 117320. [Google Scholar] [CrossRef]
- Zhang, Z.; Karimi-Maleh, H. In situ synthesis of label-free electrochemical aptasensor-based sandwich-like AuNPs/PPy/Ti3C2Tx for ultrasensitive detection of lead ions as hazardous pollutants in environmental fluids. Chemosphere 2023, 324, 138302. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, F.; Zhang, H.; Zhang, Y.; Liu, M.; Liu, Y. Universal ti3c2 MXenes based Self-Standard ratiometric fluorescence resonance energy transfer platform for highly sensitive detection of exosomes. Anal. Chem. 2018, 90, 12737–12744. [Google Scholar] [CrossRef]
- Kashefi-Kheyrabadi, L.; Koyappayil, A.; Kim, T.; Cheon, Y.; Lee, M. A MoS2@Ti3C2Tx MXene hybrid-based electrochemical aptasensor (MEA) for sensitive and rapid detection of Thyroxine. Bioelectrochemistry 2021, 137, 107674. [Google Scholar] [CrossRef]
- Lee, E.; Lee, C.E.; Han, J.H. Effects of proton irradiation on single-stranded DNA studied by using X-ray photoelectron spectroscopy. J. Korean Phys. Soc. 2016, 69, 578–583. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, C.; Yan, W.; Guo, Y.; Shuang, S.; Dong, C.; Bi, Y. Design of a facile and label-free electrochemical aptasensor for detection of atrazine. Talanta 2019, 201, 156–164. [Google Scholar] [CrossRef]
- Lu, M.; Cao, C.; Wang, F.; Liu, G. A polyethyleneimine reduced graphene oxide/gold nanocubes based electrochemical aptasensor for chloramphenicol detection using single-stranded DNA-binding protein. Mater. Des. 2021, 199, 109409. [Google Scholar] [CrossRef]
- Roushani, M.; Rahmati, Z.; Farokhi, S.; Hoseini, S.J.; Fath, R.H. The development of an electrochemical nanoaptasensor to sensing chloramphenicol using a nanocomposite consisting of graphene oxide functionalized with (3-Aminopropyl) triethoxysilane and silver nanoparticles. Mater. Sci. Eng. C 2020, 108, 110388. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, G.; Chen, S.M. Manganese oxide anchored on carbon modified halloysite nanotubes: An electrochemical platform for the determination of chloramphenicol. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126243. [Google Scholar] [CrossRef]
- Qi, X.; Teng, Z.; Yu, J.; Jia, D.; Zhang, Y.; Pan, H. A simple one-step synthesis of Fe3O4/N-rGO nanocomposite for sensitive electrochemical detection of chloramphenicol. Mater. Lett. 2023, 330, 133350. [Google Scholar] [CrossRef]
- Gao, S.; Yang, Z.; Zhang, Y.; Zhao, L.; Xing, Y.; Fei, T.; Liu, S.; Zhang, T. The synergistic effects of MoS2 and reduced graphene oxide on sensing performances for electrochemical chloramphenicol sensor. FlatChem 2022, 33, 100364. [Google Scholar] [CrossRef]
- Manjula, N.; Chen, S. Synthesis of highly electroactive nanoflowers like manganesetin oxide and electroanalytical application for chloramphenicol determination in milk and honey samples. J. Electroanal. Chem. 2021, 880, 114914. [Google Scholar] [CrossRef]
- Yang, J.; Zhong, W.; Yu, Q.; Zou, J.; Gao, Y.; Liu, S.; Zhang, S.; Wang, X.; Lu, L. MXene–AuNP-Based electrochemical aptasensor for Ultra-Sensitive detection of chloramphenicol in honey. Molecules 2022, 27, 1871. [Google Scholar] [CrossRef]
- Kang, R.; Zhang, Z.; Guo, L.; Cui, J.; Chen, Y.; Hou, X.; Wang, B.; Lin, C.; Jiang, N.; Yu, J. Enhanced thermal conductivity of epoxy composites filled with 2D transition metal carbides (MXenes) with ultralow loading. Sci. Rep. 2019, 9, 9135. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.; Hu, G.; Lv, E.; Li, C.; Wang, Z.; Li, Q.; Qian, Z.; Wang, J.; Xu, S.; Wang, R. A label-free and enzyme-free fluorescent aptasensor for amplified detection of kanamycin in milk sample based on target-triggered catalytic hairpin assembly. Food Control. 2022, 133, 108654. [Google Scholar] [CrossRef]


| Methods | Linear Range | LOD | Reference |
|---|---|---|---|
| PEI-rGO/AuNCs | 5 pM~1 μM | 2.08 pM | [40] |
| AgNPs/[NH2–Si]−f−GO | 10 pM~0.2 μM | 3.3 pM | [41] |
| Mn2O3@CCH | 0.005~91.94 μM | 0.03 μM | [42] |
| Fe3O4/N-rGO | 1~200 μM | 0.03 μM | [43] |
| MoS2-rGO | 1~55 μM | 0.6 μM | [44] |
| MSO NFs | 0.003 μM~92.21 μM | 1 nM | [45] |
| MXene-AuNP | 0.0001~10 nM | 0.03 pM | [46] |
| Ti3C2 MXene | 10 fM~1 μM | 1 fM | This work |
| Sample No. | Added (pM) | Found (pM) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|
| 1 | 10 | 10.314 | 103.14 | 2.637 |
| 2 | 1000 | 1058.791 | 105.88 | 8.342 |
| 3 | 10,000 | 10,815.439 | 108.15 | 6.953 |
| 4 | 100,000 | 96,126.457 | 96.13 | 7.736 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Xiong, S.; Zhao, P.; Dong, P.; Wu, Z. Few Layer Ti3C2 MXene-Based Label-Free Aptasensor for Ultrasensitive Determination of Chloramphenicol in Milk. Molecules 2023, 28, 6074. https://doi.org/10.3390/molecules28166074
Li F, Xiong S, Zhao P, Dong P, Wu Z. Few Layer Ti3C2 MXene-Based Label-Free Aptasensor for Ultrasensitive Determination of Chloramphenicol in Milk. Molecules. 2023; 28(16):6074. https://doi.org/10.3390/molecules28166074
Chicago/Turabian StyleLi, Fang, Shuyue Xiong, Pei Zhao, Panpan Dong, and Zijian Wu. 2023. "Few Layer Ti3C2 MXene-Based Label-Free Aptasensor for Ultrasensitive Determination of Chloramphenicol in Milk" Molecules 28, no. 16: 6074. https://doi.org/10.3390/molecules28166074






