Synthesis of Ag3PO4/Ag/g-C3N4 Composite for Enhanced Photocatalytic Degradation of Methyl Orange
Abstract
:1. Introduction
2. Results and Discussion
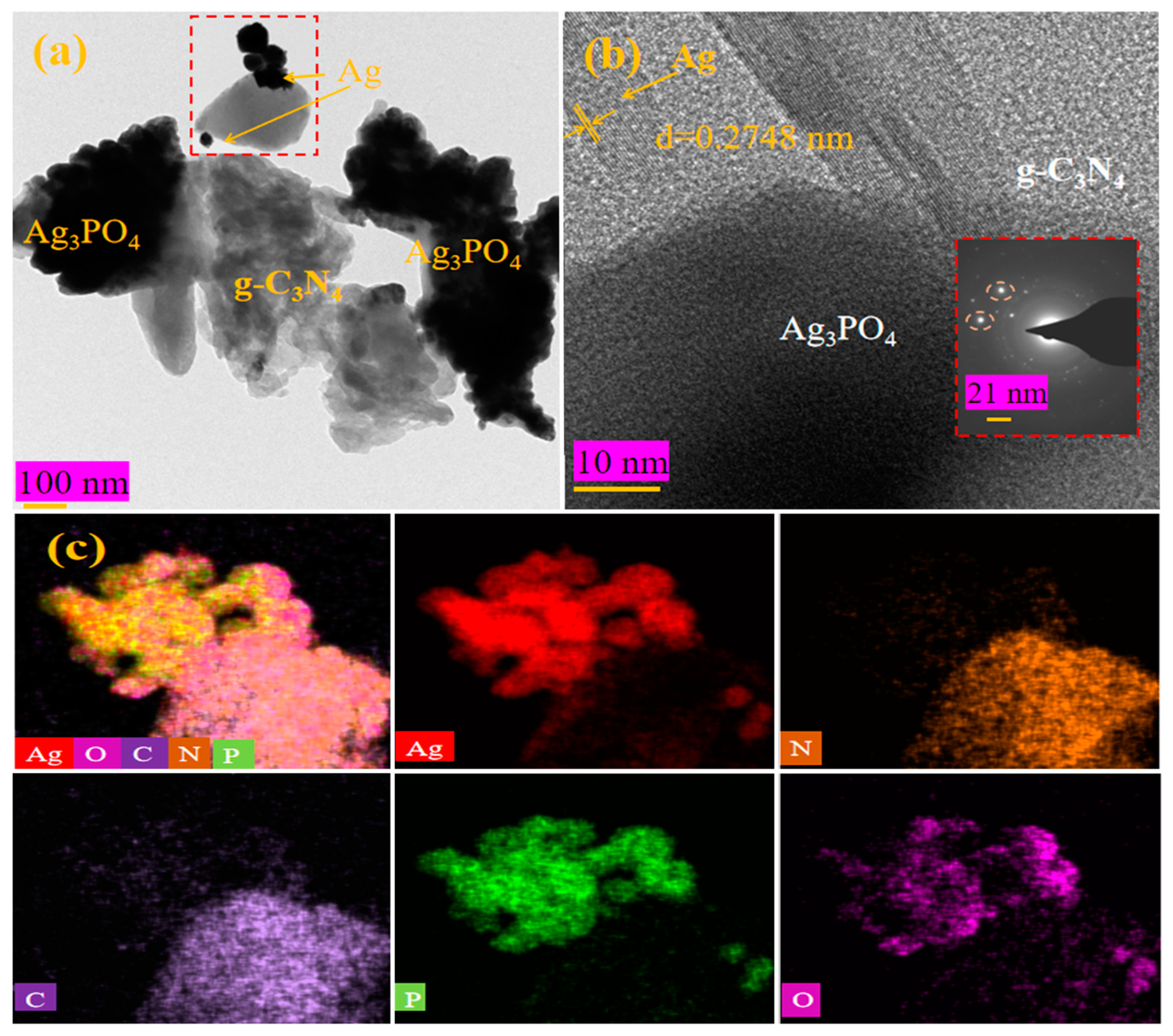
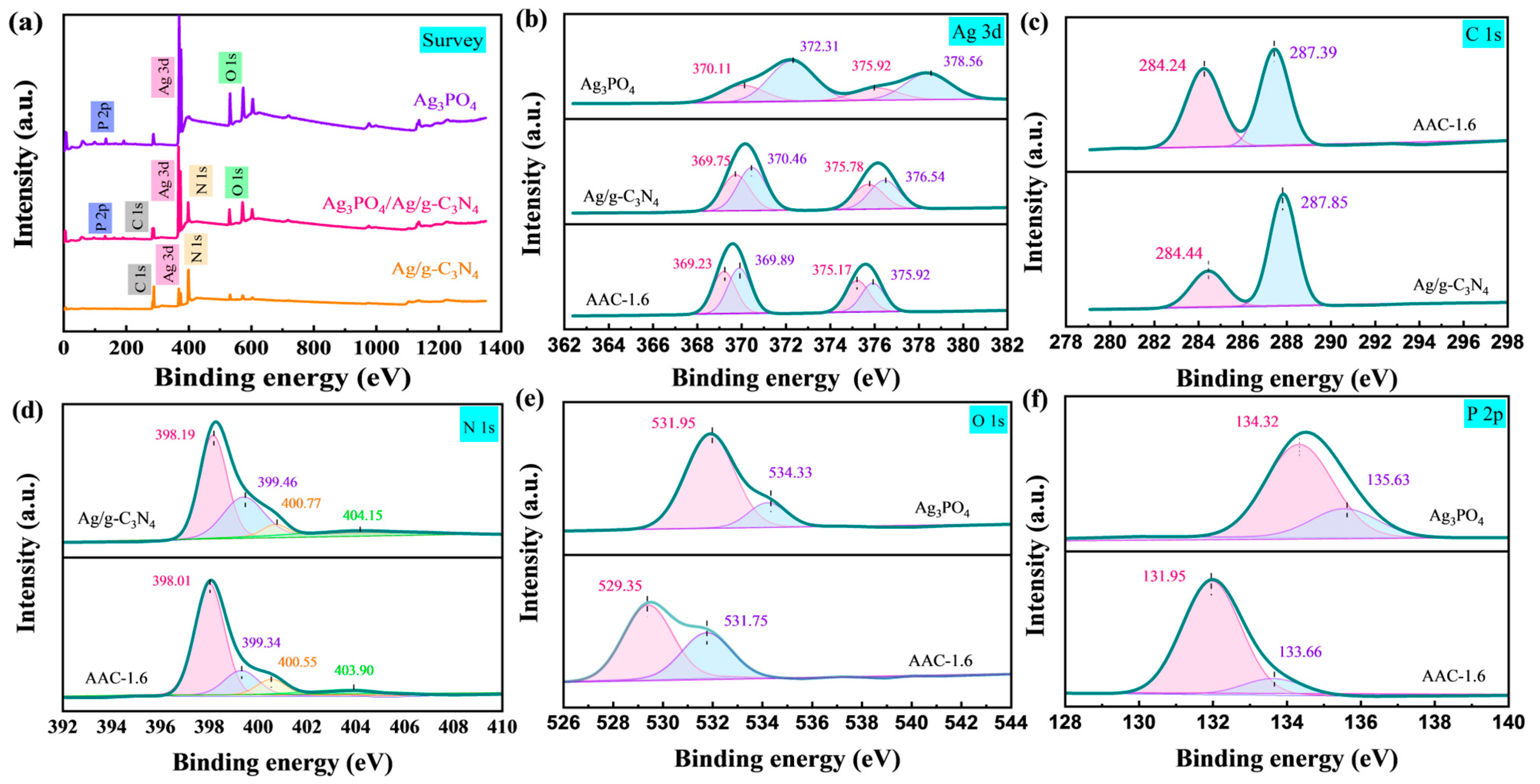
2.1. Structure and Morphology
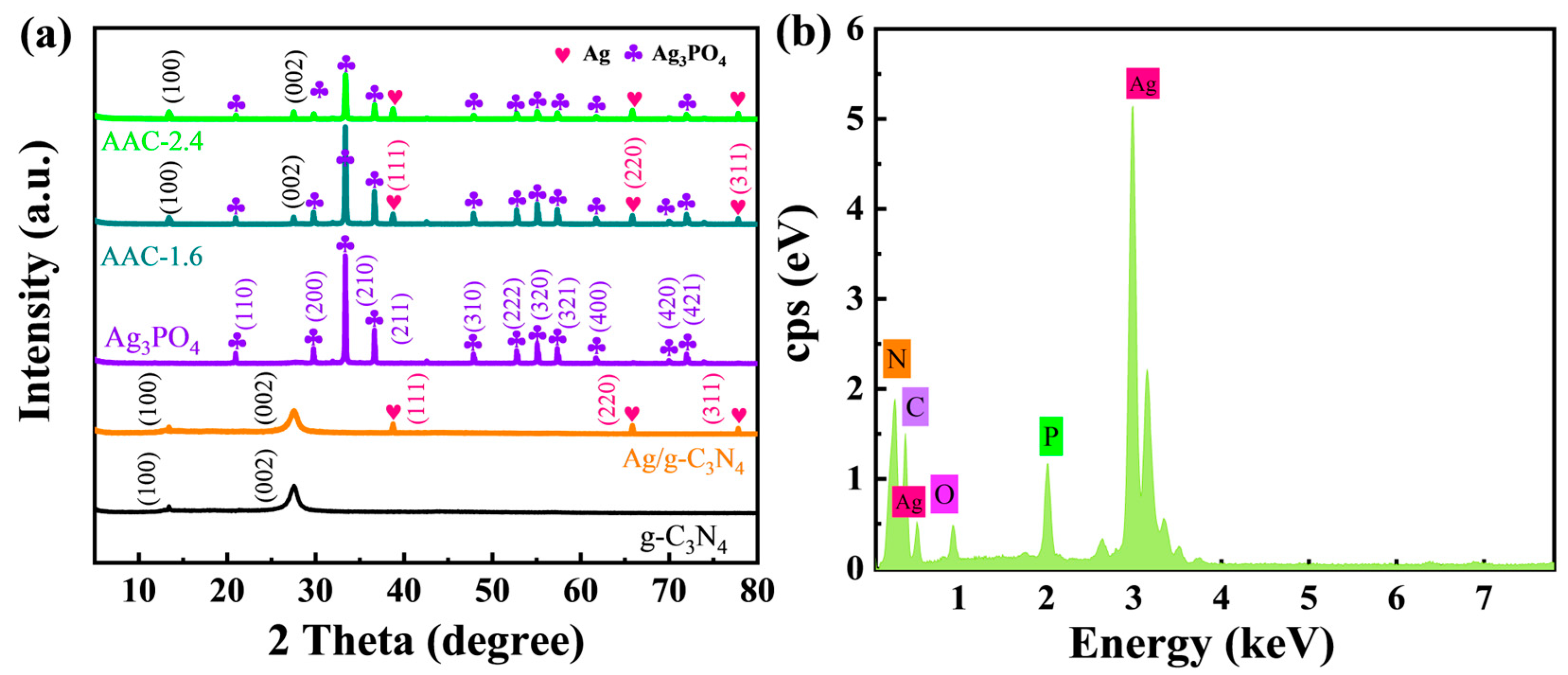
 ”, correspond to the (111), (200), and (311) crystal planes of metallic Ag. In the AAC-1.6 sample, Ag3PO4 is labeled by “
”, correspond to the (111), (200), and (311) crystal planes of metallic Ag. In the AAC-1.6 sample, Ag3PO4 is labeled by “ ” [4,26]. The characteristic peaks of Ag, g-C3N4, and Ag3PO4 can be seen in Figure 1a, indicating that there were no other peaks in the sample, which contains only Ag, g-C3N4, and Ag3PO4. This indicates the high purity of the AAC-1.6 sample. Moreover, it can be seen from the EDS (energy-dispersive X-ray spectrometry) spectrum (Figure 1b) that the AAC-1.6 composite is composed of Ag, C, N, O, and P elements. The above suggests that the high purity of the AAC-1.6 sample is very beneficial for subsequent photocatalytic degradation experiments.
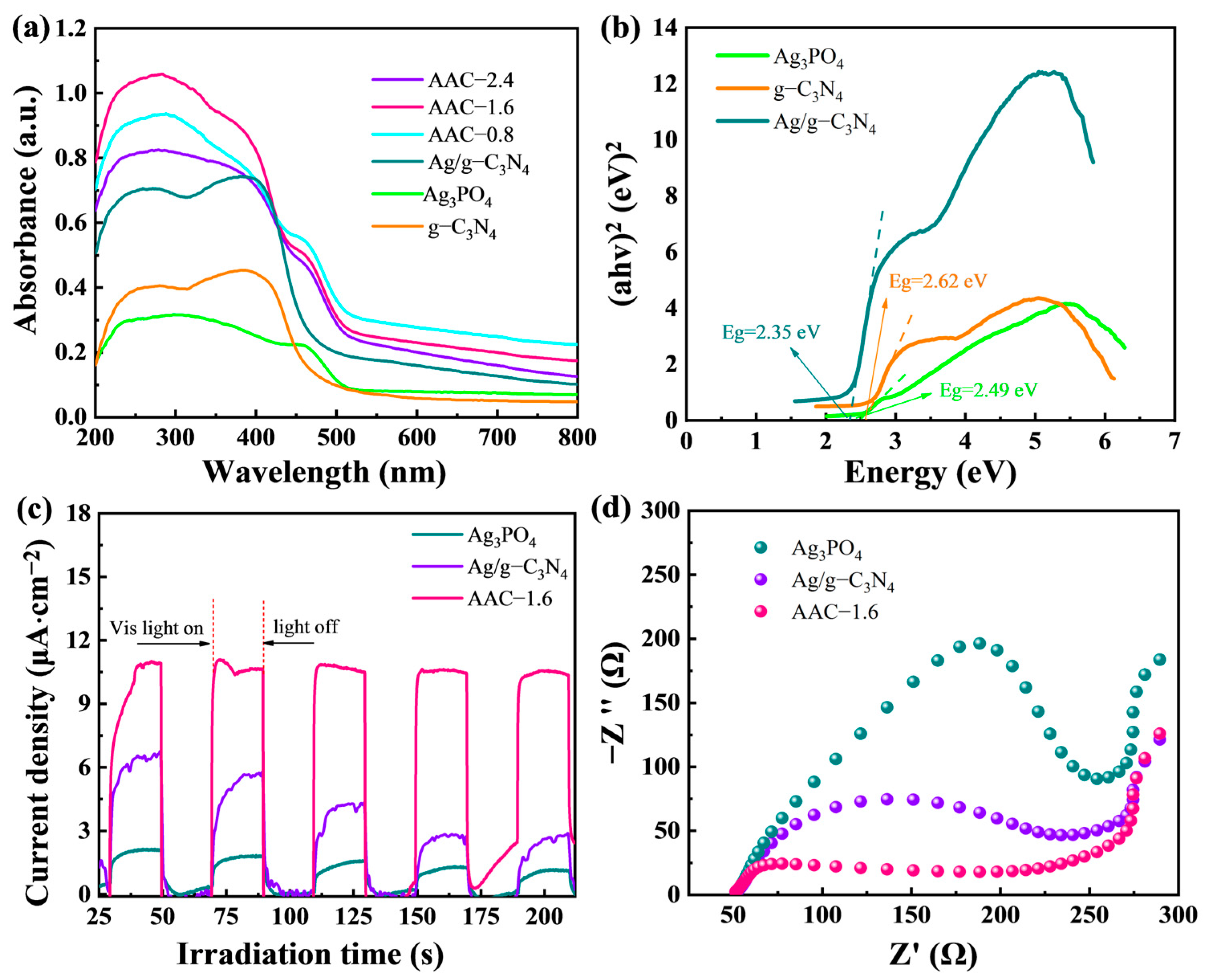
” [4,26]. The characteristic peaks of Ag, g-C3N4, and Ag3PO4 can be seen in Figure 1a, indicating that there were no other peaks in the sample, which contains only Ag, g-C3N4, and Ag3PO4. This indicates the high purity of the AAC-1.6 sample. Moreover, it can be seen from the EDS (energy-dispersive X-ray spectrometry) spectrum (Figure 1b) that the AAC-1.6 composite is composed of Ag, C, N, O, and P elements. The above suggests that the high purity of the AAC-1.6 sample is very beneficial for subsequent photocatalytic degradation experiments.2.2. Photoelectric Properties of Samples
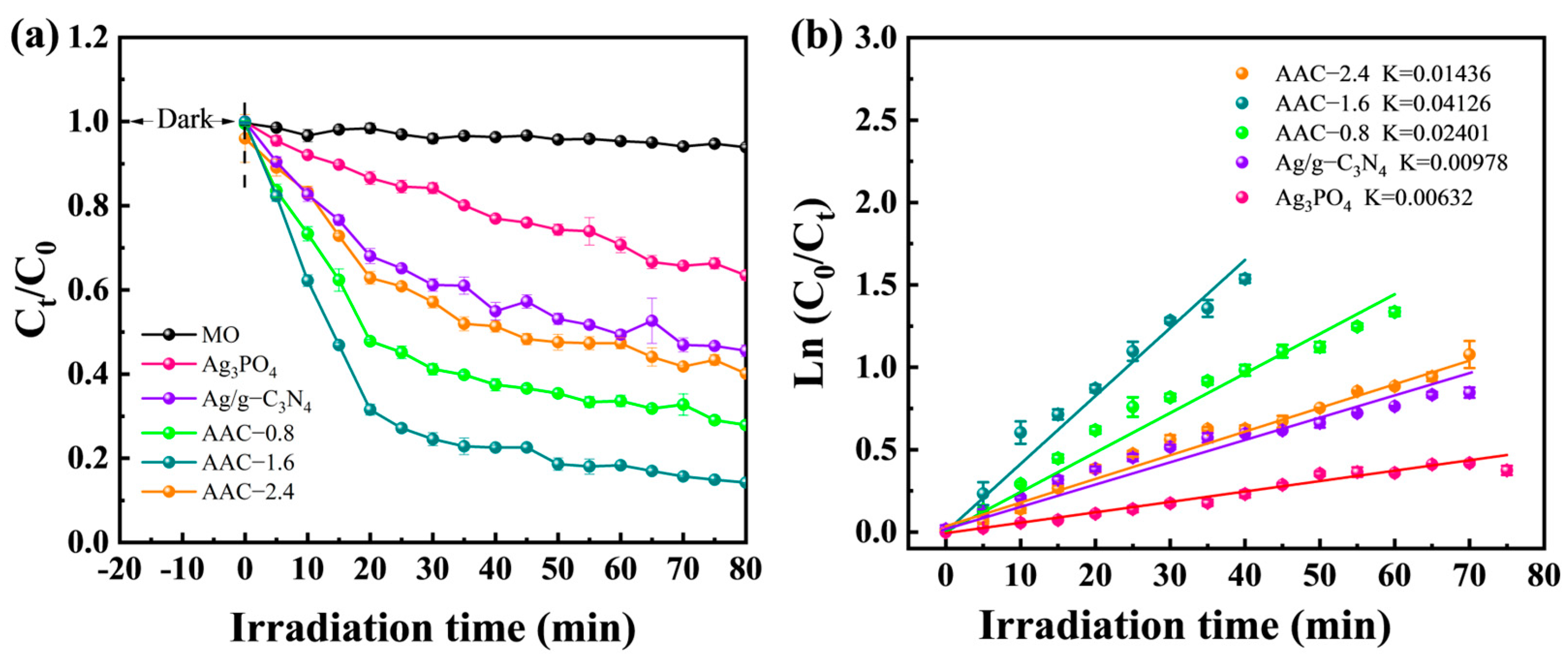
2.3. Photocatalytic Activity Test
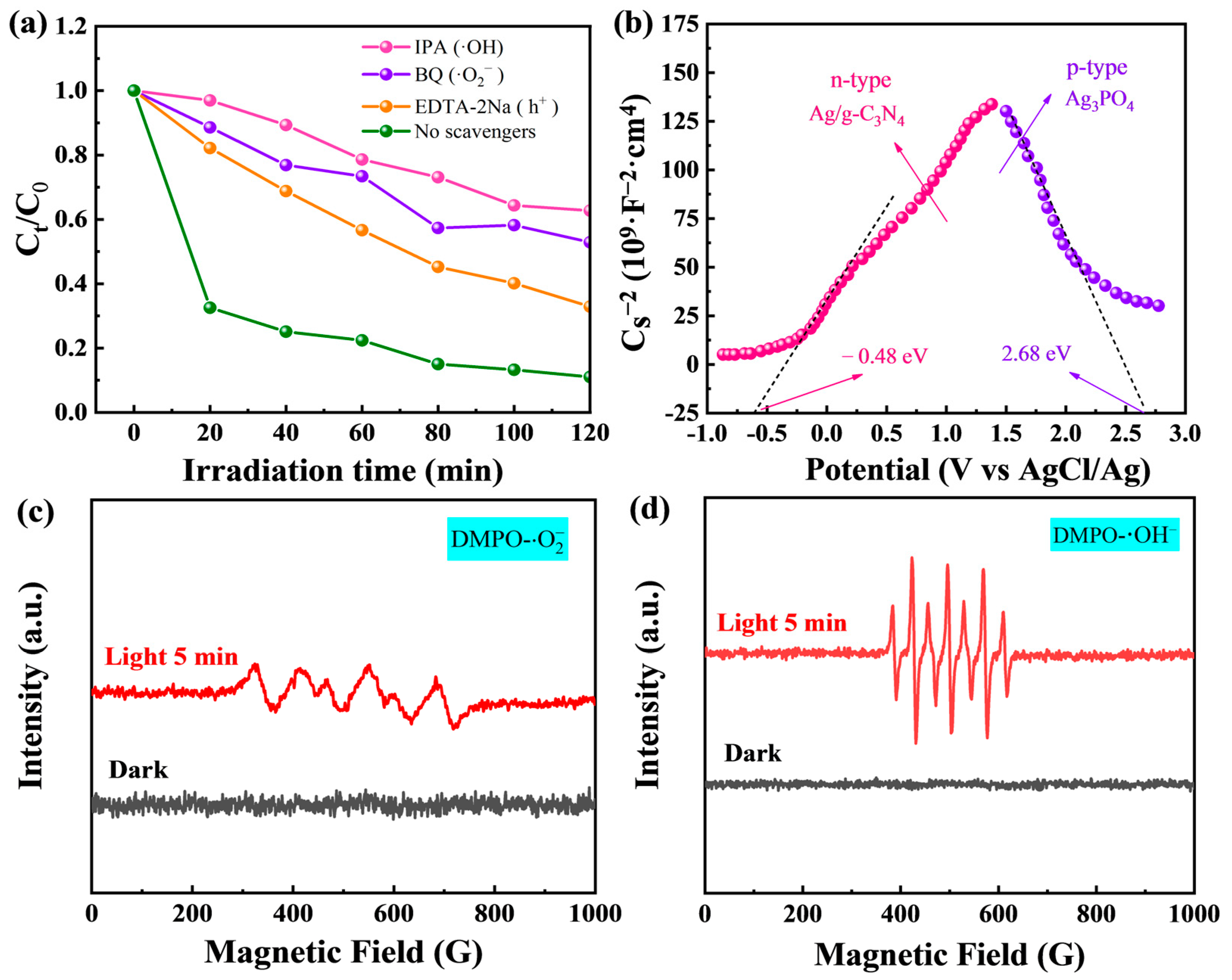
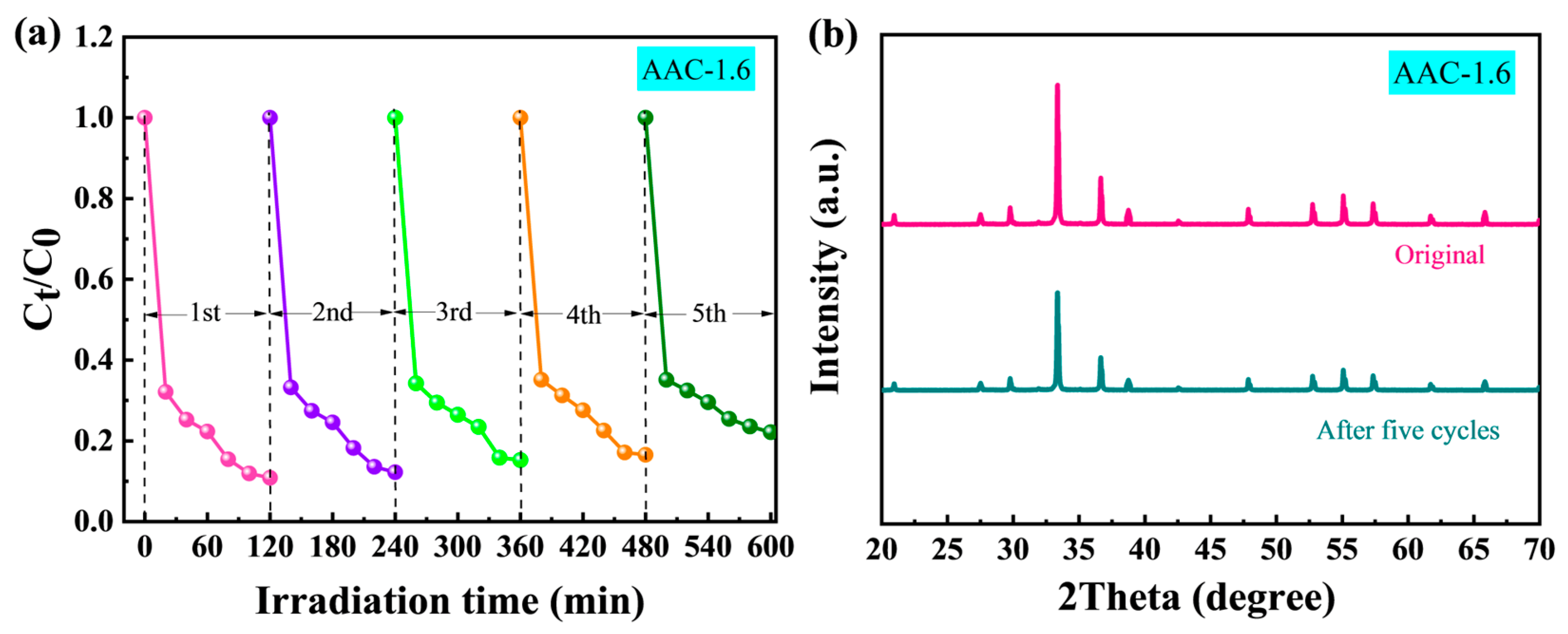
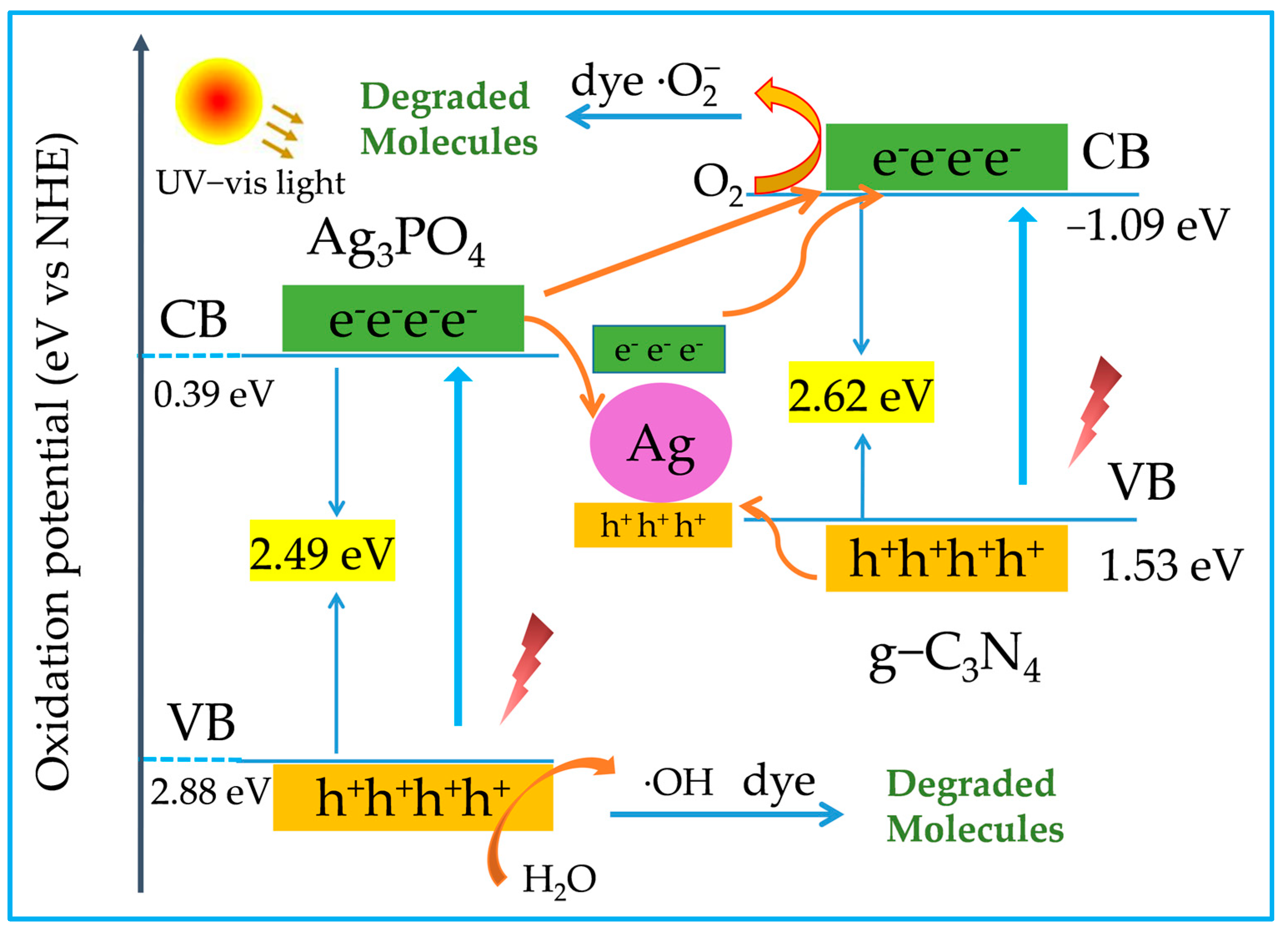
2.4. Enhancement Mechanism and Stability
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Samples
3.2.1. Preparation of Ag/g-C3N4
3.2.2. Preparation of Ag3PO4/Ag/g-C3N4
3.3. Analytical Characterization
3.4. Measurement of Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, X.; Wang, J.; Dong, Y.M.; Li, H.X.; Xia, Y.M.; Wang, H.J. One-step synthesis of Bi2MoO6/reduced graphene oxide aerogel composite with enhanced adsorption and photocatalytic degradation performance for methylene blue. Mat. Sci. Semicon. Proc. 2018, 88, 214–223. [Google Scholar] [CrossRef]
- Deng, X.Y.; Zhang, Q.; Wang, L.; Han, J.; Wu, Y.J.; Sun, Z.Y.; Li, W.B.; Li, X.B.; Xu, L.K.; Feng, C. Fabrication of Ag-modified porous ZnMgO nanorods with enhanced photocatalytic performance. J. Mater. Sci. Mater. Electron. 2018, 29, 16962–16970. [Google Scholar] [CrossRef]
- Wu, J.J.; Huang, F.Q.; Lü, X.J.; Chen, P. One-pot synthesis of BiSbO4 nanophotocatalyst with enhanced visible-light performance. CrystEngComm 2011, 13, 3920–3924. [Google Scholar] [CrossRef]
- Shen, Y.Z.; Zhu, Z.D.; Wang, X.G.; Khan, A.; Gong, J.Y.; Zhang, Y.R. Synthesis of Z-scheme g-C3N4/Ag/Ag3PO4 composite for enhanced photocatalytic degradation of phenol and selective oxidation of gaseous isopropanol. Mater. Res. Bull. 2018, 107, 407–415. [Google Scholar] [CrossRef]
- Chai, B.; Li, J.; Xu, Q. Reduced Graphene Oxide Grafted Ag3PO4 Composites with Efficient Photocatalytic Activity under Visible-Light Irradiation. Ind. Eng. Chem. Res. 2014, 53, 8744–8752. [Google Scholar] [CrossRef]
- Dong, P.; Wang, Y.; Cao, B.; Xin, S.; Guo, L.; Zhang, J.; Li, F. Ag3PO4/reduced graphite oxide sheets nanocomposites with highly enhanced visible light photocatalytic activity and stability. Appl. Catal. B Environ. 2013, 132, 45–53. [Google Scholar] [CrossRef]
- Wang, H.; Bai, Y.S.; Yang, J.T.; Lang, X.F.; Li, J.H.; Guo, L. A Facile Way to Rejuvenate Ag3PO4 as a Recyclable Highly Efficient Photocatalyst. Chem.-Eur. J. 2012, 18, 5524–5529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, H.; Ming, H.; Li, H.; Zhang, L.; Liu, Y.; Kang, Z. Carbon quantum dots/Ag3PO4 complex photocatalysts with enhanced photocatalytic activity and stability under visible light. J. Mater. Chem. 2012, 22, 10501–10506. [Google Scholar] [CrossRef]
- Zhang, R.; Han, Q.; Li, Y.; Cai, Y.F.; Zhang, T.Q.; Liu, Y.; Zhu, X.Y. Fabrication of a Ag3PO4/Reduced graphene oxide/BiOBr ternary photocatalyst for enhanced visible-light photocatalytic activity and stability. J. Alloys Compd. 2019, 810, 151868. [Google Scholar] [CrossRef]
- Katsumata, H.; Sakai, T.; Suzuki, T.; Kaneco, S. Highly Efficient Photocatalytic Activity of g-C3N4/Ag3PO4 Hybrid Photocatalysts through Z-Scheme Photocatalytic Mechanism under Visible Light. Ind. Eng. Chem. Res. 2014, 53, 8018–8025. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability. Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Huang, D.; Wang, H.; Wu, Y. Photocatalytic Aerobic Oxidation of Biomass-Derived 5-HMF to DFF over MIL-53(Fe)/g-C3N4 Composite. Molecules 2022, 27, 8537. [Google Scholar] [CrossRef]
- Rawal, S.B.; Sung, S.D.; Lee, W.I. Novel Ag3PO4/TiO2 composites for efficient decomposition of gaseous 2-propanol under visible-light irradiation. Catal. Commun. 2011, 17, 131–135. [Google Scholar] [CrossRef]
- Lin, F.; Zhou, S.; Wang, G.; Wang, J.; Gao, T.; Su, Y.; Wong, C.P. Electrostatic self-assembly combined with microwave hydrothermal strategy: Construction of 1D/1D carbon nanofibers/crystalline g-C3N4 heterojunction for boosting photocatalytic hydrogenproduction. Nano Energy 2022, 99, 107432. [Google Scholar] [CrossRef]
- Kowalki´nska, M.; Fiszka Borzyszkowska, A.; Grzegórska, A.; Karczewski, J.; Głuchowski, P.; Łapiński, M.; Sawczak, M.; Zieli´nska-Jurek, A. Pilot-scale studies of WO3/S-doped g-C3N4 heterojunction toward photocatalytic NOx removal. Materials 2022, 15, 633. [Google Scholar] [CrossRef]
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Tiago, B.; William, P.H.; Vincent, W.H.; Lau, B.; Lotsch, V.; Volker, B. Thermodynamic Equilibria in Carbon Nitride Photocatalyst Materials and Conditions for the Existence of Graphitic Carbon Nitride g-C3N4. Chem. Mater. 2017, 29, 4445–4453. [Google Scholar]
- Wang, X.; Hai, G.; Li, B.; Luan, Q.; Dong, W.; Wang, G. Construction of Dual-Z-scheme WS2-WO3·H2O/g-C3N4 catalyst for photocatalytic H2 evolution under visible light. Chem. Eng. J. 2021, 426, 130822. [Google Scholar] [CrossRef]
- Truong, H.B.; Huy, B.T.; Lee, Y.I.; Nguyen, H.T.; Cho, J.; Hur, J. Magnetic visible-light activated photocatalyst CuFe2O4/Bi2WO6/mpgC3N4 for the treatment of natural organic matter. Chem. Eng. J. 2023, 453, 139777. [Google Scholar] [CrossRef]
- Qaraah, F.A.; Mahyoub, S.A.; Hezam, A.; Qaraah, A.; Xin, F.; Xiu, G. Synergistic effect of hierarchical structure and S-scheme heterojunction over O-doped g-C3N4/N-doped Nb2O5 for highly efficient photocatalytic CO2 reduction. Appl. Catal. B Environ. 2022, 315, 121585. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B. Constructing S-scheme 2D/0D g-C3N4/TiO2 NPs/MPs heterojunction with 2D-Ti3AlC2 MAX cocatalyst for photocatalytic CO2 reduction to CO/CH4 in fixed-bed and monolith photoreactors. J. Mater. Sci. Technol. 2022, 106, 195–210. [Google Scholar] [CrossRef]
- Chen, S.K.; Wang, C.C.; Chen, Y.; Li, L.; Wen, G.L. Preparation and photocatalytic degradation of magnetic Ag2S/Ag/CoFe1.95Sm0.05O4 Z-type heterojunction. J. Inorg. Mater. 2022, 37, 1329–1336. [Google Scholar] [CrossRef]
- Aschauer, U. Charge transfer observed in light-activated catalyst particles. Nature 2022, 610, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Usman, Q.; Zeb, H.J.; Ahmad, B.R.; Ali, R.; Ghazanfar, N.; Walid, N.; Muhammad, I. Photocatalysis vs adsorption by metal oxide nanoparticles. J. Mater. Sci. Technol. 2022, 131, 122–166. [Google Scholar]
- Tian, K.; Hu, L.M.; Li, L.T.; Zheng, Q.Z.; Xin, Y.J.; Zhang, G.S. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment. Chin. Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Yu, W.; Chen, J.; Shang, T.; Chen, L.; Gu, L.; Peng, T. Direct Z-scheme g-C3N4/WO3 photocatalyst with atomically defined junction for H2 production. Appl. Catal. B Environ. 2017, 219, 693–704. [Google Scholar] [CrossRef]
- Rezaei, K.R.; Emad, D.; Mohseni-Bandpi, A.; Rezaei, L.; Esrafili, A.; Kakavandi, B.; Azari, A. Nitrate adsorption by synthetic activated carbon magnetic nanoparticles: Kinetics, isotherms and thermodynamic studies. Desalin Water Treat. 2016, 57, 16445–16455. [Google Scholar] [CrossRef]
- Pongsaton, A.; Sumetha, S. Photocatalytic degradation of dyes by AgBr/Ag3PO4 and the ecotoxicities of their degraded products. Chin. J. Catal. 2016, 37, 711–719. [Google Scholar]
- Shen, X.F.; Yang, J.Y.; Zheng, T.; Wang, Q.; Zhuang, H.F.; Zheng, R.N.; Shan, S.D.; Li, S.J. Plasmonic p-n heterojunction of Ag/Ag2S/Ag2MoO4 with enhanced Vis-NIR photocatalytic activity for purifying wastewater. Sep. Purif. Technol. 2020, 251, 117347. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Lou, Z.; Zhang, X.; Qin, X.; Dai, Y.; Zheng, Z.; Wang, X. Synthesis of highly efficient Ag@AgCl plasmonic photocatalysts with various structures. Chem. Eur. J. 2010, 16, 538–544. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, L.; Lu, H.; Li, Y.; Hu, C.; Yu, H. One-pot pyridine-assisted synthesis of visible-light-driven photocatalyst Ag/Ag3PO4. Appl. Catal. B Environ. 2012, 115, 245–252. [Google Scholar] [CrossRef]
- Lv, J.; Dai, K.; Lu, L.; Geng, L.; Liang, C.; Zhu, G. Cu/Ag/Ag3PO4 ternary composite: A hybrid alloy-semiconductor heterojunction structure with visible light photocatalytic properties. J. Alloys Compd. 2016, 682, 778–784. [Google Scholar] [CrossRef]
- Wang, H.L.; Wu, D.J.; Zhou, J.B. Gasified rice husk based RHAC/NiCo2S4 composite for high performance asymmetric supercapacitor. J. Alloys. Compd. 2019, 811, 152073. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, J.; Zhou, Y.; Song, C.; Tian, Q.; Xu, J.; Wong, C. Enhancing pseu docapacitive kinetics of nanostructured MnO2 through anchoring onto biomass-derived porous carbon. Appl. Surf. Sci. 2018, 440, 1027–1036. [Google Scholar] [CrossRef]
- Bhaskar, A.; Deepa, M.; Ramakrishna, M.; Rao, T.N. Poly(3,4-ethylenedioxythiophene) sheath over a SnO2 hollow spheres/graphene oxide hybrid for a durable anode in Li-ion batteries. J. Phys. Chem. C 2014, 118, 7296–7306. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, D.; Li, L.; Li, N.; Li, F.; Cheng, H. Nanosize SnO2 confifined in the porous shells of carbon cages for kinetically effificient and long-term lithium storage. Nanoscale 2013, 5, 1576–1582. [Google Scholar] [CrossRef]
- Liu, N.; Huang, W.Y.; Zhang, X.D.; Tang, L.; Wang, L.; Wang, Y.X.; Wu, M.H. Ultrathin graphene oxide encapsulated in uniform MIL-88A(Fe) for enhanced visible light-driven photodegradation of RhB. Appl. Catal. B Environ. 2018, 221, 119–128. [Google Scholar] [CrossRef]
- Mu, X.X.; Jiang, J.F.; Chao, F.F.; Lou, Y.B.; Chen, J.X. Ligand modification of UiO-66 with an unusual visible light photocatalytic behavior for RhB degradation. Dalton Trans. 2018, 6, 1895–1902. [Google Scholar] [CrossRef]
- Mohammad, D.; Zhang, H.H.; Naghdabadi, R.; Hu, Y.H. Athermodynamically consistent large deformation theory coupling photochemical reaction and electrochemistry for light-responsive gels. J. Mech. Phys. Solids 2018, 116, 239–266. [Google Scholar]
- Wu, Q.P.; Wang, F.T.; Niu, C.H.; Li, Y.; Yao, W.F. A novel molecular sieve supporting material for enhancing activity and stability of Ag3PO4 photocatalyst. Appl. Surf. Sci. 2016, 378, 552–563. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Jiang, R.; Huang, S.H.; Yao, J.; Fu, F.Q.; Li, J.B. Novel magnetic NiFe2O4/multi-walled carbon nanotubes hybrids: Facile synthesis, characterization, and application to the treatment of dyeing wastewater. Ceram Int. 2015, 41, 11625–11631. [Google Scholar] [CrossRef]
- Guo, J.G.; Liu, Y.; Hao, Y.J.; Li, Y.L.; Wang, X.J.; Liu, R.H.; Li, F.T. Comparison of importance between separation efficiency and valence band position: The case of heterostructured Bi3 O4Br/α-Bi2O3 photocatalysts. Appl. Catal. B Environ. 2018, 224, 841–853. [Google Scholar] [CrossRef]
- Tang, H.; Fu, Y.H.; Chang, S.F.; Xie, S.Y.; Tang, G. Construction of Ag3PO4/Ag2MoO4 Z-scheme heterogeneous photocatalyst for the remediation of organic pollutants. Chin. J. Catal. 2017, 38, 337–347. [Google Scholar] [CrossRef]
- Wu, J.; Tian, Y.; Li, D.; Han, X.Y.; Liu, J.; Feng, Y.J. Enhanced photocatalytic CO2 reduction and 2,4-dichlorophenol degradation of TiO2 nanotubes via bi-directionally controlling electrons and holes. Chemosphere 2019, 226, 704–714. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.R.; Ma, Y.L.; Jin, Z.L. Noble-Metal-Free Visible Light Driven Hetero-structural Ni/ZnxCd1−xS Photocatalyst for Efficient Hydrogen Production. Catal Lett. 2019, 149, 1788–1799. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Hwang, S.H.; Lim, S.S. Characterization of DHDP, a novel aldose reductase inhibitor isolated from Lysimachia christinae. J. Funct. Foods 2017, 37, 241–248. [Google Scholar] [CrossRef]
- Fan, H.B.; Ren, Q.F.; Wang, S.L.; Jin, Z.; Ding, Y. Synthesis of the Ag/Ag3PO4/diatomite composites and their enhanced photocatalytic activity driven by visible light. J. Alloy Compd. 2019, 775, 845–852. [Google Scholar] [CrossRef]
- Gan, L.; Xu, L.J.; Qian, K. Preparation of core-shell structured CoFe2O4 incorporated Ag3PO4 nanocomposites for photocatalytic degradation of organic dyes. Mater. Des. 2016, 109, 354–360. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, M.; Meng, Y.; Chen, S.; Yang, S. Magnetic CoFe1.95Y0.05O4-Decorated Ag3PO4 as Superior and Recyclable Photocatalyst for Dye Degradation. Materials 2023, 16, 4659. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Meng, Y.; Liu, Q.; Xu, M.; Hu, Y.; Chen, S. Synthesis of Ag3PO4/Ag/g-C3N4 Composite for Enhanced Photocatalytic Degradation of Methyl Orange. Molecules 2023, 28, 6082. https://doi.org/10.3390/molecules28166082
Liu Q, Meng Y, Liu Q, Xu M, Hu Y, Chen S. Synthesis of Ag3PO4/Ag/g-C3N4 Composite for Enhanced Photocatalytic Degradation of Methyl Orange. Molecules. 2023; 28(16):6082. https://doi.org/10.3390/molecules28166082
Chicago/Turabian StyleLiu, Qingwang, Ying Meng, Qiman Liu, Mai Xu, Yunhu Hu, and Shikun Chen. 2023. "Synthesis of Ag3PO4/Ag/g-C3N4 Composite for Enhanced Photocatalytic Degradation of Methyl Orange" Molecules 28, no. 16: 6082. https://doi.org/10.3390/molecules28166082
APA StyleLiu, Q., Meng, Y., Liu, Q., Xu, M., Hu, Y., & Chen, S. (2023). Synthesis of Ag3PO4/Ag/g-C3N4 Composite for Enhanced Photocatalytic Degradation of Methyl Orange. Molecules, 28(16), 6082. https://doi.org/10.3390/molecules28166082






