Theoretical Insights into a Near-Infrared Fluorescent Probe NI-VIS Based on the Organic Molecule for Monitoring Intracellular Viscosity
Abstract
:1. Introduction
2. Conformation Search
- (1)
- Using Confab [36] to obtain initial conformations of probe NI-VIS;
- (2)
- The batch structural optimization was conducted using Crest to invoke xtb program under the GFN2-xTB method [37];
- (3)
- Invoke isostat in Molclus [38] program to screen out the several stable probe conformations with the local lowest free energy;
- (4)
- The corresponding ground and excited state of different probe conformation’s structure optimization and vibrational frequency analysis on the stable probe conformations’ structure obtained from step (3) were conducted by using the ORCA program [39] under CAM-B3LYP/def2-TZVPD, a functional which was testified to be suitable for the excited state optimization with charge transfer character, with D3 dispersion correction and GCP correction to remove artificial overbinding effects from BSSE [40,41,42,43,44,45]. The functional and basis set combination wB2GP-PLYP/def2-TZVPD was used in a single point energy calculation to obtain the free energy with high precision [46,47,48,49,50,51,52,53,54]. Most of the figures in this work were rendered by means of VMD 1.9.3 software [55] and the analyses were finished by using the Multiwfn 3.8(dev) code [56].
3. Results and Discussion
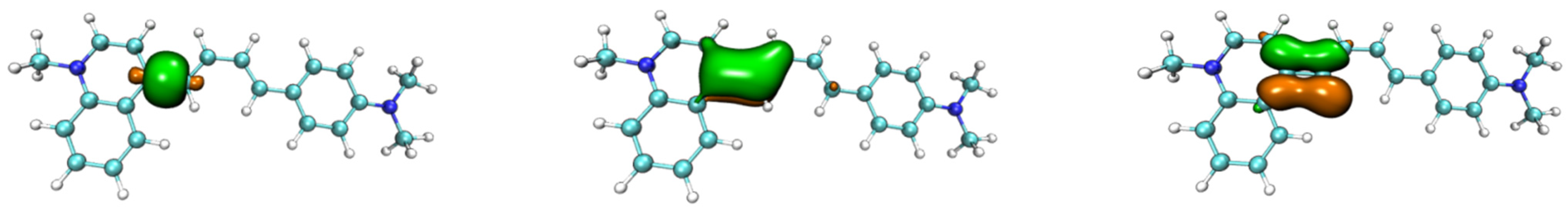
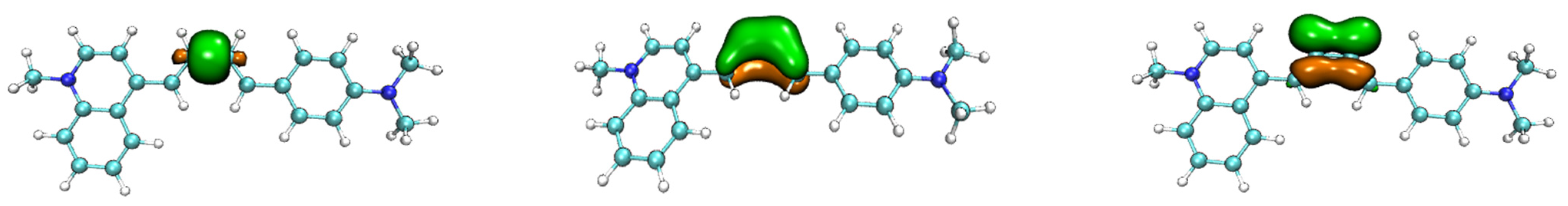
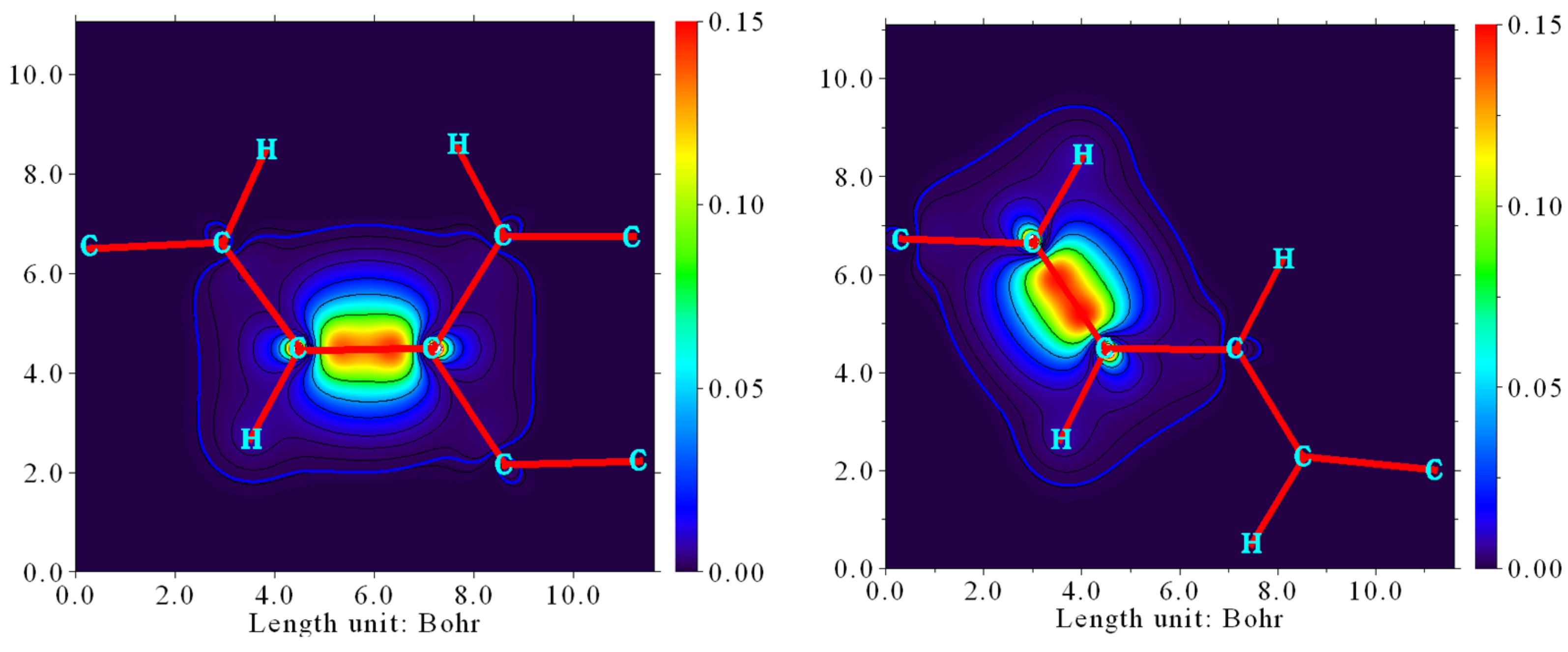
4. Densities of States (DOS)
5. Fluorescent Properties
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Puchkov, E.O. Intracellular Viscosity: Methods of Measurements and Role in Metabolism. Biol. Membr. 2014, 31, 3–13. [Google Scholar] [CrossRef]
- Kuimova, M.K.; Botchway, S.W.; Parker, A.W.; Balaz, M.; Collins, H.A.; Anderson, H.L.; Suhling, K.; Ogilby, P.R. Imaging intracellular viscosity of a single cell during photoinduced cell death. Nat. Chem. 2009, 1, 69–73. [Google Scholar] [CrossRef]
- Garcia-Seisdedos, H.; Heidenreich, M.; Levy, E.D. Not Going with the Flow: How Cells Adapt Internal Physics. Cell 2020, 183, 1462–1463. [Google Scholar] [CrossRef]
- Begum, H.M.; Shen, K. Intracellular and microenvironmental regulation of mitochondrial membrane potential in cancer cells. Wires Mech. Dis. 2023, 15, e1595. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.; Uthaman, S.; Lee, C.-S.; Kim, Y.; Pillarisetti, S.; Hwang, H.S.; Park, I.-K.; Huh, K.M. Tumor intracellular microenvironment-responsive nanoparticles for magnetically targeted chemotherapy. J. Ind. Eng. Chem. 2022, 111, 121–128. [Google Scholar] [CrossRef]
- Lin, K.-H.; Chiang, J.-C.; Ho, Y.-H.; Yao, C.-L.; Lee, H. Lysophosphatidic Acid and Hematopoiesis: From Microenvironmental Effects to Intracellular Signaling. Int. J. Mol. Sci. 2020, 21, 2015. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Mayernik, L.; Moin, K.; Sloane, B.F. Acidosis and proteolysis in the tumor microenvironment. Cancer Metastasis Rev. 2019, 38, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Sulerud, T.; Pelletier, J.; Tomschik, M.; Noren, B.E.; Kloxin, A.M.; Oakey, J.S.; Gatlin, J.C. Elucidating the mechanics of aster positioning using labile hydrogel microenvironments. Mol. Biol. Cell 2017, 28, 4832–4841. [Google Scholar]
- Chen, B.; Dai, W.; He, B.; Zhang, H.; Wang, X.; Wang, Y.; Zhang, Q. Current Multistage Drug Delivery Systems Based on the Tumor Microenvironment. Theranostics 2017, 7, 538–558. [Google Scholar] [CrossRef]
- Xie, D.; Ma, W.; Wang, C.; Zhang, W.; Ding, Z. Mitochondria-targeted fluorescent probe for imaging viscosity in hepatic ischemia-reperfusion injury cell model. Chem. Commun. 2023, 59, 1030–1033. [Google Scholar] [CrossRef]
- Samanta, S.K.; Maiti, K.; Halder, S.; Guria, U.N.; Mandal, D.; Jana, K.; Mahapatra, A.K. A ‘double locked’ ratiometric fluorescent probe for detection of cysteine in a viscous system and its application in cancer cells. Org. Biomol. Chem. 2023, 21, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yin, C.; Zhang, W.; Zhang, Y.; Huo, F. Mitochondrial-Targeting Near-Infrared Fluorescent Probe for Visualizing Viscosity in Drug-Induced Cells and a Fatty Liver Mouse Model. Anal. Chem. 2022, 94, 5069–5074. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lei, T.; Zhang, J.; Gong, L.; Yang, Y.; Ma, X.; Wen, Y.; Du, H.; Qi, D.; Bian, Y.; et al. A porphyrin-triazatruxene dyad for ratiometric two-photon fluorescent sensing of intracellular viscosity. J. Mater. Chem. B 2022, 10, 5487–5492. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-H.; Cui, W.-L.; Yang, Y.-H.; Wang, J.-Y. Viscosity-Sensitive Solvatochromic Fluorescent Probes for Lipid Droplets Staining. Biosensors 2022, 12, 851. [Google Scholar] [CrossRef] [PubMed]
- Pittman, M.; Ali, A.M.; Chen, Y. How sticky? How tight? How hot? Imaging probes for fluid viscosity, membrane tension and temperature measurements at the cellular level. Int. J. Biochem. Cell Biol. 2022, 153, 106329. [Google Scholar] [CrossRef]
- Pan, X.; Wang, C.; Zhao, C.; Cheng, T.; Zheng, A.; Cao, Y.; Xu, K. Assessment of cancer cell migration using a viscosity-sensitive fluorescent probe. Chem. Commun. 2022, 58, 4663–4666. [Google Scholar] [CrossRef]
- Li, F.; Zhou, B.-Z.; Yao, W.; Sun, S.-K.; Miao, J.-Y.; Zhao, B.-X.; Lin, Z.-M. Dual-response and lysosome-targeted fluorescent probe for viscosity and sulfur dioxide derivatives. Anal. Chim. Acta 2023, 1239, 340721. [Google Scholar] [CrossRef]
- Jo, S.; Kim, S.; Lee, Y.; Kim, G.; Kim, S.; Lee, S.; Lee, T.S. Synthesis of a dual-emissive pyrene-based fluorescent probe for imaging intracellular viscosity. J. Photochem. Photobiol. A-Chem. 2022, 433, 114147. [Google Scholar] [CrossRef]
- Fu, M.; Wang, K.; Ma, Q.; Zhu, J.; Bian, M.; Zhu, Q. A novel dual-functional fluorescent probe for imaging viscosity and cysteine in living system. Org. Biomol. Chem. 2022, 20, 672–677. [Google Scholar] [CrossRef]
- Maleckaite, K.; Dodonova, J.; Toliautas, S.; Zilenaite, R.; Jurgutis, D.; Karabanovas, V.; Tumkevicius, S.; Vysniauskas, A. Designing a Red-Emitting Viscosity-Sensitive BODIPY Fluorophore for Intracellular Viscosity Imaging. Chem.-A Eur. J. 2021, 27, 16768–16775. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, B.; Wang, N.; Yu, C.; Yang, N.; Chen, J.; Wu, Q.; Zhang, C.; Li, L.; Huang, W. Mitochondria-targeted fluorescent probe based on vibration-induced emission for real-time monitoring mitophagy-specific viscosity dynamic. Chin. Chem. Lett. 2020, 31, 2897–2902. [Google Scholar] [CrossRef]
- Ma, C.; Sun, W.; Xu, L.; Qian, Y.; Dai, J.; Zhong, G.; Hou, Y.; Liu, J.; Shen, B. A minireview of viscosity-sensitive fluorescent probes: Design and biological applications. J. Mater. Chem. B 2020, 8, 9642–9651. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Shen, W.; Chen, Y.; Yi, W.; Cai, C.; Zhu, L.; Zhu, Q. A highly sensitive red-emitting probe for the detection of viscosity changes in living cells, zebrafish, and human blood samples. J. Mater. Chem. B 2020, 8, 1310–1315. [Google Scholar] [CrossRef]
- Chen, L.; Feng, Y.; Dang, Y.; Zhong, C.; Chen, D. A deep-red emission fluorescent probe with long wavelength absorption for viscosity detection and live cell imaging. Anal. Bioanal. Chem. 2020, 412, 7819–7826. [Google Scholar] [CrossRef]
- Zhang, G.; Ni, Y.; Zhang, D.; Li, H.; Wang, N.; Yu, C.; Li, L.; Huang, W. Rational design of NIR fluorescence probes for sensitive detection of viscosity in living cells. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2019, 214, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Ning, P.; Dong, P.; Geng, Q.; Bai, L.; Ding, Y.; Tian, X.; Shao, R.; Li, L.; Meng, X. A two-photon fluorescent probe for viscosity imaging in vivo. J. Mater. Chem. B 2017, 5, 2743–2749. [Google Scholar] [CrossRef]
- Lee, S.-C.; Heo, J.; Ryu, J.-W.; Lee, C.-L.; Kim, S.; Tae, J.-S.; Rhee, B.-O.; Kim, S.-W.; Kwon, O.P. Pyrrolic molecular rotors acting as viscosity sensors with high fluorescence contrast. Chem. Commun. 2016, 52, 13695–13698. [Google Scholar] [CrossRef]
- Peng, X.; Yang, Z.; Wang, J.; Fan, J.; He, Y.; Song, F.; Wang, B.; Sun, S.; Qu, J.; Qi, J.; et al. Fluorescence Ratiometry and Fluorescence Lifetime Imaging: Using a Single Molecular Sensor for Dual Mode Imaging of Cellular Viscosity. J. Am. Chem. Soc. 2011, 133, 6626–6635. [Google Scholar] [CrossRef]
- Haidekker, M.A.; Brady, T.P.; Lichlyter, D.; Theodorakis, E.A. A ratiometric fluorescent viscosity sensor. J. Am. Chem. Soc. 2006, 128, 398–399. [Google Scholar] [CrossRef]
- Yin, X.; Li, Y.; Zhu, Y.; Jing, X.; Li, Y.; Zhu, D. A highly sensitive viscosity probe based on ferrocene-BODIPY dyads. Dalton Trans. 2010, 39, 9929–9935. [Google Scholar] [CrossRef]
- Kim, K.; Jo, C.; Easwaramoorthi, S.; Sung, J.; Kim, D.H.; Churchill, D.G. Crystallographic, Photophysical, NMR Spectroscopic and Reactivity Manifestations of the “8-Heteroaryl Effect” in 4,4-Difluoro-8-(C4H3X)-4-bora-3a,4a-diaza-s-indacene (X=O, S, Se) (BODIPY) Systems. Inorg. Chem. 2010, 49, 4881–4894. [Google Scholar] [CrossRef] [PubMed]
- Kee, H.L.; Kirmaier, C.; Yu, L.; Thamyongkit, P.; Youngblood, W.J.; Calder, M.E.; Ramos, L.; Noll, B.C.; Bocian, D.F.; Scheidt, W.R.; et al. Structural Control of the Photodynamics of Boron? Dipyrrin Complexes. J. Phys. Chem. B 2005, 109, 20433–20443. [Google Scholar] [CrossRef]
- Ikejiri, M.; Mori, K.; Miyagi, R.; Konishi, R.; Chihara, Y.; Miyashita, K. A hybrid molecule of a GFP chromophore analogue and cholestene as a viscosity-dependent and cholesterol-responsive fluorescent sensor. Org. Biomol. Chem. 2017, 15, 6948–6958. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Ning, P.; Feng, Y.; Ding, Y.; Bai, L.; Li, L.; Yu, H.; Meng, X. Two-Photon Fluorescent Probe for Monitoring Autophagy via Fluorescence Lifetime Imaging. Anal. Chem. 2018, 90, 7122–7126. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Hu, W.; Liu, Z. A Mitochondrial-Targeting Near-Infrared Fluorescent Probe for Visualizing and Monitoring Viscosity in Live Cells and Tissuese. Anal. Chem. 2019, 91, 10302–10309. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Vandermeersch, T.; Flynn, C.J.; Maguire, A.R.; Hutchison, G.R. Confab—Systematic generation of diverse low-energy conformers. J. Cheminform. 2011, 3, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB-An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. Molclus Program, Version 1.9.9.3. Available online: https://www.keinsci.com/research/molclus.html (accessed on 11 February 2021).
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. PCCP 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Verma, P.; Cramer, C.J.; Gagliardi, L.; Truhlar, D.G. Combining Wave Function Methods with Density Functional Theory for Excited States. Chem. Rev. 2018, 118, 7249–7292. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Parker, K.A.; Truhlar, D.G. Dual-Functional Tamm-Dancoff Approximation: A Convenient Density Functional Method that Correctly Describes S-1/S-0 Conical Intersections. J. Phys. Chem. Lett. 2017, 8, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, L.; Truhlar, D.G.; Li Manni, G.; Carlson, R.K.; Hoyer, C.E.; Bao, J.L. Multiconfiguration Pair-Density Functional Theory: A New Way To Treat Strongly Correlated Systems. Acc. Chem. Res. 2017, 50, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.S.; Li, S.L.; Truhlar, D.G. Perspective: Kohn-Sham density functional theory descending a staircase. J. Chem. Phys. 2016, 145, 130901. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Paez, M.; Goerigk, L. Assessing the Tamm-Dancoff approximation, singlet-singlet, and singlet-triplet excitations with the latest long-range corrected double-hybrid density functionals. J. Chem. Phys. 2020, 153, 064106. [Google Scholar] [CrossRef] [PubMed]
- Goerigk, L.; Grimme, S. Double-hybrid density functionals. Wiley Interdiscip. Rev.-Comput. Mol. Sci. 2014, 4, 576–600. [Google Scholar] [CrossRef]
- Casanova-Páez, M.; Dardis, M.B.; Goerigk, L. ωB2PLYP and ωB2GPPLYP: The First Two Double-Hybrid Density Functionals with Long-Range Correction Optimized for Excitation Energies. J. Chem. Theory Comput. 2019, 15, 4735–4744. [Google Scholar] [CrossRef]
- Liu, Y.-l.; Huang, H.; Peng, Y.-j. Fluorescent probe for simultaneous detection of human serum albumin and sulfite: A theoretical analysis. J. Mol. Struct. 2022, 1255, 132441. [Google Scholar] [CrossRef]
- Jia, A.; Huang, H.; Zuo, Z.-f.; Peng, Y.-j. Electronic structure and interaction in CH4@C-60: A first-principle investigation. J. Mol. Model. 2022, 28, 179. [Google Scholar] [CrossRef]
- Peng, Y.-J.; Huang, H.; Wang, C.-J. DFT investigation on electronic structure, chemical bonds and optical properties of Cu-6(SR)(6) nanocluster. Chem. Phys. Lett. 2021, 780, 138898. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Y.-L.; Huang, H.; Bai, G.; Peng, Y.-J. Theoretical investigation on FRET strategy of ratio metric fluorescent probe sensing hydrogen sulfide. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2023, 289, 122223. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Y.; Sun, S.H.; Liu, Y.T.; Shi, Q.Q.; Lv, J.J.; Peng, Y.J. Thiophene and diaminobenzo-(1,2,5-thiadiazol)- based DAD-type near-infrared fluorescent probe for nitric oxide: A theoretical research. Front. Chem. 2023, 10, 990979. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, G.; Peng, Y.; Chen, Z.; Gao, X.; Cheng, L.; Mei, X. Development of ratiometric sensing and white light-harvesting materials based on all-copper nanoclusters. Nanoscale Adv. 2019, 1, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K.K. VMD—Visual molecular dynamics. J. Mol. Graph. 1995, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
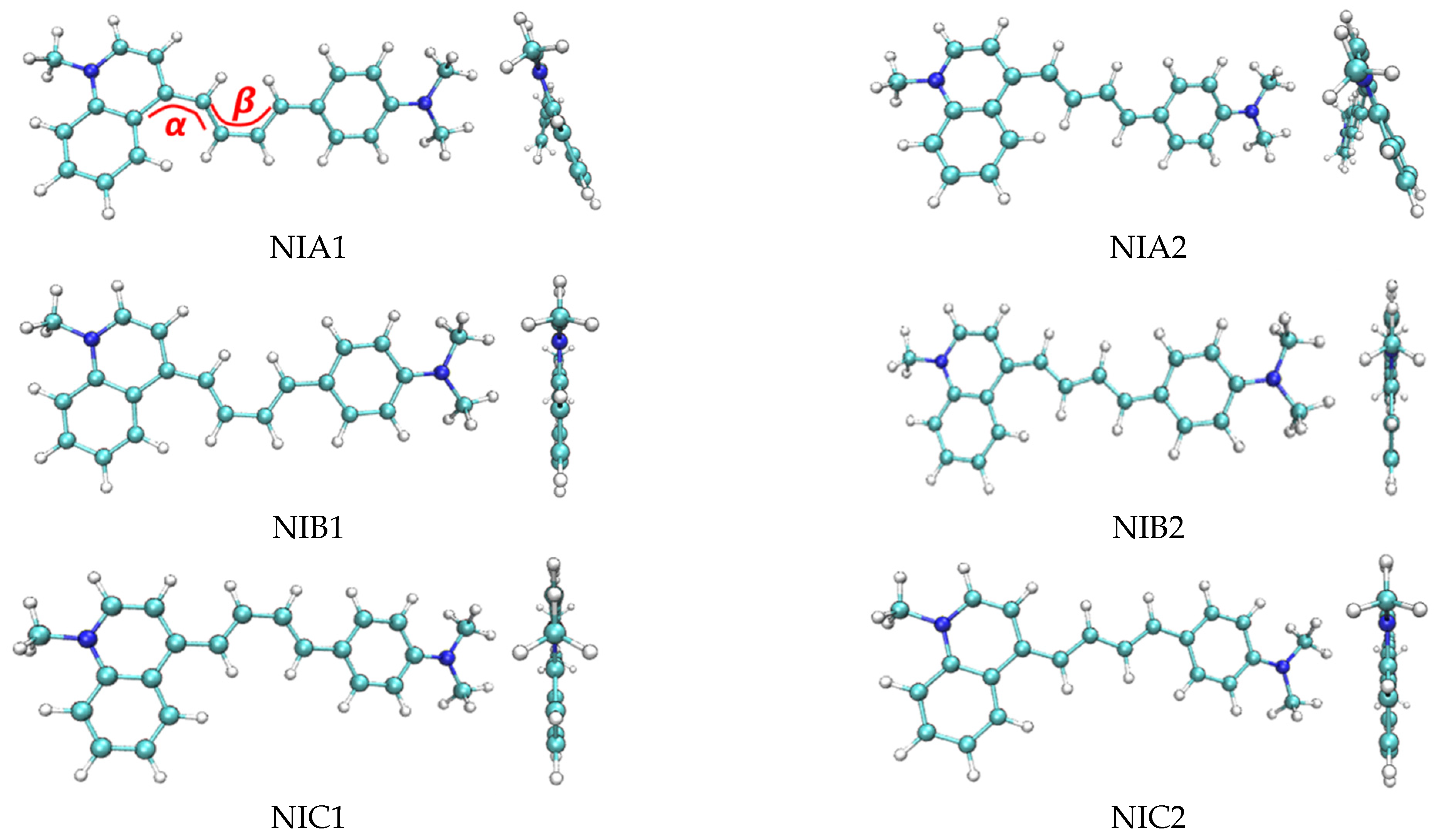


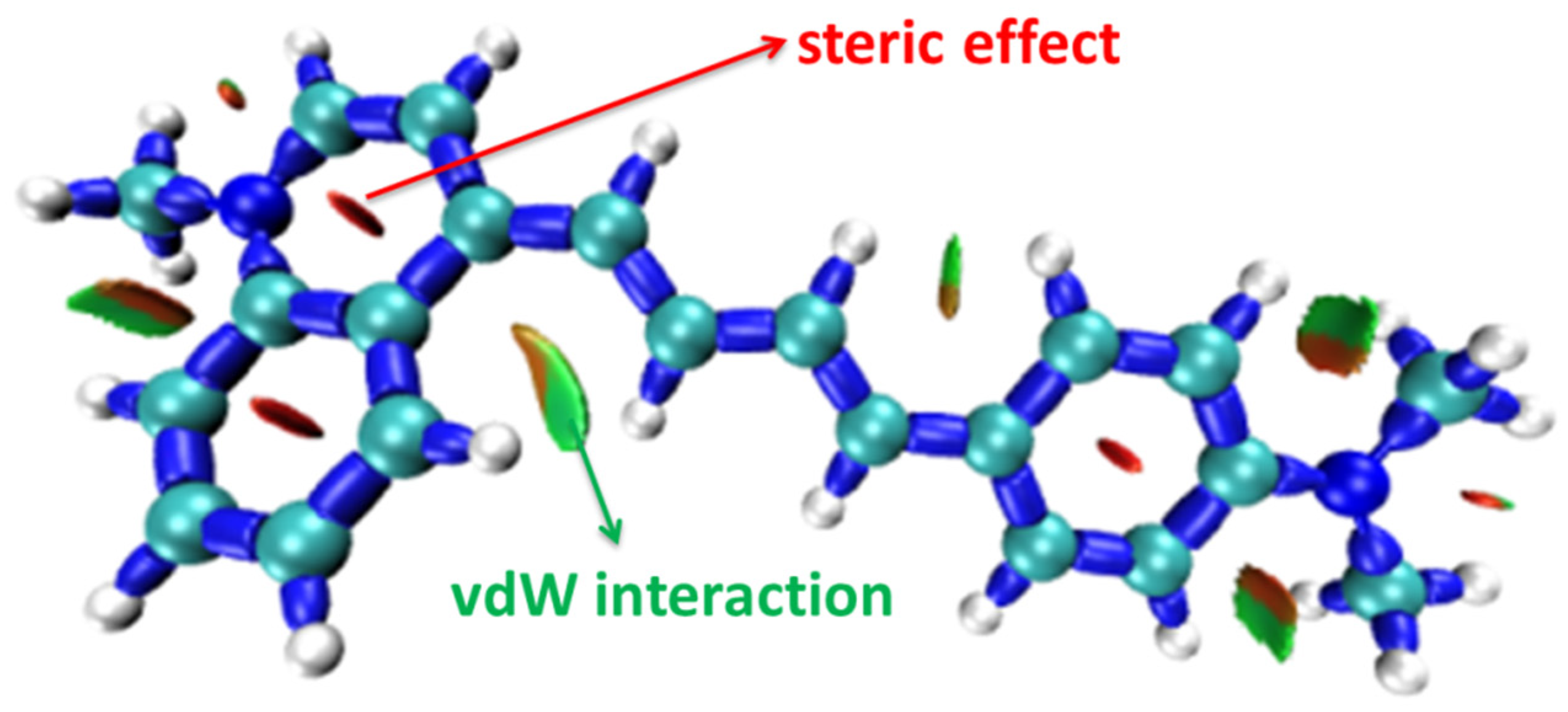
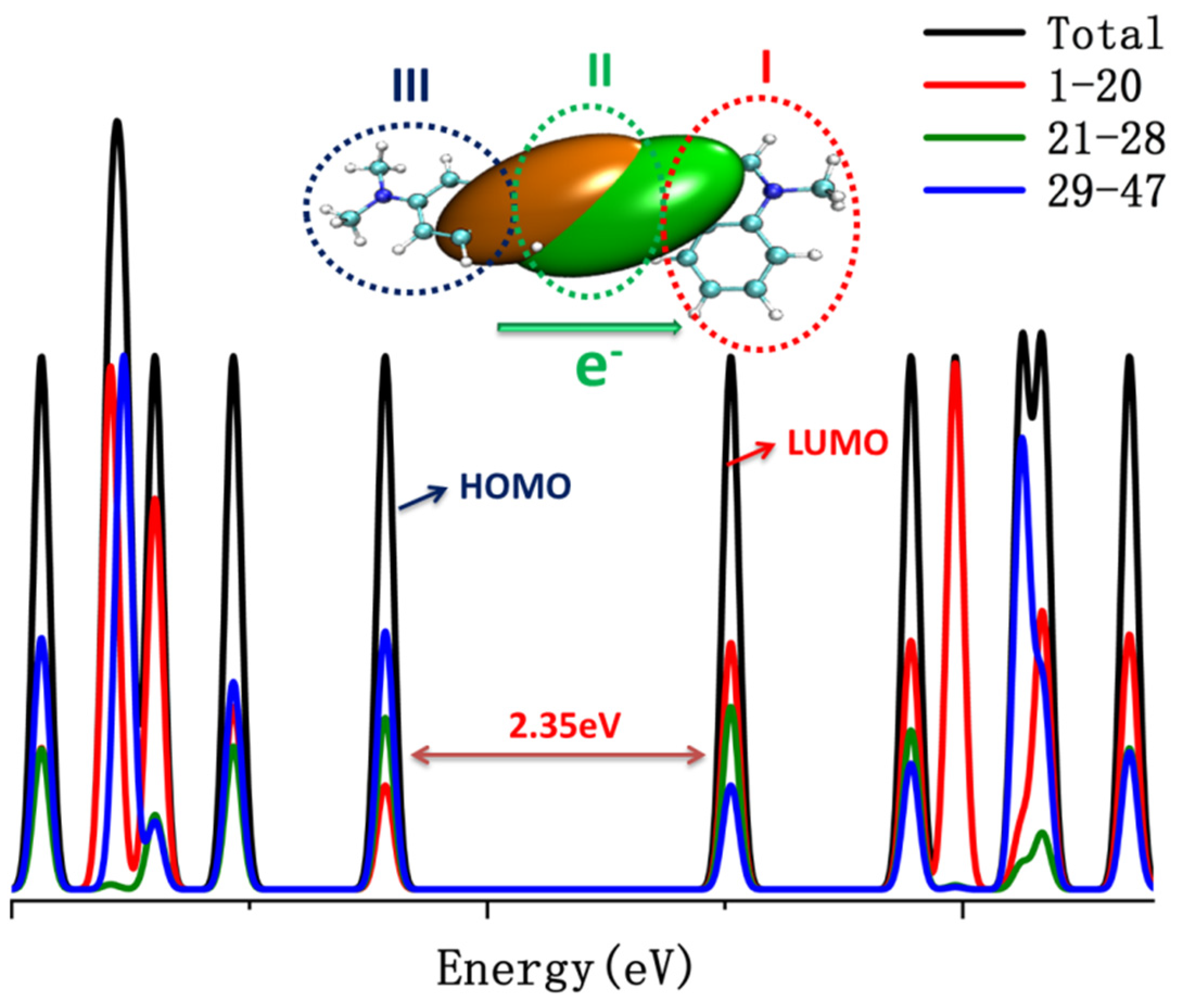
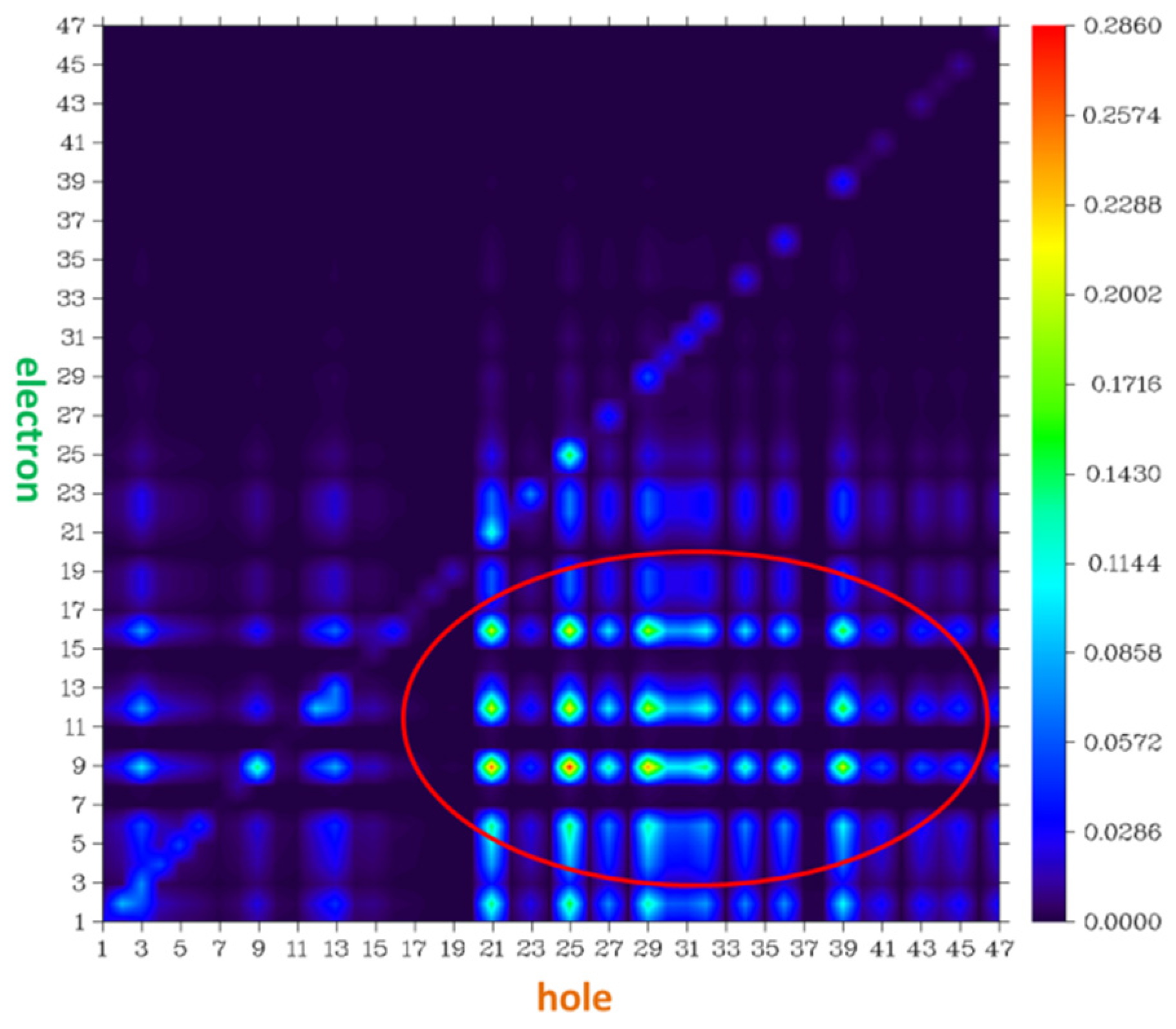

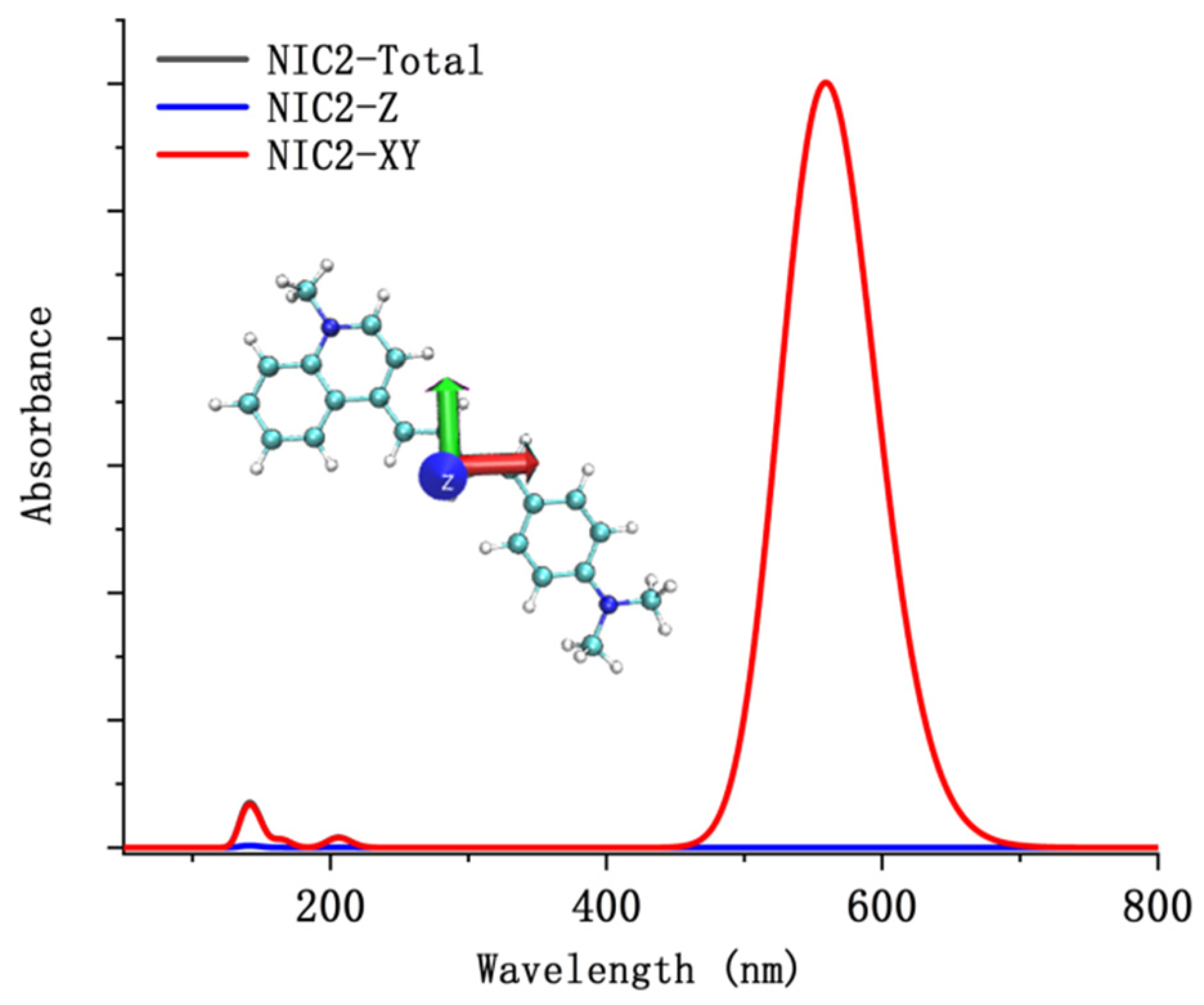
| α | β | ΔG (kcal/mol) NIC2 Taken as Reference | |
|---|---|---|---|
| NIA1 | 24° | 0° | 1.79 |
| NIA2 | 24° | 180° | 0.88 |
| NIB1 | 0° | 0° | 2.10 |
| NIB2 | 0° | 180° | 1.05 |
| NIC1 | 180° | 0° | 1.17 |
| NIC2 | 180° | 180° | 0 |
| Probe | Electronic Transition a | Excitation Energy | Oscillator Strength | Composition b | CI c |
|---|---|---|---|---|---|
| NIA1 | S0 → S1 | 548 nm | 2.0151 | H → L+1 H → L H-1 → L | 0.1002 0.6941 0.1124 |
| NIA2 | S0 → S1 | 553 nm | 1.8795 | H-1 → L H → L | 0.1005 0.6825 |
| NIB1 | S0 → S1 | 537 nm | 0.9696 | H → L H-1 → L | 0.6941 0.1127 |
| NIB2 | S0 → S1 | 545 nm | 1.4352 | H → L H-1 → L | 0.6501 0.1141 |
| NIC1 | S0 → S1 | 518 nm | 1.8625 | H → L H-1 → L | 0.6786 0.1064 |
| NIC2 | S0 → S1 | 527 nm | 1.9992 | H → L H-1 → L | 0.6875 0.1129 |
| Probe | Electronic Transition a | Emission Energy | Oscillator Strength | Composition b | CI c |
|---|---|---|---|---|---|
| NIA1 | S1 → S0 | 662 nm | 2.1046 | H → L | 0.6835 |
| NIA2 | S1 → S0 | 658 nm | 2.0135 | H → L | 0.6932 |
| NIB1 | S1 → S0 | 651 nm | 2.2160 | H → L | 0.7067 |
| NIB2 | S1 → S0 | 640 nm | 2.2504 | H → L | 0.6665 |
| NIC1 | S1 → S0 | 637 nm | 2.2312 | H → L | 0.7153 |
| NIC2 | S1 → S0 | 645 nm | 2.3014 | H → L | 0.7018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Huang, H.; Liu, Y.; Zhao, X. Theoretical Insights into a Near-Infrared Fluorescent Probe NI-VIS Based on the Organic Molecule for Monitoring Intracellular Viscosity. Molecules 2023, 28, 6105. https://doi.org/10.3390/molecules28166105
Peng Y, Huang H, Liu Y, Zhao X. Theoretical Insights into a Near-Infrared Fluorescent Probe NI-VIS Based on the Organic Molecule for Monitoring Intracellular Viscosity. Molecules. 2023; 28(16):6105. https://doi.org/10.3390/molecules28166105
Chicago/Turabian StylePeng, Yongjin, He Huang, Yuling Liu, and Xiaoyan Zhao. 2023. "Theoretical Insights into a Near-Infrared Fluorescent Probe NI-VIS Based on the Organic Molecule for Monitoring Intracellular Viscosity" Molecules 28, no. 16: 6105. https://doi.org/10.3390/molecules28166105
APA StylePeng, Y., Huang, H., Liu, Y., & Zhao, X. (2023). Theoretical Insights into a Near-Infrared Fluorescent Probe NI-VIS Based on the Organic Molecule for Monitoring Intracellular Viscosity. Molecules, 28(16), 6105. https://doi.org/10.3390/molecules28166105






