Enhancing Water Treatment Performance of Porous Polysulfone Hollow Fiber Membranes through Atomic Layer Deposition
Abstract
:1. Introduction
2. Results and Discussion
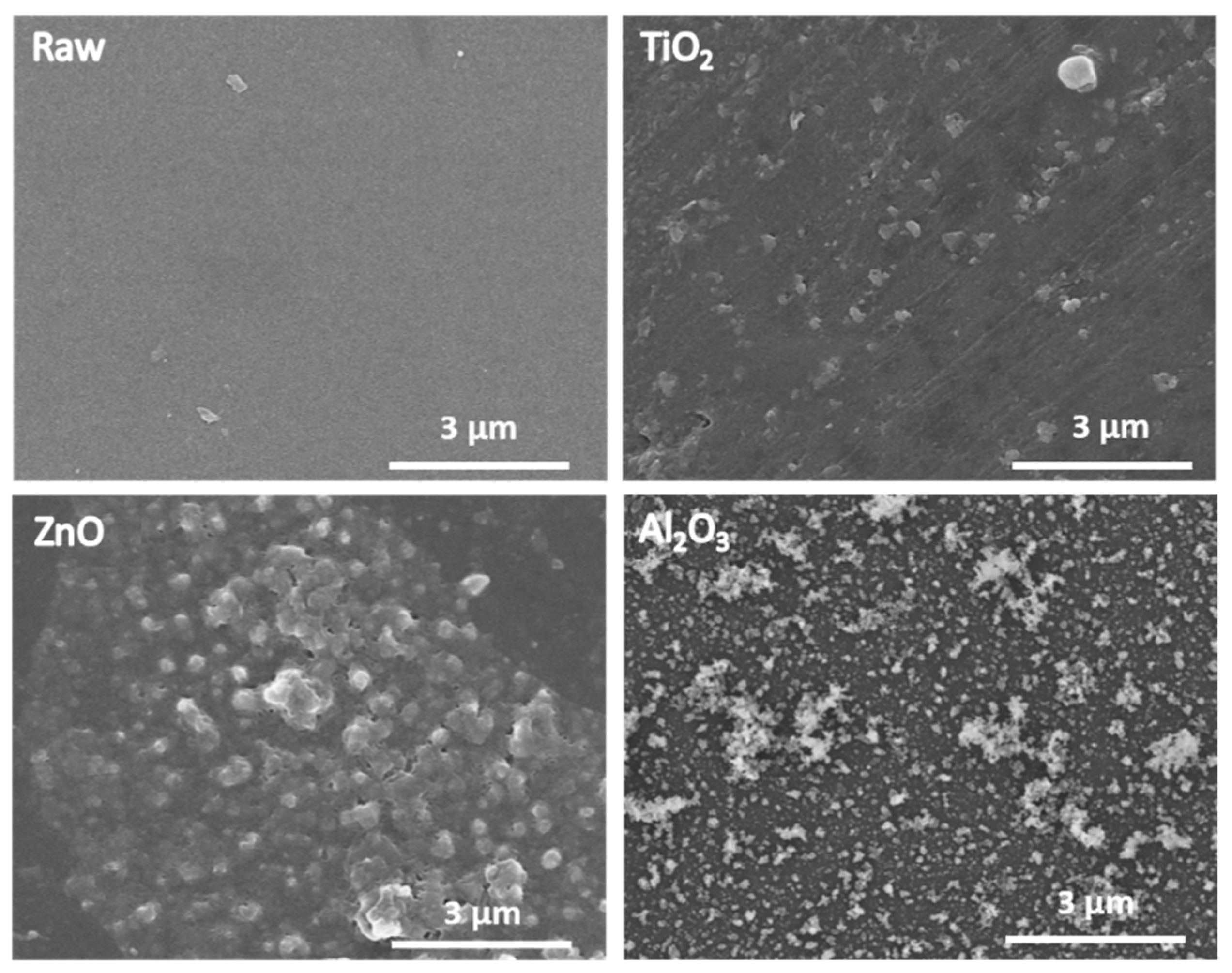
2.1. Structural, Physical, and Chemical Characterizations
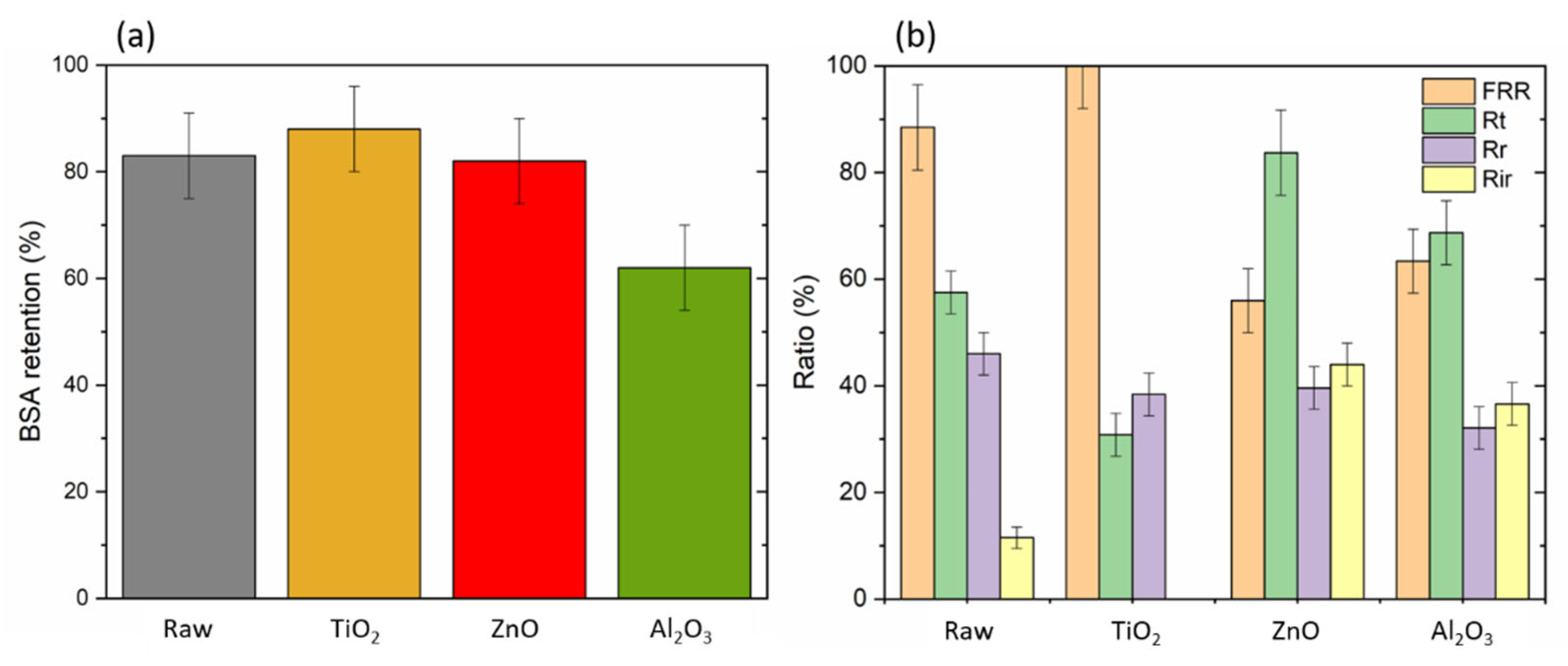
2.2. Membrane Performances
3. Materials and Methods
3.1. Materials
3.2. Oxide Deposition on PSF HF Membranes
3.3. Characterizations
3.4. Filtration Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nicolaisen, B. Developments in membrane technology for water treatment. Desalination 2003, 153, 355–360. [Google Scholar] [CrossRef]
- Kheirieh, S.; Asghari, M.; Afsari, M. Application and modification of polysulfone membranes. Rev. Chem. Eng. 2018, 34, 657–693. [Google Scholar] [CrossRef]
- Ganj, M.; Asadollahi, M.; Mousavi, S.A.; Bastani, D.; Aghaeifard, F. Surface modification of polysulfone ultrafiltration membranes by free radical graft polymerization of acrylic acid using response surface methodology. J. Polym. Res. 2019, 26, 231. [Google Scholar] [CrossRef]
- Mu, Y.; Feng, H.; Wang, S.; Zhang, S.; Luan, J.; Zhang, M.; Yu, Z.; Wang, G. Combined strategy of blending and surface modification as an effective route to prepare antifouling ultrafiltration membranes. J. Colloid Interface Sci. 2021, 589, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Y.; Zhang, X.; Hu, X.; Wang, C.; Yang, Y. Preparation and characterization of PSF-TiO2 hybrid hollow fiber UF membrane by sol–gel method. J. Polym. Res. 2020, 27, 376. [Google Scholar] [CrossRef]
- Pakkala, A.; Putkonen, M. Atomic Layer Deposition. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; ISBN 9780815520313. [Google Scholar]
- Yang, X.; Martinson, A.B.F.; Elam, J.W.; Shao, L.; Darling, S.B. Water treatment based on atomically engineered materials: Atomic layer deposition and beyond. Matter 2021, 4, 3515–3548. [Google Scholar] [CrossRef]
- Lee, J.; Kim, I.S. Environmental Science membrane surface engineering methods for water treatment: A short review. Environ. Sci. Water Res. Technol. 2020, 6, 1765–1785. [Google Scholar] [CrossRef]
- Weber, M.; Julbe, A.; Ayral, A.; Miele, P.; Bechelany, M. Atomic Layer Deposition for Membranes: Basics, Challenges, and Opportunities. Chem. Mater. 2018, 30, 7368–7390. [Google Scholar] [CrossRef]
- Li, F.; Li, L.; Liao, X.; Wang, Y. Precise pore size tuning and surface modifications of polymeric membranes using the atomic layer deposition technique. J. Memb. Sci. 2011, 385–386, 1–9. [Google Scholar] [CrossRef]
- Wang, H.; Wei, M.; Zhong, Z.; Wang, Y. Atomic-layer-deposition-enabled thin-film composite membranes of polyimide supported on nanoporous anodized alumina. J. Memb. Sci. 2017, 535, 56–62. [Google Scholar] [CrossRef]
- Feng, J.; Xiong, S.; Wang, Z.; Cui, Z.; Sun, S.P.; Wang, Y. Atomic layer deposition of metal oxides on carbon nanotube fabrics for robust, hydrophilic ultrafiltration membranes. J. Memb. Sci. 2018, 550, 246–253. [Google Scholar] [CrossRef]
- Jia, X.; Low, Z.; Chen, H.; Xiong, S.; Wang, Y. Atomic layer deposition of Al2O3 on porous polypropylene hollow fibers for enhanced membrane performances. Chin. J. Chem. Eng. 2018, 26, 695–700. [Google Scholar] [CrossRef]
- Nikkola, J.; Sievänen, J.; Raulio, M.; Wei, J.; Vuorinen, J.; Tang, C.Y. Surface modi fi cation of thin fi lm composite polyamide membrane using atomic layer deposition method. J. Memb. Sci. 2014, 450, 174–180. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Tian, Y.; Zhang, J.; Zhan, W.; Zhao, J.; Ding, Y.; Zuo, W. Hydrophilic modification of polyvinylidene fluoride membranes by ZnO atomic layer deposition using nitrogen dioxide/diethylzinc functionalization. J. Memb. Sci. 2016, 514, 241–249. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Wang, Z.; Huang, J.; Wang, Y. PVDF membranes with simultaneously enhanced permeability and selectivity by breaking the tradeoff effect via atomic layer deposition of TiO2. J. Memb. Sci. 2013, 442, 57–64. [Google Scholar] [CrossRef]
- Casetta, J.; Ortiz, D.G.; Pochat-Bohatier, C.; Bechelany, M.; Miele, P. Atomic layer deposition of TiO2 on porous polysulfone hollow fibers membranes for water treatment. Sep. Purif. Technol. 2023, 312, 123377. [Google Scholar] [CrossRef]
- Cabasso, I.; Klein, E.; Smith, J.K.; South, G. Polysulfone Hollow Fibers. I. Spinning and Properties. 1976, 20, 2377–2394. [Google Scholar]
- Janaki, A.C.; Sailatha, E.; Gunasekaran, S. Synthesis, Characteristics and Antimicrobial activity of ZnO nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 144, 17–22. [Google Scholar] [CrossRef]
- Viter, R.; Iatsunskyi, I.; Fedorenko, V.; Tumenas, S.; Balevicius, Z.; Ramanavicius, A.; Balme, S.; Nowaczyk, G.; Jurga, S.; Bechelany, M. Enhancement of Electronic and Optical Properties of Enhancement of Electronic and Optical Properties of ZnO/Al2O3 Nanolaminate Coated Electrospun Nanofibers. J. Phys. Chem. 2016, 120, 5124–5132. [Google Scholar] [CrossRef]
- Galiano, F.; Song, X.; Marino, T.; Boerrigter, M.; Saoncella, O.; Simone, S.; Faccini, M.; Chaumette, C.; Drioli, E.; Figoli, A. Novel photocatalytic PVDF/Nano-TiO2 hollow fibers for Environmental remediation. Polymers 2018, 10, 1134. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Effect of TiO2 nanoparticles on the performance of polyphenylsulfone biomaterial for orthopaedic implants. J. Mater. Chem. B 2014, 2, 7502–7514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lu, S.; Zhang, L.; Meng, Q.; Shen, C.; Zhang, J. Novel polysulfone hybrid ultrafiltration membrane prepared with TiO2-g-HEMA and its antifouling characteristics. J. Memb. Sci. 2013, 436, 163–173. [Google Scholar] [CrossRef]
- Lin, Y.; Loh, C.H.; Shi, L.; Fan, Y.; Wang, R. Preparation of high-performance Al2O3/PES composite hollow fiber UF membranes via facile in-situ vapor induced hydrolyzation. J. Memb. Sci. 2017, 539, 65–75. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P.; Zheng, Q. Preparation and properties of polysulfone/TiO2 composite ultrafiltration membranes. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 879–887. [Google Scholar] [CrossRef]
- Wu, X.; Hao, P.; He, F.; Yao, Z.; Zhang, X. Applied Surface Science Molecular dynamics simulations of BSA absorptions on pure and formate-contaminated rutile (1 1 0) surface. Appl. Surf. Sci. 2020, 533, 147574. [Google Scholar] [CrossRef]
- Membranes, B.C. Selective Separation of Similarly Sized Proteins with Tunable Nanoporous. ACS Nano 2013, 7, 768–776. [Google Scholar]
- Kubiak-ossowska, K.; Tokarczyk, K.; Jachimska, B.; Mulheran, P.A. Bovine Serum Albumin Adsorption at a Silica Surface Explored by Simulation and Experiment. J. Phys. Chem. B 2017, 121, 3975–3986. [Google Scholar] [CrossRef]
- Abba, M.U.; Man, H.C.; Azis, S.; Idris, A.I.; Hamzah, M.H.; Yunos, K.F.; Katibi, K.K. Novel PVDF-PVP Hollow Fiber Membrane Augmented with TiO2 Nanoparticles: Preparation, Characterization and Application for Copper Removal from Leachate. Nanomaterials 2021, 11, 399. [Google Scholar] [CrossRef]
- Mahdi, N.; Kumar, P.; Goswami, A.; Perdicakis, B.; Shankar, K. Robust Polymer Nanocomposite Membranes Incorporating Discrete TiO2 Nanotubes for Water Treatment. Nanomaterials 2019, 9, 1186. [Google Scholar] [CrossRef]
- Li, T.; Xiao, Y.; Guo, D.; Shen, L.; Li, R.; Jiao, Y.; Xu, Y.; Lin, H. Journal of Colloid and Interface Science In-situ coating TiO2 surface by plant-inspired tannic acid for fabrication of thin film nanocomposite nanofiltration membranes toward enhanced separation and antibacterial performance. J. Colloid Interface Sci. 2020, 572, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.; Bellare, J. Efficiently improved oil/water separation using high flux and superior antifouling polysulfone hollow fiber membranes modified with functionalized carbon nanotubes/graphene oxide nanohybrid. J. Environ. Chem. Eng. 2019, 7, 102944. [Google Scholar] [CrossRef]
- Xia, Z.; Rozyyev, V.; Mane, A.U.; Elam, W.; Darling, S.B. Surface Zeta Potential of ALD-Grown Metal-Oxide Films. Langmuir 2021, 37, 11618–11624. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, Y.; Feng, X.; Hu, X.; Zhang, Y.; Liu, L. Incorporation of oleic acid-modified Ag @ ZnO core-shell nanoparticles into thin film composite membranes for enhanced antifouling and antibacterial properties. J. Memb. Sci. 2020, 602, 117956. [Google Scholar] [CrossRef]
- Chaaya, A.A.; Viter, R.; Baleviciute, I.; Bechelany, M.; Ramanavicius, A.; Europeen, I.; Montpellier, U.; Bataillon, P.E. Tuning Optical Properties of Al2O3/ZnO Nanolaminates Synthesized by Atomic Layer Deposition. J. Phys. Chem. C 2014, 118, 3811–3819. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H. Novel polysulfone ultrafiltration membranes incorporating polydopamine functionalized graphene oxide with enhanced flux and fouling resistance. J. Memb. Sci. 2021, 620, 118900. [Google Scholar] [CrossRef]


| Atomic Percentages (%) | |||||
|---|---|---|---|---|---|
| Membranes | C | O | Ti | Zn | Al |
| Raw | 86.8 ± 8.6 | 9.9 ± 0.9 | / | / | / |
| TiO2 | 87.2 ± 8.7 | 9.9 ± 0.9 | ≤0.1 | / | / |
| ZnO | 62.4 ± 6.2 | 34.3 ± 3.4 | / | 1.7 ± 0.2 | / |
| Al2O3 | 48.3 ± 4.8 | 47.8 ± 4.8 | / | / | 3.2 ± 0.3 |
| Oxides | Young’s Modulus (MPa) | Strength at Break (N) | Elongation at Break (mm) |
|---|---|---|---|
| Raw | 132 ± 5 | 2.10 ± 0.02 | 55 ± 5 |
| TiO2 | 143 ± 6 | 1.84 ± 0.05 | 62 ± 7 |
| ZnO | 157 ± 5 | 1.31 ± 0.17 | 49 ± 8 |
| Al2O3 | 132 ± 3 | 1.12 ± 0.05 | 38 ± 5 |
| Oxide | TiO2 | ZnO | Al2O3 |
|---|---|---|---|
| Precursor 1 | TiCl4 | DEZ | TMA |
| Pulse time 1 (s) | 0.5 | 0.4 | 0.4 |
| Precursor 2 | H2O | H2O | H2O |
| Pulse time 2 (s) | 2 | 2 | 2 |
| Temperature (°C) | 100 | 100 | 100 |
| Exposure time for both precursors (s) | 10 | 30 | 40 |
| Purge time for both precursors (s) | 60 | 40 | 60 |
| Argon mass flow during purge (sccm) | 100 | 100 | 100 |
| Number of cycles | 20 | 10 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casetta, J.; Pochat-Bohatier, C.; Cornu, D.; Bechelany, M.; Miele, P. Enhancing Water Treatment Performance of Porous Polysulfone Hollow Fiber Membranes through Atomic Layer Deposition. Molecules 2023, 28, 6133. https://doi.org/10.3390/molecules28166133
Casetta J, Pochat-Bohatier C, Cornu D, Bechelany M, Miele P. Enhancing Water Treatment Performance of Porous Polysulfone Hollow Fiber Membranes through Atomic Layer Deposition. Molecules. 2023; 28(16):6133. https://doi.org/10.3390/molecules28166133
Chicago/Turabian StyleCasetta, Jeanne, Céline Pochat-Bohatier, David Cornu, Mikhael Bechelany, and Philippe Miele. 2023. "Enhancing Water Treatment Performance of Porous Polysulfone Hollow Fiber Membranes through Atomic Layer Deposition" Molecules 28, no. 16: 6133. https://doi.org/10.3390/molecules28166133





