Hirsutine, an Emerging Natural Product with Promising Therapeutic Benefits: A Systematic Review
Abstract
:1. Introduction
2. Results
2.1. Botanical Sources of Hirsutine
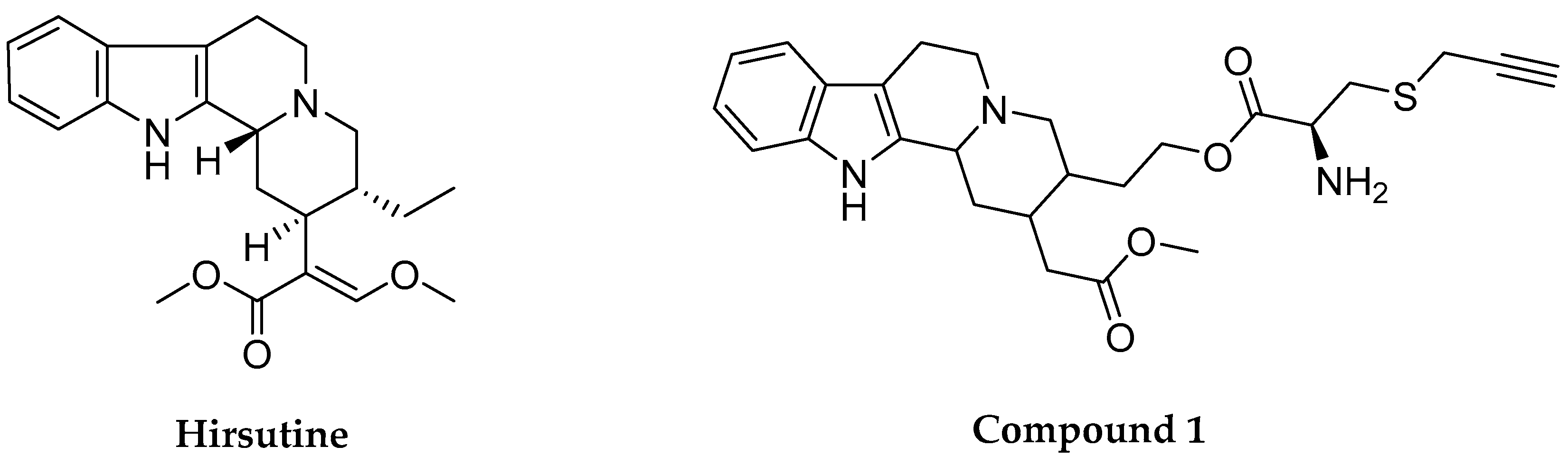
2.2. Physicochemical and Biopharmaceutical Profiles
2.3. Pharmacological Profile of Hirsutine
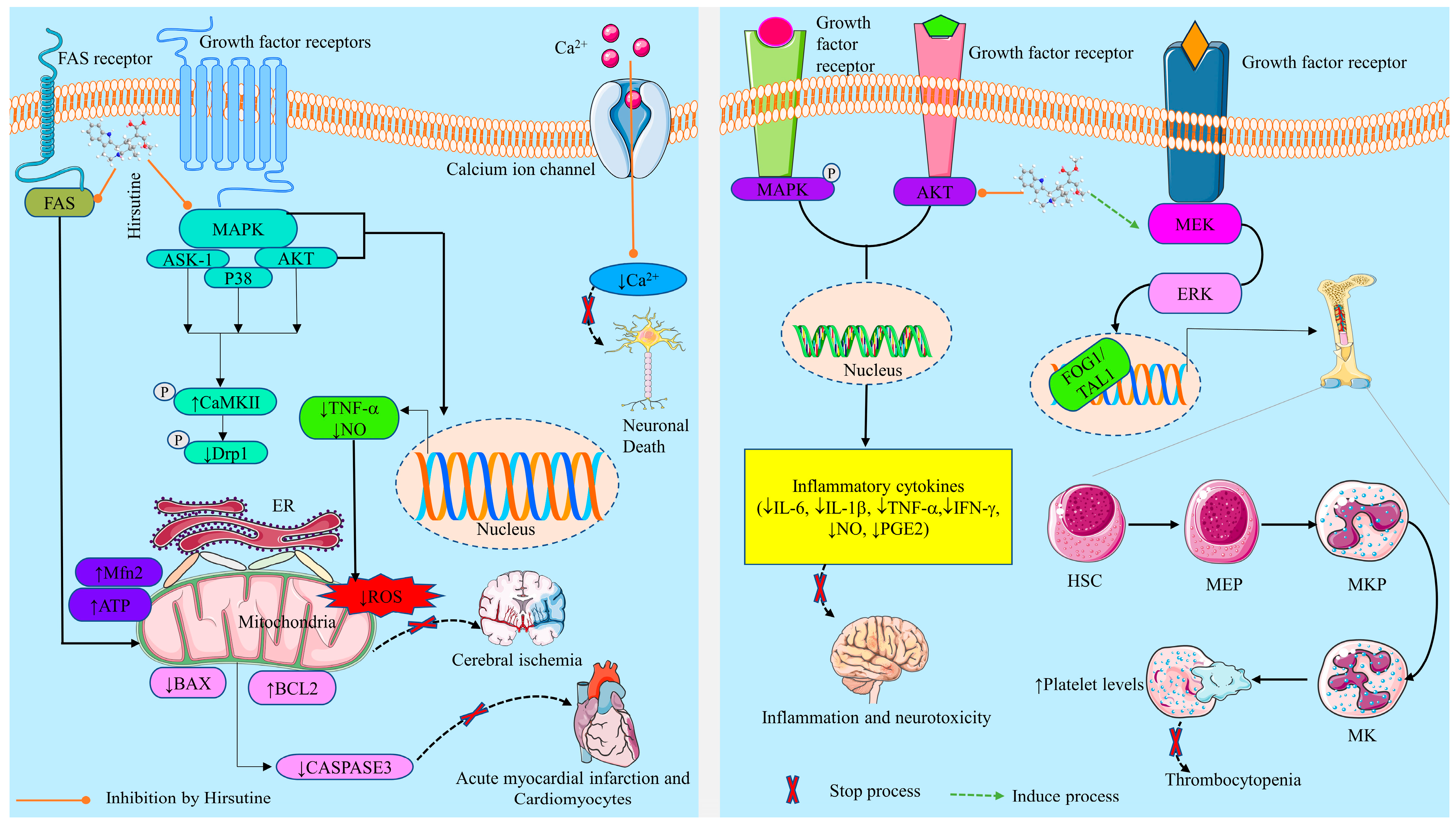
2.3.1. Neurobiological Effects
Prevention of Neuroinflammation and Neurotoxicity
Prevention of Neuronal Cell Death
2.3.2. Cardioprotective Activity
2.3.3. Antiviral Activity
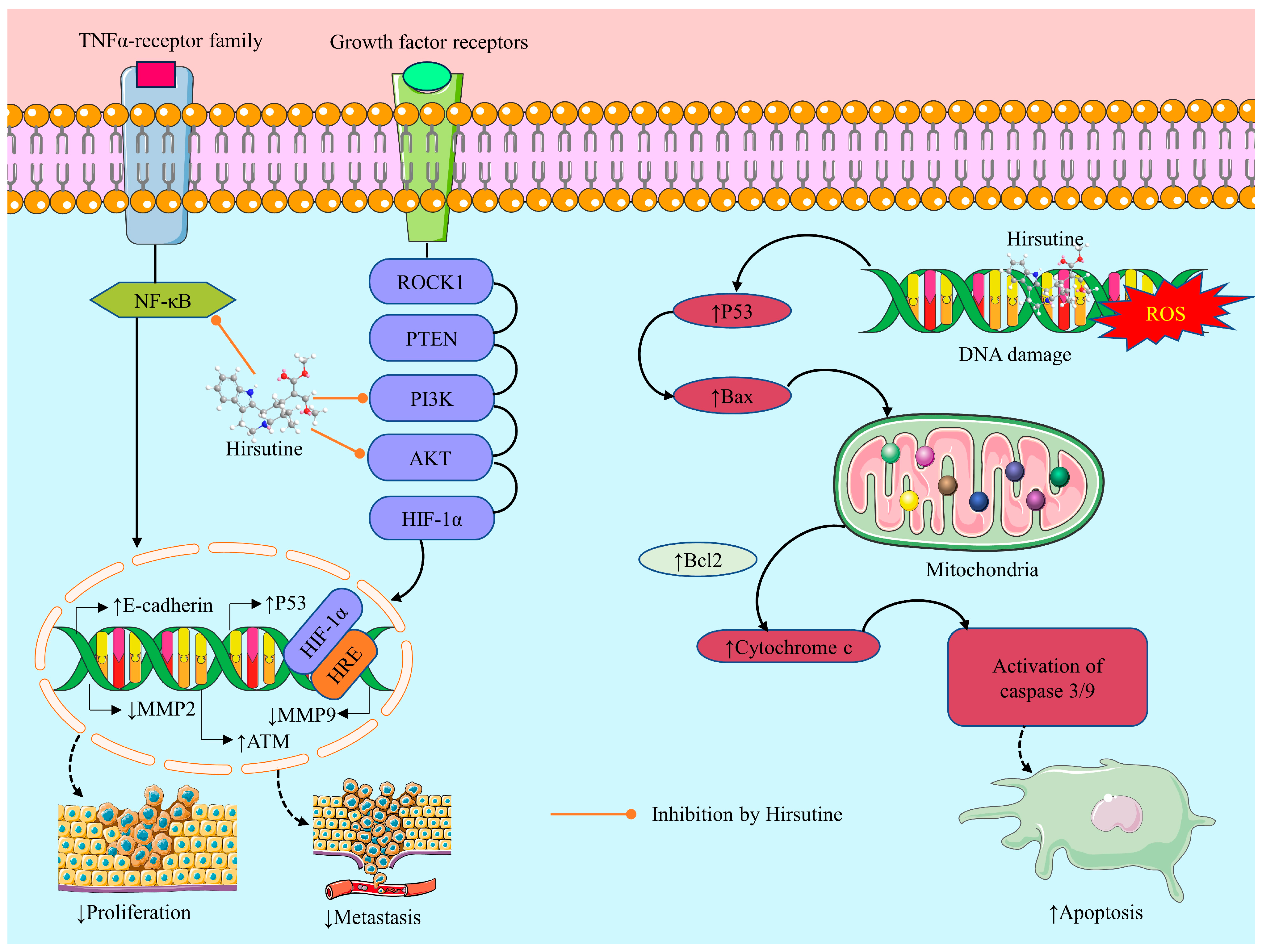
2.3.4. Anticancer Activity of Hirsutine: Underlying Mechanisms
Induction of Oxidative Stress
Cytotoxicity
Apoptotic Effect
Inhibition of Cell Migration and Invasion
Anti-Proliferative Effect
Genotoxic Effect
2.3.5. Effects on Thrombocytopenia
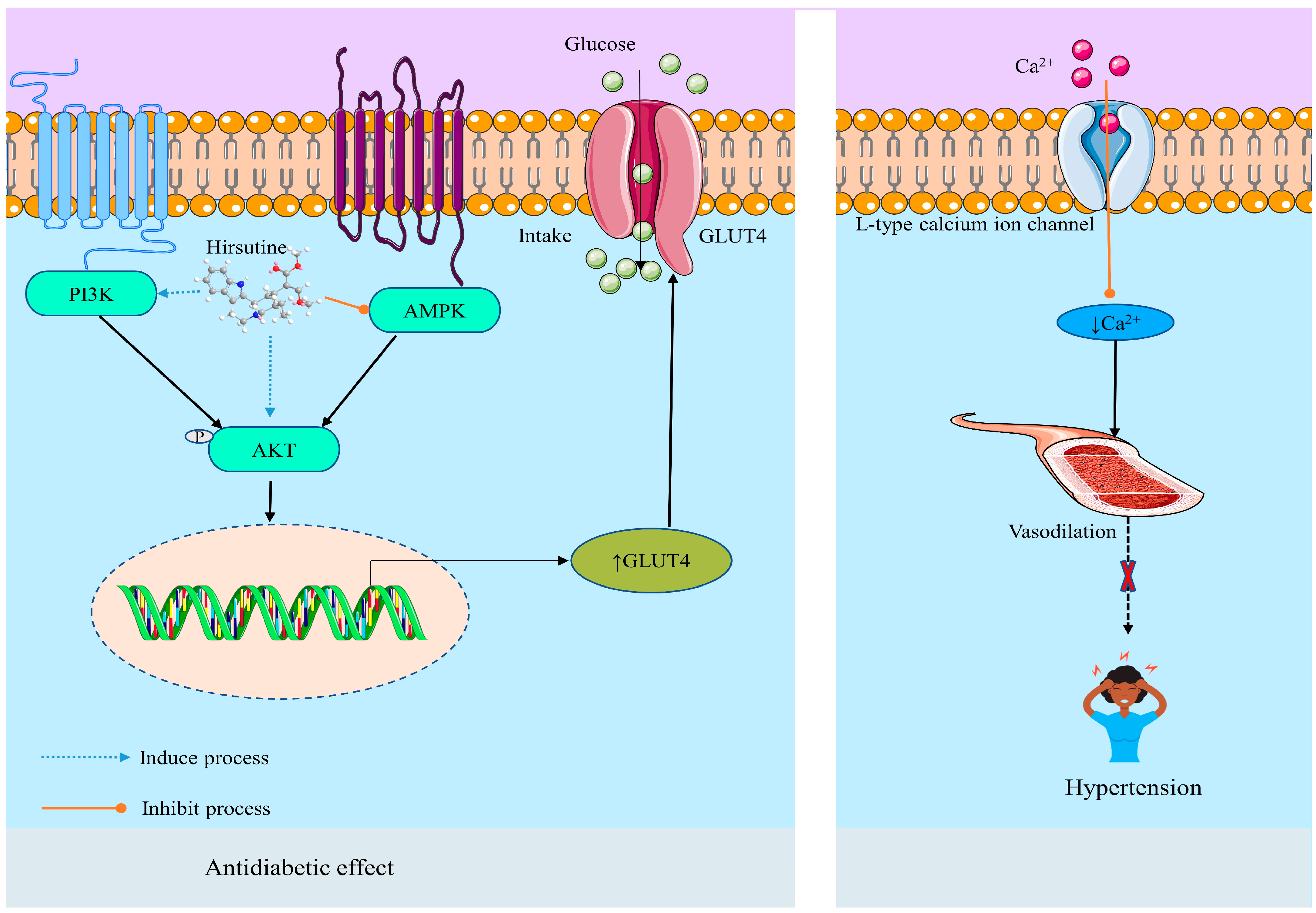
2.3.6. Metabolic Disease and Disorders
Antihypertensive Effect
Anti-Diabetic Effect
| Related Disease/Effect | Test Medium/Cell Line/Test System | Compound/ Dose (R/A)/IC50/ Concentration/ Course Interval | Possible Mechanism | Reference |
|---|---|---|---|---|
| Inflammation | Rats, LPS-induced preeclampsia | 35, 70, and 140 mg/kg b.w. | ↓ TNF-α, and ↓ IFN-γ, ↓ IL-6, ↓ IL-1β | [18] |
| Rat brain microglia (LPS-induced inflammation, 10 µg/mL), in vitro | - | ↓ NO, ↓ PGE2 and ↓ IL-1β, ↓ ROS, ↓ phosphorylation of the MAPK, ↓ Akt signaling proteins. | [29] | |
| Thrombocytopenia | The Kunming thrombocytopenia mouse model was established by X-ray irradiation, in vivo | - | ↑ MKD/MKM of K562 and Meg01 cells, ↑ platelet levels, ↑ MKD via activation of MEK-ERK-FOG1/TAL1 signaling | [35] |
| Neuronal death | Rat cerebellar granule cells (glutamate-induced neuronal death), in vitro | 10−4–3 × 10−4 M | ↓ Ca2+ influx | [36] |
| Myocardial ischemia-reperfusion | Sprague Dawley Rat Model | 5, 10, and 20 mg/kg (p.o.) | ↓ Myocardial infarct size, ↑ cardiac function, ↓ LDH, ↓ ROS, ↓ apoptosis, ↑ myocardial ATP, ↑ Mfn2 expression, ↓ p-Drp1, ↑ p-CaMKII, ↓ AKT/ASK-1/p38 MAPK pathway | [28] |
| Cardiomyocytes cell death | Neonatal rat cardiomyocytes treated with hypoxia | 0.1, 1, and 10 μΜ | ↓ Bax, ↓ Fas, ↓ caspase-3. ↑ Bcl-2. | [31] |
| Hypertension/ negative chronotropic/ antiarrhythmia | In male SD rats, in vitro, vasodilatation induced by the NO/cyclic GMP pathway | IC50 = 1.129×10−9 ± 0.5025 | ↓ Ca2+ influx, no effect on K+ channel | [14] |
| Male Japanese white rabbits | 0.1 to 10 μM | ↓ Influx of Ca2+ via voltage-dependent Ca2+ channels | [27] | |
| Male Wistar rats | 30 μM | ↓ Intracellular Ca2+ influx | [32] | |
| Aortic arteries of Wistar male rats, in vitro | 10−6 to 3 × 10−5 M | ↓ Ca2+ influx | [112] | |
| Male Sprague-Dawley rats | 3–300 µM, y 60 mM KCl (IC50 = 20–30 µM) | ↑ Ca2+, ↑ KCl | [33] | |
| Diabetes | Male C57BL/6 J mice, high-fat diet-induced diabetes, in vivo, n = 9 | 5, 10, and 20 mg/kg (p.o) | ↓ Ca2+, ↓ glucose tolerance, ↑ glucose uptake, ↑ glycolysis,↑ phosphatidylinositol 3-kinase (PI3K)/Akt pathways | [26] |
| HepG2 and H9c2 cells, high-glucose and high-insulin (HGHI) incubation, in vitro | 0.325 μM | ↑ p-Akt, ↑ GLUT4 activity, ↓ AMPK. | ||
| Antiviral activity | Human lung carcinoma cells (A549) and baby hamster kidney cells (BHK-21), DENV-1 (02-20 strain), DENV-2 (16681 strain), DENV-3 (09-59 strain), and DENV-4 (09-48 strain) | 10 µM | ↓ Ca2+, ↓ viral particle assembly, ↓ budding, or release step. | [19] |
| Influenza A virus (subtype H3N2), in vitro | EC50 = 0.4–0.57 µg/mL | ↓ Replication of the strains of Influenza A | [82] | |
| Antitumor | Jurkat clone E6-1 cells, evaluated by CCK8 assay, in vitro | 10, 25, and 50 μM for 48 h | ↓ Cell proliferation, ↑ pro-apoptotic Bax, cleaved-caspase3, cleaved-caspase9 and Cyt C proteins, ↓ Bcl-2 | [25] |
| Lung cancer | A549 xenograft mouse model, NCI-H1299, and LO2 cells | 60–80 μM | ↑ Apoptosis, ↑ ROCK1 and PTEN,↓ PI3K/Akt, ↑ caspase-3 | [34] |
| Breast cancer | MCF-10A, MCF-7 and MDA-MB-231 cells | 160 μM/L HSN for 24, 48, and 72 h | ↑ Apoptosis, ↓ Bax, ↓ Bcl-2, opening MPTP, releasing Cyt C from mitochondria, and activating caspase 9 and caspase 3. | [23] |
| MCF-7 | IC50 = 62.82 μM/L | Inhibits hypoxia, ↓ migration, and ↓ invasion, ↓ HIF-1α, ↓ snail, ↓ MMP-9, ↑ E-cadherin | [24] | |
| MCF-7 cell line, ataxia telangiectasia mutated (ATM) pathway | (50 µM) for 24 h | ↑ Cell apoptosis by inducing DNA damage, ↑ ATM pathway, ↑ p53-independent DNA damage response, ↑ ROS, ↓ metastasis of breast cancer cells | [21] | |
| HER2-positive/p53-mutated MDA-MB-453 and BT474 cell lines, in vitro | 6.25, 12.5, 25, and 50 μM | ↑ Cytotoxicity, ↑ apoptosis, ↑ DNA damage response | [22] | |
| Mouse mammary carcinoma 4T1 cells, in vitro | (25 µM) for 24 h | ↓ NF-κB, ↓ migration and invasion. ↓ MMP-2, MMP-9, and ↓ NF-κB signaling pathways | [20] |
3. Toxicological Profile
4. Methodology
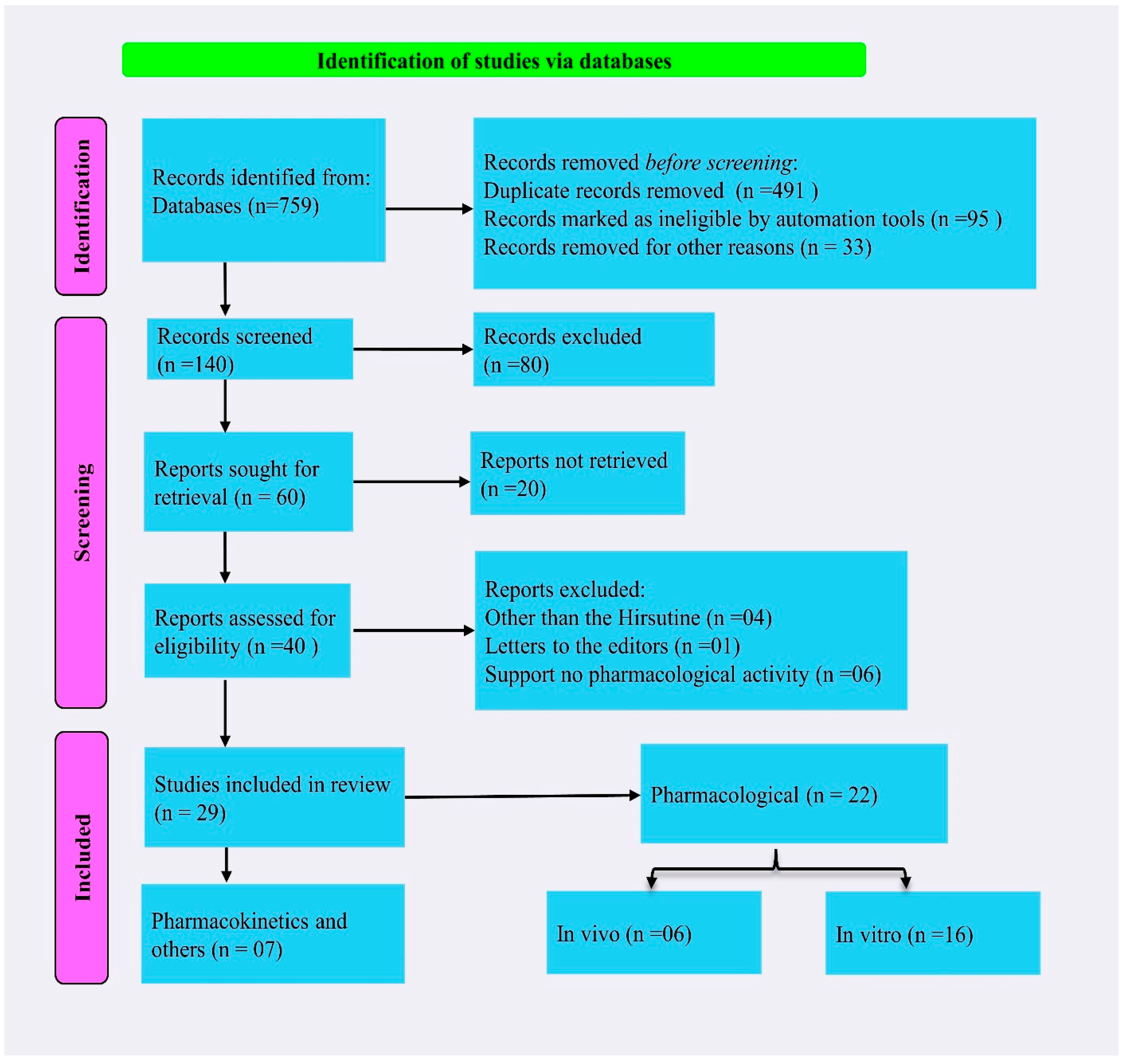
4.1. Literature Searching Strategy
4.2. Inclusion and Exclusion Criteria
4.3. In Silico ADME Prediction
4.4. Database Reports
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, D.D.; Chu, M.; Oza, U.; Rajgarhia, V. The value of natural products to future pharmaceutical discovery. Nat. Prod. Rep. 2007, 24, 1225–1244. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Drug discovery and development from natural products: The way forward. In Proceedings of the 11th NAPRECA Symposium Book of Proceedings, Antananarivo, Madagascar, 9–12 August 2005; Volume 1, pp. 56–69. [Google Scholar]
- Kinghorn, A.D.; Pan, L.; Fletcher, J.N.; Chai, H. The Relevance of Higher Plants in Lead Compound Discovery Programs. J. Nat. Prod. 2011, 74, 1539–1555. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Doak, B.C.; Over, B.; Giordanetto, F.; Kihlberg, J. Oral druggable space beyond the rule of 5: Insights from drugs and clinical candidates. Chem. Biol. 2014, 21, 1115–1142. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Majlesi, M.; Rafieian-Kopaei, M. Herbal versus synthetic drugs; beliefs and facts. J. Nephropharmacol. 2015, 4, 27–30. [Google Scholar]
- Bahmani, M.; Rafieian-Kopaei, M.; Hassanzadazar, H.; Saki, K.; Karamati, S.A.; Delfan, B. A review on most important herbal and synthetic antihelmintic drugs. Asian Pac. J. Trop. Med. 2014, 7, S29–S33. [Google Scholar] [CrossRef]
- Nisar, B.; Sultan, A.; Rubab, S.L. Comparison of Medicinally Important Natural Products versus Synthetic Drugs—A Short Commentary. Nat. Prod. Chem. Res. 2018, 6, 308. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Siam, M.S.H.; Ahamed, M.R.; Roy, U.K.; Hossain, M.I.; Rokonuzzman, M.; Islam, T.; Sharafat, R.; Bappi, M.H.; Mia, M.N.; et al. Toxicity Analysis of Some Frequently Used Food Processing Chemicals Using Allium cepa Biomonitoring System. Biology 2023, 12, 637. [Google Scholar] [CrossRef]
- Mohr, K.I. History of Antibiotics Research. In How to Overcome the Antibiotic Crisis: Facts, Challenges, Technologies and Future Perspectives, 1st ed.; Springer: Cham, Switzerland, 2016; pp. 237–272. [Google Scholar] [CrossRef]
- Ligon, B.L. Penicillin: Its Discovery and Early Development. Semin. Pediatr. Infect. Dis. 2004, 15, 52–57. [Google Scholar] [CrossRef]
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef]
- Xu, D.; Xu, Z. Indole Alkaloids with Potential Anticancer Activity. Curr. Top. Med. Chem. 2020, 20, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Yang, S.N.; Ma, F.F.; Gu, X.F.; Zhu, Y.C.; Zhu, Y.Z. The Novel Analogue of Hirsutine as an Anti-Hypertension and Vasodilatory Agent Both In Vitro and In Vivo. PLoS ONE 2015, 10, e0119477. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of Alkaloids (Indole Alkaloids, Isoquinoline Alkaloids, Tropane Alkaloids). In Recent Advances in Natural Products Analysis; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 505–567. [Google Scholar] [CrossRef]
- Nakazawa, T.; Banba, K.I.; Hata, K.; Nihei, Y.; Hoshikawa, A.; Ohsawa, K. Metabolites of Hirsuteine and Hirsutine, the Major Indole Alkaloids of Uncaria Rhynchophylla, in Rats. Biol. Pharm. Bull. 2006, 29, 1671–1677. [Google Scholar] [CrossRef]
- Ozaki, Y. Nihon Yakurigaku Zasshi. Folia Pharmacol. Jpn. 1989, 94, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Z.; Xiao, X.M. Evaluation of the Effects of Uncaria Rhynchophylla Alkaloid Extract on LPS-Induced Preeclampsia Symptoms and Inflammation in a Pregnant Rat Model. Braz. J. Med. Biol. Res. 2019, 52, e8273. [Google Scholar] [CrossRef] [PubMed]
- Hishiki, T.; Kato, F.; Tajima, S.; Toume, K.; Umezaki, M.; Takasaki, T.; Miura, T. Hirsutine, an Indole Alkaloid of Uncaria rhynchophylla, Inhibits Late Step in Dengue Virus Lifecycle. Front. Microbiol. 2017, 8, 1674. [Google Scholar] [CrossRef]
- Lou, C.; Takahashi, K.; Irimura, T.; Saiki, I.; Hayakawa, Y. Identification of Hirsutine as an Anti-Metastatic Phytochemical by Targeting NF-κB Activation. Int. J. Oncol. 2014, 45, 2085–2091. [Google Scholar] [CrossRef]
- Lou, C.; Yokoyama, S.; Abdelhamed, S.; Saiki, I.; Hayakawa, Y. Targeting the Ataxia Telangiectasia Mutated Pathway for Effective Therapy against Hirsutine-Resistant Breast Cancer Cells. Oncol. Lett. 2016, 12, 295–300. [Google Scholar] [CrossRef]
- Lou, C.; Yokoyama, S.; Saiki, I.; Hayakawa, Y. Selective Anticancer Activity of Hirsutine against HER2 Positive Breast Cancer Cells by Inducing DNA Damage. Oncol. Rep. 2015, 33, 2072–2076. [Google Scholar] [CrossRef]
- Huang, Q.W.; Zhai, N.N.; Huang, T.; Li, D.M. Hirsutine induces apoptosis of human breast cancer MDA-MB-231 cells through mitochondrial pathway. Sheng Li Xue Bao 2018, 70, 40–46. [Google Scholar]
- Huang, W.Q.; Chen, S.K. Effect of hirsutine on hypoxia-induced migration and invasion abilities in human breast cancer MCF-7 cells. Chin. J. Pathophysiol. 2017, 33, 2009–2014. [Google Scholar]
- Meng, J.; Su, R.; Wang, L.; Yuan, B.; Li, L. Inhibitory Effect and Mechanism of Action (MOA) of Hirsutine on the Proliferation of T-Cell Leukemia Jurkat Clone E6-1 Cells. PeerJ 2021, 9, e10692. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, M.; Sun, W.; Li, Q.; Xi, H.; Qiu, Y.; Wang, R.; Ding, Q.; Wang, Z.; Yu, Y.; et al. Hirsutine ameliorates hepatic and cardiac insulin resistance in high-fat diet-induced diabetic mice and in vitro models. Pharmacol. Res. 2022, 177, 105917. [Google Scholar] [CrossRef] [PubMed]
- Masumiya, H.; Saitoh, T.; Tanaka, Y.; Horie, S.; Aimi, N.; Takayama, H.; Tanaka, H.; Shigenobu, K. Effects of Hirsutine and Dihydrocorynantheine on the Action Potentials of Sino-Atrial Node, Atrium and Ventricle. Life Sci. 1999, 65, 2333–2341. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, Y.; Zhang, W.; Pan, X.; Liu, J.; Chen, Q.; Chen, J. Hirsutine ameliorates myocardial ischemia-reperfusion injury through improving mitochondrial function via CaMKII pathway. Clin. Exp. Hypertens. 2023, 45, 2192444. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Nam, K.N.; Woo, B.C.; Kim, K.P.; Kim, S.O.; Lee, E.H. Hirsutine, an indole alkaloid of Uncaria rhynchophylla, inhibits inflammation-mediated neurotoxicity and microglial activation. Mol. Med. Rep. 2013, 7, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Suk, K.; Kim, S.Y.; Leem, K.; Kim, Y.O.; Park, S.Y.; Hur, J.; Baek, J.; Lee, K.J.; Zheng, H.Z.; Kim, H. Neuroprotection by Methanol Extract of Uncaria rhynchophylla against Global Cerebral Ischemia in Rats. Life Sci. 2002, 70, 2467–2480. [Google Scholar] [CrossRef]
- Wu, L.X.; Gu, X.F.; Zhu, Y.C.; Zhu, Y.Z. Protective Effects of Novel Single Compound, Hirsutine on Hypoxic Neonatal Rat Cardiomyocytes. Eur. J. Pharmacol. 2011, 650, 290–297. [Google Scholar] [CrossRef]
- Horie, S.; Yano, S.; Aimi, N.; Sakai, S.; Watanabe, K. Effects of hirsutine, an antihypertensive indole alkaloid from Uncaria rhynchophylla, on intracellular calcium in rat thoracic aorta. Life Sci. 1992, 50, 491–498. [Google Scholar] [CrossRef]
- Zhang, W.B.; Chen, C.X.; Sim, S.M.; Kwan, C.Y. In Vitro Vasodilator Mechanisms of the Indole Alkaloids Rhynchophylline and Isorhynchophylline, Isolated from the Hook of Uncaria Rhynchophylla (Miquel). Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 232–238. [Google Scholar] [CrossRef]
- Zhang, R.; Li, G.; Zhang, Q.; Tang, Q.; Huang, J.; Hu, C.; Liu, Y.; Wang, Q.; Liu, W.; Gao, N.; et al. Hirsutine Induces mPTP-Dependent Apoptosis through ROCK1/PTEN/PI3K/GSK3β Pathway in Human Lung Cancer Cells. Cell Death Dis. 2018, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Lin, J.; Wang, L.; Shen, X.; Li, J.; Wu, A.; Yue, L.; Wei, L.; Ye, Y.; Yang, J.; et al. Hirsutine, a novel megakaryopoiesis inducer, promotes thrombopoiesis via MEK/ERK/FOG1/TAL1 signaling. Phytomedicine 2022, 102, 154150. [Google Scholar] [CrossRef]
- Shimada, Y.; Goto, H.; Itoh, T.; Sakakibara, I.; Kubo, M.; Sasaki, H.; Terasawa, K. Evaluation of the Protective Effects of Alkaloids Isolated from the Hooks and Stems of Uncaria sinensis on Glutamate-Induced Neuronal Death in Cultured Cerebellar Granule Cells from Rats. J. Pharm. Pharmacol. 1999, 51, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Devi, G.; Sudhakar, K.; Vasupradaa, A.P.; Sravya, V.; Manasa, V.; Yasaswini, E. Medicinal Plants in India and Their Antioxidant Potential—A Review. Rev. Geintec-Gest. Inov. E Tecnol. 2021, 11, 1397–1405. [Google Scholar]
- Chirumbolo, S. Plant Phytochemicals as New Potential Drugs for Immune Disorders and Cancer Therapy: Really a Promising Path? J. Sci. Food Agric. 2012, 92, 1573–1577. [Google Scholar] [CrossRef]
- Uramova, S.; Kubatka, P.; Dankova, Z.; Kapinova, A.; Zolakova, B.; Samec, M.; Zubor, P.; Zulli, A.; Valentova, V.; Kwon, T.K.; et al. Plant Natural Modulators in Breast Cancer Prevention: Status Quo and Future Perspectives Reinforced by Predictive, Preventive, and Personalized Medical Approach. EPMA J. 2018, 9, 403–419. [Google Scholar] [CrossRef]
- Park, H.J. Chemistry and Pharmacological Action of Caffeoylquinic Acid Derivatives and Pharmaceutical Utilization of Chwinamul (Korean Mountainous Vegetable). Arch. Pharmacal Res. 2010, 33, 1703–1720. [Google Scholar] [CrossRef]
- Li, Y.J.; Sun, A.S.; Yu, L.M.; Wu, Q. Inhibition of Rhynchophylline on Cultured Vascular Smooth Muscle Cells Proliferation Induced by Angiotensin II. Chin. Pharm. J. 2008, 143, 1621–1624. [Google Scholar]
- Kim, T.J.; Lee, J.H.; Lee, J.J.; Yu, J.Y.; Hwang, B.Y.; Ye, S.K.; Shujuan, L.; Gao, L.; Pyo, M.Y.; Yun, Y.P. Corynoxeine Isolated from the Hook of Uncaria rhynchophylla Inhibits Rat Aortic Vascular Smooth Muscle Cell Proliferation through the Blocking of Extracellular Signal-Regulated Kinase 1/2 Phosphorylation. Biol. Pharm. Bull. 2008, 31, 2073–2078. [Google Scholar] [CrossRef]
- Gao, J.; Inagaki, Y.; Liu, Y. Research Progress on Flavonoids Isolated from Traditional Chinese Medicine in Treatment of Alzheimer’s Disease. Intractable Rare Dis. Res. 2013, 2, 3–10. [Google Scholar] [CrossRef]
- Gai, Y.; Yang, N.; Chen, J. Inhibitory Activity of 8 Alkaloids on P-gp and Their Distribution in Chinese Uncaria Species. Nat. Prod. Commun. 2020, 15, 1934578X20973506. [Google Scholar] [CrossRef]
- Laus, G.; Brössner, D.; Keplinger, K. Alkaloids of Peruvian Uncaria tomentosa. Phytochemistry 1997, 45, 855–860. [Google Scholar] [CrossRef]
- Reichel, A.; Lienau, P. Pharmacokinetics in Drug Discovery: An Exposure-Centred Approach to Optimising and Predicting Drug Efficacy and Safety. Handb. Exp. Pharmacol. 2016, 232, 235–260. [Google Scholar] [CrossRef] [PubMed]
- Bhuia, M.S.; Kamli, H.; Islam, T.; Sonia, F.A.; Kazi, M.A.; Siam, M.S.H.; Rahman, N.; Bappi, M.H.; Mia, M.N.; Hossen, M.M.; et al. Antiemetic Activity of Trans-Ferulic Acid Possibly through Muscarinic Receptors Interaction Pathway: In Vivo and In Silico Study. Results Chem. 2023, 6, 101014. [Google Scholar] [CrossRef]
- Chow, S.C. Bioavailability and Bioequivalence in Drug Development. Wiley Interdiscip. Rev. Comput. Stat. 2014, 6, 304. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Islam, T.; Rokonuzzman, M.; Shamsh Prottay, A.A.; Akter, F.; Hossain, M.I.; Chowdhury, R.; Kazi, M.A.; Khalipha, A.B.R.; Coutinho, H.D.M.; et al. Modulatory Effects of Phytol on the Antiemetic Property of Domperidone, Possibly through the D2 Receptor Interaction Pathway: In Vivo and In Silico Studies. 3 Biotech 2023, 13, 116. [Google Scholar] [CrossRef]
- Jia, C.Y.; Li, J.Y.; Hao, G.F.; Yang, G.F. A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discov. Today 2020, 25, 248–258. [Google Scholar] [CrossRef]
- Lucas, A.J.; Sproston, J.L.; Barton, P.; Riley, R.J. Estimating Human ADME Properties, Pharmacokinetic Parameters and Likely Clinical Dose in Drug Discovery. Expert Opin. Drug Discov. 2019, 14, 1313–1327. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, J.; Chen, L. Tissue Distribution of Hirsutine and Hirsuteine in Mice by Ultrahigh-Performance Liquid Chromatography-Mass Spectrometry. J. Anal. Methods Chem. 2020, 2020, 7204315. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, J.J.; Xu, J.; Feng, F.; Qu, W. Medicinal Uses, Phytochemistry and Pharmacology of the Genus Uncaria. J. Ethnopharmacol. 2015, 173, 48–80. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Walter, L.; Neumann, H. Role of Microglia in Neuronal Degeneration and Regeneration. Semin. Immunopathol. 2009, 31, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Bhuia, M.S.; Rahaman, M.M.; Islam, T.; Bappi, M.H.; Sikder, M.I.; Hossain, K.N.; Akter, F.; Al Shamsh Prottay, A.; Rokonuzzman, M.; Gürer, E.S.; et al. Neurobiological Effects of Gallic Acid: Current Perspectives. Chin. Med. 2023, 18, 27. [Google Scholar] [CrossRef]
- Skrzypczak-Wiercioch, A.; Sałat, K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules 2022, 27, 5481. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.F.; Olivon, V.C.; Mestriner, F.L.A.C.; Zanotto, C.Z.; Ferreira, R.G.; Ferreira, N.S.; Silva, C.A.A.; Luiz, J.P.M.; Alves, J.V.; Fazan, R.; et al. Acute Increase in O-GlcNAc Improves Survival in Mice with LPS-Induced Systemic Inflammatory Response Syndrome. Front. Physiol. 2020, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Nakanishi, S.; Nakajima, Y.; Masu, M.; Ueda, Y.; Nakahara, K.; Watanabe, D.; Yamaguchi, S.; Kawabata, S.; Okada, M. Glutamate Receptors: Brain Function and Signal Transduction. Brain Res. Brain Res. Rev. 1998, 26, 230–235. [Google Scholar] [CrossRef]
- Murrough, J.W.; Abdallah, C.G.; Mathew, S.J. Targeting Glutamate Signalling in Depression: Progress and Prospects. Nat. Rev. Drug Discov. 2017, 16, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Madji Hounoum, B.; Blasco, H.; Coque, E.; Vourc’h, P.; Emond, P.; Corcia, P.; Andres, C.R.; Raoul, C.; Mavel, S. The Metabolic Disturbances of Motoneurons Exposed to Glutamate. Mol. Neurobiol. 2018, 55, 7669–7676. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Mai, D.; Qu, S. Molecular Mechanisms of Glutamate Toxicity in Parkinson’s Disease. Front. Neurosci. 2020, 14, 585584. [Google Scholar] [CrossRef] [PubMed]
- Sribnick, E.A.; Del Re, A.M.; Ray, S.K.; Woodward, J.J.; Banik, N.L. Estrogen Attenuates Glutamate-Induced Cell Death by Inhibiting Ca2+ Influx through L-Type Voltage-Gated Ca2+ Channels. Brain Res. 2009, 1276, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Kristián, T.; Siesjö, B.K. Calcium in Ischemic Cell Death. Stroke 1998, 29, 705–718. [Google Scholar] [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics—2015 Update: A Report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef]
- Neri, M.; Riezzo, I.; Pascale, N.; Pomara, C.; Turillazzi, E. Ischemia/Reperfusion Injury following Acute Myocardial Infarction: A Critical Issue for Clinicians and Forensic Pathologists. Mediators Inflamm. 2017, 2017, 7018393. [Google Scholar] [CrossRef]
- Frank, A.; Bonney, M.; Bonney, S.; Weitzel, L.; Koeppen, M.; Eckle, T. Myocardial ischemia reperfusion injury: From basic science to clinical bedside. Semin. Cardiothorac. Vasc. Anesth. 2012, 16, 123–132. [Google Scholar] [CrossRef]
- Cadenas, S.; Aragones, J.; Landazuri, M.O. Mitochondrial reprogramming through cardiac oxygen sensors in ischaemic heart disease. Cardiovasc. Res. 2010, 88, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, H.; Zhang, J.; Yan, M. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway. Chem. Biol. Interact. 2018, 284, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Nojima, T.; Fujisaki, N.; Tsukahara, K.; Yamamoto, H.; Yamada, T.; Aokage, T.; Yumoto, T.; Osako, T.; Nakao, A. Therapeutic Strategies for Ischemia Reperfusion Injury in Emergency Medicine. Acute Med. Surg. 2020, 7, e501. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, N.; Cardosa, J.; Hanley, K.A.; Holmes, E.C.; Weaver, S.C. Fever from the Forest: Prospects for the Continued Emergence of Sylvatic Dengue Virus and Its Impact on Public Health. Nat. Rev. Microbiol. 2011, 9, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, S.; Rodrigo, C.; Rajapakse, A. Treatment of Dengue Fever. Infect. Drug Resist. 2012, 5, 103–112. [Google Scholar] [CrossRef]
- Messer, W.B.; Vitarana, U.T.; Sivananthan, K.; Elvtigala, J.; Preethimala, L.D.; Ramesh, R.; Withana, N.; Gubler, D.J.; De Silva, A.M. Epidemiology of Dengue in Sri Lanka before and after the Emergence of Epidemic Dengue Hemorrhagic Fever. Am. J. Trop. Med. Hyg. 2002, 66, 765–773. [Google Scholar] [CrossRef]
- Gupta, E.; Dar, L.; Kapoor, G.; Broor, S. The Changing Epidemiology of Dengue in Delhi, India. Virol. J. 2006, 3, 92. [Google Scholar] [CrossRef]
- Peteranderl, C.; Herold, S.; Schmoldt, C. Human Influenza Virus Infections. Semin. Respir. Crit. Care Med. 2016, 37, 487–500. [Google Scholar] [CrossRef]
- Takayama, H.; Iimura, Y.; Kitajima, M.; Aimi, N.; Konno, K.; Inoue, H.; Fujiwara, M.; Mizuta, T.; Yokota, T.; Shigeta, S.; et al. Discovery of Anti-Influenza A Virus Activity of a Corynanthe-Type Indole Alkaloid, Hirsutine, In Vitro and the Structure-Activity Relationship of Natural and Synthetic Analogs. Bioorg. Med. Chem. Lett. 1997, 7, 3145–3148. [Google Scholar] [CrossRef]
- Van Loenhout, J.; Peeters, M.; Bogaerts, A.; Smits, E.; Deben, C. Oxidative Stress-Inducing Anticancer Therapies: Taking a Closer Look at Their Immunomodulating Effects. Antioxidants 2020, 9, 1188. [Google Scholar] [CrossRef]
- Islam, M.T.; Martorell, M.; González-Contreras, C.; Villagran, M.; Mardones, L.; Tynybekov, B.; Docea, A.O.; Abdull Razis, A.F.; Modu, B.; Calina, D.; et al. An Updated Overview of Anticancer Effects of Alternariol and Its Derivatives: Underlying Molecular Mechanisms. Front. Pharmacol. 2023, 14, 1099380. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The Role of Cellular Reactive Oxygen Species in Cancer Chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Bhuia, M.S.; Wilairatana, P.; Chowdhury, R.; Rakib, A.I.; Kamli, H.; Shaikh, A.; Coutinho, H.D.M.; Islam, M.T. Anticancer Potentials of the Lignan Magnolin: A Systematic Review. Molecules 2023, 28, 3671. [Google Scholar] [CrossRef] [PubMed]
- Ozben, T. Oxidative Stress and Apoptosis: Impact on Cancer Therapy. J. Pharm. Sci. 2007, 96, 2181–2196. [Google Scholar] [CrossRef]
- Chaudhry, G.E.; Akim, A.M.; Sung, Y.Y.; Muhammad, T.S.T. Cancer and Apoptosis. Methods Mol. Biol. 2022, 2543, 191–210. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Role of Bcl-2 Family Proteins in Apoptosis: Apoptosomes or Mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Yuan, X.; Ou, Y.; Zhu, X.; Cheng, Z.; Zhang, P.; Wu, X.; Meng, Y.; Zhang, L. The Relationship between the Bcl-2/Bax Proteins and the Mitochondria-Mediated Apoptosis Pathway in the Differentiation of Adipose-Derived Stromal Cells into Neurons. PLoS ONE 2016, 11, e0163327. [Google Scholar] [CrossRef]
- Vara, J.Á.F.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. PI3K/Akt Signalling Pathway and Cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef]
- Kim, E.H.; Song, H.S.; Yoo, S.H.; Yoon, M. Tumor Treating Fields Inhibit Glioblastoma Cell Migration, Invasion and Angiogenesis. Oncotarget 2016, 7, 65125–65136. [Google Scholar] [CrossRef] [PubMed]
- Bhuia, M.S.; Chowdhury, R.; Sonia, F.A.; Kamli, H.; Shaikh, A.; El-Nashar, H.A.S.; El-Shazly, M.; Islam, M.T. Anticancer Potential of the Plant-Derived Saponin Gracillin: A Comprehensive Review of Mechanistic Approaches. Chem. Biodivers. 2023, e202300847. [Google Scholar] [CrossRef] [PubMed]
- Appert-Collin, A.; Hubert, P.; Crémel, G.; Bennasroune, A. Role of ErbB Receptors in Cancer Cell Migration and Invasion. Front. Pharmacol. 2015, 6, 283. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-Signaling Pathway in Cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an Active Player in Human Cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Tcherkezian, J.; Roux, P.P. The expanding role of mTOR in cancer cell growth and proliferation. Mutagenesis 2015, 30, 169–176. [Google Scholar] [CrossRef]
- Pui, C.H.; Relling, M.V. Can the Genotoxicity of Chemotherapy be Predicted? Lancet 2004, 364, 917–918. [Google Scholar] [CrossRef]
- Fox, J.T.; Sakamuru, S.; Huang, R.; Teneva, N.; Simmons, S.O.; Xia, M.; Tice, R.R.; Austin, C.P.; Myung, K. High-throughput genotoxicity assay identifies antioxidants as inducers of DNA damage response and cell death. Proc. Natl. Acad. Sci. USA 2012, 109, 5423–5428. [Google Scholar] [CrossRef]
- Gauer, R.L.; Braun, M.M. Thrombocytopenia. Am. Fam. Physician 2012, 85, 612–622. [Google Scholar]
- Izak, M.; Bussel, J.B. Management of thrombocytopenia. F1000Prime Rep. 2014, 6, 45. [Google Scholar] [CrossRef]
- Yang, F.; Lai, J.; Deng, J.; Du, J.; Du, X.; Zhang, X.; Wang, Y.; Huang, Q.; Xu, Q.; Yang, G.; et al. The Application of Ethnomedicine in Modulating Megakaryocyte Differentiation and Platelet Counts. Int. J. Mol. Sci. 2023, 24, 3168. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, T.; Liu, S.; Mo, Q.; Jiang, N.; Chen, Q.; Yang, J.; Han, Y.W.; Chen, J.P.; Huang, F.H.; et al. Discovery of a Novel Megakaryopoiesis Enhancer, Ingenol, Promoting Thrombopoiesis through PI3K-Akt Signaling Independent of Thrombopoietin. Pharmacol. Res. 2022, 177, 106096. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.P.; Kauppi, M.; Metcalf, D.; Hyland, C.D.; Josefsson, E.C.; Lebois, M.; Zhang, J.G.; Baldwin, T.M.; Di Rago, L.; Hilton, D.J.; et al. Mpl Expression on Megakaryocytes and Platelets Is Dispensable for Thrombopoiesis but Essential to Prevent Myeloproliferation. Proc. Natl. Acad. Sci. USA 2014, 111, 5884–5889. [Google Scholar] [CrossRef] [PubMed]
- Flack, J.M.; Peters, R.; Shafi, T.; Alrefai, H.; Nasser, S.A.; Crook, E. Prevention of hypertension and its complications: Theoretical basis and guidelines for treatment. J. Am. Soc. Nephrol. 2003, 14, S92–S98. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Mohaupt, M. Kalzium und Blutdruck [Calcium and Blood Pressure]. Ther. Umsch. Rev. Ther. 2007, 64, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Villa-Etchegoyen, C.; Lombarte, M.; Matamoros, N.; Belizán, J.M.; Cormick, G. Mechanisms Involved in the Relationship between Low Calcium Intake and High Blood Pressure. Nutrients 2019, 11, 1112. [Google Scholar] [CrossRef]
- Oparil, S.; Schmieder, R.E. New Approaches in the Treatment of Hypertension. Circ. Res. 2015, 116, 1074–1095. [Google Scholar] [CrossRef]
- Yano, S.; Horiuchi, H.; Horie, S.; Aimi, N.; Sakai, S.I.; Watanabe, K. Ca2+ Channel Blocking Effects of Hirsutine, an Indole Alkaloid from Uncaria Genus, in the Isolated Rat Aorta. Planta Med. 1991, 57, 403–405. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Taylor, R. Insulin Resistance and Type 2 Diabetes. Diabetes 2012, 61, 778. [Google Scholar] [CrossRef]
- Shahid, M.S.; Ibrahim, M.; Rahman, M.M.; Islam, T.; Bhuia, M.S.; Zaman, S.; Islam, M.T. Phytochemical Group Test and Pharmacological Investigations of Persicaria barbata (L.) H. Hara. Phytopharmacol. Res. J. 2023, 2, 1–15. [Google Scholar]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef]
- Guengerich, F.P. Mechanisms of Drug Toxicity and Relevance to Pharmaceutical Development. Drug Metab. Pharmacokinet. 2011, 26, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, S. Toxicological Screening. J. Pharmacol. Pharmacother. 2011, 2, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Dorato, M.A.; Buckley, L.A. Toxicology Testing in Drug Discovery and Development. Curr. Protoc. Toxicol. 2007, 19, 19.1. [Google Scholar] [CrossRef]
- Matsuura, H.N.; Fett-Neto, A.G. Plant Alkaloids: Main Features, Toxicity, and Mechanisms of Action. Plant Toxins 2015, 2, 1–15. [Google Scholar]
- Kuete, V. Health Effects of Alkaloids from African Medicinal Plants. In Toxicological Survey of African Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 611–633. [Google Scholar]
- Szabo, I.; Zoratti, M.; Biasutto, L. Targeting Mitochondrial Ion Channels for Cancer Therapy. Redox Biol. 2021, 42, 101846. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness, and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
| Plants | Parts | References |
|---|---|---|
| Uncaria rhynchophylla (Miquel) | Bark | [25] |
| Dried hooks | [32] | |
| U. hirsuta U. lancifolia, U. scandens, U. macrophylla U. homomalla, U. laevigata, U. sessilifructus, U. yunnanensis U. lanosa, U. rhynchophylloides, | Stems and hooks | [44] |
| U. sinensis | Stems and hooks | [36] |
| Uncaria tomentosa | Leaves and roots | [45] |
| Mitragyna hirsuta | Leaves and root | https://pubchem.ncbi.nlm.nih.gov/taxonomy/371154, accessed on 30 April 2023 |
| Parameter (s) | Values/Status |
|---|---|
| Physicochemical properties | |
| Molecular mass | 368.5 g/mol |
| Number of heavy atoms | 27 |
| Number of aromatic heavy atoms | 9 |
| Number of rotatable bonds | 5 |
| Number H-bond acceptors | 4 |
| Number H-bond donors | 1 |
| Molar Refractivity | 110.39 |
| TPSA | 54.56 Å2 |
| Lipophilicity | |
| Log Po/w (MLOGP) | 2.35 |
| Water Solubility | |
| Solubility class | Moderately soluble |
| Pharmacokinetics | |
| GI absorption | High |
| BBB permeant | Yes |
| P-gp substrate | Yes |
| CYP1A2 inhibitor | No |
| CYP2C19 inhibitor | No |
| Drug-likeness | |
| Lipinski | Yes; 0 violation |
| Bioavailability Score | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuia, M.S.; Wilairatana, P.; Ferdous, J.; Chowdhury, R.; Bappi, M.H.; Rahman, M.A.; Mubarak, M.S.; Islam, M.T. Hirsutine, an Emerging Natural Product with Promising Therapeutic Benefits: A Systematic Review. Molecules 2023, 28, 6141. https://doi.org/10.3390/molecules28166141
Bhuia MS, Wilairatana P, Ferdous J, Chowdhury R, Bappi MH, Rahman MA, Mubarak MS, Islam MT. Hirsutine, an Emerging Natural Product with Promising Therapeutic Benefits: A Systematic Review. Molecules. 2023; 28(16):6141. https://doi.org/10.3390/molecules28166141
Chicago/Turabian StyleBhuia, Md. Shimul, Polrat Wilairatana, Jannatul Ferdous, Raihan Chowdhury, Mehedi Hasan Bappi, Md Anisur Rahman, Mohammad S. Mubarak, and Muhammad Torequl Islam. 2023. "Hirsutine, an Emerging Natural Product with Promising Therapeutic Benefits: A Systematic Review" Molecules 28, no. 16: 6141. https://doi.org/10.3390/molecules28166141
APA StyleBhuia, M. S., Wilairatana, P., Ferdous, J., Chowdhury, R., Bappi, M. H., Rahman, M. A., Mubarak, M. S., & Islam, M. T. (2023). Hirsutine, an Emerging Natural Product with Promising Therapeutic Benefits: A Systematic Review. Molecules, 28(16), 6141. https://doi.org/10.3390/molecules28166141












