Electrochemical Biosensor Based on Horseradish Peroxidase and Black Phosphorene Quantum Dot Modified Electrode
Abstract
:1. Introduction
2. Results and Discussion
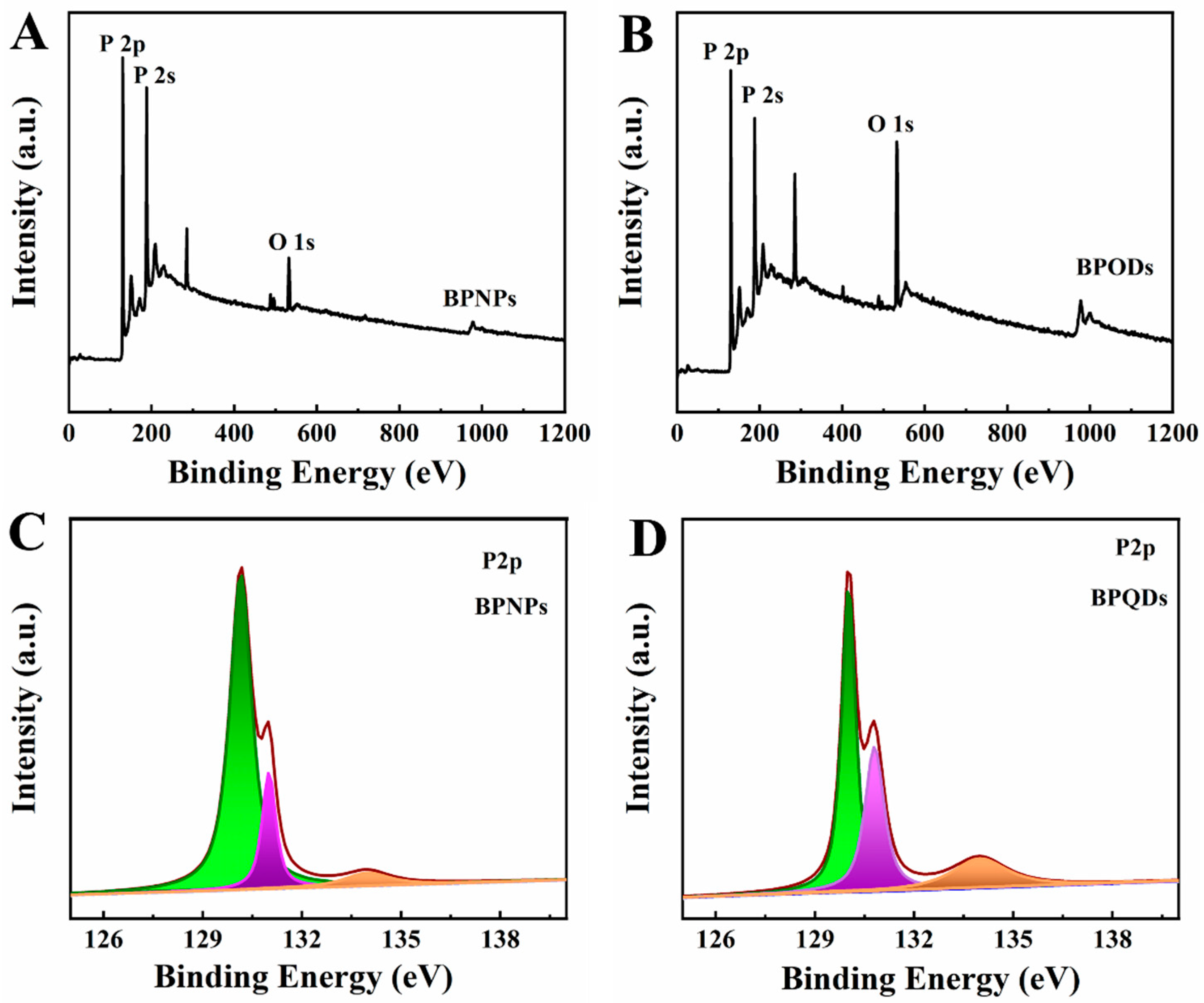
2.1. Characterizations
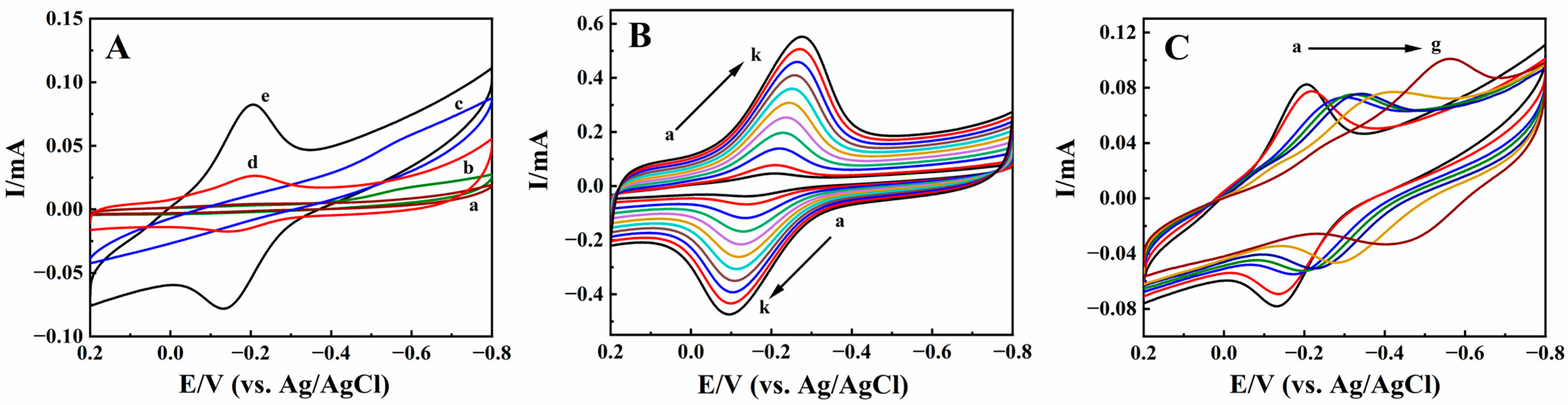
2.2. Direct Electrochemistry
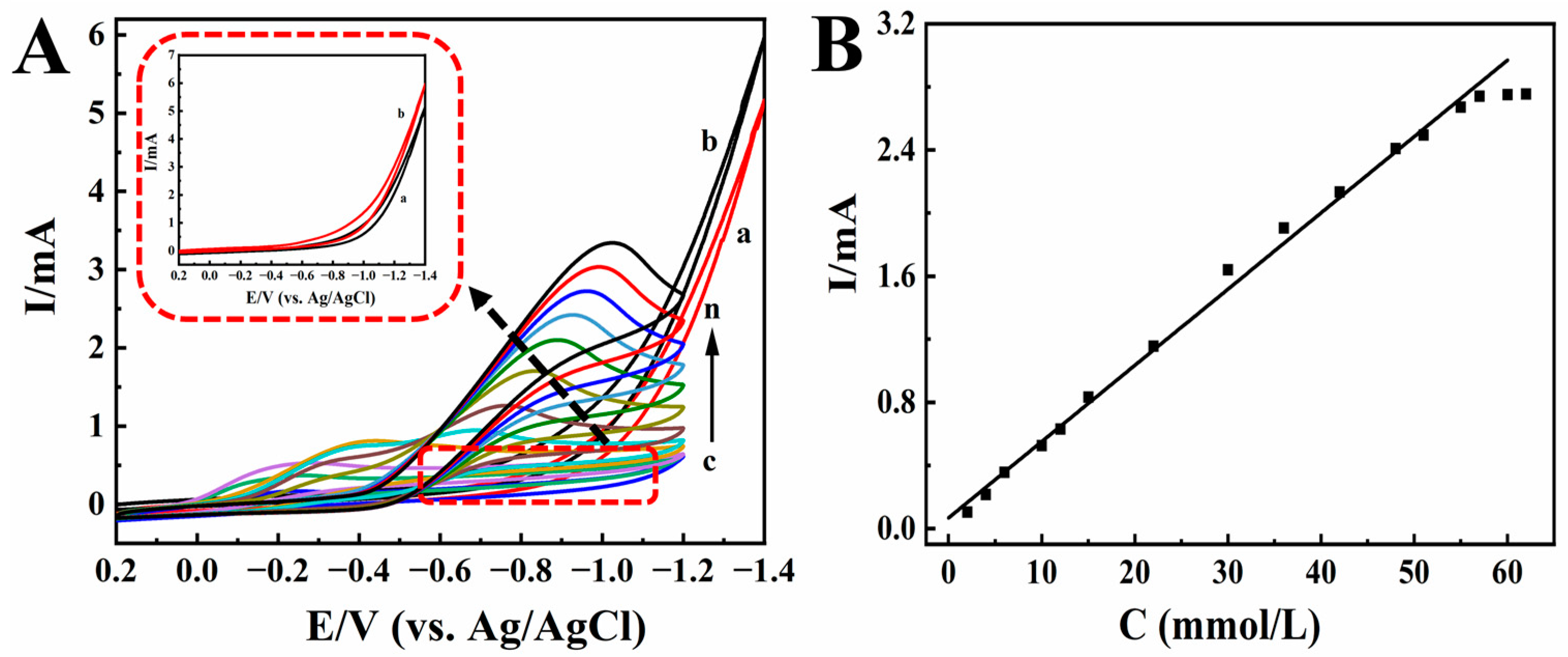
2.3. Electrocatalytic Performances of TCA and KBrO3
2.4. Analytical Applications
2.5. Interference Test
2.6. Stability and Repeatability of the Modified Electrode
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Apparatus
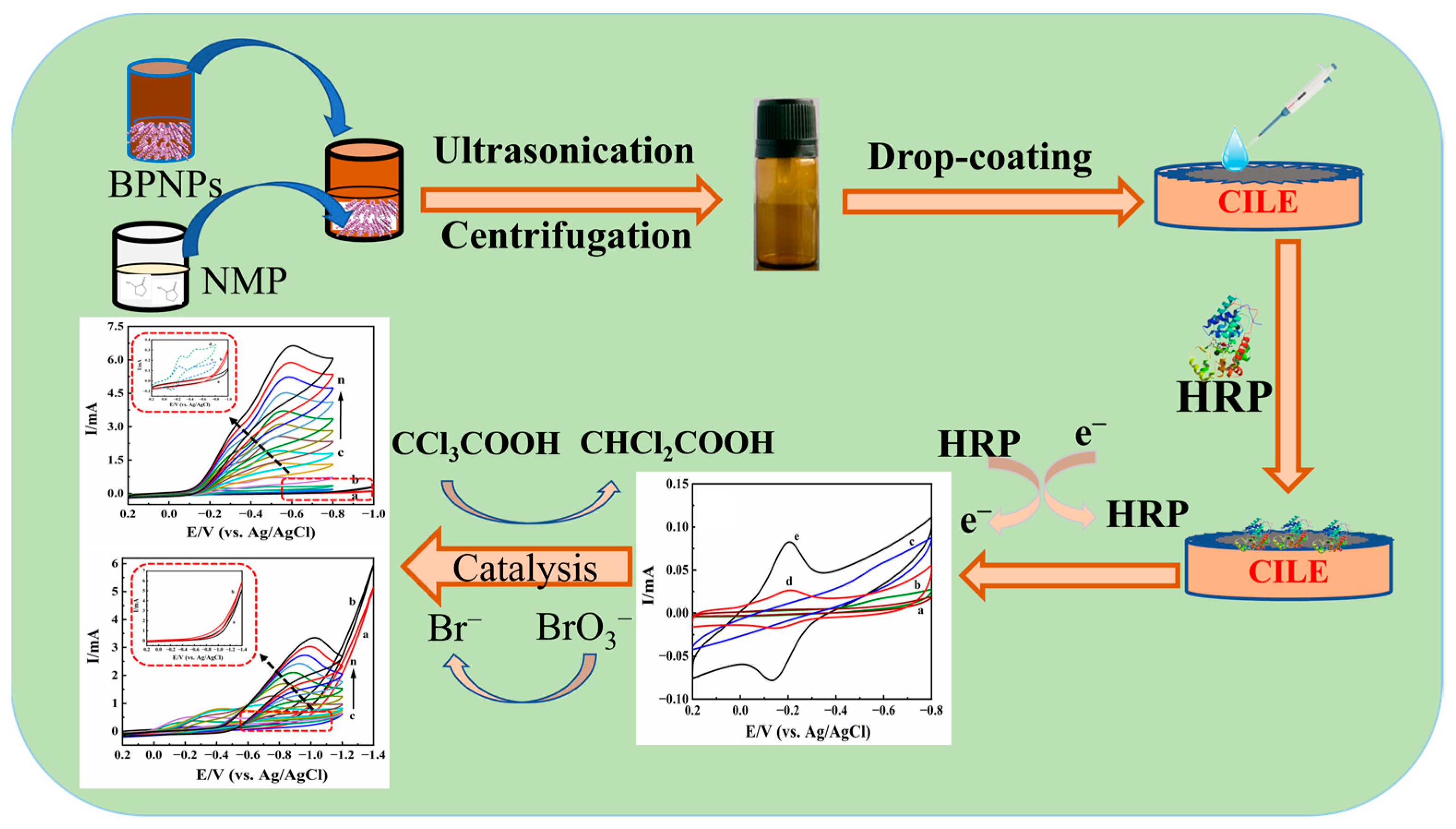
3.3. Preparation of BPQDs
3.4. Preparation of BPQDs Modified Electrode
3.5. Samples Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gusmao, R.; Sofer, Z.; Pumera, M.B. Black phosphorus rediscovered: From bulk material to monolayers. Angew Chem. Int. Ed. 2017, 56, 8052–8072. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.X.; Xia, Z.H.; Guo, S.J. Recent advances on black phosphorus for biomedicine and biosensing. Adv. Funct. Mater. 2019, 29, 1900318. [Google Scholar] [CrossRef]
- Pang, J.B.; Bachmatiuk, A.; Yin, Y. Applications of phosphorene and black phosphorus in energy conversion and storage devices. Adv. Energy Mater. 2018, 8, 1702093. [Google Scholar] [CrossRef]
- Luo, S.; Zhao, J.; Zou, J. Self-standing polypyrrole/black phosphorus laminated film: Promising electrode for flexible supercapacitor with enhanced capacitance and cycling stability. ACS Appl. Mater. Interfaces 2018, 10, 3538–3548. [Google Scholar] [CrossRef]
- Huang, Y.H.; Yan, L.J.; Wang, B.; Zhu, L.; Shao, B.; Niu, Y.Y.; Zhang, X.P.; Yin, P.; Ge, Y.Q.; Sun, W.; et al. Recent applications of black phosphorus and its related composites in electrochemistry and bioelectrochemistry: A mini review. Electrochem. Commun. 2021, 19, 107095. [Google Scholar] [CrossRef]
- Abate, Y.; Akinwande, D.; Gamage, S.; Wang, H.; Snure, M.; Poudel, N.; Cronin, S.B. Recent progress on stability and passivation of black phosphorus. Adv Mater. 2018, 30, 1704749. [Google Scholar] [CrossRef]
- Liu, H.; Neal, A.T.; Ye, P.D. Channel length scaling of MoS2 mosfets. ACS Nano 2012, 6, 8563–8569. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.Y.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Han, X.P.; Han, J.P.; Liu, C.; Sun, J. Promise and challenge of phosphorus in science, technology, and application. Adv. Funct. Mater. 2018, 28, 1803471. [Google Scholar] [CrossRef]
- Wu, S.X.; Hui, K.S.; Hui, K.N. 2D black phosporus: From preparation to applications for electrochemical energy stroage. Adv. Sci. 2018, 5, 1700491. [Google Scholar] [CrossRef]
- Brent, J.R.; Savjani, N.; Lewis, E.A.; Haigh, S.J.; Lewis, D.J.; O’Brien, P. Production of few-layer phosphorene by liquid exfoliation of black phosphorus. Chem. Commun. 2014, 50, 13338–13341. [Google Scholar] [CrossRef]
- Feng, Q.L.; Liu, H.Y.; Zhu, M.J.; Shang, J.; Liu, D.; Cui, X.Q.; Shen, D.Q.; Kou, L.Z.; Mao, D.; Zheng, J.B.; et al. Electrostatic funcitonalization and passivation of water exfoliated few-layer black phosphorus by poly dimethyldiallyl ammonium chloride and its ultrafast laser application. ACS Appl. Mater. Interfaces 2018, 10, 9679–9687. [Google Scholar] [CrossRef]
- Lei, W.Y.; Liu, G.; Zhang, J.; Liu, M.H. Black phosphorus nanostructures: Recent advances in hybridization, doping and functionalization. Chem. Soc. Rev. 2017, 46, 3492–3509. [Google Scholar] [CrossRef]
- Li, X.Y.; Niu, X.L.; Zhao, W.S.; Chen, W.; Yin, C.X.; Men, Y.L.; Li, G.J.; Sun, W. Black phosphorene and PEDOT: PSS-modified electrode for electrochemistry of hemoglobin. Electrochem. Commun. 2018, 86, 68–71. [Google Scholar] [CrossRef]
- Liu, H.; Neal, A.T.; Zhu, Z. Phosphorene: An unexplored 2D semiconductor with a high hole mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.P.; Xiao, G.S.; Zuo, X.X. Two-dimensional black phosphorus: An emerging anode material for lithium-ion batteries. Nano-micro Lett. 2020, 12, 120. [Google Scholar] [CrossRef]
- Lee, H.U.; Park, S.Y.; Lee, S.C.; Choi, S.; Seo, S. Black phosphorus (BP) nanodots for potential biomedical applications. Small 2016, 12, 214–219. [Google Scholar] [CrossRef]
- Du, J.; Zhang, M.; Guo, Z.; Chen, J.; Zhu, X.; Hu, G.; Peng, P.; Zheng, Z.; Zhang, H. Phosphorene quantum dot saturable absorbers for ultrafast fiber lasers. Sci. Rep. 2017, 7, 42357. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Liang, X.; Chen, Q.; Miao, Q.W.; Chen, X.L.; Zhang, X.D.; Mei, L. Surgical tumor-derived personalized photothermal vaccine formulation for cancer immunotherapy. ACS Nano 2019, 13, 2956–2968. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Phosphorene and black phosphorus for sensing and biosensing. Trends Anal. Chem. 2017, 93, 1–6. [Google Scholar] [CrossRef]
- Ding, H.C.; Zhang, L.; Tang, Z.R.; Dong, Y.P.; Chu, X.F. Black phosphorus quantum dots doped ZnO nanoparticles as efficient electrode materials for sensitive hydrogen peroxide detection. J. Electroanal. Chem. 2018, 824, 161–168. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, K.J.; Dong, Y.P. Electrogenerated chemiluminescence of Ru(bpy)32+ at a black phosphorus quantum dot modified electrode and its sensing application. Analyst 2018, 143, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Bull, R.J.; Sanchez, I.M.; Nelson, M.A.; Larson, J.L.; Lansing, A.J. Liver tumor induction in B6C3F1 mice by dichloroacetate and trichloroacetate. Toxicology 1990, 63, 341–359. [Google Scholar] [CrossRef]
- Dojlido, J.; Zbiec, E.; Swietlik, R. Formation of the haloacetic acids during ozonation and chlorination of water in warsaw waterworks, (Poland). Water Res. 1999, 33, 3111–3118. [Google Scholar] [CrossRef]
- Ferreira, A.M.C.; Laespada, M.E.F.; Pavon, J.L.P.; Cordero, B.M. In situ derivatization coupled to microextraction by packed sorbent and gas chromatography for the automated determination of haloacetic acids in chlorinated water. J. Chromatogr. A 2013, 1318, 35–42. [Google Scholar] [CrossRef]
- Prieto-Blanco, M.C.; Alpendurada, M.F.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D.; Machado, S.; Gonçalves, C. Improving methodological aspects of the analysis of five regulated haloacetic acids in water samples by solid-phase extraction, ion-pair liquid chromatography and electrospray tandem mass spectrometry. Talanta 2012, 94, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Avila, V.; Goor, T.V.D.; Gas, B.; Coufal, P. Separation of haloacetic acids in water by capillary zone electrophoresis with direct UV detection and contactless conductivity detection. J. Chromatogr. A 2003, 993, 143–152. [Google Scholar] [CrossRef]
- Kim, S.; Collins, L.B.; Boysen, G.; Swenberg, J.A.; Gold, A.; Ball, L.M.; Bradford, B.U.; Rusyn, I. Liquid chromatography electrospray ionization tandem mass spectrometry analysis method for simultaneous detection of trichloroacetic acid, dichloroacetic acid, S-(1,2-dichlorovinyl)glutathione and S-(1,2-dichlorovinyl)-L-cysteine. Toxicology 2009, 262, 230–238. [Google Scholar] [CrossRef]
- Hautman, D.P.; Munch, D.J.; Frebis, C.; Wagner, H.P.; Pepich, B.V. Review of the methods of the US environmental protection agency for bromate determination and validation of method 317.0 for disinfection by-product anions and low-level bromate. J. Chromatgr. A 2001, 920, 221–229. [Google Scholar] [CrossRef] [PubMed]
- GB 5749–85; National Standard of the People’s Republic of China–Standards for Drinking Water Quality. State Bureau of Quality and Technical Supervision: Beijing, China, 2022.
- Shiddiky, M.J.A.; Torriero, A.A.J. Application of ionic liquids in electrochemical sensing systems. Biosens. Bioelectron. 2011, 26, 1775–1787. [Google Scholar] [CrossRef]
- Opallo, M.; Lesniewski, A. A review on electrodes modified with ionic liquids. J. Electroanal. Chem. 2011, 656, 2–16. [Google Scholar] [CrossRef]
- Sun, W.; Gao, R.F.; Li, X.Q.; Wang, D.D.; Jiao, K. Fabrication and Electrochemical behavior of hemoglobin modified carbon ionic liquid electrode. Electroanalysis 2008, 20, 1048–1054. [Google Scholar] [CrossRef]
- Chen, X.H.; Guo, H.; Yi, J.; Hu, J.Q. Fabrication of AucoreCo3O4shell/PAA/HRP composite film for direct electrochemistry and hydrogen peroxide sensor applications. Sens. Mater. 2009, 21, 433–444. [Google Scholar]
- Zhang, X.; Xie, H.M.; Liu, Z.D.; Tan, C.L.; Luo, Z.M.; Li, H.; Lin, J.D.; Sun, L.Q.; Chen, W.; Xu, Z.C.; et al. Black phosphorus quantum dots. Angew. Chem. Int. Ed. 2015, 54, 3653–3657. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cen, Y.; Huang, J.Y.; Li, X.J.; Zhang, H.; Geng, Y.F.; Yakobson, B.I.; Du, Y.; Tian, X.Q. Zinc oxide-black phosphorus composites for ultrasensitive nitrogen dioxide sensing. Nanoscale Horiz. 2018, 3, 525–531. [Google Scholar] [CrossRef]
- Yi, J.Q.; Chen, X.P.; Weng, Q.H.; Zhou, Y.; Han, Z.Z.; Chen, J.H.; Li, C.Y. A simple electrochemical pH sensor based on black phosphorus nanosheets. Electrochem. Commun. 2020, 118, 106796. [Google Scholar] [CrossRef]
- Barman, S.C.; Sharifuzzaman, M.; Zahed, M.A.; Park, C.; Yoon, S.H.; Zhang, S.P.; Kim, H.; Yoon, H.; Park, J.Y. A highly selective and stable cationic polyelectrolyte encapsulated black phosphorene based impedimetric immunosensor for interleukin-6 biomarker detection. Biosens. Bioelectron. 2021, 186, 113287. [Google Scholar] [CrossRef]
- Hu, C.X.; Xiao, Q.; Zhao, M.; Dun, G.H.; Wu, H.R.; Li, X.Y.; Yang, Q.Q.; Sun, B.; Wang, Q.; Zhang, H.L. Polymer ionic liquid stabilized black phosphorus for environmental robust flexible optoelectronics. Adv. Funct. Mater. 2018, 28, 1805311. [Google Scholar] [CrossRef]
- Yang, J.; Pan, Z.H.; Zhong, J.; Li, S.; Wang, J.; Chen, P.Y. Electrostatic self-assembly of heterostructured black phosphorus-MXene nanocomposites for flexible microsuperacpacitors with high rate performance. Energy Storage Mater. 2021, 36, 257–264. [Google Scholar] [CrossRef]
- Li, K.X.; Qiao, X.J.; Zhao, H.Y.; He, Y.P.; Sheng, Q.L.; Yue, T.L. Ultrasensitive and label-free electrochemical aptasensor based on carbon dots-black phosphorus nanohybrid for the detection of Ochratoxins A. Microchem. J. 2021, 168, 106378. [Google Scholar] [CrossRef]
- Oldham, K.B. Analytical expressions for the reversible Randles-Sevick function. J. Electroanal. Chem. 1979, 105, 373–375. [Google Scholar] [CrossRef]
- Niu, X.L.; Weng, W.J.; Yin, C.X.; Niu, Y.Y.; Li, G.J.; Dong, R.X.; Men, Y.L.; Sun, W. Black phosphorene modified glassy carbon electrode for the sensitive voltammetric detecion of rutin. J. Electroanal. Chem. 2018, 811, 78–83. [Google Scholar] [CrossRef]
- Laviron, E. Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J. Electroanal. Chem. 1974, 52, 355–393. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Niu, Y.Y.; Liu, J.; Chen, W.; Yin, C.X.; Weng, W.J.; Li, X.Y.; Wang, X.L.; Li, G.J.; Sun, W. A direct electron transfer biosensor based on a horseradish peroxidase and gold nanotriangle modified electrode and electrocatalysis. Anal. Methods 2018, 10, 5297–5304. [Google Scholar] [CrossRef]
- Chen, W.; Weng, W.J.; Yin, C.X.; Niu, X.L.; Li, G.J.; Xie, H.; Liu, J.; Sun, W. Fabrication of an electrochemical biosnesor based on Nafion/horseradish peroxidase/Co3O4NP/CILE and its electrocatalysis. Int. J. Electrochem. Sci. 2018, 13, 4741–4752. [Google Scholar] [CrossRef]
- Lavanya, N.; Radhakrishnan, S.; Sekar, C. Fabrication of hydrogen peroxide biosensor based on Ni doped SnO2 nanoparticles. Biosens. Bioelectron. 2012, 36, 41–47. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, R.W.; Shang, L.B.; Zhu, Z.Q.; Li, G.X. Electron transfer reactivity and the catalytic activity of hemoglobin incorporated in dimethylaminoethyl methacrylate film. J. Braz. Chem. Soc. 2005, 16, 1195–1199. [Google Scholar] [CrossRef]
- Yan, L.J.; Hu, T.X.; Li, X.Q.; Ding, F.Z.; Wang, B.; Wang, B.L.; Zhang, B.X.; Shi, F.; Sun, W. Graphdiyne and ionic liquid composite modified gold electrode for sensitive voltammetric analysis of rutin. Electroanalysis 2022, 34, 286–293. [Google Scholar] [CrossRef]
- Niu, Y.Y.; Xie, H.; Luo, G.L.; Weng, W.J.; Ruan, C.X.; Li, G.J.; Sun, W. Electrochemical performance of myoglobin based on TiO2-doped carbon nanofiber decorated electrode and its applications in biosensing. RSC Adv. 2019, 9, 4480–4487. [Google Scholar] [CrossRef]
- Niu, Y.Y.; Zou, R.Y.; Yones, H.A.; Li, X.B.; Li, X.Y.; Niu, X.L.; Chen, Y.; Li, P.; Sun, W. Electrochemical behavior of horseradish peroxidase on WS2 nanosheet-modified electrode and electrocatalytic investigation. J. Chin. Chem. Soc. 2018, 65, 1127–1135. [Google Scholar] [CrossRef]
- Kamin, R.A.; Wilson, G.S. Rotating ring-disk enzyme electrode for biocatalysis kinetic studies and characterization of the immobilized enzyme layer. Anal. Chem. 1980, 52, 1198–1205. [Google Scholar] [CrossRef]
- Cheng, H.; Sun, Y.X.; Shao, B.; Deng, Y.; Zhang, X.P.; Li, G.J.; Sun, W. Application of gold nanoparticles and nanodimond modified electrode for hemoglobin electrochemistry. Int. J. Electrochem. Sci. 2020, 15, 11416–11426. [Google Scholar] [CrossRef]
- Dai, H.; Xu, H.F.; Wu, X.P.; Lin, Y.Y.; Wei, M.D.; Chen, G.N. Electrochemical behavior of thionine at titanate nanotubes-based modified electrode: A sensing platform for the detection of trichloroacetic acid. Talanta 2010, 81, 1461–1466. [Google Scholar] [CrossRef]
- Zhan, T.R.; Wang, X.J.; Li, X.J.; Song, Y.; Hou, W.G. Hemoglobin immobilized in exfoliated Co2Al LDH-graphene nanocomposite film: Direct electrochemistry and electrocatalysisi toward trichloroacetic acid. Sens. Actuators B 2016, 228, 101–108. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Y.P.; Guo, S.X.; Zhai, J.; Bond, A.M.; Zhang, J. Phosphomolybdate@poly (diallyldimethylammonium chloride)-reduced graphene oxide modified electrode for highly efficient electrocatalytic reduction of bromate. J. Electroanal. Chem. 2014, 727, 69–77. [Google Scholar] [CrossRef]
- He, P.L.; Hu, N.F.; Zhou, G. Assembly of electroactive layer-by-layer films of hemoglobin and polycationic poly(diallyldimethylammonium). Biomacromolecules 2002, 3, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, R.R.; Jian, F.F.; Wang, K.F. An electrochemical sensing platform based on a new Cd(II)-containing ionic liquid for the determination of trichloroacetic acid. Ionics 2010, 16, 661–666. [Google Scholar] [CrossRef]
- Li, X.Y.; Xie, H.; Luo, G.L.; Niu, Y.Y.; Li, X.B.; Xi, Y.R.; Xiong, Y.; Chen, Y.; Sun, W. Electrochemistry and electrocatalysis of hemoglobin based on graphene quantum dots modified electrode. Curr. Anal. Chem. 2018, 14, 308–315. [Google Scholar] [CrossRef]
- Zhou, D.D.; Ding, L.; Cui, H.; An, H.; Zhai, J.P.; Li, Q. Fabrication of high dispersion Pd/MWNTs nanocomposite and its electrocatalytic performance for bromate determination. Chem. Eng. J. 2012, 200–202, 32–38. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Z.N.; Lu, W.J.; Zhou, X.F.; Ma, H.Y. Fabrication of free-standing nanoporous silver by selectively dissolving gold from gold-silver alloys via a novel converse dealloying method. Electrochem. Commun. 2011, 13, 1086–1089. [Google Scholar] [CrossRef]
- Casella, I.G.; Contursi, M. Electrochemical and spectroscopic characterization of a tungsten electrode as a sensitive amperometric sensor of small inorganic ions. Electrochim. Acta 2005, 50, 4146–4154. [Google Scholar] [CrossRef]
- Najafi, M.; Darabi, S.; Tadjarodi, A.; Imani, M. Determination of trichloroacetic acid (TCAA) using CdO nanoparticles modified carbon paste electrode. Electroanalysis 2013, 25, 487–492. [Google Scholar] [CrossRef]
- Salimi, A.; MamKhezri, H.; Hallaj, R.; Zandi, S. Modification of glassy carbon electrode with multi-walled carbon nanotubes and iron (III)-porphyrin film: Application to chlorate, bromate and iodate detection. Electrochim. Acta 2007, 52, 6097–6105. [Google Scholar] [CrossRef]
- Li, X.Y.; Luo, G.L.; Xie, H.; Niu, Y.Y.; Li, X.B.; Zou, R.Y.; Xi, Y.Y.; Xiong, Y.; Sun, W. Voltammetric sensing performances of a carbon ionic liquid electrode modified with black phosphorene and hemin. Microchim. Acta 2019, 186, 304. [Google Scholar] [CrossRef] [PubMed]






| Modified Electrodes | TCA | Ref. | Modified Electrodes | KBrO3 | Ref. | ||
|---|---|---|---|---|---|---|---|
| Linear Range (mmol/L) | Detection Limit (mmol/L) | Linear Range (mmol/L) | Detection Limit (mmol/L) | ||||
| Nafion/Mb/TiO2@CNF/CILE | 5.0–105.0 | 1.6 | [52] | PdNPs/PANI/SBA-15/GCE | 0.008–40.0 | 0.005 | [31] |
| TH/TNTs/CS/GCE | 0.015–1.5 | / | [56] | Nafion/Hb/AuNPs/ND/CILE | 0.01–12.0 | 0.0033 | [55] |
| CTS/ELDH-GR-Hb/CILE | 5.0–135.0 | 1.506 | [57] | rGO-PDDA/PMo12/GCE | 0.02–10.0 | / | [58] |
| Hb/PDDA/PGE | 3.92–58.4 | 1.98 | [59] | Cd-IL/CPE | 5.0–20.0 | 3.0 | [60] |
| Nafion/Hb/GQD/CILE | 6.0–100.0 | 2.0 | [61] | MWCNT/Pd/GCE | 1.0–40.0 | / | [62] |
| np-Ag electrode | 2.5–22.5 | 0.25 | [63] | Tungsten oxide electrode | 0.3–45.0 | 0.1 | [64] |
| CdOMCPE | 0.23–0.003 | 0.0023 | [65] | Fe(III)P/MWCNTs/GCE | 0.5–3.5 | / | [66] |
| Nafion/HRP/BPQDs/CILE | 4.0–600.0 | 0.03 | This work | Nafion/HRP/BPQDs/CILE | 2.0–57.0 | 0.18 | This work |
| Samples | Added (mmol/L) | Total (mmol/L) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Medical facial peel solution | 10.00 | 20.90 | 97.0 | 3.21 |
| 20.00 | 33.01 | 109.1 | 3.67 | |
| 30.00 | 43.54 | 107.8 | 2.81 | |
| Flour supernatant | 2.00 | 1.98 | 99.0 | 2.13 |
| 4.00 | 4.12 | 103.0 | 3.36 | |
| 6.00 | 5.84 | 97.3 | 2.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Shi, F.; Wang, L.; Zhang, S.; Yan, L.; Zhang, X.; Sun, W. Electrochemical Biosensor Based on Horseradish Peroxidase and Black Phosphorene Quantum Dot Modified Electrode. Molecules 2023, 28, 6151. https://doi.org/10.3390/molecules28166151
Li X, Shi F, Wang L, Zhang S, Yan L, Zhang X, Sun W. Electrochemical Biosensor Based on Horseradish Peroxidase and Black Phosphorene Quantum Dot Modified Electrode. Molecules. 2023; 28(16):6151. https://doi.org/10.3390/molecules28166151
Chicago/Turabian StyleLi, Xiaoqing, Fan Shi, Lisi Wang, Siyue Zhang, Lijun Yan, Xiaoping Zhang, and Wei Sun. 2023. "Electrochemical Biosensor Based on Horseradish Peroxidase and Black Phosphorene Quantum Dot Modified Electrode" Molecules 28, no. 16: 6151. https://doi.org/10.3390/molecules28166151
APA StyleLi, X., Shi, F., Wang, L., Zhang, S., Yan, L., Zhang, X., & Sun, W. (2023). Electrochemical Biosensor Based on Horseradish Peroxidase and Black Phosphorene Quantum Dot Modified Electrode. Molecules, 28(16), 6151. https://doi.org/10.3390/molecules28166151






