Electron Beam Irradiation Alters the Physicochemical Properties of Chickpea Proteins and the Peptidomic Profile of Its Digest
Abstract
:1. Introduction
2. Results
2.1. The Effect of EBI on the Amino Acid Residues of CPC
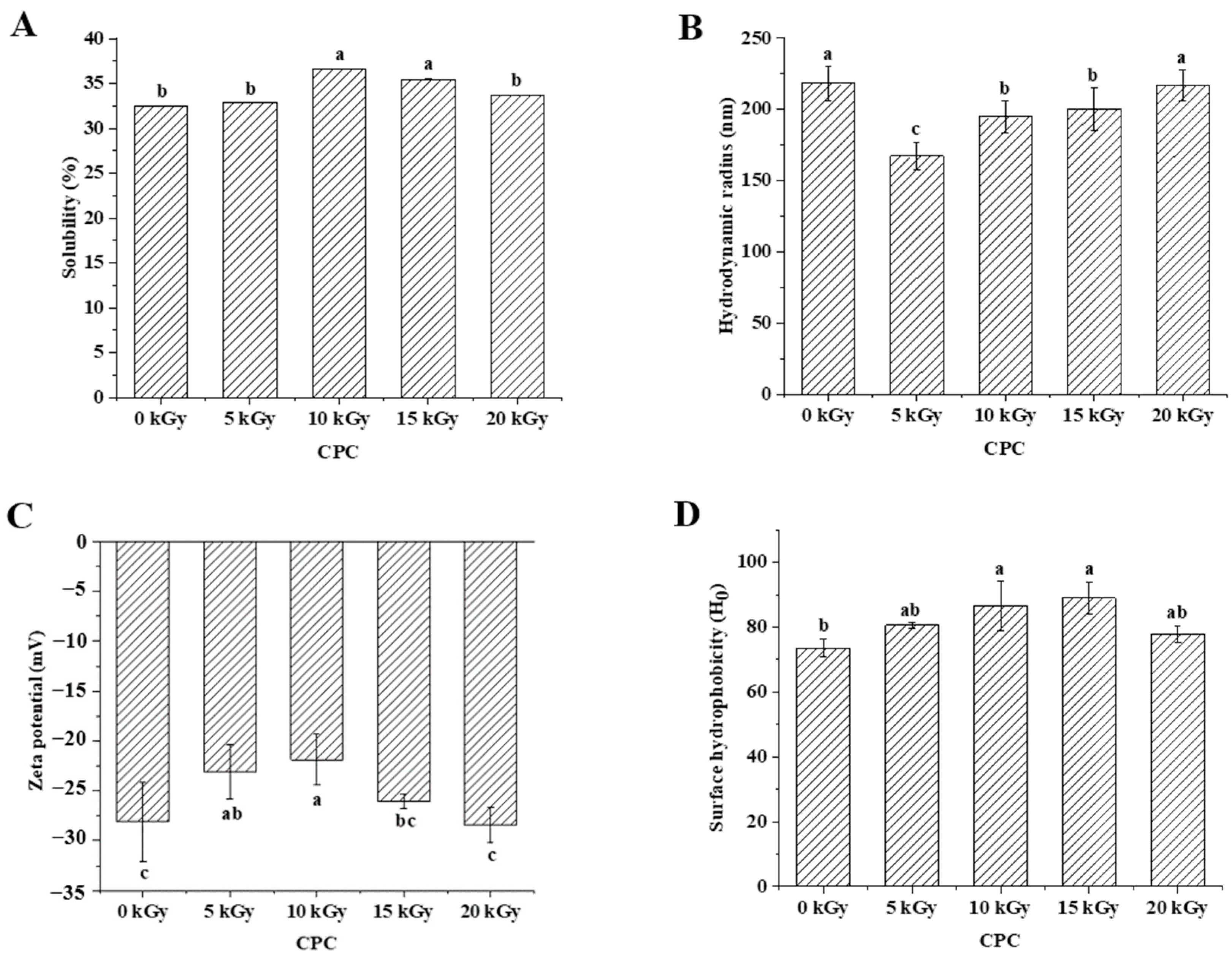
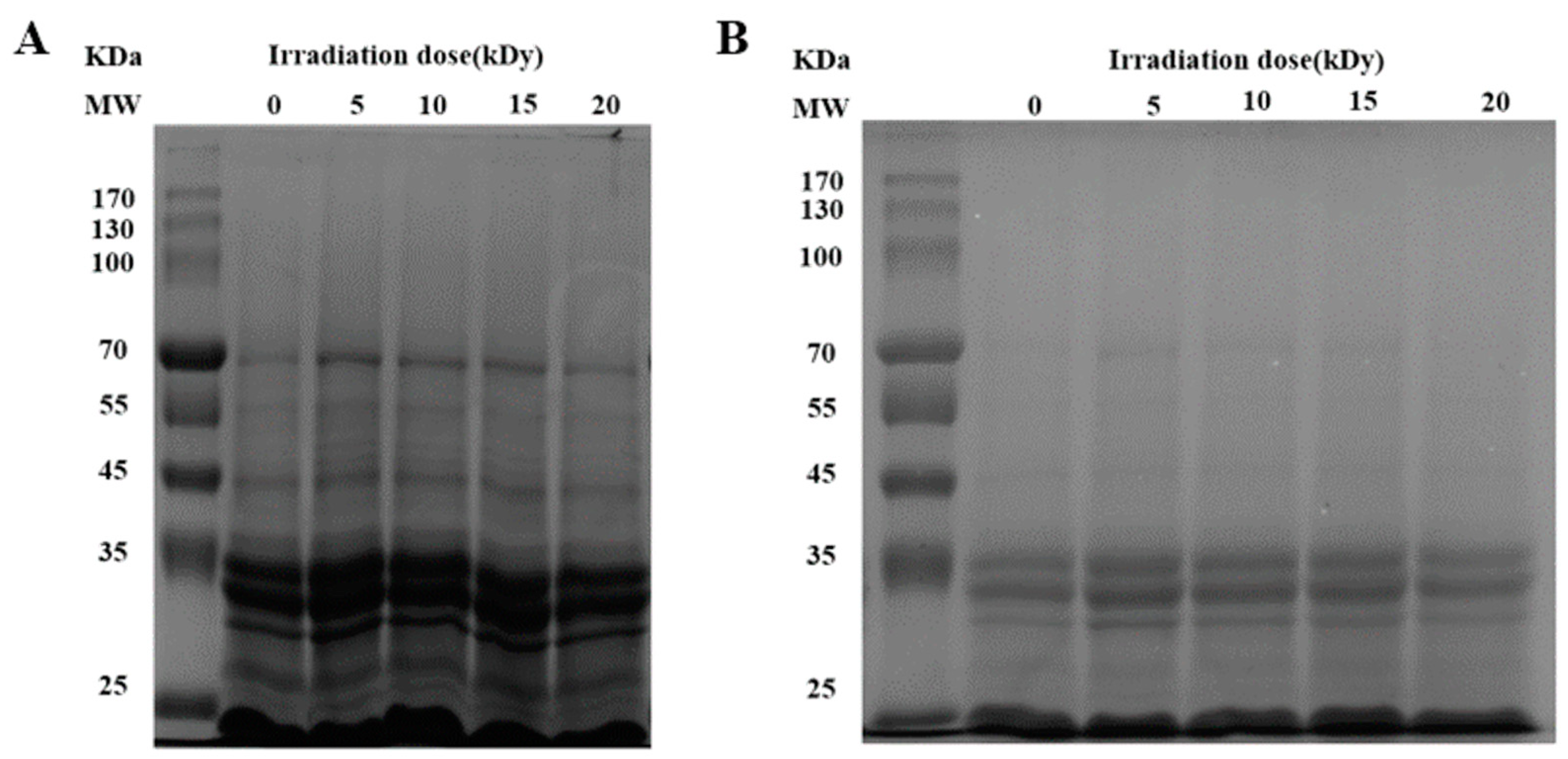
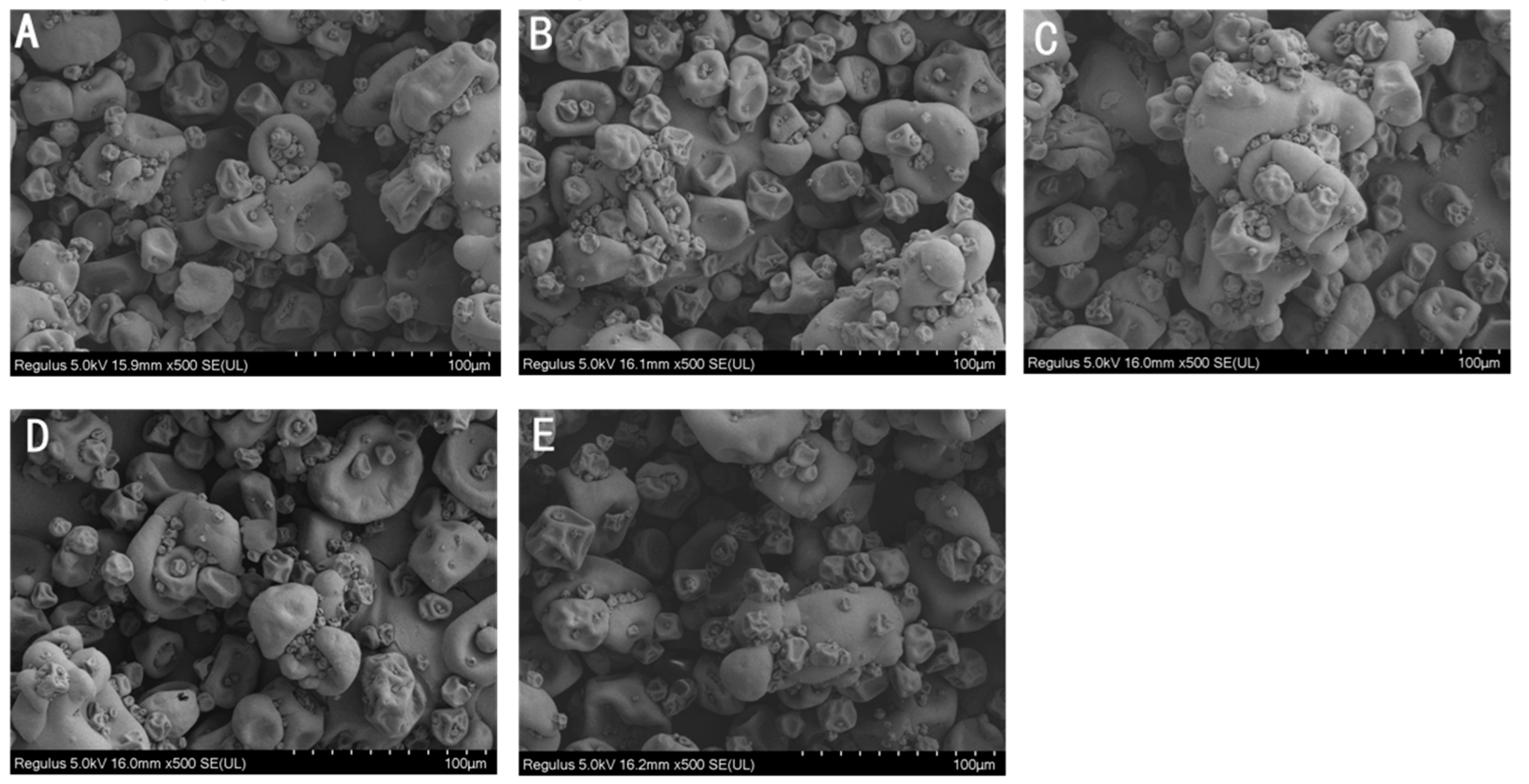
2.2. The Effect of EBI on the Physicochemical Properties of Proteins in CPC
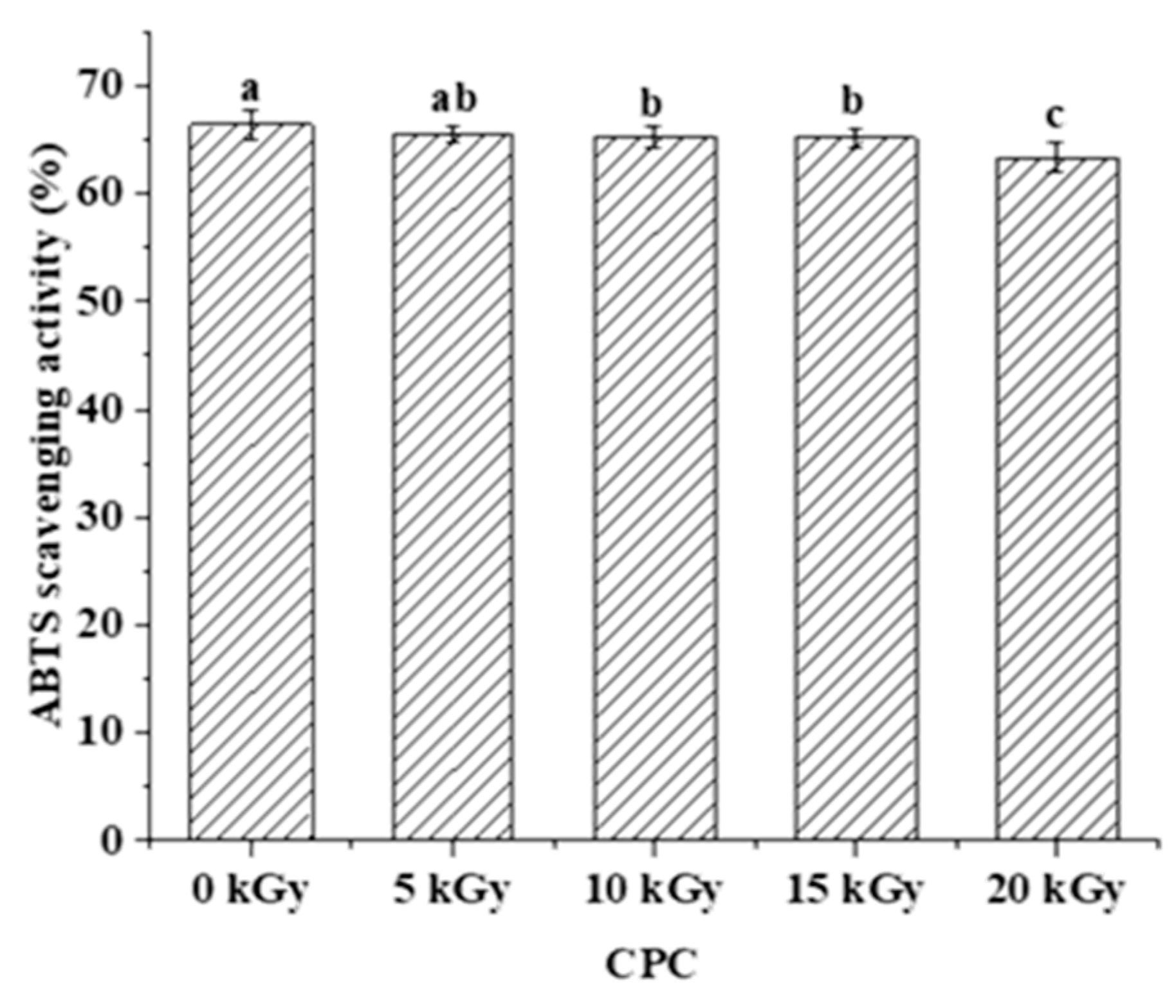
2.3. The Effect of EBI on the Peptidomics of CPC
3. Discussion
3.1. Oxidative Modification of Chickpea Protein
3.2. Physicochemical Properties of Irradiated Chickpea Protein
3.3. Characterization of the Peptides of In-Vitro Digested Chickpea protein
4. Materials and Methods
4.1. Materials and Electron Beam Irradiation
4.2. Amino Acid Content
4.3. Oxidative Modification of Amino Acids
4.3.1. Sulfhydryl Content
4.3.2. Carbonyl Content
4.4. Solubility of CPC
4.5. Particle Size and Zeta Potential of CPC
4.6. Surface Hydrophobicity
4.7. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4.8. SDS Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.9. Scanning Electron Microscopy (SEM)
4.10. Intrinsic Fluorescence Spectroscopy (IFS)
4.11. In Vitro Digestion of CPC
4.11.1. In Vitro Digestibility
4.11.2. Peptidomic Analysis
4.11.3. ABTS Radical Scavenging Capacity
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Felix, M.; Cermeno, M.; Romero, A.; FitzGerald, R.J. Characterisation of the bioactive properties and microstructure of chickpea protein-based oil in water emulsions. Food Res. Int. 2019, 121, 577–585. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108 (Suppl. S1), S11–S26. [Google Scholar] [CrossRef] [PubMed]
- Glusac, J.; Isaschar-Ovdat, S.; Fishman, A. Transglutaminase modifies the physical stability and digestibility of chickpea protein-stabilized oil-in-water emulsions. Food Chem. 2020, 315, 126301. [Google Scholar] [CrossRef]
- Bi, C.H.; Chi, S.Y.; Zhou, T.; Zhang, J.Y.; Wang, X.Y.; Li, J.; Shi, W.T.; Tian, B.; Huang, Z.G.; Liu, Y. Effect of low-frequency high-intensity ultrasound (HIU) on the physicochemical properties of chickpea protein. Food Res. Int. 2022, 159, 111474. [Google Scholar] [CrossRef]
- Perovic, M.N.; Pajin, B.S.; Antov, M.G. The effect of enzymatic pretreatment of chickpea on functional properties and antioxidant activity of alkaline protein isolate. Food Chem. 2022, 374, 131809. [Google Scholar] [CrossRef]
- Khrisanapant, P.; Kebede, B.; Leong, S.Y.; Oey, I. Effects of Hydrothermal Processing on Volatile and Fatty Acids Profile of Cowpeas (Vigna unguiculata), Chickpeas (Cicer arietinum) and Kidney Beans (Phaseolus vulgaris). Molecules 2022, 27, 8204. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, L.M.; Huerta-Ocampo, J.A.; Ruiz-Cruz, S.; Cinco-Moroyoqui, F.J.; Wong-Corral, F.J.; Rascon-Valenzuela, L.A.; Robles-Garcia, M.A.; Gonzalez-Vega, R.I.; Rosas-Burgos, E.C.; Corella-Madueno, M.A.G.; et al. Evaluation of Quality, Antioxidant Capacity, and Digestibility of Chickpea (Cicer arietinum L. cv Blanoro) Stored under N(2) and CO(2) Atmospheres. Molecules 2021, 26, 2773. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Guo, F.; Pei, M.; Tsao, R.; Wang, X.; Jiang, L.; Sun, Y.; Xiong, H. Anti-inflammatory effect of lentil hull (Lens culinaris) extract via MAPK/NF-κB signaling pathways and effects of digestive products on intestinal barrier and inflammation in Caco-2 and Raw264.7 co-culture. J. Funct. Foods 2022, 92, 105044. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Gonzalez de Mejia, E. Optimization, identification, and comparison of peptides from germinated chickpea (Cicer arietinum) protein hydrolysates using either papain or ficin and their relationship with markers of type 2 diabetes. Food Chem. 2022, 374, 131717. [Google Scholar] [CrossRef]
- Acevedo Martinez, K.A.; Gonzalezde Mejia, E. Comparison of five chickpea varieties, optimization of hydrolysates production and evaluation of biomarkers for type 2 diabetes. Food Res. Int. 2021, 147, 110572. [Google Scholar] [CrossRef]
- Farkas, J.; Mohácsi-Farkas, C. History and future of food irradiation. Trends Food Sci. Technol. 2011, 22, 121–126. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Sun, D.; Li, Y.; Chen, Z. Effect of enzymolysis-assisted electron beam irradiation on structural characteristics and antioxidant activity of rice protein. J. Cereal Sci. 2019, 89, 102789. [Google Scholar] [CrossRef]
- Wang, L.; Ding, Y.; Zhang, X.; Li, Y.; Wang, R.; Luo, X.; Li, Y.; Li, J.; Chen, Z. Effect of electron beam on the functional properties and structure of defatted wheat germ proteins. J. Food Eng. 2017, 202, 9–17. [Google Scholar] [CrossRef]
- Steele, J.H. Food irradiation: A public health challenge for the 21st century. Clin. Infect. Dis. 2001, 33, 376–377. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.; He, J.; Wu, Z.; Sun, Y.; Pan, D.; Tian, H.; Xia, Q. Combining e-beam irradiation and modified atmosphere packaging as a preservation strategy to improve physicochemical and microbiological properties of sauced duck product. Food Control 2022, 136, 108889. [Google Scholar] [CrossRef]
- Wei, Q.; Mei, J.; Xie, J. Application of electron beam irradiation as a non-thermal technology in seafood preservation. LWT 2022, 169, 113994. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, X.; Liang, Y.; He, M.; Geng, M.; Huang, Y.; Teng, F.; Li, Y. Effects of electron beam irradiation pretreatment on the structural and functional properties of okara protein. Innov. Food Sci. Emerg. Technol. 2022, 79, 103049. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Zhang, W.; Han, S.; Zhang, T.; Liu, B. Electron beam irradiation-induced structural changes increase the antioxidant activities of egg white protein. LWT 2019, 111, 846–852. [Google Scholar] [CrossRef]
- Joshisaha, A.; Reddy, K.S.; Petwal, V.C.; Dwivedi, J. Identification of novel mutants through Electron beam and Gamma irradiation in chickpea (Cicer arietinum L.). J. Food Legumes 2015, 28, 1–6. [Google Scholar]
- Zhang, X.; Wang, L.; Lu, H.; Zong, Z.; Chen, Z.; Li, Y.; Luo, X.; Li, Y. Preservation of hydrogen peroxide-induced oxidative damage in HepG-2 cells by rice protein hydrolysates pretreated with electron beams. Sci. Rep. 2020, 10, 8415. [Google Scholar] [CrossRef]
- Miron, L.; Montevecchi, G.; Bruggeman, G.; Macavei, L.I.; Maistrello, L.; Antonelli, A.; Thomas, M. Functional properties and essential amino acid composition of proteins extracted from black soldier fly larvae reared on canteen leftovers. Innov. Food Sci. Emerg. Technol. 2023, 87, 103407. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; He, J.; Xu, Y.; Guo, X. Effects of high-pressure homogenization on the physicochemical, foaming, and emulsifying properties of chickpea protein. Food Res. Int. 2023, 170, 112986. [Google Scholar] [CrossRef] [PubMed]
- Soglia, F.; Petracci, M.; Ertbjerg, P. Novel DNPH-based method for determination of protein carbonylation in muscle and meat. Food Chem. 2016, 197 Pt A, 670–675. [Google Scholar] [CrossRef]
- Jun, S.; Yaoyao, M.; Hui, J.; Obadi, M.; Zhongwei, C.; Bin, X. Effects of single- and dual-frequency ultrasound on the functionality of egg white protein. J. Food Eng. 2020, 277, 109902. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Z.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Effects of soluble aggregates sizes on rheological properties of soybean protein isolate under high temperature. LWT 2023, 182, 114793. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, N.; Li, Y.; Cheng, S.; Jiang, C.; Lin, S. Effects of electron beam irradiation (EBI) on structure characteristics and thermal properties of walnut protein flour. Food Res. Int. 2017, 100 Pt 1, 850–857. [Google Scholar] [CrossRef]
- Huang, X.; Yan, C.; Lin, M.; He, C.; Xu, Y.; Huang, Y.; Zhou, Z. The effects of conjugation of walnut protein isolate with polyphenols on protein solubility, antioxidant activity, and emulsifying properties. Food Res. Int. 2022, 161, 111910. [Google Scholar] [CrossRef]
- Boachie, R.T.; Okagu, O.D.; Abioye, R.; Huttmann, N.; Oliviero, T.; Capuano, E.; Fogliano, V.; Udenigwe, C.C. Lentil Protein and Tannic Acid Interaction Limits in Vitro Peptic Hydrolysis and Alters Peptidomic Profiles of the Proteins. J. Agric. Food Chem. 2022, 70, 6519–6529. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Safavi, M.; Hadizadeh, M. In vitro and in silico studies of novel synthetic ACE-inhibitory peptides derived from Saccharomyces cerevisiae protein hydrolysate. Bioorg. Chem. 2019, 87, 647–654. [Google Scholar] [CrossRef]
- Pillai, S.D.; Shayanfar, S. Electron Beam Technology and Other Irradiation Technology Applications in the Food Industry. Top Curr Chem 2017, 375, 6. [Google Scholar] [CrossRef]
- Feng, X.; Jo, C.; Nam, K.C.; Ahn, D.U. Impact of electron-beam irradiation on the quality characteristics of raw ground beef. Innov. Food Sci. Emerg. Technol. 2019, 54, 87–92. [Google Scholar] [CrossRef]
- Lv, M.; Mei, K.; Zhang, H.; Xu, D.; Yang, W. Effects of electron beam irradiation on the biochemical properties and structure of myofibrillar protein from Tegillarca granosa meat. Food Chem. 2018, 254, 64–69. [Google Scholar] [CrossRef]
- Kumari, S.; Gupta, O.P.; Mishra, C.B.; Thimmegowda, V.; Krishnan, V.; Singh, B.; Sachdev, A.; Dahuja, A. Gamma irradiation, an effective strategy to control the oxidative damage of soy proteins during storage and processing. Radiat. Phys. Chem. 2020, 177, 109134. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Morgan, P.E.; Davies, M.J. Quantification of protein modification by oxidants. Free. Radic. Biol. Med. 2009, 46, 965–988. [Google Scholar] [CrossRef]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estevez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mubango, E.; Dou, P.; Bao, Y.; Tan, Y.; Luo, Y.; Li, X.; Hong, H. Insight into the protein oxidation impact on the surface properties of myofibrillar proteins from bighead carp. Food Chem. 2023, 411, 135515. [Google Scholar] [CrossRef]
- Kato, A.; Nakai, S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Bba-Biomembranes 1980, 624, 13–20. [Google Scholar] [CrossRef]
- Dogbevi, M.K.; Vachon, C.; Lacroix, M. Effect of gamma irradiation on the microbiological quality and on the functional properties of proteins in dry red kidney beans (Phaseolus vulgaris). Radiat. Phys. Chem. 2000, 57, 265–268. [Google Scholar] [CrossRef]
- Duque-Estrada, P.; Berton-Carabin, C.C.; Nieuwkoop, M.; Dekkers, B.L.; Janssen, A.E.M.; van der Goot, A.J. Protein Oxidation and In Vitro Gastric Digestion of Processed Soy-Based Matrices. J. Agric. Food Chem. 2019, 67, 9591–9600. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Guo, A. Animal and Plant Protein Oxidation: Chemical and Functional Property Significance. Foods 2020, 10, 40. [Google Scholar] [CrossRef]
- Sante-Lhoutellier, V.; Aubry, L.; Gatellier, P. Effect of oxidation on in vitro digestibility of skeletal muscle myofibrillar proteins. J. Agric. Food Chem. 2007, 55, 5343–5348. [Google Scholar] [CrossRef]
- Arcan, I.; Yemenicioğlu, A. Antioxidant activity of protein extracts from heat-treated or thermally processed chickpeas and white beans. Food Chem 2007, 103, 301–312. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, B.; Li, B.; Wang, C.; Luo, Y. Preparation and identification of peptides and their zinc complexes with antimicrobial activities from silver carp (Hypophthalmichthys molitrix) protein hydrolysates. Food Res. Int. 2014, 64, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Sun, J.; Yao, K.; Xu, M.; Zhou, X. Nondestructive detection and visualization of protein oxidation degree of frozen-thawed pork using fluorescence hyperspectral imaging. Meat Sci. 2022, 194, 108975. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Shi, T.; Xiong, Z.; Yuan, L.; Hong, H.; Gao, R.; Bao, Y. Oxidation affects dye binding of myofibrillar proteins via alteration in net charges mediated by a reduction in isoelectric point. Food Res. Int. 2023, 163, 112204. [Google Scholar] [CrossRef]
- Ji, H.; Dong, S.; Han, F.; Li, Y.; Chen, G.; Li, L.; Chen, Y. Effects of Dielectric Barrier Discharge (DBD) Cold Plasma Treatment on Physicochemical and Functional Properties of Peanut Protein. Food Bioprocess Technol. 2017, 11, 344–354. [Google Scholar] [CrossRef]
- Gulseren, I.; Guzey, D.; Bruce, B.D.; Weiss, J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason. Sonochem. 2007, 14, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, F.; Wang, R.; Wang, L.; Zhang, H.; Chen, Z. Solubilization by freeze-milling of water-insoluble subunits in rice proteins. Food Funct. 2015, 6, 423–430. [Google Scholar] [CrossRef]
- Wang, H.; Ke, B.; Wang, W.; Guo, J.; Ying, W.; Ma, S.; Jiang, H. A novel method for the component identification of human blood products: Mass spectrometric analysis of human fibrinogen digested after SDS-PAGE in-gel digestion. J. Chromatogr. B 2023, 1226, 123718. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Chen, Z.; Li, Y.; Luo, X.; Li, Y. Effect of electron beam irradiation on the structural characteristics and functional properties of rice proteins. RSC Adv. 2019, 9, 13550–13560. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, M.; Liu, H.; Li, Q.; Xue, D.; Nian, Y.; Zhao, D.; Shan, K.; Dai, C.; Li, C. Ultrasound treatment can increase digestibility of myofibrillar protein of pork with modified atmosphere packaging. Food Chem. 2022, 377, 131811. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhou, G.; Li, L.; Xu, X.; Yu, X.; Bai, Y.; Li, C. Effect of cooking on in vitro digestion of pork proteins: A peptidomic perspective. J. Agric. Food Chem. 2015, 63, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Osorio, D.; Rondón-Villarreal, P.; Torres, R. Peptides: A Package for Data Mining of Antimicrobial Peptides. R J. 2015, 7, 4–14. [Google Scholar] [CrossRef]
- Xing, Z.; Li, J.; Zhang, Y.; Gao, A.; Xie, H.; Gao, Z.; Chu, X.; Cai, Y.; Gu, C. Peptidomics comparison of plant-based meat alternatives and processed meat after in vitro digestion. Food Res. Int. 2022, 158, 111462. [Google Scholar] [CrossRef]


| Amino Acid Content g/100 g | 0 kGy | 5 kGy | 10 kGy | 15 kGy | 20 kGy |
|---|---|---|---|---|---|
| asp | 8.51 ± 0.09 a | 8.54 ± 0.07 a | 8.55 ± 0.12 a | 8.41 ± 0.04 a | 8.52 ± 0.13 a |
| glu | 13.37 ± 0.17 a | 13.46 ± 0.12 a | 13.50 ± 0.21 a | 13.27 ± 0.09 a | 13.44 ± 0.21 a |
| ser | 2.98 ± 0.08 b | 3.07 ± 0.07 ab | 3.15 ± 0.10 a | 3.12 ± 0.03 a | 3.16 ± 0.01 a |
| his | 1.69 ± 0.05 a | 1.73 ± 0.07 a | 1.68 ± 0.15 a | 1.68 ± 0.03 a | 1.65 ± 0.09 a |
| gly | 2.66 ± 0.03 a | 2.68 ± 0.03 a | 2.67 ± 0.03 a | 2.62 ± 0.04 a | 2.65 ± 0.04 a |
| thr | 2.1 ± 0.02 a | 2.12 ± 0.01 a | 2.13 ± 0.05 a | 2.1 ± 0.06 a | 2.15 ± 0.04 a |
| arg | 6.08 ± 0.09 b | 6.16 ± 0.04 ab | 6.21 ± 0.08 a | 6.11 ± 0.03 ab | 6.19 ± 0.05 ab |
| ala | 2.89 ± 0.02 a | 2.90 ± 0.03 a | 2.91 ± 0.04 a | 2.88 ± 0.02 a | 2.92 ± 0.04 a |
| tyr | 1.67 ± 0.06 b | 1.78 ± 0.03 a | 1.72 ± 0.03 ab | 1.71 ± 0.05 ab | 1.71 ± 0.03 ab |
| cys-s | 0.26 ± 0.01 a | 0.26 ± 0.03 a | 0.30 ± 0.03 a | 0.29 ± 0.03 a | 0.29 ± 0.01 a |
| val | 3.65 ± 0.04 a | 3.64 ± 0.04 a | 3.66 ± 0.05 a | 3.58 ± 0.05 a | 3.63 ± 0.05 a |
| met | 0.97 ± 0.05 a | 0.97 ± 0.09 a | 1.00 ± 0.07 a | 0.98 ± 0.05 a | 0.97 ± 0.03 a |
| phe | 4.31 ± 0.06 b | 4.35 ± 0.03 ab | 4.36 ± 0.06 ab | 4.29 ± 0.02 b | 4.41 ± 0.07 a |
| ile | 3.45 ± 0.03 ab | 3.47 ± 0.00 a | 3.46 ± 0.03 a | 3.39 ± 0.04 b | 3.44 ± 0.04 ab |
| leu | 5.48 ± 0.07 a | 5.53 ± 0.04 a | 5.55 ± 0.07 a | 5.47 ± 0.03 a | 5.54 ± 0.06 a |
| lys | 4.33 ± 0.03 ab | 4.39 ± 0.07 ab | 4.39 ± 0.02 ab | 4.32 ± 0.01 b | 4.41 ± 0.07 a |
| pro | 2.93 ± 0.38 a | 2.78 ± 0.13 a | 3.17 ± 0.31 a | 3.01 ± 0.25 a | 2.94 ± 0.11 a |
| β-Sheet | Random Coils | α-Helix | β-Turn | |
|---|---|---|---|---|
| 0 kGy | 51.69 ± 0.93 b | 2.95 ± 0.30 b | 21.69 ± 0.16 a | 23.69 ± 1.07 a |
| 5 kGy | 56.96 ± 2.81 a | 2.76 ± 0.53 b | 21.20 ± 1.47 ab | 19.08 ± 0.82 b |
| 10 kGy | 57.10 ± 0.79 a | 4.29 ± 0.50 a | 20.53 ± 0.12 ab | 18.08 ± 1.17 b |
| 15 kGy | 57.15 ± 1.25 a | 2.07 ± 0.19 b | 18.41 ± 0.53 c | 22.38 ± 0.53 a |
| 20 kGy | 54.32 ± 0.60 ab | 2.11 ± 0.89 b | 19.60 ± 0.22 bc | 23.98 ± 0.07 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Kong, Y.; Xu, W.; Yang, Z.; Bao, Y. Electron Beam Irradiation Alters the Physicochemical Properties of Chickpea Proteins and the Peptidomic Profile of Its Digest. Molecules 2023, 28, 6161. https://doi.org/10.3390/molecules28166161
Zhang Y, Kong Y, Xu W, Yang Z, Bao Y. Electron Beam Irradiation Alters the Physicochemical Properties of Chickpea Proteins and the Peptidomic Profile of Its Digest. Molecules. 2023; 28(16):6161. https://doi.org/10.3390/molecules28166161
Chicago/Turabian StyleZhang, Yaqi, Yunfei Kong, Wanjun Xu, Zhen Yang, and Yulong Bao. 2023. "Electron Beam Irradiation Alters the Physicochemical Properties of Chickpea Proteins and the Peptidomic Profile of Its Digest" Molecules 28, no. 16: 6161. https://doi.org/10.3390/molecules28166161
APA StyleZhang, Y., Kong, Y., Xu, W., Yang, Z., & Bao, Y. (2023). Electron Beam Irradiation Alters the Physicochemical Properties of Chickpea Proteins and the Peptidomic Profile of Its Digest. Molecules, 28(16), 6161. https://doi.org/10.3390/molecules28166161







