Identification of Efficacy-Associated Markers to Discriminate Flos Chrysanthemum and Flos Chrysanthemi Indici Based on Fingerprint–Activity Relationship Modeling: A Combined Evaluation over Chemical Consistence and Quality Consistence
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chromatographic Fingerprints and the Analysis of Similarity
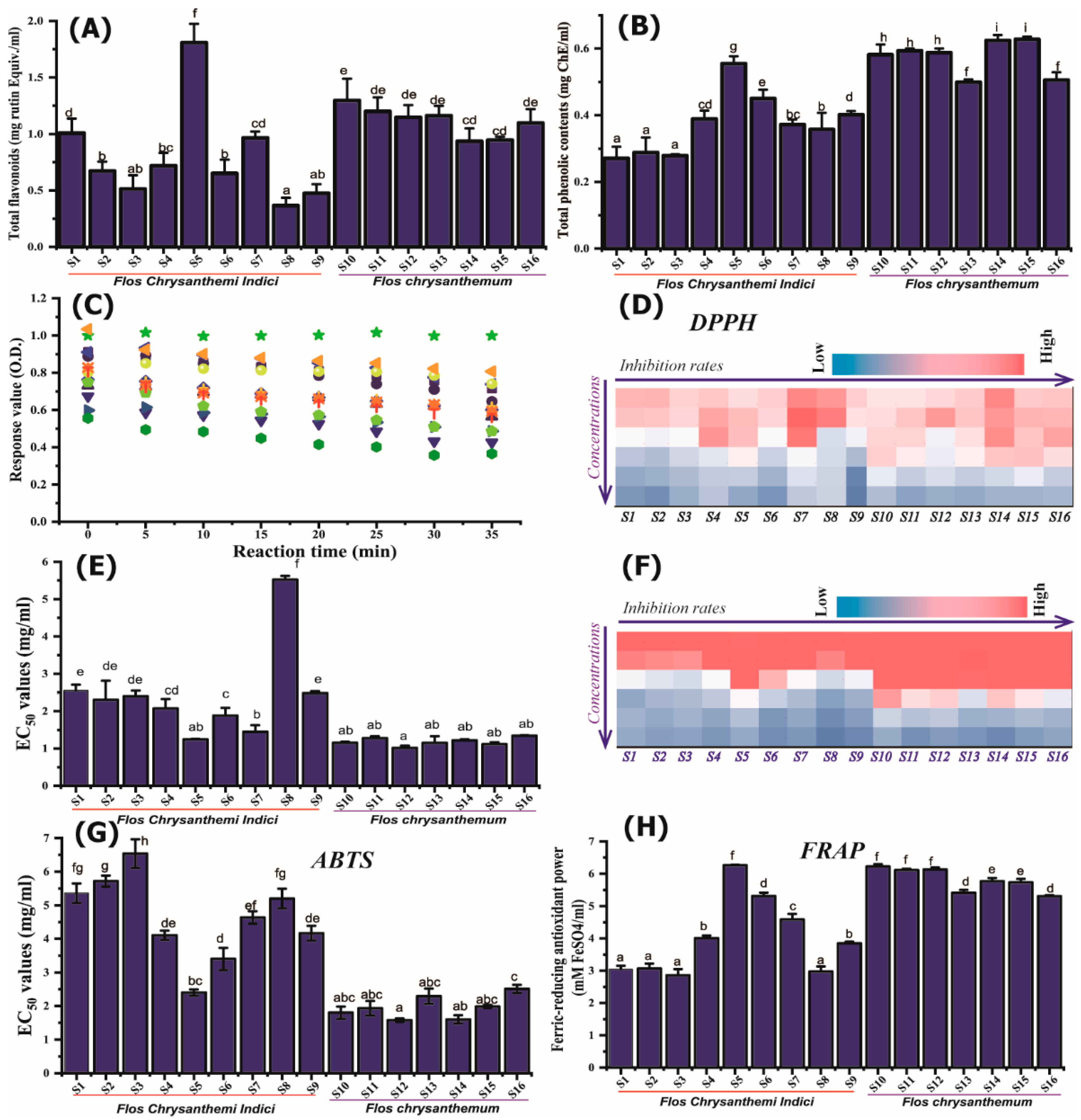
2.2. In Vitro Antioxidant Activity Characterization Based on Chemical Methodologies
2.3. Cellular-Based Antioxidant Activity Characterization
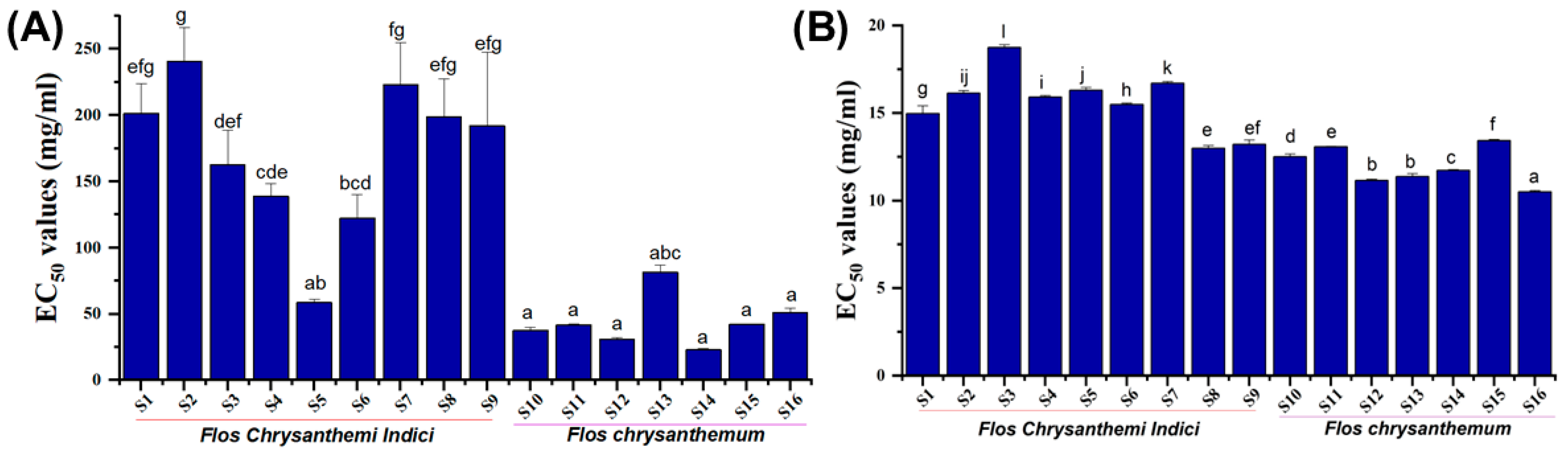
2.4. Glucosidase Inhibitory Activity and Lipase-Inhibitory Activity
2.5. Identification of Potential Bioactive Markers by PLSR and ANN
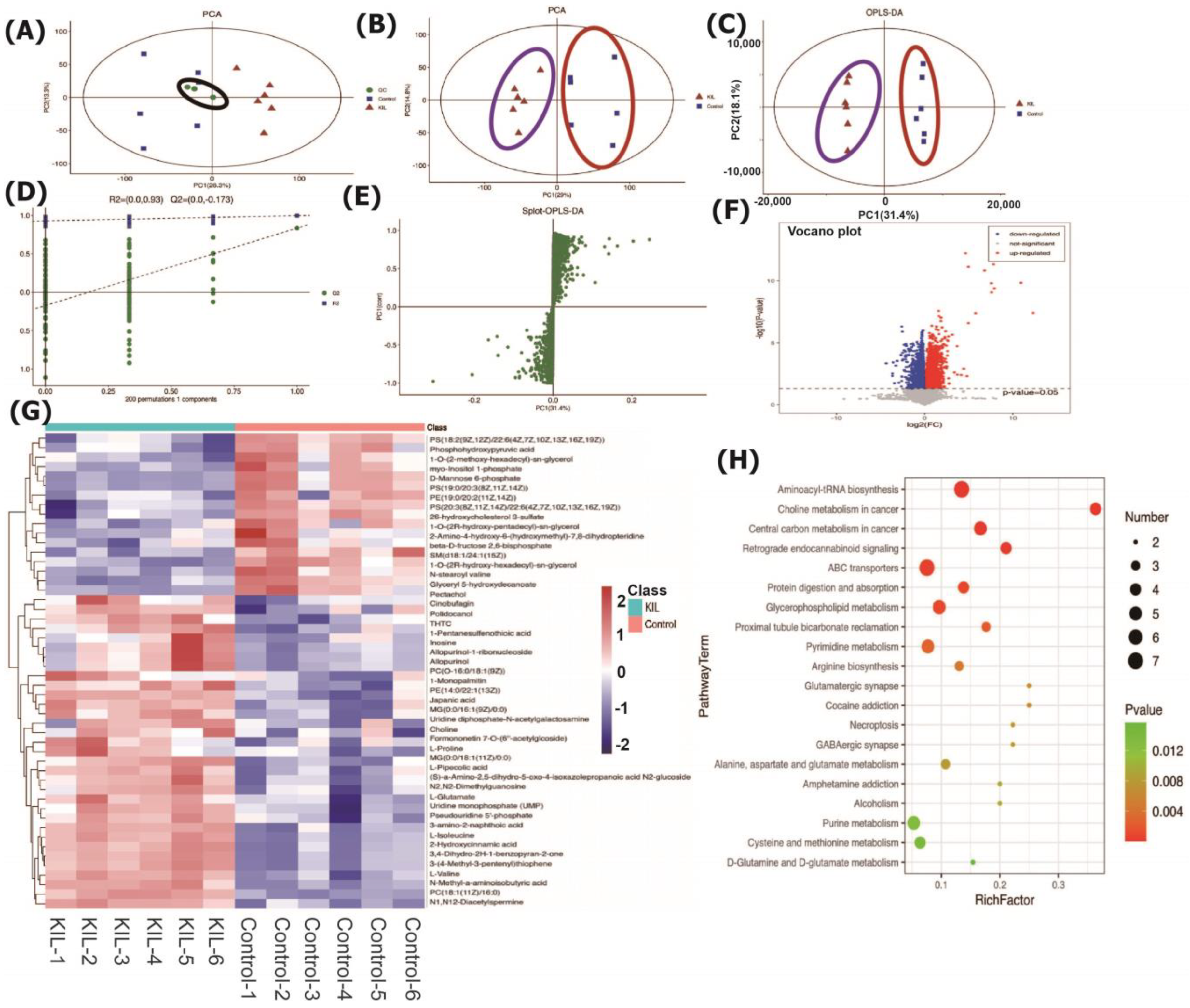
2.6. Effects of the Identified Bioactive Markers on the Cell Metabolome
3. Materials and Methods
3.1. Materials, Sample Preparation, and Chemicals
3.2. Chromatographic Conditions
3.3. Determination of Antioxidant Components
3.4. Chemical Evaluation of In Vitro Antioxidant Activities
3.5. Cellular-Based Antioxidant Capacity Evaluation
3.6. Measurement of α-Glucosidase Inhibitory Activities
3.7. Measurement of Lipase-Inhibitory Activities
3.8. Modeling of Fingerprint–Activity Relationship
3.9. Metabolomic Analysis
3.10. Data Processing and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, F.; Xiong, Z.-Y.; Li, P.; Yang, H.; Gao, W.; Li, H.-J. From chemical consistency to effective consistency in precise quality discrimination of Sophora flower-bud and Sophora flower: Discovering efficacy-associated markers by fingerprint-activity re-lationship modeling. J. Pharm. Biomed. Anal. 2017, 132, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Scotti, F.; Reich, E.; Kirchhof, R.; Booker, A.; Heinrich, M. Chrysanthemum species used as food and medicine: Understanding quality differences on the global market. S. Afr. J. Bot. 2022, 148, 123–134. [Google Scholar] [CrossRef]
- Liang, F.; Hu, C.; He, Z.; Pan, Y. An arabinogalactan from flowers of Chrysanthemum morifolium: Structural and bioactivity studies. Carbohydr. Res. 2014, 387, 37–41. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M. Identification of the phenolic components of chrysanthemum flower (Chrysanthemum morifolium Ramat). Food Chem. 2010, 120, 319–326. [Google Scholar] [CrossRef]
- Yuan, H.; Luo, J.; Lyu, M.; Jiang, S.; Qiu, Y.; Tian, X.; Liu, L.; Liu, S.; Ouyang, Y.; Wang, W. An integrated approach to Q-marker discovery and quality assessment of edible Chrysanthemum flowers based on chromatogram–effect relationship and bioin-formatics analyses. Ind. Crops Prod. 2022, 188, 115745. [Google Scholar] [CrossRef]
- Chu, Q.; Fu, L.; Guan, Y.; Ye, J. Determination and Differentiation of Flos Chrysanthemum Based on Characteristic Elec-trochemical Profiles by Capillary Electrophoresis with Electrochemical Detection. J. Agric. Food Chem. 2004, 52, 7828–7833. [Google Scholar] [CrossRef]
- Maitreesophone, P.; Khine, H.E.E.; Nealiga, J.Q.L.; Kongkatitham, V.; Panuthai, P.; Chaotham, C.; Likhitwitayawuid, K.; Sritularak, B. α-Glucosidase and pancreatic lipase inhibitory effects and anti-adipogenic activity of dendrofalconerol B, a bisbibenzyl from Dendrobium harveyanum. S. Afr. J. Bot. 2022, 146, 187–195. [Google Scholar] [CrossRef]
- Yang, C.; Zheng, Y.; Green, B.D.; Zhou, C.; Pan, D.; Cao, J.; Wang, L.; Cai, Z.; Xia, Q. Volatilome evolution during storage and in vitro starch digestibility of high-power ultrasonication pretreated wholegrain Oryza sativa L. Food Res. Int. 2022, 162, 112127. [Google Scholar] [CrossRef]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, S.M.I.; Devkota, H.P.; Wada, M.; Kishimoto, N.; Moriuchi, M.; Shuto, T.; Misumi, S.; Kai, H.; Watanabe, T. Free radical scavenging, α-glucosidase inhibitory and lipase inhibitory activities of eighteen Sudanese medicinal plants. BMC Complement. Altern. Med. 2018, 18, 282. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, X.; Ni, H.; Li, P.; Li, H.-J. Discovery of quality control markers from traditional Chinese medicines by fingerprint-efficacy modeling: Current status and future perspectives. J. Pharm. Biomed. Anal. 2018, 159, 296–304. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Yang, L.; Wang, Y.; Jin, W.; Li, J.; Zhang, Z. Comprehensive Quality Evaluation of Polygonatum cyrtonema and Its Processed Product: Chemical Fingerprinting, Determination and Bioactivity. Molecules 2023, 28, 4341. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, X.; Yang, L.; Zhang, J.; Sun, G. Monitoring quality consistency of Ixeris sonchifolia (Bunge) Hance injection by integrating UV spectroscopic fingerprints, a multi-wavelength fusion fingerprint method, antioxidant activities and UHPLC/Q-TOF-MS. RSC Adv. 2016, 6, 87616–87627. [Google Scholar] [CrossRef]
- Yuan, J.-h.; Cai, Z.-C.; Chen, C.-H.; Wu, N.; Yin, S.-X.; Wang, W.-X.; Chen, H.-J.; Zhou, Y.-Y.; Li, L.; Liu, X.-H. A study for quality evaluation of Taxilli Herba from different hosts based on fingerprint-activity relationship modeling and multivariate statistical analysis. Arab. J. Chem. 2022, 15, 103933. [Google Scholar] [CrossRef]
- Tian, D.; Yang, Y.; Yu, M.; Han, Z.-Z.; Wei, M.; Zhang, H.-W.; Jia, H.-M.; Zou, Z.-M. Anti-inflammatory chemical constituents of Flos Chrysanthemi Indici determined by UPLC-MS/MS integrated with network pharmacology. Food Funct. 2020, 11, 6340–6351. [Google Scholar] [CrossRef]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100255. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, Y.; Li, D.; Chen, Y. Chrysanthemum indicum L.: A comprehensive review of its botany, phytochemistry and pharmacology. Am. J. Chin. Med. 2020, 48, 871–897. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zheng, Y.; Wang, L.; Chen, X. Proposing signaling molecules as key optimization targets for intensifying the phy-tochemical biosynthesis induced by emerging nonthermal stress pretreatments of plant-based foods: A focus on gam-ma-aminobutyric acid. J. Agric. Food Chem. 2023. [Google Scholar] [CrossRef]
- Martinelli, E.; Granato, D.; Azevedo, L.; Gonçalves, J.E.; Lorenzo, J.M.; Munekata, P.E.; Simal-Gandara, J.; Barba, F.J.; Carrillo, C.; Rajoka, M.S.R.; et al. Current perspectives in cell-based approaches towards the definition of the antioxidant activity in food. Trends Food Sci. Technol. 2021, 116, 232–243. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.). Food Chem. 2017, 217, 773–781. [Google Scholar] [CrossRef]
- Wang, A.; Hou, K.; Mu, G.; Ma, C.; Tuo, Y. Antioxidative effect of soybean milk fermented by Lactobacillus plantarum Y16 on 2,2–azobis (2-methylpropionamidine) dihydrochloride (ABAP)-damaged HepG2 cells. Food Biosci. 2021, 44, 101120. [Google Scholar] [CrossRef]
- Xia, Q.; Green, B.D.; Zhu, Z.; Li, Y.; Gharibzahedi, S.M.T.; Roohinejad, S.; Barba, F.J. Innovative processing techniques for altering the physicochemical properties of wholegrain brown rice (Oryza sativa L.)—Opportunities for enhancing food quality and health attributes. Crit. Rev. Food Sci. Nutr. 2019, 59, 3349–3370. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef]
- Shawul, A.A.; Chakma, S.; Melesse, A.M. The response of water balance components to land cover change based on hy-drologic modeling and partial least squares regression (PLSR) analysis in the Upper Awash Basin. J. Hydrol. Reg. Stud. 2019, 26, 100640. [Google Scholar] [CrossRef]
- Jia, J.; Deng, H.; Duan, J.; Zhao, J. Analysis of the major drivers of the ecological footprint using the STIRPAT model and the PLS method—A case study in Henan Province, China. Ecol. Econ. 2009, 68, 2818–2824. [Google Scholar] [CrossRef]
- Ruan, Y.; Cai, Z.; Deng, Y.; Pan, D.; Zhou, C.; Cao, J.; Chen, X.; Xia, Q. An untargeted metabolomic insight into the high-pressure stress effect on the germination of wholegrain Oryza sativa L. Food Res. Int. 2021, 140, 109984. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Wang, L.; Xu, C.; Mei, J.; Li, Y. Effects of germination and high hydrostatic pressure processing on mineral elements, amino acids and antioxidants in vitro bioaccessibility, as well as starch digestibility in brown rice (Oryza sativa L.). Food Chem. 2017, 214, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zheng, Y.; Wang, L.; Zhou, C.; Wang, J.; He, J.; Sun, Y.; Cao, J.; Pan, D.; Xia, Q. Contribution of process-induced molten-globule state formation in duck liver protein to the enhanced binding ability of (E,E)-2,4-heptadienal. J. Sci. Food Agric. 2023, 103, 3334–3345. [Google Scholar] [CrossRef]
- Xia, Q.; Zheng, Y.; Liu, Z.; Cao, J.; Chen, X.; Liu, L.; Yu, H.; Barba, F.J.; Pan, D. Nonthermally driven volatilome evolution of food matrices: The case of high pressure processing. Trends Food Sci. Technol. 2020, 106, 365–381. [Google Scholar] [CrossRef]
- Xia, Q.; Mei, J.; Yu, W.; Li, Y. High hydrostatic pressure treatments enhance volatile components of pre-germinated brown rice revealed by aromatic fingerprinting based on HS-SPME/GC–MS and chemometric methods. Food Res. Int. 2017, 91, 103–114. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. PCA as a Practical Indicator of OPLS-DA Model Reliability. Curr. Metabolomics 2016, 4, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gong, X.; Quan, Y.; Zhou, Y.; Li, Y.; Peng, C. A Cell-Based Metabonomics Approach to Investigate the Varied Influences of Chrysophanol-8-O-β-D-Glucoside with Different Concentrations on L-02 Cells. Front. Pharmacol. 2019, 9, 1530. [Google Scholar] [CrossRef] [PubMed]
- García-Cañaveras, J.C.; Jiménez, N.; Gómez-Lechón, M.J.; Castell, J.V.; Donato, M.T.; Lahoz, A. LC-MS untargeted metab-olomic analysis of drug-induced hepatotoxicity in HepG2 cells. Electrophoresis 2015, 36, 2294–2302. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Dion, M.Z.; Wang, Y.J.; Bregante, D.; Chan, W.; Andersen, N.; Hilderbrand, A.; Leiske, D.; Salisbury, C.M. The Use of a 2,2′-Azobis (2-Amidinopropane) Dihydrochloride Stress Model as an Indicator of Oxidation Susceptibility for Monoclonal Antibodies. J. Pharm. Sci. 2018, 107, 550–558. [Google Scholar] [CrossRef]
- Uraipong, C.; Zhao, J. Rice bran protein hydrolysates exhibit strong in vitro α-amylase, β-glucosidase and ACE-inhibition activities. J. Sci. Food Agric. 2016, 96, 1101–1110. [Google Scholar] [CrossRef]
- Maqsood, M.; Ahmed, D.; Atique, I.; Malik, W. Lipase inhibitory activity of Lagenaria siceraria fruit as a strategy to treat obesity. Asian Pac. J. Trop. Med. 2017, 10, 305–310. [Google Scholar] [CrossRef]
- Han, C.; Zheng, Y.; Zhou, C.; Liang, Y.; Huang, S.; Sun, Y.; Cao, J.; He, J.; Barba, F.J.; Liu, J.; et al. Pressurization-induced molten globule-like protein as a regulatory target for off-flavor controlling: Thermodynamic evidence and molecular modeling. Food Control 2023, 154, 110022. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Zheng, Y.; Hong, H.; Liu, L.; Chen, X.; Xia, Q. Identification of Efficacy-Associated Markers to Discriminate Flos Chrysanthemum and Flos Chrysanthemi Indici Based on Fingerprint–Activity Relationship Modeling: A Combined Evaluation over Chemical Consistence and Quality Consistence. Molecules 2023, 28, 6254. https://doi.org/10.3390/molecules28176254
Liu F, Zheng Y, Hong H, Liu L, Chen X, Xia Q. Identification of Efficacy-Associated Markers to Discriminate Flos Chrysanthemum and Flos Chrysanthemi Indici Based on Fingerprint–Activity Relationship Modeling: A Combined Evaluation over Chemical Consistence and Quality Consistence. Molecules. 2023; 28(17):6254. https://doi.org/10.3390/molecules28176254
Chicago/Turabian StyleLiu, Feng, Yuanrong Zheng, Huijie Hong, Lianliang Liu, Xiaojia Chen, and Qiang Xia. 2023. "Identification of Efficacy-Associated Markers to Discriminate Flos Chrysanthemum and Flos Chrysanthemi Indici Based on Fingerprint–Activity Relationship Modeling: A Combined Evaluation over Chemical Consistence and Quality Consistence" Molecules 28, no. 17: 6254. https://doi.org/10.3390/molecules28176254






