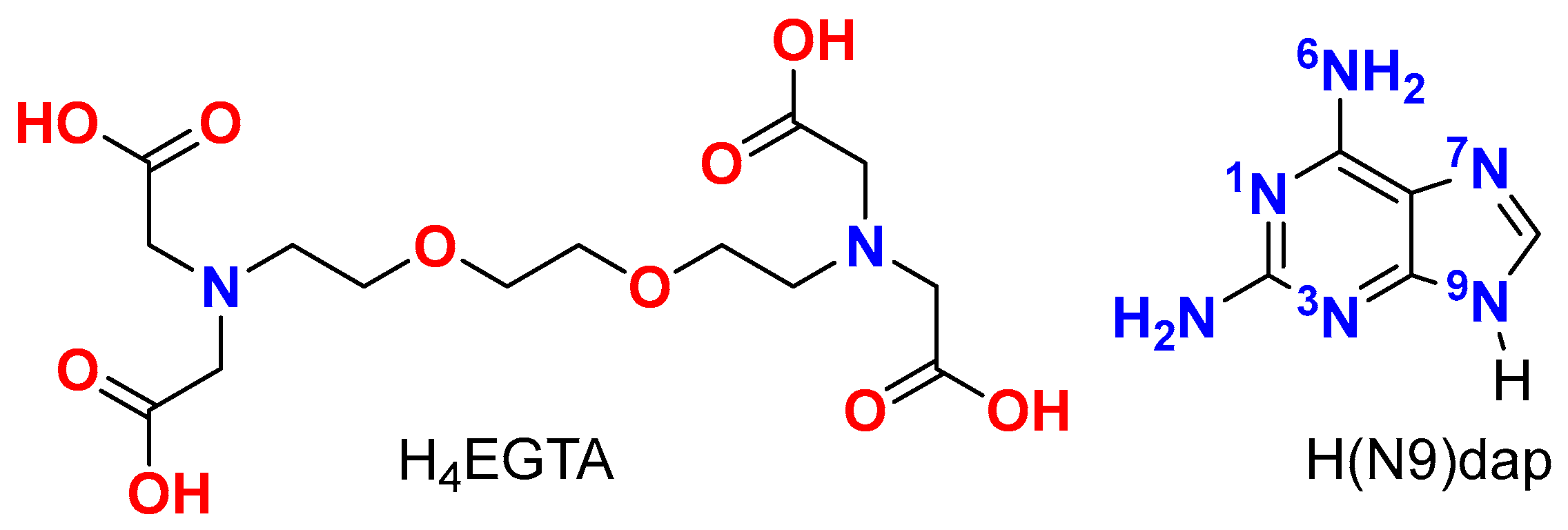
H(N3)dap (Hdap = 2,6-Diaminopurine) Recognition by Cu2(EGTA): Structure, Physical Properties, and Density Functional Theory Calculations of [Cu4(μ-EGTA)2(μ-H(N3)dap)2(H2O)2]·7H2O
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Synthesis of the Binary Precursor and the New Compound 1
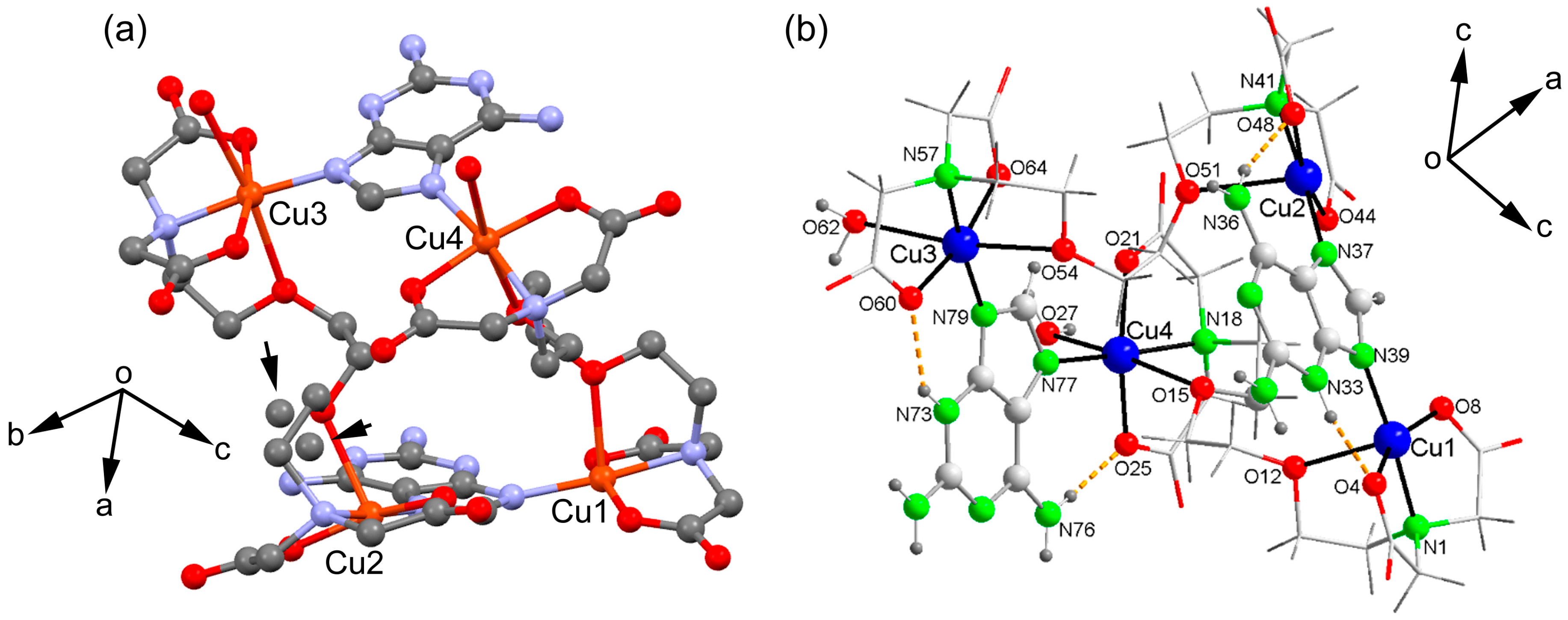
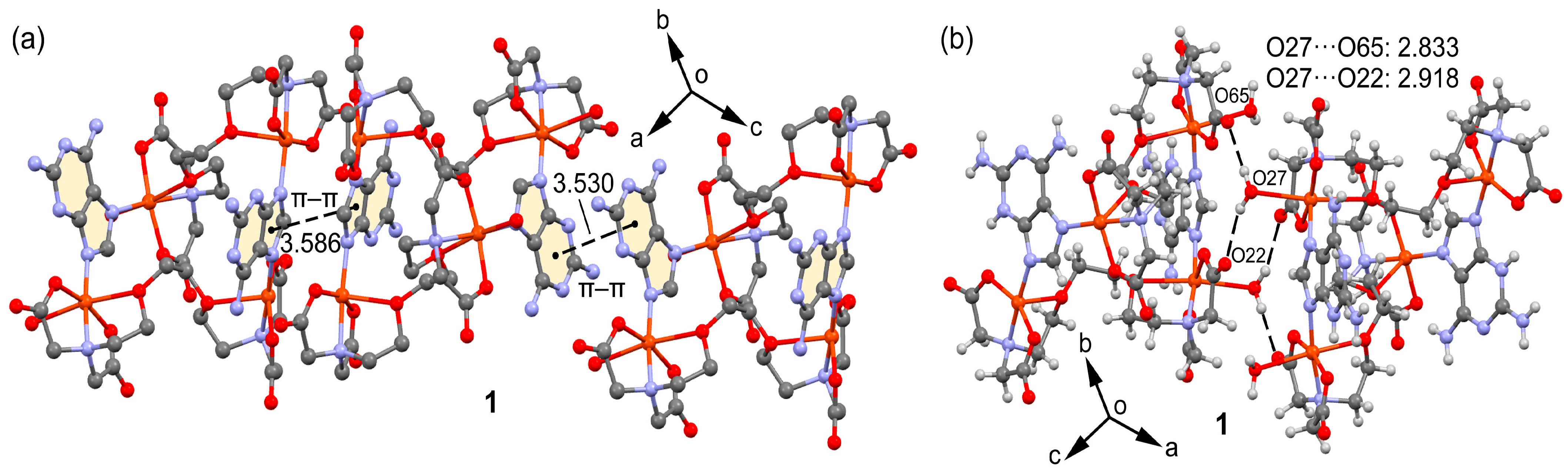
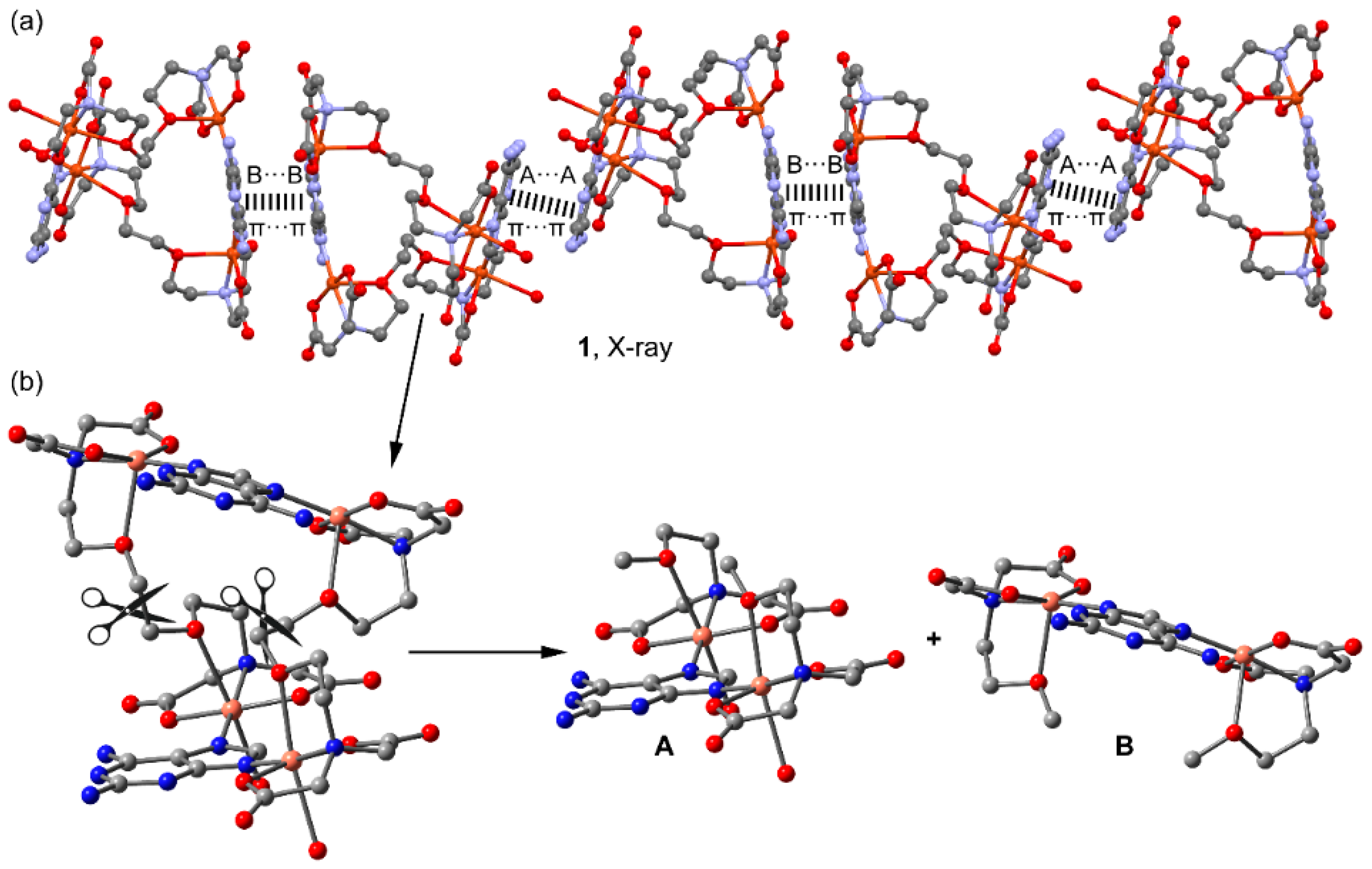
2.2. Molecular and Supramolecular Structures in the Crystal
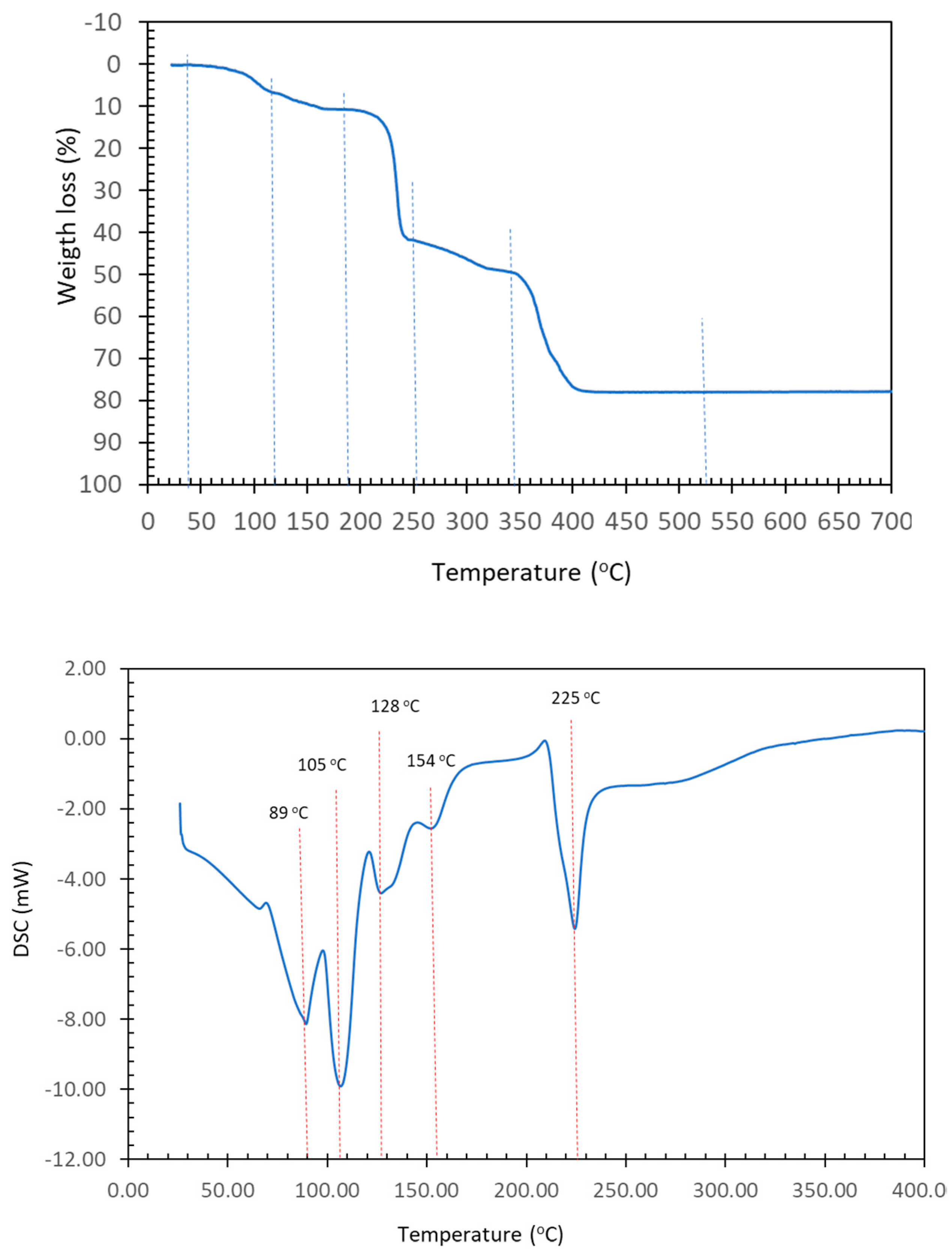
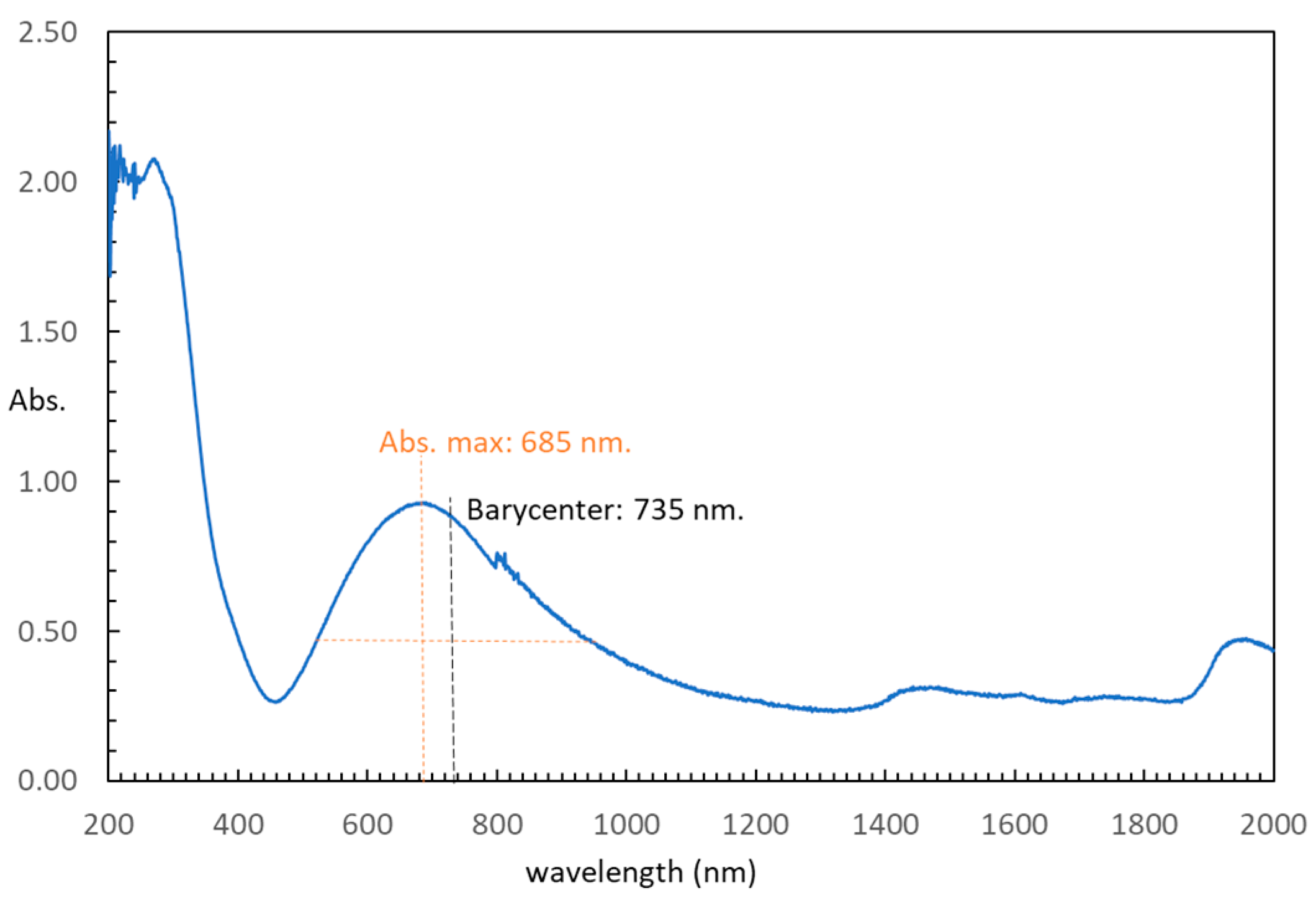
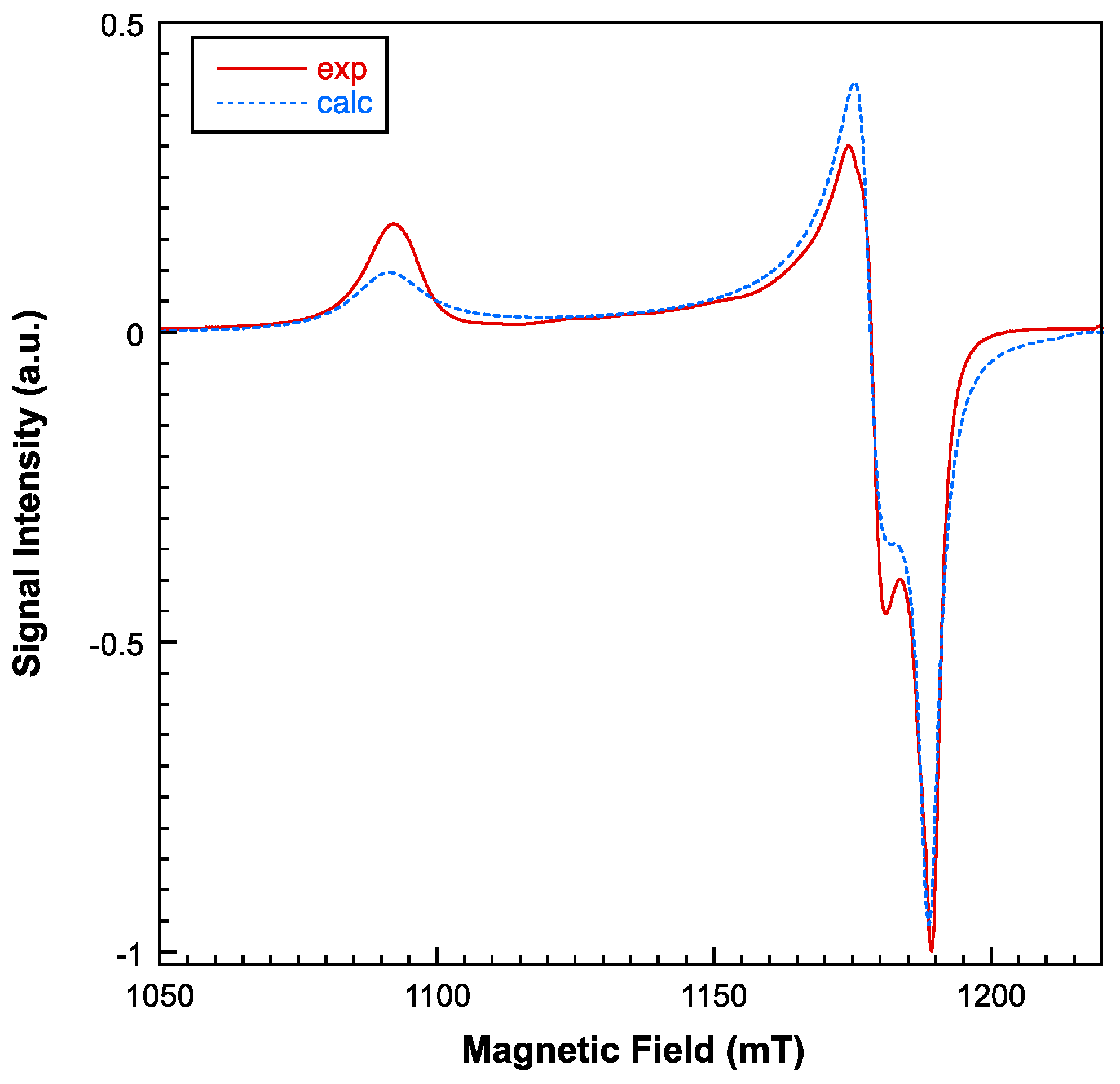
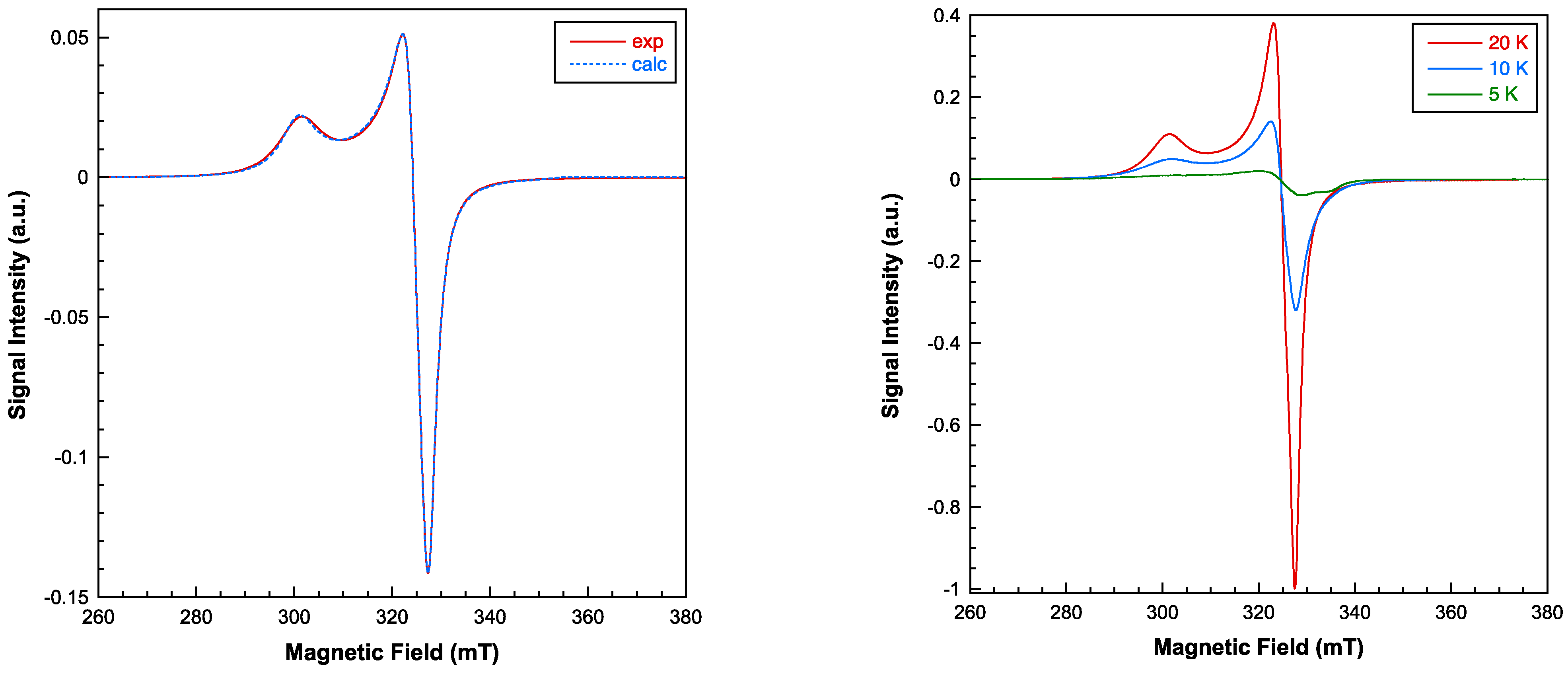
2.3. Physical Properties
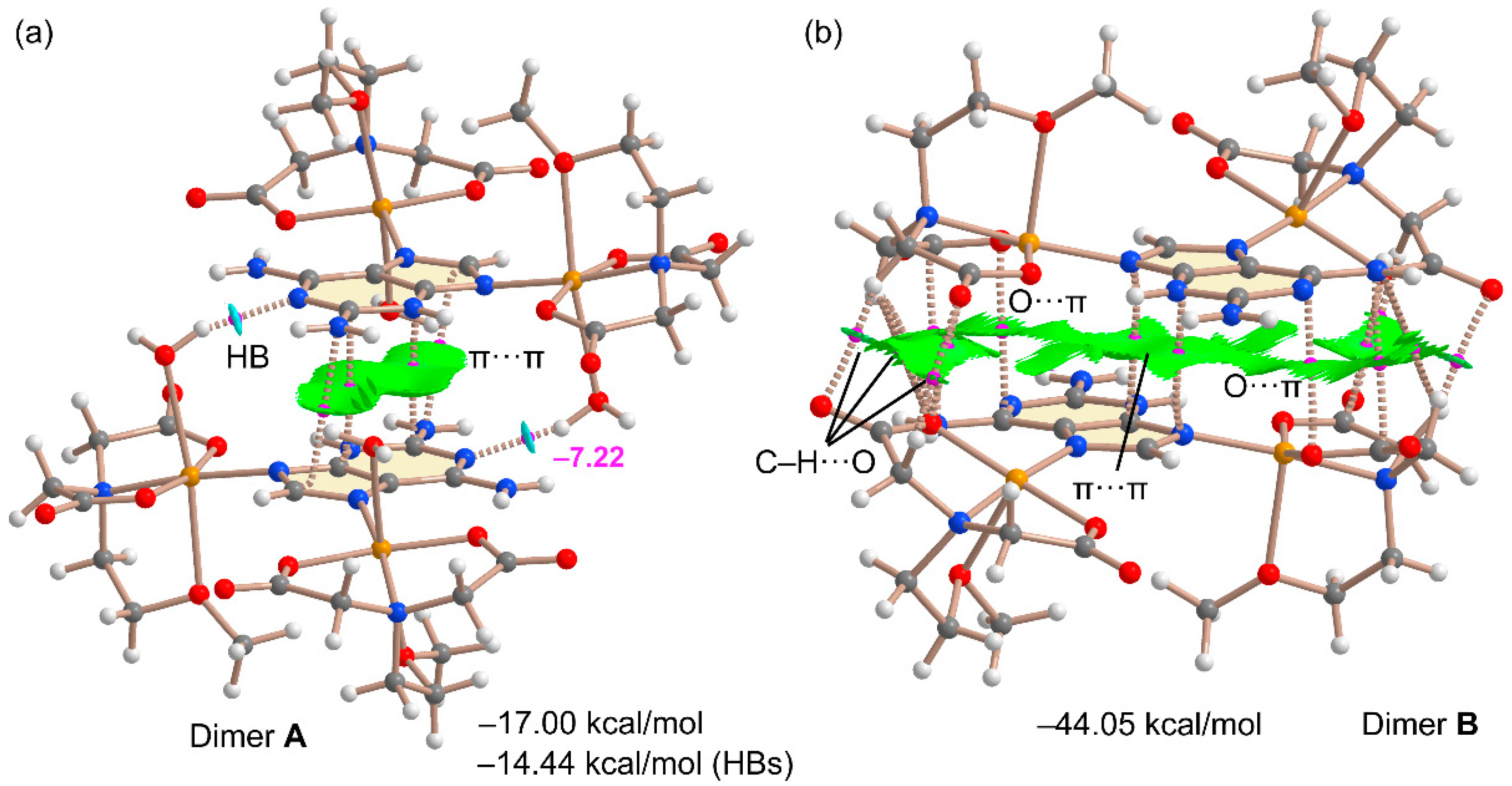
2.4. DFT Calculations
3. Materials and Methods
3.1. Reagents and Synthesis for the Dicopper(II)-EGTA Chelate and the New Compound 1
3.2. Physical Measurements
3.3. Crystallography
3.4. Computational Details
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rosales-Martínez, C.; Matilla-Hernádez, A.; Choquesillo-Lazarte, D.; Frontera, A.; Castiñeiras, A.; Niclós-Gutiérrez, J. The copper(II)-thiodiacetate (tda) chelate as efficient receptor of N9-(2-hydroxyethyl)adenine (9heade). Synthesis, molecular and crystal structures, physical properties and DFT calculations of [Cu(tda)(9heade)(H2O)]·2H2O. Molecules 2023, 28, 5830. [Google Scholar] [CrossRef] [PubMed]
- García-Rubiño, M.E.; Matilla-Hernández, A.; Frontera, A.; Lezama, L.; Niclós-Gutiérrez, J.; Choquesillo-Lazarte, D. Dicopper(II)-EDTA Chelate as a bicephalic receptor model for a synthetic adenine nucleoside. Pharmaceuticals 2021, 14, 426. [Google Scholar] [CrossRef]
- Bendich, A.; Furst, S.S.; Brown, G.B. On the role of 2,6-diaminopurine in the biosynthesis of nucleic acid guanine. J. Biol. Chem. 1950, 185, 423–433. [Google Scholar] [CrossRef]
- Szabla, R.; Zdrowowicz, M.; Spisz, P.; Green, N.J.; Stadlbauer, P.; Kruse, H.; Šponer, J.; Rak, J. 2,6-diaminopurine promotes repair of DNA lesions under prebiotic conditions. Nat. Commun. 2021, 12, 3018. [Google Scholar] [CrossRef]
- Madaoui, M.; Datta, D.; Wassarman, K.; Zlatev, I.; Egli, M.; Ross, B.S.; Manoharan, M. A Chemical Approach to Introduce 2,6-Diaminopurine and 2-Aminoadenine Conjugates into Oligonucleotides without Need for Protecting Groups. Org. Lett. 2022, 24, 6111–6116. [Google Scholar] [CrossRef]
- Kim, H.-J.; Benner, S.A. Prebiotic stereoselective synthesis of purine and noncanonical pyrimidine nucleotide from nucleobases and phosphorylated carbohydrates. PNAS 2017, 114, 11315–11320. [Google Scholar] [CrossRef]
- Martina, M.G.; Giammarino, F.; Vicenti, I.; Groaz, E.; Rozenski, J.; Incerti, M.; Sannio, F.J.; Docquier, D.; Zazzi, M.; Radi, M. Nucleoside Derivatives of 2,6-diaminopurine Antivirals: Base-Modified Nucleosides with Broad-Spectrum Antimicrobial Properties. ChemMedChem 2023, 18, e202300200. [Google Scholar] [CrossRef]
- XiaoHua, X.; Zhiyuan, S.; Ramachandra, S.H. A versatile synthetic precursor for introduction of specific N6-modifications in 2,6-diaminopurine nucleosides: N2-acetyl-2′,3′,5′-tri-O-acetyl-N6-(1,2,4-triazol-1-yl)-2,6-diaminopurine -9-ribofuranoside. In Proceedings of the ECSOC-3, The Third International Electronic Conference on Synthetic Organic Chemistry, Basel, Switzerland, 1–30 September 1999. [Google Scholar]
- Krečmerová, M.; Jansa, P.; Dračínský, M.; Sázelová, P.; Kašička, V.; Neyts, J.; Auwerx, J.; Kiss, E.; Goris, N.; Stepan, G.; et al. 9-[2-(R)-(phosphonomethoxy)propyl]-2,6-diaminopurine (R)-PMPDAP and its prodrugs: Optimized preparation, including identification of by-products formed, and antiviral evaluation in vitro. Bioorg. Med. Chem. 2013, 21, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Furman, P.A.; Jeffrey, J.; Kiefer, L.L.; Feng, J.Y.; Anderson, K.S.; Borroto-Esoda, K.; Hill, E.; Copeland, W.C.; Chu, C.K.; Sommadossi, J.-P.; et al. Mechanism of action of 1-β-d-2,6-diaminopurine dioxolane, a prodrug of the human immunodeficiency virus type 1 Inhibitor 1-β-d-dioxolane guanosine. Antimicrob. Agents Chemother. 2001, 45, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, A.; Kierzek, E.; Pasternak, K.; Turner, D.H.; Kierzek, R. A chemical synthesis of LNA-2,6-diaminopurine riboside, and the influence of 2′-O-methyl-2,6-diaminopurine and LNA-2,6-diaminopurine ribosides on the thermodynamic properties of 2′-O-methyl RNA/RNA heteroduplexes. Nucleic Acids Res. 2007, 35, 4055–4063. [Google Scholar] [CrossRef]
- Schauer, C.K.; Anderson, O.P. Calcium-selective ligands. 1. Structure of 3,12-bis (carboxymethyl)-6,9-dioxa-3,12-diazatetradecanedioic acid (H4egta). Acta Crystallogr. 1986, C42, 760–762. [Google Scholar] [CrossRef]
- Yerly, F.; Hardcastle, K.I.; Helm, L.; Aime, S.; Botta, M.; Merbach, A.E. Molecular dynamics simulation of [Gd(egta)(H2O)]− in aqueous solution: Internal motions of the poly(amino carboxylate) and water ligands, and rotational correlation times. Chem. Eur. J. 2002, 8, 1031–1039. [Google Scholar] [CrossRef]
- Schauer, C.K.; Anderson, O.P. Highly polydentate ligands. 5. Structures of alkaline-earth complexes of the calcium-selective ligand EGTA4- (H4EGTA = 3.12- bis (carboxymethyl)-6,9-dioxa-3,12-diazatetradecanedioic acid). Inorg. Chem. 1988, 27, 3118–3130. [Google Scholar] [CrossRef]
- Gaur, R. Selective anionic dye adsorption, topology and luminescence study of structurally diverse cadmium(II) coordination polymers. Inorg. Chem. Front. 2019, 6, 278–286. [Google Scholar] [CrossRef]
- Alarcón-Payer, C.; Bugella-Altamirano, E.; Choquesillo-Lazarte, D.; Castineiras, A.; González-Pérez, J.M.; Niclós-Gutiérrez, J. Dinuclear EGTA-copper(II) chelates with imidazole as auxiliary ligand. Inorg. Chem. Commun. 2006, 9, 903–906. [Google Scholar] [CrossRef]
- Gaur, R.; Roy, S.; Kallem, P.; Banat, F. Experimental and theoretical investigation of hydrogen bonded supramolecular assemblies through water molecules in a copper(II)-EGTA complex. J. Mol. Struct. 2022, 1265, 133400. [Google Scholar] [CrossRef]
- Escriva, E.; Server-Carrio, J.; Garcia-Lozano, J.; Folgado, J.-V.; Sapina, F.; Lezama, L. Exchange interactions through hydrogen-bond bridges. Crystal structure, spectroscopic characterisation and magnetic properties of the complex [{Cu(en)}2(μ-egta)]·4H2O (H4egta = 3,12-bis(carboxymethyl)-6,9-dioxa-3,12-diazatetradecanedionic acid). Inorg. Chim. Acta 1998, 279, 58–64. [Google Scholar] [CrossRef]
- Wu, Y.-P.; Wang, Y.-Y.; Wang, C.-J.; Li, D.-S.; Guo, C.-Y.; Wen, G.-L.; Shi, Q.-Z. From 1D polymeric chain to two-fold parallel interpenetration of (4,4) net: Synthesis and characterization of two new copper(II) complexes derived from highly polydentate aminopolycarboxylate ligand. Chin. J. Chem. 2008, 26, 1233–1238. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Chen, Y.-G.; Liu, S.-X. Weakley sandwich-type anion in organic–inorganic hybrid compound with 3D framework. Inorg. Chem. Commun. 2013, 29, 45–48. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Chen, Y.-G. A flexible chelate ligand in dodecatungstoborate-based supramolecular compounds. J. Inclusion Phenom. Macrocyclic Chem. 2014, 79, 171–176. [Google Scholar] [CrossRef]
- Yang, E.-C.; Chan, Y.-N.; Liu, H.; Wang, Z.-C.; Zhao, X.-J. Unusual polymeric ZnII/CdII complexes with 2,6-diaminopurine by synergistic coordination of nucleobases and polycarboxylate anions: Binding behavior, self-assembled pattern of the nucleobase, and luminscent properties. Cryst. Growth Des. 2009, 9, 4933–4944. [Google Scholar] [CrossRef]
- González-Pérez, J.M.; Choquesillo-Lazarte, D.; Domínguez-Martín, A.; El Bakkali, H.; García-Rubiño, M.E.; Pérez-Toro, I.; Vílchez-Rodríguez, E.; Castiñeiras, A.; Marina Nurchi, V.; Niclós-Gutiérrez, J. Molecular recognition between adenine or 2,6-diaminopurine and copper(II) chelates with N,O2,S-tripodal tetradentate chelators having thioether or disulfide donor groups. J. Inorg. Biochem. 2015, 151, 75–86. [Google Scholar] [CrossRef]
- Xu, L.-L.; Zhang, H.-F.; Li, M.; Ng, S.W.; Feng, J.-H.; Mao, J.-G.; Li, D. Chiroptical Activity from an Achiral Biological Metal−Organic Framework. J. Am. Chem. Soc. 2018, 140, 11569–11572. [Google Scholar] [CrossRef]
- Zhao, H.-K.; Yang, H.-W.; Wang, X.-G.; Ding, B.; Liu, Z.-Y.; Zhao, X.-J.; Yang, E.-C. An unusual 2D polymeric Co(II) complex with 2,6-diaminopurine: Synthesis, crystal structure, and magnetic behavior. Inorg. Chem. Commun. 2019, 107, 107483. [Google Scholar] [CrossRef]
- Bai, J.-P.; Huang, Y.-L.; Xie, M.; Zhao, Y.; Luo, D.; Li, Y.Y.; Lu, W.; Li, D. Reversible multiphase transition in a BioMOF and its distinctive luminescence turn-on in alcohol vapor. ACS Appl. Mater. Interfaces 2019, 11, 38503–38509. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Eshtiagh-Hosseini, H.; Alipour, M.; Frontera, A. Recent developments in the crystal engineering of diverse coordination modes (0–12) for Keggin-type polyoxometalates in hybrid inorganic–organic architectures. Coord. Chem. Rev. 2014, 275, 1–18. [Google Scholar] [CrossRef]
- Taleghani, S.; Mirzaei, M.; Eshtiagh-Hosseini, H.; Frontera, A. Tuning the topology of hybrid inorganic–organic materials based on the study of flexible ligands and negative charge of polyoxometalates: A crystal engineering perspective. Coord. Chem. Rev. 2016, 309, 84–106. [Google Scholar] [CrossRef]
- Arefian, M.; Mirzaei, M.; Eshtiagh-Hosseini, H.; Frontera, A. A survey of the different roles of polyoxometalates in their interaction with amino acids, peptides and proteins. Dalton Trans. 2017, 46, 6812–6829. [Google Scholar] [CrossRef]
- Mirzaei, M.; Eshtiagh-Hosseini, H.; Bolouri, Z.; Rahmati, Z.; Esmaeilzadeh, A.; Hassanpoor, A.; Bauza, A.; Ballester, P.; Barceló-Oliver, M.; Mague, J.T.; et al. Rationalization of Noncovalent Interactions within Six New MII/8-Aminoquinoline Supramolecular Complexes (MII = Mn, Cu, and Cd): A Combined Experimental and Theoretical DFT Study. Cryst. Growth Des. 2015, 15, 1351–1361. [Google Scholar] [CrossRef]
- Mirzaei, M.; Eshtiagh-Hosseini, H.; Lotfian, N.; Salimi, A.; Bauzá, A.; Van Deun, R.; Decadt, R.; Barceló-Oliver, M.; Frontera, A. Syntheses, structures, properties and DFT study of hybrid inorganic–organic architectures constructed from trinuclear lanthanide frameworks and Keggin-type polyoxometalates. Dalton Trans. 2014, 43, 1906–1916. [Google Scholar] [CrossRef]
- Addison, A.W.; Nageswara Rao, T.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[l,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Blackman, A.G.; Schenk, E.B.; Jelley, R.E.; Krensked, E.H.; Gahan, L.R. Five-coordinate transition metal complexes and the value of τ5: Observations and caveats. Dalton Trans. 2020, 49, 14798–14806. [Google Scholar] [CrossRef]
- Sutton, D. Electronic Spectra of Transition Metal Complexes: An Introductory Text; McGraw-Hill Book Co.: New York, NY, USA; London, UK, 1968. [Google Scholar]
- Calvo, R.; Mesa, M.A. EPR study of electronic and magnetic properties of bis(DL-α-amino-n-butyrato)copper(II). A layered magnetic system. Phys. Rev. B 1983, 28, 1244–1248. [Google Scholar] [CrossRef]
- Hathaway, B.J. A new look at the stereochemistry and electronic properties of complexes of the copper(II) ion. Struct. Bonding 1984, 57, 55–118. [Google Scholar]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Qin, T.; Zhang, X.; Li, D.; Dong, X.; Qi, N.; Shang, Y.; Sakiyamad, H.; Afzal, M.; Alarifi, A. Temperature modulation on functional coordination polymers with tetracarboxylate linker: Syntheses, structural traits, and magnetism. J. Mol. Struct. 2023, 1291, 136074. [Google Scholar] [CrossRef]
- Bruker. APEX3 Software; v2018.7-2; Bruker AXS Inc.: Madison, WI, USA, 2019. [Google Scholar]
- Sheldrick, G.M. SADABS v. 2.03, Program for Empirical Absorption Correction of Area Detector Data; University of Goettingen: Goettingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Wilson, A.J.C. International Tables for Crystallography; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; Volume C. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155. [Google Scholar] [CrossRef]
- Hahn, T. (Ed.) International Tables for Crystallography. Volume A, Space-Group Symmetry; International Union of Crystallography by Kluwer Academic Publishers: Dordrecht, The Netherlands; London, UK, 2002. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–11. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154122. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893928. [Google Scholar] [CrossRef]
- Keith, T.A. TK Gristmill Software, version 13.05.06; AIMAll: Overland Park, KS, USA, 2013. [Google Scholar]
- Contreras-Garcia, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.P.; Beratan, D.N.; Yang, W. NCIPLOT: A program for plotting non-covalent interaction regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef]
- Sadhukhan, D.; Maiti, M.; Pilet, G.; Bauzá, A.; Frontera, A.; Mitra, S. Hydrogen Bond, π–π, and CH–π Interactions Governing the Supramolecular Assembly of Some Hydrazone Ligands and Their MnII Complexes—Structural and Theoretical Interpretation. Eur. J. Inorg. Chem. 2015, 11, 1958–1972. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Bauzá, A.; Amini, M.; Molins, E.; Mague, J.T.; Frontera, A. On the importance of tetrel bonding interactions in lead (II) complexes with (iso)nicotinohydrazide based ligands and several anions. Dalton Trans. 2016, 45, 10708–10716. [Google Scholar] [CrossRef]



| Empirical formula | C38H70Cu4N16O29 | |
| Formula weight | 1469.24 | |
| Temperature | 298(2) K | |
| Wavelength | 1.54178 Å | |
| Crystal system | Triclinic | |
| Space group | P | |
| Unit cell dimensions | a = 12.9272(4) Å | α = 104.372(1)° |
| b = 13.1727(4) Å | β = 94.015(2)° | |
| c = 19.6298(6) Å | γ = 95.213(2)° | |
| Volume | 3209.80(17) Å3 | |
| Z | 2 | |
| Calculated density | 1.520 Mg/m3 | |
| Absorption coefficient | 2.396 mm–1 | |
| F(000) | 1516 | |
| Crystal size | 0.10 × 0.10 × 0.09 mm | |
| θ range for data collection (°) | 2.334 to 66.591. | |
| Limiting indices | 23 | |
| Reflections collected/unique | 23,305/10,915 [Rint = 0.0287] | |
| Completeness to theta = 66.591° | 96.2% | |
| Absorption correction | Semi-empirical from equivalents | |
| Max. and min. transmission | 1.000 and 0.714 | |
| Refinement method | Full-matrix least-squares on F2 | |
| Data/restraints/parameters | 10,915/1/742 | |
| Goodness-of-fit on F2 | 1.038 | |
| Final R indices [I > 2σ(I)] | R1 = 0.0354, | wR2 = 0.0956 |
| R indices (all data) | R1 = 0.0394 | wR2 = 0.0986 |
| Largest diff. peak and hole | 0.462 and −0.429 e·Å−3 | |
| CCSD code | 2287840 | |
| Cu(1)-N(1) | 2.000(2) | Cu(2)-N(37) | 1.973(2) |
| Cu(1)-O(4) | 1.934(2) | Cu(2)-N(41) | 2.004(3) |
| Cu(1)-O(8) | 1.930(2) | Cu(2)-O(44) | 1.939(2) |
| Cu(1)-N(39) | 1.961(2) | Cu(2)-O(48) | 1.951(2) |
| Cu(1)-O(12) | 2.337(2) | Cu(2)-O(51) | 2.424(2) |
| N(39)-Cu(1)-N(1) | θ = 175.67(10) | N(37)-Cu(2)-N(41) | θ = 174.95(10) |
| O(8)-Cu(1)-O(4) | φ = 158.40(11) | O(44)-Cu(2)-O(48) | φ = 163.30(10) |
| τ = (θ − φ)/60 * | 0.29 | τ = (θ − φ)/60 | 0.19 |
| Cu(3)-N(57) | 1.997(2) | Cu(4)-N(77) | 1.9801(2) |
| Cu(3)-O(60) | 1.969(2) | Cu(4)-N(18) | 2.010(2) |
| Cu(3)-O(64) | 1.955(2) | Cu(4)-O(21) | 1.959(2) |
| Cu(3)-N(79) | 1.944(2) | Cu(4)-O(25) | 1.959(2) |
| Cu(3)-O(54) | 2.417(2) | Cu(4)-O(27) | 2.499(2) |
| Cu(3)-O(62) | 2.552(3) | Cu(4)-O(15) | 2.561(2) |
| N(79)-Cu(3)-N(57) | 172.71(8) | O(27)-Cu(4)-O(15) | 172.58(6) |
| O(54)-Cu(3)-O(62) | 171.82(8) | N(77)-Cu(4)-N(18) | 170.55(8) |
| O(64)-Cu(3)-O(60) | 167.33(7) | O(21)-Cu(4)-O(25) | 166.11(6) |
| D-H···A | d(D···A) | <(D-H···A) |
|---|---|---|
| O(27)-H(27A)···O(65)#1 | 2.833(3) | 159.4 |
| O(27)-H(27B)···O(22)#1 | 2.917(3) | 142.7 |
| N(33)-H(33)···O(4) | 2.760(3) | 138.6 |
| N(36)-H(36A)···O(26)#2 | 2.938(3) | 159.4 |
| N(36)-H(36B)···O(48) | 2.844(4) | 160.3 |
| O(62)-H(62B)···N(71)#3 | 2.947(3) | 162.0 |
| N(72)-H(72A)···O(22)#4 | 2.751(3) | 133.2 |
| N(73)-H(73)···O(60) | 2.781(2) | 140.3 |
| N(76)-H(76B)···O(25) | 2.825(3) | 170.4 |
| Compound § | Formula * | N3-H···O (Å, °) | N6-H···O (Å, °) | Ref. |
|---|---|---|---|---|
| 1 (this work) | [Cu4(μ-EGTA)2(μ-H(N3)dap)2(H2O)2]·7H2O | 2.760(3), 139 2.781(2), 140 | 2.844(4), 139 2.825(3), 170.4 | - |
| QUDKEG | [Zn2(μ2-Hdap)(tp)2]n (3D MOF) | 2.739(2), 173 | 3.003(2), 162 2.912(2), 155 | [22] |
| QUDKIK | {[Zn2(μ2-Hdap)(tm)(μ2-OH)]·3H2O}n (1D polymer) | 2.559(2), 176 | 2.935(3), 172 | [22] |
| MULCED | [Cu2(BCBC)2(μ2-N7,N9)Hdap)(H2O)2]·4H2O | 2.732(7), 140 | 2.774(7), 169 | [23] |
| FINDAC | [Zn(FDC)(μ2-N7,N9)Hdap)]·0.5H2O (3D MOF) | N/A | N/A | [24] |
| KOZNAR | [Co(Hdap)(ip)]n (2D layers) | N/A | N/A | [25] |
| Step or R | Temp. (°C) | Time (min) | Weight Loss (%) Exp. Calc. | Evolved Gases or Residue (R) | |
|---|---|---|---|---|---|
| 1 | 30–115 | 0–12 | 6.905 | 8.098 | ~6 H2O *, CO2 (t *) |
| 2 | 115–185 | 12–17 | 3.681 | 4.049 | ~3 H2O, CO2 (t *) |
| (1 + 2) | (30–185) | (0–17) | (10.586) | 11.035 | 9 H2O |
| 3 | 185–255 | 17–23 | 31.177 | - | CO2, H2O |
| 4 | 255–335 | 23–32 | 7.128 | - | CO2, CO, H2O |
| 5 | 335–450 | 32–45 | 28.922 | - | CO2, CO, H2O, N2O, NO, NO2, CH4 (t *) |
| R | 520 | 95 | 19.532 | 17.879 | 4 CuO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi, H.; García-Rubiño, M.E.; Choquesillo-Lazarte, D.; Castiñeiras, A.; Lezama, L.; Frontera, A.; Niclós-Gutiérrez, J. H(N3)dap (Hdap = 2,6-Diaminopurine) Recognition by Cu2(EGTA): Structure, Physical Properties, and Density Functional Theory Calculations of [Cu4(μ-EGTA)2(μ-H(N3)dap)2(H2O)2]·7H2O. Molecules 2023, 28, 6263. https://doi.org/10.3390/molecules28176263
Mousavi H, García-Rubiño ME, Choquesillo-Lazarte D, Castiñeiras A, Lezama L, Frontera A, Niclós-Gutiérrez J. H(N3)dap (Hdap = 2,6-Diaminopurine) Recognition by Cu2(EGTA): Structure, Physical Properties, and Density Functional Theory Calculations of [Cu4(μ-EGTA)2(μ-H(N3)dap)2(H2O)2]·7H2O. Molecules. 2023; 28(17):6263. https://doi.org/10.3390/molecules28176263
Chicago/Turabian StyleMousavi, Homa, María Eugenia García-Rubiño, Duane Choquesillo-Lazarte, Alfonso Castiñeiras, Luis Lezama, Antonio Frontera, and Juan Niclós-Gutiérrez. 2023. "H(N3)dap (Hdap = 2,6-Diaminopurine) Recognition by Cu2(EGTA): Structure, Physical Properties, and Density Functional Theory Calculations of [Cu4(μ-EGTA)2(μ-H(N3)dap)2(H2O)2]·7H2O" Molecules 28, no. 17: 6263. https://doi.org/10.3390/molecules28176263
APA StyleMousavi, H., García-Rubiño, M. E., Choquesillo-Lazarte, D., Castiñeiras, A., Lezama, L., Frontera, A., & Niclós-Gutiérrez, J. (2023). H(N3)dap (Hdap = 2,6-Diaminopurine) Recognition by Cu2(EGTA): Structure, Physical Properties, and Density Functional Theory Calculations of [Cu4(μ-EGTA)2(μ-H(N3)dap)2(H2O)2]·7H2O. Molecules, 28(17), 6263. https://doi.org/10.3390/molecules28176263







