Role of Delocalization, Asymmetric Distribution of π-Electrons and Elongated Conjugation System for Enhancement of NLO Response of Open Form of Spiropyran-Based Thermochromes
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
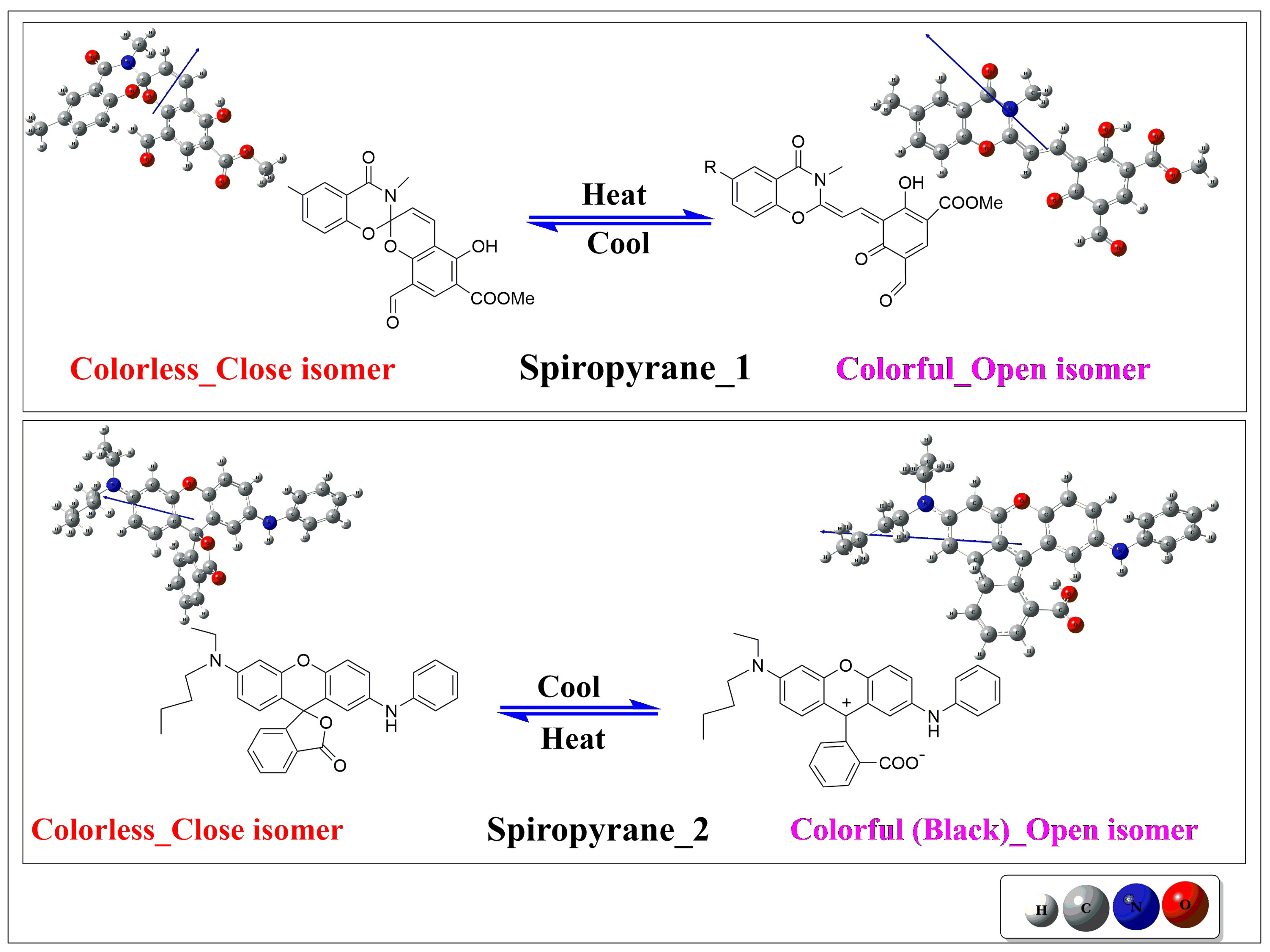
3.1. Geometric and Structural Properties of Close and Open Isomers of Spiropyranes
3.2. NBO Charge Analysis of Close and Open Isomers of Spiropyranes (1 & 2)
3.3. Dipole Moment of Close and Open and Isomers of Spiropyranes (1 & 2)
3.4. Frontier Molecular Orbitals (FMOs) Analysis of Close and Open Isomers of Spiropyranes (1 & 2)
3.5. Linear and Nonlinear Optical (NLO) Properties of Close and Open Isomers of Spiropyranes (1 & 2)
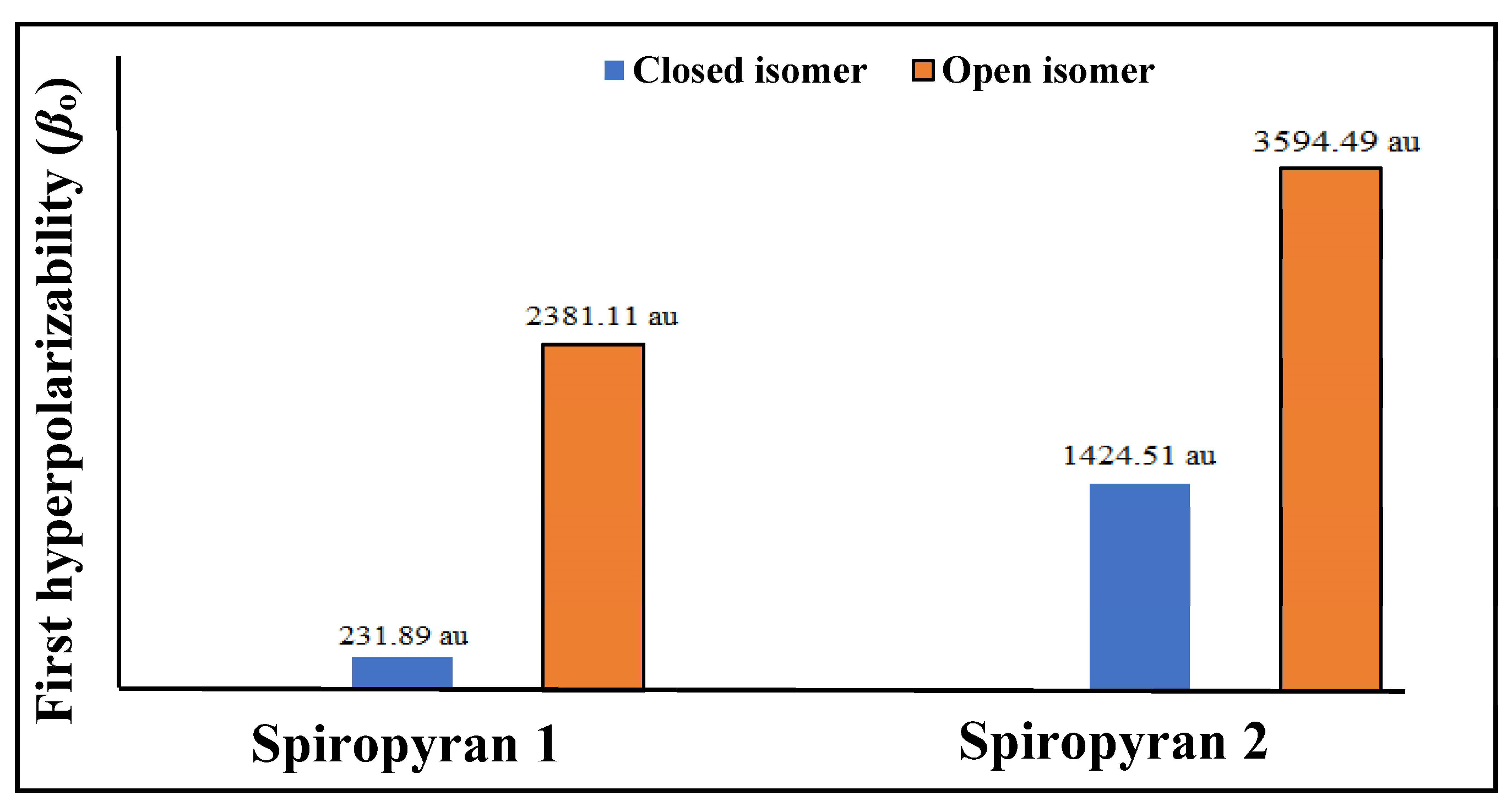
3.5.1. Polarizability (αo) and First Hyperpolarizability (βo) Analyses
3.5.2. Two-Level Model Analysis of Close and Open Isomers of Spiropyranes 1 & 2
3.5.3. Frequency-Dependent First Hyperpolarizability of Close and Open Isomers of Spiropyranes 1 & 2
3.5.4. Frequency-Dependent Second Hyperpolarizability and Quadratic Nonlinear Refractive Index Analysis of Spiropyranes 1 & 2
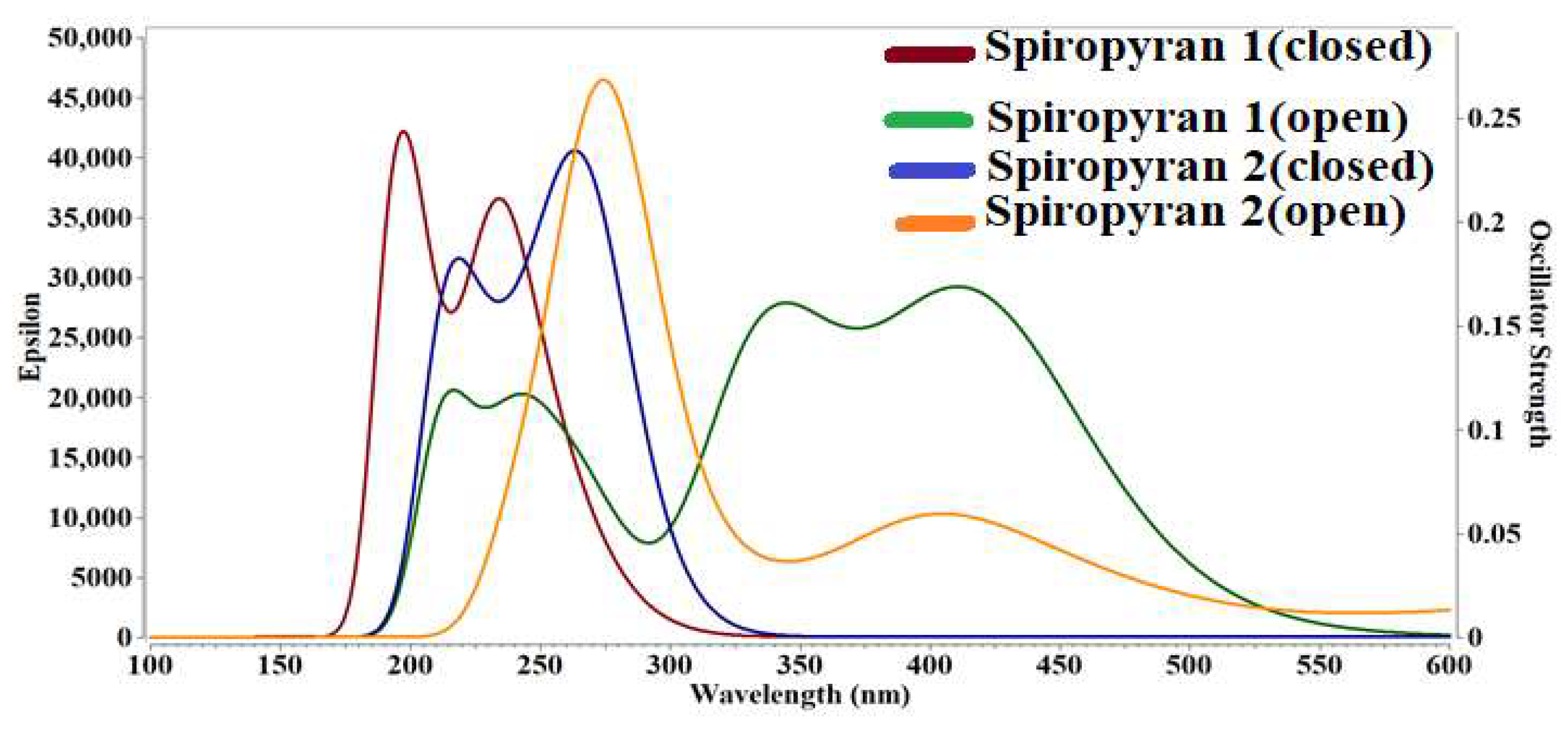
3.6. Absorption Spectra for Close and Open Isomers of Spiropyranes 1 & 2
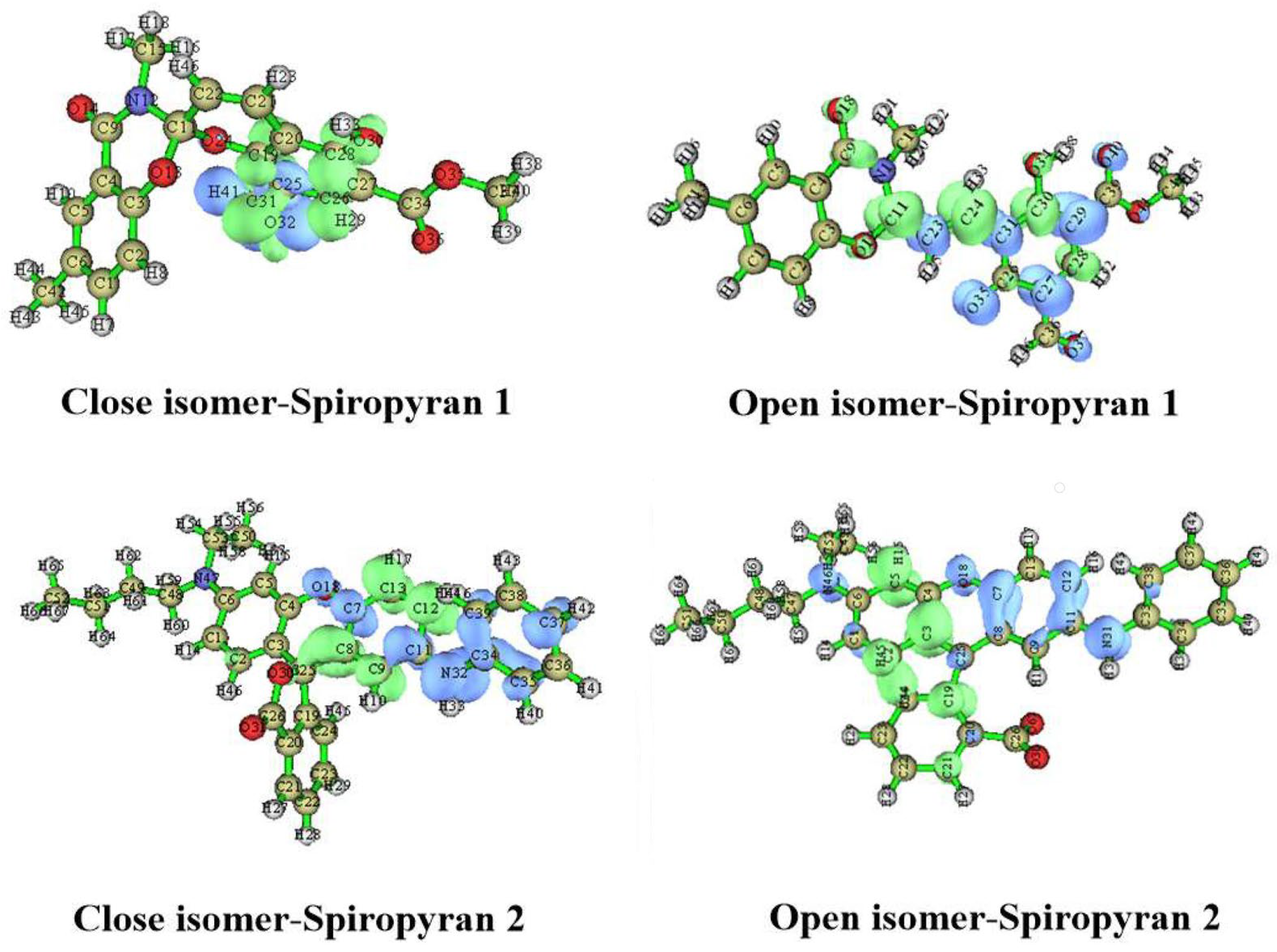
3.7. The Charge-Transfer Distance or Density Difference between Ground and Excited States for Close and Open Isomers of Spiropyranes 1 & 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chowdhury, M.A.; Joshi, M.; Butola, B.S. Photochromic and Thermochromic Colorants in Textile Applications. J. Eng. Fibers Fabr. 2014, 9, 107–123. [Google Scholar] [CrossRef]
- Study, A.S.A.; Francesco, B.Y. Thermal and Photochemical Interconversion of Spiropyrans and Merocyanines. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1984, 80, 1513–1527. [Google Scholar]
- Kao, H.-L.; Mohan, M.; Schmandt, C.; Paradiso, J.A.; Vega, K. ChromoSkin. In Proceedings of the 2016 CHI Conference Extended Abstracts on Human Factors in Computing Systems, San Jose, CA, USA, 7–12 May 2016; Association for Computing Machinery: New York, NY, USA; pp. 3703–3706. [Google Scholar]
- Lewit, E.M. An Evaluation of a Plastic Strip Thermometer. J. Am. Med. Assoc. 1982, 247, 321. [Google Scholar] [CrossRef]
- Dawson, T.L. Changing Colours: Now You See Them, Now You Don’t. Color. Technol. 2010, 126, 177–188. [Google Scholar] [CrossRef]
- Yoo, W.J.; Seo, J.K.; Jang, K.W.; Heo, J.Y.; Moon, J.S.; Park, J.-Y.; Park, B.G.; Lee, B. Fabrication and Comparison of Thermochromic Material-Based Fiber-Optic Sensors for Monitoring the Temperature of Water. Opt. Rev. 2011, 18, 144–148. [Google Scholar] [CrossRef]
- Dzisah, P.; Sadoh, A.; Ravindra, N.M. 3D Printing of Polymer-Based Gasochromic, Thermochromic and Piezochromic Sensors. In Proceedings of the TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings, San Antonio, TX, USA, 10–14 March 2019; pp. 1545–1561. [Google Scholar]
- Yang, W.-J.; Yang, P.P.T. Literature Survey on Biomedical Applications of Thermography. Biomed. Mater. Eng. 1992, 2, 7–18. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Fang, C.; Chen, J.; Wang, Z. Discoloration Mechanism, Structures and Recent Applications of Thermochromic Materials via Different Methods: A Review. J. Mater. Sci. Technol. 2018, 34, 2225–2234. [Google Scholar] [CrossRef]
- Seeboth, A.; Lötzsch, D.; Ruhmann, R.; Muehling, O. Thermochromic Polymers—Function by Design. Chem. Rev. 2014, 114, 3037–3068. [Google Scholar] [CrossRef]
- Panák, O.; Držková, M.; Kaplanová, M.; Novak, U.; Klanjšek Gunde, M. The Relation between Colour and Structural Changes in Thermochromic Systems Comprising Crystal Violet Lactone, Bisphenol A, and Tetradecanol. Dye. Pigment. 2017, 136, 382–389. [Google Scholar] [CrossRef]
- Towns, A.D. Thermochromic Composite Materials Formulated from Spirolactone Colour Formers. In Proceedings of the ChemiChromics USA 99, New Orleans, LA, USA, 20 January 1999; pp. 1171–1182. [Google Scholar]
- Rosario, R.; Gust, D.; Hayes, M.; Jahnke, F.; Springer, J.; Garcia, A.A. Photon-Modulated Wettability Changes on Spiropyran-Coated Surfaces. Langmuir 2002, 18, 8062–8069. [Google Scholar] [CrossRef]
- Lewis, K.L.; Pitt, A.M.; Wyatt-Davies, T.; Milward, J.R. Thin Film Thermochromic Materials for Non-Linear Optical Devices. MRS Online Proc. Libr. 1994, 374, 105. [Google Scholar] [CrossRef]
- Huggins, K.E.; Son, S.; Stupp, S.I. Two-Dimensional Supramolecular Assemblies of a Polydiacetylene. 1. Synthesis, Structure, and Third-Order Nonlinear Optical Properties. Macromolecules 1997, 30, 5305–5312. [Google Scholar] [CrossRef]
- Nasr, M.; Gomaa, H.M.; Yahia, I.S.; Saleh, H.A. Novel Thermochromic (TC) and Electrochromic (EC) Characteristics of the V4O7 Liquid Crystal for LCDs and Versatile Optoelectronic Applications. J. Mol. Liq. 2021, 330, 115620. [Google Scholar] [CrossRef]
- Drozdowski, D.; Gągor, A.; Stefańska, D.; Zarȩba, J.K.; Fedoruk, K.; Mączka, M.; Sieradzki, A. Three-Dimensional Methylhydrazinium Lead Halide Perovskites: Structural Changes and Effects on Dielectric, Linear, and Nonlinear Optical Properties Entailed by the Halide Tuning. J. Phys. Chem. C 2022, 126, 1600–1610. [Google Scholar] [CrossRef]
- Fan, H.; Lin, C.; Chen, K.; Peng, G.; Li, B.; Zhang, G.; Long, X.; Ye, N. (NH4)Bi2(IO3)2F5: An Unusual Ammonium-Containing Metal Iodate Fluoride Showing Strong Second Harmonic Generation Response and Thermochromic Behavior. Angew. Chem. Int. Ed. 2020, 59, 5268–5272. [Google Scholar] [CrossRef]
- Mączka, M.; Zaręba, J.K.; Gągor, A.; Stefańska, D.; Ptak, M.; Roleder, K.; Kajewski, D.; Soszyński, A.; Fedoruk, K.; Sieradzki, A. [Methylhydrazinium]2PbBr4, a Ferroelectric Hybrid Organic–Inorganic Perovskite with Multiple Nonlinear Optical Outputs. Chem. Mater. 2021, 33, 2331–2342. [Google Scholar] [CrossRef]
- Parthasarathy, V.; Pandey, R.; Das, P.K.; Castet, F.; Blanchard-Desce, M. Linear and Nonlinear Optical Properties of Tricyanopropylidene-Based Merocyanine Dyes: Synergistic Experimental and Theoretical Investigations. ChemPhysChem 2018, 19, 187–197. [Google Scholar] [CrossRef]
- Previtali, A.; He, W.; Forni, A.; Malpicci, D.; Lucenti, E.; Marinotto, D.; Carlucci, L.; Mercandelli, P.; Ortenzi, M.A.; Terraneo, G.; et al. Tunable Linear and Nonlinear Optical Properties from Room Temperature Phosphorescent Cyclic Triimidazole-Pyrene Bio-Probe. Chem. A Eur. J. 2021, 27, 16690–16700. [Google Scholar] [CrossRef]
- Sliwa, M.; Létard, S.; Malfant, I.; Nierlich, M.; Lacroix, P.G.; Asahi, T.; Masuhara, H.; Yu, P.; Nakatani, K. Design, Synthesis, Structural and Nonlinear Optical Properties of Photochromic Crystals: Toward Reversible Molecular Switches. Chem. Mater. 2005, 17, 4727–4735. [Google Scholar] [CrossRef]
- Tamaoki, N.; Van Keuren, E.; Matsuda, H.; Hasegawa, K.; Yamaoka, T. Photoreversible Optical Nonlinearities of Polymeric Films Containing Spiropyran with Long Alkyl Chains. Appl. Phys. Lett. 1996, 69, 1188–1190. [Google Scholar] [CrossRef]
- Berkovic, G.; Krongauz, V.; Weiss, V. Spiropyrans and Spirooxazines for Memories and Switches. Chem. Rev. 2000, 100, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Aubert, V.; Guerchais, V.; Ishow, E.; Hoang-Thi, K.; Ledoux, I.; Nakatani, K.; Le Bozec, H. Efficient Photoswitching of the Nonlinear Optical Properties of Dipolar Photochromic Zinc(II) Complexes. Angew. Chem. Int. Ed. 2008, 47, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Guerchais, V.; Ordronneau, L.; Le Bozec, H. Recent Developments in the Field of Metal Complexes Containing Photochromic Ligands: Modulation of Linear and Nonlinear Optical Properties. Coord. Chem. Rev. 2010, 254, 2533–2545. [Google Scholar] [CrossRef]
- Jing, L.-X.; Wang, L.; Ye, J.-T.; Chen, Z.-Z.; Chen, H.; Qiu, Y.-Q. Theoretical Investigation on Second-Order Nonlinear Optical Properties of Ruthenium Alkynyl–Dihydroazulene/Vinylheptafulvene Complexes. J. Mol. Graph. Model. 2017, 77, 363–371. [Google Scholar] [CrossRef]
- Okuno, K.; Shigeta, Y.; Kishi, R.; Nakano, M. Photochromic Switching of Diradical Character: Design of Efficient Nonlinear Optical Switches. J. Phys. Chem. Lett. 2013, 4, 2418–2422. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Tkachev, V.V.; Lukyanov, B.S.; Mukhanov, E.L.; Rostovtseva, I.A.; Lukyanova, M.B.; Shilov, G.V.; Strekal, N.D.; Aldoshin, S.M.; Minkin, V.I. Synthesis, Structure and Photochromic Properties of Novel Highly Functionalized Spiropyrans of 1,3-Benzoxazin-4-One Series. J. Mol. Struct. 2018, 1161, 18–25. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Dong, N.; Li, Z. Fabrication and Characterization of Reversible Thermochromic Wood Veneers. Sci. Rep. 2017, 7, 16933. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, UK, 2009. [Google Scholar]
- Ozhogin, I.V.; Dorogan, I.V.; Lukyanov, B.S.; Mukhanov, E.L.; Tkachev, V.V.; Chernyshev, A.V.; Lukyanova, M.B.; Aldoshin, S.M.; Minkin, V.I. New Spiropyrans Based on 1,3-Benzoxazine-2-One: Acid Catalyzed Synthesis and Theoretical Insight into the Photochromic Activity. Tetrahedron Lett. 2016, 57, 2382–2385. [Google Scholar] [CrossRef]
- Plaquet, A.; Guillaume, M.; Champagne, B.; Castet, F.; Ducasse, L.; Pozzo, J.-L.; Rodriguez, V. In Silico Optimization of Merocyanine-Spiropyran Compounds as Second-Order Nonlinear Optical Molecular Switches. Phys. Chem. Chem. Phys. 2008, 10, 6223. [Google Scholar] [CrossRef]
- Vaez, Z.; Javanbakht, V. Synthesis, Characterization and Photocatalytic Activity of ZSM-5/ZnO Nanocomposite Modified by Ag Nanoparticles for Methyl Orange Degradation. J. Photochem. Photobiol. A Chem. 2020, 388, 112064. [Google Scholar] [CrossRef]
- Dennington, R.I.; Keith, T.; Millam, J. GaussView, Version 5.0.8; Semichem, Inc.: Shawnee Mission, KS, USA, 2008. [Google Scholar]
- Reed, A.E.; Weinhold, F. Natural Bond Orbital Analysis of Near-Hartree–Fock Water Dimer. J. Chem. Phys. 1983, 78, 4066–4073. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S. A Benzothiazolinic Spiropyran for Highly Selective, Sensitive and Visible Light Controlled Detection of Copper Ions in Aqueous Solution. J. Photochem. Photobiol. A Chem. 2020, 390, 112265. [Google Scholar] [CrossRef]
- Hou, X.-F.; Chen, X.-M.; Bisoyi, H.K.; Qi, Q.; Xu, T.; Chen, D.; Li, Q. Light-Driven Aqueous Dissipative Pseudorotaxanes with Tunable Fluorescence Enabling Deformable Nano-Assemblies. ACS Appl. Mater. Interfaces 2023, 15, 11004–11015. [Google Scholar] [CrossRef]
- Kosar, N.; Wajid, S.; Ayub, K.; Gilani, M.A.; Mahmood, T. First, Second and Third Order NLO Response of Alkaline Earth Metals Doped C6O6Li6 Organometallic Complexes. Chem. Phys. 2023, 570, 111894. [Google Scholar] [CrossRef]
- Kosar, N.; Wajid, S.; Ayub, K.; Mahmood, T. Excellent Static and Dynamic Hyperpolarizabilities of TM@C6O6Li6 (TM=Sc, Ti, V, Cr and Mn) Complexes to Prove Their NLO Applications. Optik 2023, 276, 170660. [Google Scholar] [CrossRef]
- Menzonatto, T.G.; Lopes, J.F. The Role of Intramolecular Interactions on the Stability of the Conformers of a Spiropyran Derivative. Chem. Phys. 2022, 562, 111654. [Google Scholar] [CrossRef]
- Dorogan, I.V.; Minkin, V.I. Theoretical Modeling of Electrocyclic 2H-Pyran and 2H-1,4-Oxazine Ring Opening Reactions in Photo- and Thermochromic Spiropyrans and Spirooxazines. Chem. Heterocycl. Compd. 2016, 52, 730–735. [Google Scholar] [CrossRef]
- Kudernac, T.; Katsonis, N.; Browne, W.R.; Feringa, B.L. Nano-Electronic Switches: Light-Induced Switching of the Conductance of Molecular Systems. J. Mater. Chem. 2009, 19, 7168. [Google Scholar] [CrossRef]
- Mukhanov, E.L.; Dorogan, I.V.; Chernyshev, A.V.; Bezuglyi, S.O.; Ozhogin, I.V.; Lukyanova, M.B.; Lukyanov, B.S. Theoretical and Experimental Study of New Photochromic Bis-Spiropyrans with Hydroxyethyl and Carboxyethyl Substituents. Int. J. Photoenergy 2013, 2013, 752949. [Google Scholar] [CrossRef]
- van Gisbergen, S.J.A.; Snijders, J.G.; Baerends, E.J. Accurate Density Functional Calculations on Frequency-Dependent Hyperpolarizabilities of Small Molecules. J. Chem. Phys. 1998, 109, 10657–10668. [Google Scholar] [CrossRef]
- Therssen, E.; Bouvier, Y.; Schoemaecker-Moreau, C.; Mercier, X.; Desgroux, P.; Ziskind, M.; Focsa, C. Determination of the Ratio of Soot Refractive Index Function E(m) at the Two Wavelengths 532 and 1064 Nm by Laser Induced Incandescence. Appl. Phys. B 2007, 89, 417–427. [Google Scholar] [CrossRef]
- Tonnelé, C.; Castet, F. Nonlinear Optical Properties of Spirocyclohexadine Photochromes: Insights from DFT Calculations. Photochem. Photobiol. Sci. 2019, 18, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Hättig, C.; Christiansen, O.; Jørgensen, P. Frequency-Dependent Second Hyperpolarizabilities Using Coupled Cluster Cubic Response Theory. Chem. Phys. Lett. 1998, 282, 139–146. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, H.-L.; Su, Z.-M. A Novel Acid-Controlled Second-Order Nonlinear Optical Switch Based on Dimethyldihydropyrene/Cyclophanediene Photoswitch. J. Mater. Chem. C 2022, 10, 12338–12349. [Google Scholar] [CrossRef]
- Bibi, T.; Jadoon, T.; Ayub, K. Two State “ON–OFF” NLO Switch Based on Coordination Complexes of Iron and Cobalt Containing Isomeric Ligand: A DFT Study. RSC Adv. 2022, 12, 23204–23214. [Google Scholar] [CrossRef]
- Roohi, H.; Rostami, T. Mechanism of the Photo Triggered Ring-Opening Reaction of Spiropyran Derivatives (SP-X1-7; X1-7= H, NO2, CF3, CN, OH, OMe and NMe2) in the Gas Phase and Various Solvent Media: A GD3-TD-DFT Approach. J. Photochem. Photobiol. A Chem. 2020, 392, 112410. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]


| Compounds | Isomer | bC-O | Erel | Eact | |e| | |
|---|---|---|---|---|---|---|
| Carbon Atom | Oxygen Atom | |||||
| Spiropyran 1 | close | 1.43 | 0.00 | 12.74 | 0.75 | −0.59 |
| open | 4.11 | 5.78 | 0.64 | −0.63 | ||
| Spiropyran 2 | close | 1.36 | 0.29 | 14.83 | 0.37 | −0.57 |
| open | 4.30 | 0.00 | −0.05 | −0.65 | ||
| Compounds | Isomer | EH | EL | GH-L | µ | αo | βo |
|---|---|---|---|---|---|---|---|
| spiropyran 1 | close | −8.74 | −0.38 | 8.36 | 6.489 | 276 | 231.89 |
| open | −7.75 | −1.41 | 6.34 | 8.783 | 350 | 2381.11 | |
| spiropyran 2 | close | −7.25 | 0.25 | 7.51 | 6.258 | 413 | 1424.51 |
| open | −9.61 | −5.35 | 4.26 | 10.223 | 460 | 3594.49 |
| Compounds | Isomer | ΔE | ƒo | λmax | Δµ |
|---|---|---|---|---|---|
| spiropyran 1 | close | 6.40 | 0.54 | 194 | 6.488 |
| open | 2.97 | 0.68 | 418 | 8.789 | |
| spiropyran 2 | close | 4.62 | 0.41 | 269 | 6.259 |
| open | 4.55 | 0.53 | 272 | 10.227 |
| Compound | Isomer | Frequency | βo (0; 0, 0) | βo (−ω; ω, 0) | βo (−2ω; ω, ω) |
|---|---|---|---|---|---|
| Spiropyran 1 | close | 0 | 231.9 | ||
| 5.63 × 1014 Hz (0.09 au) | 359.6 | 4984.1 | |||
| 2.82 × 1014 Hz (0.04 au) | 255.9 | 317.9 | |||
| open | 0 | 2373.2 | |||
| 5.63 × 1014 Hz (0.09 au) | 6324.6 | 6698.7 | |||
| 2.82 × 1014 Hz (0.04 au) | 2971.4 | 5125.1 | |||
| Spiropyran 2 | close | 0 | 1424.5 | ||
| 5.63 × 1014 Hz (0.09 au) | 2097.8 | 51,645.1 | |||
| 2.82 × 1014 Hz (0.04 au) | 1565.4 | 1893.9 | |||
| open | 0 | 3594.5 | |||
| 5.63 × 1014 Hz (0.09 au) | 22,805.2 | 54,124.8 | |||
| 2.82 × 1014 Hz (0.04 au) | 8774.1 | 23,919.6 |
| Compounds | Isomers | Frequency (in Hz) | γ(0; 0, 0, 0) | γ(−ω; ω, ω, 0) | γ(−2ω; ω, ω, 0) | n2 |
|---|---|---|---|---|---|---|
| Spiropyran 1 | close | 0 | 3564.7 | |||
| 5.63 × 1014 Hz (0.09 au) | 5095.1 | 3037.8 | 3.23 × 10−18 | |||
| 2.82 × 1014 Hz (0.04 au) | 3909.5 | 4636.6 | 1.01 × 10−18 | |||
| open | 0 | 6907.9 | ||||
| 5.63 × 1014 Hz (0.09 au) | 52,842.8 | 2398.2 | 1.99 × 10−17 | |||
| 2.82 × 1014 Hz (0.04 au) | 9030.3 | 23,299.4 | 3.25 × 10−18 | |||
| Spiropyran 2 | close | 0 | 11,533.7 | |||
| 5.63 × 1014 Hz (0.09 au) | 20,011.3 | 1027.6 | 1.11 × 10−17 | |||
| 2.82 × 1014 Hz (0.04 au) | 13,033.1 | 1713.9 | 3.45 × 10−18 | |||
| open | 0 | 27,098.8 | ||||
| 5.63 × 1014 Hz (0.09 au) | 126,583.9 | 11,287.0 | 2.21 × 10−17 | |||
| 2.82 × 1014 Hz (0.04 au) | 120,760.7 | 33,722.1 | 1.50 × 10−17 |
| Compounds | Isomer | ΔE | ƒo | λmax | Δµ | µ | αo | βo |
|---|---|---|---|---|---|---|---|---|
| spiropyran 1 | close | 5.26 | 0.60 | 236 | 8.94 | 8.94 | 373 | 1242.20 |
| open | 5.03 | 0.51 | 246 | 11.74 | 11.73 | 476 | 4876.61 | |
| spiropyran 2 | close | 4.57 | 0.62 | 271 | 8.36 | 8.36 | 553 | 2844.07 |
| open | 4.60 | 0.89 | 270 | 13.91 | 13.91 | 640 | 3578.72 |
| Compounds | Parameters | Ex. 1 | Ex. 2 | Ex. 3 | Ex. 4 | Ex. 5 |
|---|---|---|---|---|---|---|
| spiropyran 1-close isomer | S | 0.21 | 0.51 | 0.45 | 0.53 | 0.17 |
| D | 1.57 | 0.26 | 0.67 | 0.23 | 2.07 | |
| spiropyran 1-open-isomer | S | 0.34 | 0.15 | 0.39 | 0.20 | 0.27 |
| D | 1.89 | 2.36 | 1.11 | 1.92 | 2.84 | |
| spiropyran 2-close isomer | S | 0.26 | 0.30 | 0.37 | 0.20 | 0.25 |
| D | 1.94 | 1.77 | 0.97 | 2.51 | 1.64 | |
| spiropyran 2-open isomer | S | 0.35 | 0.21 | 0.25 | 0.34 | 0.37 |
| D | 2.14 | 1.73 | 2.43 | 2.98 | 1.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosar, N.; Kanwal, S.; Hamid, M.H.S.A.; Ayub, K.; Gilani, M.A.; Imran, M.; Arshad, M.; Alkhalifah, M.A.; Sheikh, N.S.; Mahmood, T. Role of Delocalization, Asymmetric Distribution of π-Electrons and Elongated Conjugation System for Enhancement of NLO Response of Open Form of Spiropyran-Based Thermochromes. Molecules 2023, 28, 6283. https://doi.org/10.3390/molecules28176283
Kosar N, Kanwal S, Hamid MHSA, Ayub K, Gilani MA, Imran M, Arshad M, Alkhalifah MA, Sheikh NS, Mahmood T. Role of Delocalization, Asymmetric Distribution of π-Electrons and Elongated Conjugation System for Enhancement of NLO Response of Open Form of Spiropyran-Based Thermochromes. Molecules. 2023; 28(17):6283. https://doi.org/10.3390/molecules28176283
Chicago/Turabian StyleKosar, Naveen, Saba Kanwal, Malai Haniti S. A. Hamid, Khurshid Ayub, Mazhar Amjad Gilani, Muhammad Imran, Muhammad Arshad, Mohammed A. Alkhalifah, Nadeem S. Sheikh, and Tariq Mahmood. 2023. "Role of Delocalization, Asymmetric Distribution of π-Electrons and Elongated Conjugation System for Enhancement of NLO Response of Open Form of Spiropyran-Based Thermochromes" Molecules 28, no. 17: 6283. https://doi.org/10.3390/molecules28176283






