Enhanced Two-Step Extraction from Biomass of Two Cymbopogon Species Cultivated in Santander, Colombia
Abstract
:1. Introduction
2. Results
2.1. Essential Oil Yields
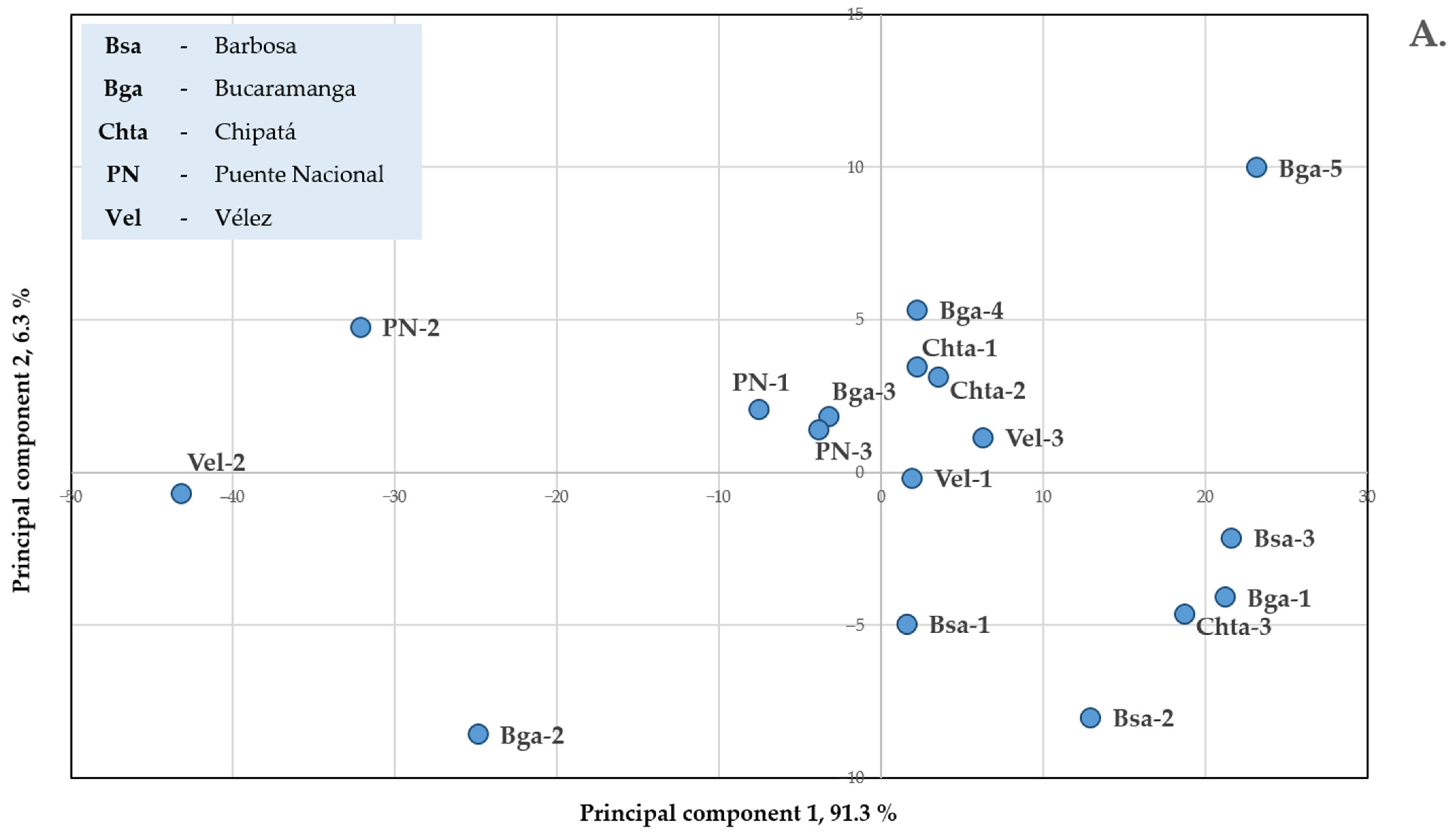
2.2. Chemical Composition of Essential Oils Distilled via MWHD from Plants Harvested from Different Santander Municipalities
2.3. Physicochemical Essential Oil Parameters
2.4. Chemical Composition of Java-Type Citronella and Palmarosa Hydroalcoholic Extracts
2.5. Antioxidant Activity of Cymbopogon sp. Hydroalcoholic Extracts
3. Discussion
4. Materials and Methods
4.1. Chemical Substances and Reagents
4.2. Vegetal Material
4.3. Essential Oil Distillation
4.3.1. Microwave Radiation-Assisted Hydrodistillation
4.3.2. Steam Distillation
4.4. Solvent Extraction
4.5. Essential Oil Physicochemical Properties
4.6. GC/FID/MS Analysis
4.7. UHPLC-ESI-Orbitrap-HRMS Analysis
4.8. Antioxidant Activity
4.8.1. ABTS+● Radical-Cation Decoloration Assay
4.8.2. Evaluation of Oxygen-Radical Absorption Capacity (ORAC)
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| AAPH | 2,2-Azobis-(2-methylpropionamidine) dihydrochloride |
| ABTS | 2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)-diammonium salt assay |
| AT | Agilent Technologies |
| df | Stationary-phase film thickness |
| I.D. | Column internal diameter |
| EIC | Extracted ion current |
| EO(s) | Essential oil(s) |
| ESI | Electrospray ionization |
| eV | Electronvolt |
| FID | Flame ionization detector |
| GC | Gas chromatography |
| GC/MS | Gas chromatography coupled to mass spectrometry |
| HCD | Higher-energy collision dissociation cell |
| HD | Hydrodistillation |
| HRMS | High-resolution mass spectrometry |
| I | Intensity (abundance) |
| ISO | International Organization for Standardization |
| LC | Liquid chromatography |
| LC/MS | Liquid chromatography coupled to mass spectrometry |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| LRIs | Linear retention indices |
| MS | Mass spectrometry or mass spectrum (spectra) |
| MWHD | Microwave-assisted hydrodistillation |
| m/z | Mass-to-charge ratio |
| ORAC | Oxygen-radical absorption capacity assay |
| S.D. | Steam distillation |
| SIM | Selected ion monitoring |
| tR | Retention time (min) |
| UHPLC | Ultra-high-performance liquid chromatography |
References
- Singh, A.; Singh, M.; Singh, K. Productivity and economic viability of a palmarosa–pigeon pea intercropping system in the subtropical climate of north India. J. Agric. Sci. 1998, 130, 149–154. [Google Scholar] [CrossRef]
- Da Silva, L.C.; De Souza, W.M.; Sá, F.A.; De Souza, M.A.; Bitencourt, R.D.; Sanavria, A.; Da Costa Angelo, I. In vitro acaricidal activity of Cymbopogon citratus, Cymbopogon nardus and Mentha arvensis against Rhipicephalus microplus (Acari: Ixodidae). Exp. Parasitol. 2020, 216, 107937. [Google Scholar] [CrossRef]
- Shasany, A.K.; Lal, R.K.; Patra, N.K.; Darokar, M.P.; Garg, A.; Kumar, S.; Khanuja, S.P.S. Phenotypic and RAPD diversity among Cymbopogon winterianus Jowitt accessions in relation to Cymbopogon nardus Rendle. Genet. Resour. Crop Evol. 2000, 47, 553–559. [Google Scholar] [CrossRef]
- Mota, T.F.; Caliene, M.D.; Conceição, M.D.; Fraga, D.B.; Brodskyn, C.I.; Moysés, F.D.; Magalhães-Júnior, J.T. Screening organic repellent compounds against Lutzomyia longipalpis (Diptera: Psychodidae) present in plant essential oils: Bioassay plus an in silico approach. Acta Trop. 2022, 229, 106367. [Google Scholar] [CrossRef] [PubMed]
- Cansian, R.L.; Staudt, A.; Bernardi, J.L.; Puton, B.M.S.; Oliveira, D.; De Oliveira, J.V.; Paroul, N. Toxicity and larvicidal activity on Aedes aegypti of citronella essential oil submitted to enzymatic esterification. Braz. J. Biol. 2021, 83, e244647. [Google Scholar] [CrossRef]
- Tran, T.H.; Pham, T.N.; Ngo, T.C.; Le, T.H.; Mai, H.C.; Do, T.S.; Tran, T.K. Formulation of a floor cleaning product using lemongrass (Cymbopogon citratus) essential oil and evaluation of foam ability and foam durability. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012132. [Google Scholar] [CrossRef]
- Plescia, M.; Ganz, R.; Saremi, M.E. Deodorant Compositions. U.S. Patent 2022/0183960 A1, 16 June 2022. [Google Scholar]
- Pham, L. Multipurpose Mosquito Repellant Composition and Method of Manufacturing the Same. U.S. Patent 11,395,496 B2, 26 July 2022. [Google Scholar]
- Jijakli, H.; Dal Maso, S.; Parisi, O. Bio-Herbicide Based on Essential Oil. U.S. Patent 2021/0251218 A1, 19 August 2021. [Google Scholar]
- Gregoris, R. Cosmetic Composition. U.S. Patent 11,013,681 B2, 25 May 2021. [Google Scholar]
- Enan, E. Synergistic Pest-Control Compositions. U.S. Patent 8,734,869 B2, 27 May 2014. [Google Scholar]
- Nandapure, S.P.; Wankhade, S.G.; Jadhao, S.M.; Bhoyar, S.M.; Wanjari, S.S.; Sarode, R.B. Quality parameters of citronella Java oil as influenced by nutrient management under inceptisols. J. Trop. Agric. 2016, 34, 579–584. [Google Scholar]
- Prakasa, R.; Singh, M.; Ganesha, R. Effect of plant spacing and application of nitrogen fertilizer on herb and essential oil yields of palmarosa (Cymbopogon martini Stapf. var. Motia). J. Agric. Sci. 1985, 104, 67–70. [Google Scholar]
- Rao, R.; Sing, K.; Kaul, P.; Bhattachapya, K. Response of palmarosa (Cymbopogon martinii (Roxb.) Wats. var. Motia Burk.) to plant spacings and nitrogen fertilizer application. J. Trop. Agric. 1990, 8, 177–183. [Google Scholar]
- Verma, R.S.; Verma, S.K.; Tandon, S.; Padalia, R.C.; Darokar, M.P. Chemical composition and antimicrobial activity of citronella Java (Cymbopogon winterianus Jowitt ex Bor) essential oil extracted by different methods. J. Essent. Oil Res. 2020, 32, 449–455. [Google Scholar] [CrossRef]
- ISO 3848; Essential Oil of Citronella Java Type. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 4727; Essential Oils of Palmarosa [Cymbopogon martini (Roxb.) W. Watson var. Motia]. International Organization for Standardization: Geneva, Switzerland, 2021.
- Boruah, T.; Barman, A.; Kalita, P.; Lahkar, J.; Deka, H. Vermicomposting of citronella bagasse and paper mill sludge mixture employing Eisenia Fetida. Bioresour. Technol. 2019, 294, 122147. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, Y.; Yadav, V.; Nigam, N.; Yadav, A.; Khare, P. Quality of bio-oil by pyrolysis of distilled spent of Cymbopogon flexuosus. J. Anal Appl Pyrol. 2015, 115, 43–50. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Shanaida, M.; Hudz, N.; Wieczorek, P.P. Phytochemical and pharmacological evaluation of the residue by-product developed from the Ocimum americanum (Lamiaceae) postdistillation waste. Foods 2021, 10, 3063. [Google Scholar] [CrossRef] [PubMed]
- Turrini, F.; Beruto, M.; Mela, L.; Curir, P.; Triglia, G.; Boggia, R.; Monroy, F. Ultrasound-assisted extraction of lavender (Lavandula angustifolia Miller, cultivar rosa) solid by-products remaining after the distillation of the essential oil. Appl. Sci. 2021, 11, 5495. [Google Scholar] [CrossRef]
- Slavov, A.; Vasileva, I.; Denev, P.; Dinkova, R.; Teneva, D.; Ognyanov, M.; Georgiev, Y. Polyphenol-rich extracts from essential oil industry wastes. Bulg. Chem. Commun. 2021, 52, 78–83. [Google Scholar]
- Iwashina, T. Flavonoid function and activity to plants and other organisms. Biol. Sci. Space. 2003, 17, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Kim, C.; Park, J.; Lee, H.; Hwang, D.Y.; Park, S.H.; Lee, H. Evaluation of the EtOAc extract of lemongrass (Cymbopogon citratus) as a potential skincare cosmetic material for acne vulgaris. J. Microbiol. Biotecnol. 2022, 32, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Costa, G.; Figueirinha, A.; Marques, C.; Pereira, P.; Neves, B.M.; Batista, M.T. Anti-inflammatory activity of Cymbopogon citratus leaves infusion via proteasome and nuclear factor-κB pathway inhibition: Contribution of chlorogenic acid. J. Ethnopharmacol. 2013, 148, 126–134. [Google Scholar] [CrossRef]
- Gebashe, F.; Aremu, A.O.; Gruz, J.; Finnie, J.F.; Van Staden, J. Phytochemical profiles and antioxidant activity of grasses used in South African traditional medicine. Plants 2020, 9, 371. [Google Scholar] [CrossRef]
- Rizk, F.M.; Ismail, S.I.; Kamel, A.S.; Rimpler, H. Constituents of plants growing in Qatar part XXVII: Flavonoids of Cymbopogonparkeri. Qatar Univ. Sci. J. 1995, 15, 33–35. [Google Scholar]
- Cheel, J.; Theoduloz, C.; Rodríguez, J.; Schmeda-Hirschmann, G. Free radical scavengers and antioxidants from lemongrass (Cymbopogon citratus (DC.) Stapf.). J. Agric. Food Chem. 2005, 53, 2511–2517. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Jalali-Heravi, M.; Zekavat, B.; Sereshti, H. Characterization of essential oil components of lranian geranium oil using gas chromatography-mass spectrometry combined with chemometric resolution techniques. J. Chromatogr. A 2006, 1114, 154–163. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Punruckvong, A.; Bean, A.R.; Forster, P.I.; Lepschi, B.J.; Rozefelds, A.C. Leaf essential oils of the genus Leptospermum (Myrtaceae) in eastern Australia. Part 7. Leptospermum petersonii, L. liversidgei and allies. Flavour Fragr. J. 2000, 15, 342–351. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; pp. 1–804. [Google Scholar]
- NIST Standard Reference Database. NIST/EPA/NIH Spectral Library with Search Program, Version 2.3; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017. [Google Scholar]
- McLafferty, F.W.; Douglas, B.S. The Wiley/NBS Registry of Mass Spectral Data, 2nd ed.; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Wishart, D.S.; Guo, A.C.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Gautam, V. HMDB 5.0: The Human Metabolome DataBase. Nucleic Acids Res. 2022, 50, D622–D631. Available online: www.hmdb.ca (accessed on 30 July 2022). [CrossRef]
- PhytoChemical Interactions Database. Available online: www.genome.jp/db/pcidb (accessed on 30 July 2022).
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Medicinal plants: Factors of influence on the content of secondary metabolites. Quim. Nova. 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Kumar, A.; Jnanesha, A.C.; Lal, R.K.; Chanotiya, C.S.; Srivastava, S.; Pant, Y. Biplot investigation for essential oil yield and chemical compositions under the Deccan Plateau region of southern India in cultivars of citronella Java (Cymbopogon winterianus Jowitt). Ind. Crops Prod. 2022, 175, 114249. [Google Scholar] [CrossRef]
- Chauhan, N.K.; Semwal, M.P.; Singh, D.; Singh, B.; Rauthan, S. Influence of various plant spacing on growth, herbage yield, essential oil yield and aroma content of palmarosa (Cymbopogon martinii Roxb.) at different harvest under agro-climatic condition of Doon valley. J. Essent. Oil-Bear. Plants 2017, 20, 1587–1593. [Google Scholar] [CrossRef]
- Camacho, S.C.; Carandang, A.P.; Camacho, L.D.; Gevaña, D.T.; Carandang, M.G.; Reynaldo, E.; Lorida, R.E.; Bandian, M.N. Economic potential of small-scale citronella (Cymbopogon winterianus) production in the Philippines. Philipp. J. Crop Sci. 2015, 40, 73–81. [Google Scholar]
- Raina, V.K.; Srivastava, S.K.; Aggarwal, K.K.; Syamasundar, K.V.; Khanuja, S.P. Essential oil composition of Cymbopogonmartinii from different places in India. Flavour Fragr. J. 2003, 18, 312–315. [Google Scholar] [CrossRef]
- Daba, A.; Tadesse, M.; Habte, G.; Negawo, A.T.; Berecha, G. Phytochemical composition of essential oils from aromatic plants inherited with bioherbicidal activity in arabica coffee production system of Ethiopia. J. Agric. Res. 2020, 10, 100368. [Google Scholar] [CrossRef]
- Romana, P.; Marimuthu, G. The essential oil from Zanthoxylum monophyllum a potential mosquito larvicide with low toxicity to the non-target fish Gambusia affinis. J. Pest Sci. 2017, 90, 369–378. [Google Scholar]
- De Lima Albuquerque, B.; Da Silva, M.; Da Silva, P.; Pimentel, C.; Da Rocha, S.; De Oliveira, J.; Navarro, D. Oviposition deterrence, larvicidal activity and docking of β-germacrene-D-4-ol obtained from leaves of Piper corcovadensis (Piperaceae) against Aedes aegypti. Ind. Crops Prod. 2020, 182, 114830. [Google Scholar] [CrossRef]
- Rajeswara, B.; Rajput, D.; Patel, R. Essential oil profiles of different parts of palmarosa [Cymbopogon martinii (Roxb.) Wats. var. motia Burk.]. J. Essent Oil Res. 2009, 21, 519–521. [Google Scholar] [CrossRef]
- Scherer, R.; Wagner, R.; Duarte, M.; Godoy, H. Composição e atividades antioxidante e antimicrobiana dos óleos essenciais de cravo-da-índia, citronela e palmarosa. Rev. Bras. Plantas Med. 2009, 11, 442–449. [Google Scholar] [CrossRef]
- Devi, M.A.; Sahoo, D.; Singh, T.B.; Rajashekar, Y. Toxicity, repellency and chemical composition of essential oils from Cymbopogon species against red flour beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). J. Food Prot. 2020, 15, 181–191. [Google Scholar]
- Pereira, M.; Andrade, F.; Queiroga, G.; Macena, G.; Oliveira, F.; Oliveira, I. Antimicrobial activity of geraniol: An integrative review. J. Essent Oil Res. 2020, 32, 187–197. [Google Scholar]
- Bard, M.; Albrecht, M.; Gupta, N.; Guynn, C.; Stillwell, W. Geraniol interferes with membrane functions in strains of Candida and Saccharomyces. J. Anal. Sci. Technol. 1988, 23, 534–538. [Google Scholar]
- Nurain, A.; Noriham, A.; Zainon, M.N.; Wan Saidatul, W.S.; Khairusy, S.Z. Comparative study of aqueous and ethanolic aromatic malaysian herbs extracts using four antioxidant activity assays. Int. J. Agric. Res. 2013, 8, 55–66. [Google Scholar] [CrossRef]
- Clain, E.; Baranauskienė, R.; Kraujalis, P.; Šipailienė, A.; Maždžierienė, R.; Kazernavičiūtė, R.; Venskutonis, P.R. Biorefining of Cymbopogon nardus from Reunion Island into essential oil and antioxidant fractions by conventional and high-pressure extraction methods. Ind. Crops Prod. 2018, 126, 158–167. [Google Scholar] [CrossRef]
- Geng, P.; Sun, J.; Zhang, M.; Li, X.; Harnly, J.M.; Chen, P. Comprehensive characterization of C-glycosyl flavones in wheat (Triticum aestivum L.) germ using UPLC-PDA-ESI/HRMSn and mass defect filtering. J. Mass Spectrom. 2016, 51, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Deng, Z.; Zhang, J.; Dong, H.; Wang, W.; Xing, B.; Liu, X. Comparison of flavonoid o-glycoside, C-glycoside and their aglycones on antioxidant capacity and metabolism during in vitro digestion and in vivo. Foods 2022, 11, 882. [Google Scholar] [CrossRef]
- Cao, J.; Yin, C.; Qin, Y.; Cheng, Z.; Chen, D. Approach to the study of flavone di-C-glycosides by high performance liquid chromatography-tandem ion trap mass spectrometry and its application to characterization of flavonoid composition in Viola yedoensis. J Mass Spectrom. 2014, 49, 1010–1024. [Google Scholar] [CrossRef] [PubMed]
- Borges, P.H.; Pedreiro, S.; Baptista, S.J.; Geraldes, C.F.; Batista, M.T.; Silva, M.M.; Figueirinha, A. Inhibition of α-glucosidase by flavonoids of Cymbopogon citratus (DC) Stapf. J. Ethnopharmacol. 2021, 280, 114470. [Google Scholar] [CrossRef]
- De Toledo, L.; Ramos, M.; Spósito, L.; Castilho, E.; Pavan, F.; Lopes, É.; De Almeida, M. Profiling the Cymbopogon nardus ethanol extract and its antifungal potential against Candida species with different patterns of resistance. J. Braz. Chem. Soc. 2020, 31, 1926–1938. [Google Scholar] [CrossRef]
- Rita, I.; Pereira, C.; Barros, L.; Ferreira, I.C. Exploring reserve lots of Cymbopogon citratus, Aloysia citrodora and Thymus x citriodorus as improved sources of phenolic compounds. Food Chem. 2018, 257, 83–89. [Google Scholar] [CrossRef]
- Tsai, T.H.; Tsai, T.H.; Chien, Y.C.; Lee, C.W.; Tsai, P.J. In vitro antimicrobial activities against cariogenic streptococci and their antioxidant capacities: A comparative study of green tea versus different herbs. Food Chem. 2008, 110, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Marques, V.; Farah, A. Chlorogenic acids and related compounds in medicinal plants and infusions. Food Chem. 2009, 113, 1370–1376. [Google Scholar] [CrossRef]
- Folador, P.; Cazarolli, L.H.; Gazola, A.C.; Reginatto, F.H.; Schenkel, E.P.; Silva, F. Potential insulin secretagogue effects of isovitexin and swertisin isolated from Wilbrandia ebracteata roots in non-diabetic rats. Fitoterapia 2010, 81, 1180–1187. [Google Scholar] [CrossRef]
- Ragone, M.I.; Sella, M.; Conforti, P.; Volonté, M.G.; Consolini, A.E. The spasmolytic effect of Aloysia citriodora, Palau (South American cedrón) is partially due to its vitexin but not isovitexin on rat duodenums. J. Ethnopharmacol. 2007, 113, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Van Hoyweghen, L.; Karalic, I.; Van Calenbergh, S.; Deforce, D.; Heyerick, A. Antioxidant flavone glycosides from the leaves of Fargesia robusta. J. Nat. Prod. 2010, 73, 1573–1577. [Google Scholar] [CrossRef]
- Shie, J.J.; Chen, C.A.; Lin, C.C.; Ku, A.F.; Cheng, T.J.R.; Fang, J.M.; Wong, C.H. Regioselective synthesis of di-C-glycosylflavones possessing anti-inflammation activities. Org. Biomol. Chem. 2010, 8, 4451–4462. [Google Scholar] [CrossRef]
- Gorzalczany, S.; Marrassini, C.; Miño, J.; Acevedo, C.; Ferraro, G. Antinociceptive activity of ethanolic extract and isolated compounds of Urtica circularis. J. Ethnopharmacol. 2011, 134, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.M.; Zucolotto, S.M.; Reginatto, F.H.; Schenkel, E.P.; De Lima, T. Neuropharmacological activity of the pericarp of Passiflora edulis flavicarpa Degener: Putative involvement of C-glycosylflavonoids. Exp. Biol. Med. 2009, 234, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Fernandes, F.A.; de Brito, E.S.; Sousa, A.D.; Narain, N. Ultrasound extraction of phenolics and anthocyanins from jabuticaba peel. Ind. Crops Prod. 2015, 69, 400–407. [Google Scholar] [CrossRef]
- ISO 279; Essential Oils: Determination of Relative Density at 20 °C. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO 875; Essential Oils: Evaluation of Miscibility in Etanol. International Organization for Standardization: Geneva, Switzerland, 1999.
- ISO 592; Essential Oils: Determination of Optical Rotation. 2nd ed. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO/TR 11018; Essential Oils: General Guidance on the Determination of Flashpoint. International Organization for Standardization: Geneva, Switzerland, 1997.
- ISO 11021; Essential Oils: Determination of Water Content. Karl Fischer Method. International Organization for Standardization: Geneva, Switzerland, 1999.
- ISO 1041; Essential Oils: Determination of Freezing Point. International Organization for Standardization: Geneva, Switzerland, 1973.
- ISO 280; Essential Oils: Determination of Refractive Index. 2nd ed. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO 1242; Essential Oils: Determination of Acid Value. International Organization for Standardization: Geneva, Switzerland, 1999.
- ISO 709; Essential Oils: Determination of Ester Value. International Organization for Standardization: Geneva, Switzerland, 2001.

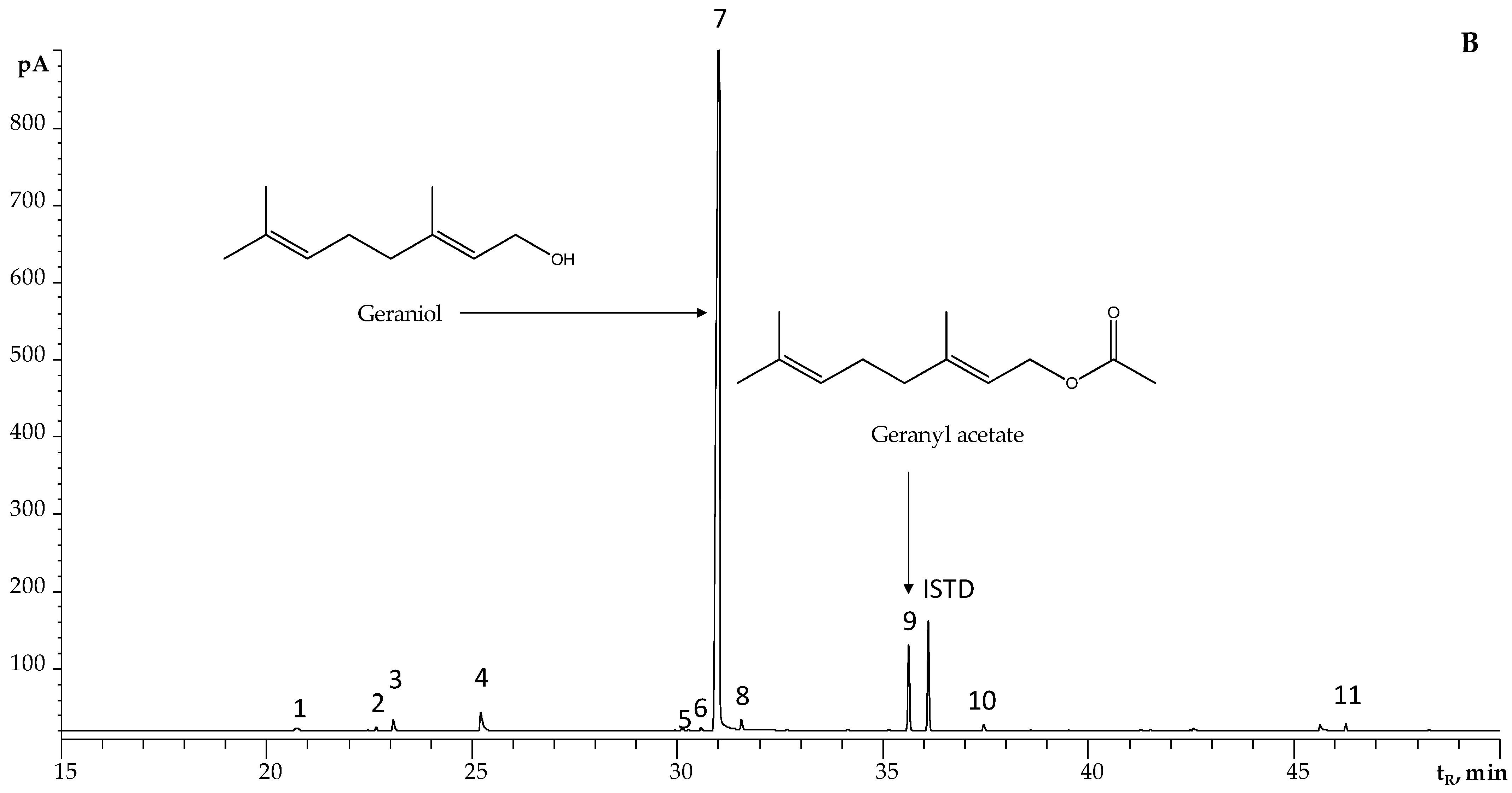


| Collection Place | Number of Harvests, n | Yield, % ± SD | |||
|---|---|---|---|---|---|
| S.D. | MWHD | ||||
| Citronella | Palmarosa | Citronella | Palmarosa | ||
| Barbosa | 3 | 0.7 ± 0.1 | 0.37 ± 0.09 | 1.0 ± 0.2 | 0.4 ± 0.1 |
| Bucaramanga | 5 | 0.7 ± 0.1 | 0.26 ± 0.05 | 0.7 ± 0.2 | 0.32 ± 0.07 |
| Chipatá | 3 | 0.8 ± 0.1 | 0.37 ± 0.08 | 0.9 ± 0.2 | 0.5 ± 0.3 |
| Puente Nacional | 3 | 0.9 ± 0.1 | 0.44 ± 0.07 | 1.0 ± 0.4 | 0.42 ± 0.09 |
| Vélez | 2–3 * | 0.9 ± 0.1 | 0.40 ± 0.08 | 1.2 ± 0.1 | 0.4 ± 0.1 |
| Average yield, % | 0.8 ± 0.1 | 0.37 ± 0.06 | 1.0 ± 0.2 | 0.4 ± 0.1 | |
| N° Figure 1A | Compound | Linear Retention Indices | GC/FID Relative Peak Area, % ± SD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DB-5MS | DB-WAX | ISO 3848:2016 [16] | Municipalities | |||||||||||
| Exp | Std | Lit | Exp | Std | Lit | Min. | Max. | Barbosa
(n = 3) | Bucaramanga
(n = 5) | Chipatá (n = 3) | Puente Nacional (n = 3) | Vélez (n = 2) | ||
| 1 | Limonene a,b,c | 1032 | 1035 | 1030 [30] | 1202 | 1203 | 1198 [30] | 2.0 | 5.0 | 3.1 ± 0.3 | 2.21 ± 0.07 | 2.7 ± 0.2 | 2.6 ± 0.3 | 3.2 ± 0.3 |
| 2 | Linalool a,b,c | 1101 | 1102 | 1099 [30] | 1552 | 1552 | 1543 [30] | 0.5 | 1.5 | 0.77 ± 0.06 | 0.43 ± 0.01 | 0.7 ± 0.1 | 0.87 ± 0.06 | 0.8 ± 0.1 |
| 3 | Citronellal a,b,c | 1158 | 1157 | 1154 [30] | 1491 | 1486 | 1475 [30] | 31 | 39 | 41 ± 4 | 43.0 ± 0.3 | 34 ± 1 | 37 ± 3 | 41 ± 1 |
| 4 | iso-Pulegol a,b,c | 1165 | 1157 | 1156 [31] | 1576 | 1584 | 1566 [32] | 0.5 | 1.7 | 0.17 ± 0.06 | 0.135 ± 0.002 | 0.17 ± 0.06 | 0.20 ± 0.01 | 0.20 ± 0.01 |
| 5 | Citronellol a,b,c | 1229 | 1230 | 1228 [30] | 1769 | 1769 | 1763 [30] | 8.5 | 13 | 14 ± 3 | 10.72 ± 0.05 | 16 ± 2 | 19 ± 2 | 15 ± 2 |
| 6 | Neral a,b | 1241 | - | 1242 [30] | 1692 | - | 1679 [30] | 0.6 ± 0.1 | 0.596 ± 0.002 | 0.7 ± 0.1 | 0.4 ± 0.1 | 0.85 ± 0.07 | ||
| 7 | Geraniol a,b,c | 1255 | 1255 | 1255 [30] | 1857 | 1855 | 1839 [30] | 20 | 25 | 24.4 ± 0.6 | 19.97 ± 0.08 | 24 ± 1 | 23 ± 4 | 23.5 ± 0.3 |
| 8 | Geranial a,b,c | 1270 | 1272 | 1270 [30] | 1740 | 1736 | 1725 [30] | 0.3 | 11 | 0.9 ± 0.1 | 0.863 ± 0.002 | 1.0 ± 0.1 | 0.7 ± 0.1 | 1.2 ± 0.1 |
| 9 | Citronellyl acetate a,b | 1345 | - | 1352 [30] | 1666 | - | 1656 [30] | 2.0 | 4.0 | 2.0 ± 0.2 | 1.33 ± 0.01 | 3.3 ± 0.1 | 2.0 ± 0.2 | 2.3 ± 0.1 |
| 10 | Eugenol a,b,c | 1353 | 1361 | 1358 [30] | 2184 | 2167 | 2163 [30] | 0.5 | 1.0 | 1.1 ± 0.1 | 1.014 ± 0.003 | 1.2 ± 0.1 | 0.9 ± 0.2 | 1.0 ± 0.1 |
| 11 | Geranyl acetate a,b,c | 1374 | 1387 | 1380 [30] | 1760 | 1759 | 1751 [30] | 2.5 | 5.5 | 2.5 ± 0.6 | 1.76 ± 0.01 | 4.7 ± 0.9 | 1.9 ± 0.2 | 2.9 ± 0.4 |
| 12 | β-Elemene a,b | 1394 | - | 1390 [30] | 1599 | - | 1591 [30] | 0.7 | 2.5 | 0.5 ± 0.1 | 0.98 ± 0.02 | 0.63 ± 0.06 | 0.57 ± 0.06 | 0.5 ± 0.1 |
| 13 | Germacrene D a,b | 1490 | - | 1481 [30] | 1719 | - | 1708 [30] | 1.5 | 3.0 | 1.2 ± 0.2 | 1.95 ± 0.01 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.25 ± 0.07 |
| 14 | δ-Cadinene a,b | 1525 | - | 1523 [30] | 1764 | - | 1756 [30] | 1.4 | 2.5 | 0.5 ± 0.1 | 1.18 ± 0.01 | 1.1 ± 0.2 | 0.3 ± 0.1 | 0.7 ± 0.1 |
| 15 | Elemol a,b | 1557 | - | 1548 [30] | 2086 | - | 2079 [30] | 1.3 | 4.0 | 2.0 ± 0.4 | 4.7 ± 0.1 | 2.3 ± 0.6 | 1.7 ± 0.3 | 1.3 ± 0.1 |
| 16 | Germacrene D-4-ol a,b | 1587 | - | 1574 [30] | 2059 | - | 2057 [30] | 3 ± 1 | 4.0 ± 0.1 | 3.1 ± 0.9 | 3.9 ± 0.8 | 2.3 ± 0.1 | ||
| 17 | α-Cadinol a,b | 1666 | - | 1652 [30] | 2243 | - | 2227 [30] | 0.3 ± 0.1 | 1.59 ± 0.02 | 0.6 ± 0.2 | 0.2 ± 0.1 | 0.45 ± 0.07 | ||
| Total GC peak area, % | 97.87 | 96.49 | 97.53 | 97.54 | 99.25 | |||||||||
| N° Figure 1B | Compound | Linear Retention Indices | GC/FID Relative Peak Area, % Mean ± SD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DB-5MS | DB-WAX | ISO 4727:2021 [17] | Municipalities | |||||||||||
| Exp | Std | Lit | Exp | Std | Lit | Min. | Max. | Barbosa (n = 3) | Bucaramanga (n = 5) | Chipatá (n = 3) | Puente Nacional (n = 3) | Vélez
(n = 3) | ||
| 1 | β-Myrcene a,b,c | 990 | 989 | 989 [30] | 1165 | 1165 | 1161 [30] | 0.1 | 0.5 | 0.23 ± 0.06 | 0.140 ± 0.003 | 0.73 ± 0.06 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| 2 | (Z)-β-Ocimene a,b | 1037 | - | 1038 [30] | 1237 | - | 1235 [30] | 0.3 ± 0.1 | 0.243 ± 0.002 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | ||
| 3 | (E)-β-Ocimene a,b | 1048 | - | 1048 [30] | 1254 | - | 1250 [30] | 0.2 | 2.0 | 1.4 ± 0.6 | 1.122 ± 0.007 | 1.6 ± 0.1 | 1.4 ± 0.2 | 1.5 ± 0.3 |
| 4 | Linalool a,b,c | 1101 | 1102 | 1099 [30] | 1550 | 1551 | 1543 [30] | 1.5 | 4.0 | 3.1 ± 0.8 | 1.720 ± 0.004 | 3.9 ± 0.5 | 3.1 ± 0.4 | 3.2 ± 0.3 |
| 5 | Nerol a,b,c | 1232 | 1230 | 1229 [30] | 1806 | 1806 | 1795 [30] | 0.2 | 1.0 | 0.2 ± 0.1 | 0.13 ± 0.03 | 0.37 ± 0.06 | 0.43 ± 0.06 | 0.43 ± 0.06 |
| 6 | Neral a,b | 1241 | - | 1242 [30] | 1688 | - | 1679 [30] | 0.05 | 0.3 | 0.23 ± 0.06 | 0.163 ± 0.001 | 0.27 ± 0.06 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| 7 | Geraniol a,b,c | 1259 | 1253 | 1255 [30] | 1858 | 1853 | 1839 [30] | 77 | 85 | 84 ± 2 | 84.4 ± 0.1 | 83 ± 3 | 87.5 ± 0.2 | 83 ± 3 |
| 8 | Geranial a,b,c | 1272 | 1271 | 1270 [30] | 1735 | 1735 | 1725 [30] | 0.1 | 0.6 | 0.7 ± 0.2 | 0.60 ± 0.04 | 0.8 ± 0.2 | 0.47 ± 0.06 | 0.6 ± 0.2 |
| 9 | Geranyl acetate a,b,c | 1377 | 1378 | 1380 [30] | 1756 | 1758 | 1751 [30] | 5 | 13 | 7 ± 1 | 5.50 ± 0.04 | 4 ± 1 | 4.9 ± 0.3 | 5 ± 1 |
| 10 | (E)-β-Caryophyllene a,b,c | 1434 | 1434 | 1420 [30] | 1609 | 1611 | 1599 [30] | 1 | 2.5 | 0.5 ± 0.1 | 0.763 ± 0.002 | 0.7 ± 0.1 | 0.37 ± 0.06 | 0.43 ± 0.06 |
| 11 | (2E,6Z-)-Farnesol a,b,c | 1718 | 1718 | 1714 [30] | 2360 | 2361 | 2359 [30] | tr | 1.5 | 0.4 ± 0.1 | 1.60 ± 0.02 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Total GC peak area, % | 97.87 | 96.49 | 97.53 | 97.54 | 99.25 | |||||||||
| Extracts/Compounds | Vegetal Material | µmol Trolox®/g Extract, Mean ± SD (n = 3) | |
|---|---|---|---|
| ORAC | ABTS+● | ||
| Java-type citronella | Before distillation | 1100 ± 40 | 71 ± 2 |
| Postdistillation waste | 1400 ± 837 | 78 ± 1 | |
| Palmarosa | Before distillation | 1300 ± 61 | 167 ± 1 |
| Postdistillation waste | 1400 ± 118 | 103 ± 7 | |
| p-Coumaric acid | 17,600 ± 704 | 8700 ± 218 | |
| Ferulic acid | 14,200 ± 167 | 8500 ± 309 | |
| 3-Caffeoyl quinic acid | 14,000 ± 132 | 2140 ± 88 | |
| 4-Caffeoyl quinic acid | 9900 ± 600 | 2080 ± 56 | |
| Luteolin | 18,000 ± 636 | 4000 ± 151 | |
| Luteolin-6-C-glucoside | 11,600 ± 240 | 3300 ± 143 | |
| Apigenin-8-C-glucoside | 10,500 ± 405 | * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, A.K.; Portillo, D.J.; Beltrán, S.B.; Sierra, L.J.; Álvarez, C.A.; Ramírez, K.J.; Martínez, J.R.; Stashenko, E.E. Enhanced Two-Step Extraction from Biomass of Two Cymbopogon Species Cultivated in Santander, Colombia. Molecules 2023, 28, 6315. https://doi.org/10.3390/molecules28176315
Romero AK, Portillo DJ, Beltrán SB, Sierra LJ, Álvarez CA, Ramírez KJ, Martínez JR, Stashenko EE. Enhanced Two-Step Extraction from Biomass of Two Cymbopogon Species Cultivated in Santander, Colombia. Molecules. 2023; 28(17):6315. https://doi.org/10.3390/molecules28176315
Chicago/Turabian StyleRomero, Angie K., Daysy J. Portillo, Sheila B. Beltrán, Lady J. Sierra, Camilo A. Álvarez, Karen J. Ramírez, Jairo R. Martínez, and Elena E. Stashenko. 2023. "Enhanced Two-Step Extraction from Biomass of Two Cymbopogon Species Cultivated in Santander, Colombia" Molecules 28, no. 17: 6315. https://doi.org/10.3390/molecules28176315
APA StyleRomero, A. K., Portillo, D. J., Beltrán, S. B., Sierra, L. J., Álvarez, C. A., Ramírez, K. J., Martínez, J. R., & Stashenko, E. E. (2023). Enhanced Two-Step Extraction from Biomass of Two Cymbopogon Species Cultivated in Santander, Colombia. Molecules, 28(17), 6315. https://doi.org/10.3390/molecules28176315







