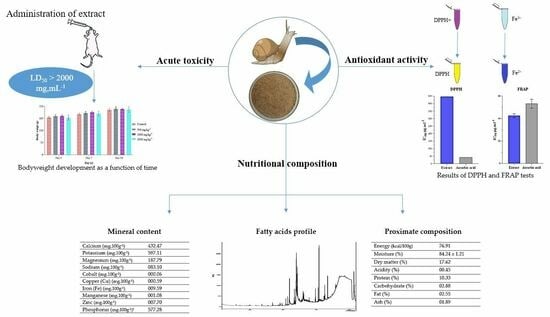
Nutritional Composition, Fatty Acids Profile, Mineral Content, Antioxidant Activity and Acute Toxicity of the Flesh of Helix aspersa Müller
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proximate Analysis
2.1.1. Moisture Content, Dry-Matter Content and Acidity Measurement
2.1.2. Protein, Total Sugar and Fat Content
2.2. Mineral Content
2.3. Calorific Content and Nutritional Value
2.4. Fatty Acids Content
2.5. Biochemical Analysis
2.6. Antioxidant Activity
2.7. Acute Toxicity
3. Materials and Methods
3.1. Snail Harvesting and Laboratory Upkeep
3.2. Preparation of H. aspersa Müller Flour
3.3. Proximate Analysis
3.3.1. Moisture Content
3.3.2. Dry-Matter Content
3.3.3. Acidity Measurement
3.3.4. Ash Content
3.3.5. Protein Content
3.3.6. Lipid Content
3.3.7. Total Carbohydrate Content
3.4. Mineral Composition
3.5. Fatty Acid Composition
3.6. Antioxidant Activity and Toxicity of the Aqueous Extract of H. aspersa Müller Flesh (HAAE)
3.6.1. Aqueous Extract of H. aspersa Müller Preparation
3.6.2. Determination of Total Phenolic Content and Total Flavonoid Content
3.6.3. Content of Reducing Sugars
3.6.4. Antioxidant Activity
- Total antioxidant capacity (TAC)
- Free radical scavenging activity (DPPH)
- Ferrous-ion-reducing antioxidant power assay (FRAP)
3.7. Evaluation of Acute Toxicity of the Aqueous Extract of H. aspersa Müller Flesh
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ha, A.W.; Kang, H.J.; Kim, S.L.; Kim, M.H.; Kim, W.K. Acute and Subacute Toxicity Evaluation of Corn Silk Extract. Prev. Nutr. Food Sci. 2018, 23, 70–76. [Google Scholar] [CrossRef]
- Meguellati, H.; Saida, O.; Saad, S.; Djemouai, N. Evaluation of Acute, Subacute Oral Toxicity and Wound Healing Activity of Mother Plant and Callus of Teucrium Polium L. Subsp. Geyrii Maire from Algeria. S. Afr. J. Bot. 2019, 127, 25–34. [Google Scholar] [CrossRef]
- Ateba, S.B.; Simo, R.V.; Mbanya, J.C.; Krenn, L.; Njamen, D. Safety Profile and Gender Specific Differences of a Methanol Extract of Eriosema laurentii (Leguminosae) in Acute and Subchronic (28 Days) Oral Toxicity Studies in Wistar Rats. Food Chem. Toxicol. 2014, 65, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhuang, Y.; Tian, W.; Sun, L. In Vivo Acute and Subacute Toxicities of Phenolic Extract from Rambutan (Nephelium lappaceum) Peels by Oral Administration. Food Chem. 2020, 320, 126618. [Google Scholar] [CrossRef]
- Luo, F.; Xing, R.; Wang, X.; Peng, Q.; Li, P. Proximate Composition, Amino Acid and Fatty Acid Profiles of Marine Snail Rapana venosa Meat, Visceral Mass and Operculum: Comparative Study of Nutritional Composition in Different Body Parts of R. venosa. J. Sci. Food Agric. 2017, 97, 5361–5368. [Google Scholar] [CrossRef]
- Sallam, A.; Nabil, E.-W. Biological and Ecological Studies on Land Snails and Their Control. Integr. Pest Manag. Pest Control. Curr. Future Tactics 2012, 1, 413–444. [Google Scholar]
- Schileyko, A.A. Family Helicidae Excluding Helicinae (Gastropoda pulmonata): Morphology, Taxonomy, and a Catalogue of Taxa. Ruthenica Russ. Malacol. J. 2013, 23, 127–162. [Google Scholar]
- Tremlova, B. Histological examination of snail meat specialities. Fleischwirtschaft 2001, 81, 96–97. [Google Scholar]
- Engmann, F. Proximate and Mineral Composition of Snail (Achatina achatina) Meat; Any Nutritional Justification for Acclaimed Health Benefits? J. Basic Appl. Sci. Res. 2013, 3, 8–15. [Google Scholar]
- Sando, D.; Grujić, R.; Meho, B.; Lisickov, K.; Vujadinović, D. Quality Indicators of Snail Meat Grown in Different Conditions. Qual. Life (Banja Luka)-Apeiron 2012, 6, 55–64. [Google Scholar] [CrossRef]
- Babalola, O.O.; Akinsoyinu, A.O. Proximate Composition and Mineral Profile of Snail Meat from Different Breeds of Land Snail in Nigeria. Pak. J. Nutr. 2001, 8, 4. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.; Olgunoglu, A.I. Fatty Acid Profile and Mineral Content of the Wild Snail (Helix pomatia) from the Region of the South of the Turkey. Eur. Food Res. Technol. 2005, 221, 547–549. [Google Scholar] [CrossRef]
- Yildirim, M.; Kebapçı, Ü.; Gümüş, B. Edible Snails (Terrestrial) of Turkey. Turk. J. Zool. 2004, 28, 329–335. [Google Scholar]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V. Snail as Mini-Livestock: Nutritional Potential of Farmed Pomacea canaliculata (Ampullariidae). Agric. Nat. Resour. 2018, 51, 504–511. [Google Scholar] [CrossRef]
- Zymantiene, J.; Jukna, V.; Jukna, C.; Zelvyte, R.; Oberauskas, V. Comparison of meat quality characteristics between commercial pigs and snails. Pol. J. Food Nutr. Sci. 2008, 58, 23–26. [Google Scholar]
- Ajayi, S.; Tewet, O.; Moriarty, C.; Awesu, M. Observations on the Biology and Nutritive Value of the African Giant Snail Archachatina marginata. Afr. J. Ecol. 2008, 16, 85–95. [Google Scholar] [CrossRef]
- Ozyurt, G.; Kuley, E.; Etyemez, M.; Ozoğul, F. Comparative Seasonal Sterol Profiles in Edible Parts of Mediterranean Fish and Shellfish Species. Int. J. Food Sci. Nutr. 2013, 64, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Piba, N.; Mamadou, K.; Adou, C.; Otchoumou, A.; Kouassi, K. Effet Du Régime et de La Teneur En Protéines Brutes Alimentaires Sur Le Rendement En Viande de l’escargot Achatina Fulica (Bowdich, 1720). Int. J. Biol. Chem. Sci. 2015, 8, 2296. [Google Scholar] [CrossRef]
- Murphy, B. Breeding and Growing Snails Commercially in Australia; Rural Industries Research Development Corporation: Barton, ACT, Austraila, 2001. [Google Scholar]
- Gomot, A. Biochemical composition of Helix snails: Influence of genetic and physiological factors. J. Mollus. Stud. 1998, 64, 173–181. [Google Scholar] [CrossRef]
- Rybska, E. Promoting IBSE using living organisms: Studying snails in the secondary science classroom. In Professional Development for Inquiry-Based Science Teaching and Learning; Springer: Berlin/Heidelberg, Germany, 2018; pp. 43–63. [Google Scholar] [CrossRef]
- Pissia, M.A.; Matsakidou, A.; Kiosseoglou, V. Raw Materials from Snails for Food Preparation. Future Foods 2021, 3, 100034. [Google Scholar] [CrossRef]
- Matusiewicz, M.; Kosieradzka, I.; Niemiec, T.; Grodzik, M.; Antushevich, H.; Strojny, B.; Gołębiewska, M. In Vitro Influence of Extracts from Snail Helix aspersa Müller on the Colon Cancer Cell Line Caco-2. Int. J. Mol. Sci. 2018, 19, 1064. [Google Scholar] [CrossRef] [PubMed]
- Grilla, A.; Lajeunesse, C.; Mcmaster, D.; Morgan, D. Feasibility of Snail Farming as a Model for Small Urban Farms to Expand into Niche Markets for Increased Profitability. Bachelor’s Thesis, Worcester Polytechnic Institute, Worcester, UK, 2016. [Google Scholar]
- Çağıltay, F.; Erkan, N.; Tosun, D.; Selçuk, A. Amino acid, fatty acid, vitamin and mineral contents of the edible garden snail (Helix aspersa). J. Fisheriessci. 2011, 5, 354–363. [Google Scholar] [CrossRef]
- Milinsk, M.C.; das Graças Padre, R.; Hayashi, C.; de Oliveira, C.C.; Visentainer, J.V.; de Souza, N.E.; Matsushita, M. Effects of Feed Protein and Lipid Contents on Fatty Acid Profile of Snail (Helix Aspersa Maxima) Meat. J. Food Compos. Anal. 2006, 19, 212–216. [Google Scholar] [CrossRef]
- Caetano, D.; Miranda, A.; Lopes, S.; Paiva, J.; Rodrigues, A.; Videira, A.; Almeida, C.M.M. Nutritional and Toxicity Profiles of Two Species of Land Snail, Theba Pisana and Otala Lactea, from Morocco. J. Food Compos. Anal. 2021, 100, 103893. [Google Scholar] [CrossRef]
- Newar, J.; Ghatak, A. Studies on the Adhesive Property of Snail Adhesive Mucus. Langmuir 2015, 31, 12155–12160. [Google Scholar] [CrossRef] [PubMed]
- Fox, B.A.; Cameron, A.G. Food Science—A Chemical Approach; Hodder and Stoughton: London, UK, 1977. [Google Scholar]
- Baby, R.L.; Hasan, I.; Kabir, K.A.; Naser, M.N. Nutrient Analysis of Some Commercially Important Molluscs of Bangladesh. J. Sci. Res. 2010, 2, 390–396. [Google Scholar] [CrossRef]
- Madejón, P.; Arrébola, J.; Madejón, E.; Burgos, P.; López-Garrido, R.; Cárcaba, A.; Cabrera, F.; Murillo, J.M. The Snail Theba Pisana as an Indicator of Soil Contamination by Trace Elements: Potential Exposure for Animals and Humans. J. Sci. Food Agric. 2013, 93, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; West, K.P. Interactions between Zinc and Vitamin A: An Update. Am. J. Clin. Nutr. 1998, 68, 435S–441S. [Google Scholar] [CrossRef]
- Drozd, Ł.; Ziomek, M.; Szkucik, K.; Paszkiewicz, W.; Maćkowiak-Dryka, M.; Bełkot, Z.; Gondek, M. Selenium, Copper, and Zinc Concentrations in the Raw and Processed Meat of Edible Land Snails Harvested in Poland. J. Vet. Res. 2017, 61, 293–298. [Google Scholar] [CrossRef]
- EU Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union 2011, 304, 18–63.
- Zhao, F.; Zhuang, P.; Song, C.; Shi, Z.; Zhang, L. Amino Acid and Fatty Acid Compositions and Nutritional Quality of Muscle in the Pomfret, Pampus Punctatissimus. Food Chem. 2010, 118, 224–227. [Google Scholar] [CrossRef]
- Vujkovic, G.; Karlovic; Vujkovic, I.; Vorosbaranyi, I.; Jovanovic, B. Composition of Muscle Tissue Lipids of Silver Carp and Bighead Carp. J. Am. Oil Chem. Soc. 1999, 76, 475–480. [Google Scholar] [CrossRef]
- HMSO. Nutritional Aspects of Cardiovascular Disease. Report of the Cardiovascular Review Group Committee on Medical Aspects of Food Policy. Rep. Health Soc. Subj. 1994, 46, 1–186. [Google Scholar]
- Keys, A.; Menotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The diet and 15-year death rate in the Seven Countries Study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Harrison, R.A.; Summerbell, C.D.; Moore, H.; Worthington, H.V.; Ness, A.; Capps, N.; Smith, G.D.; Riemersma, R.; Ebrahim, S. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Syst. Rev. 2004, 4, CD003177. [Google Scholar] [CrossRef] [PubMed]
- Psota, T.L.; Gebauer, S.K.; Kris-Etherton, P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am. J. Cardiol. 2006, 21, 3–18. [Google Scholar] [CrossRef]
- Berbert, A.A.; Kondo, C.R.; Almendra, C.L.; Matsuo, T.; Dichi, I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition 2005, 21, 131–136. [Google Scholar] [CrossRef]
- Kouachi, M.; Naimi, D.; Djadouri, D.; Bendahra, I. Preventive effect of Helix aspersa crude extract against chemo-induced colonic damages in rats. Int. J. Pharm. Sci. Res. 2017, 8, 2458–2468. [Google Scholar] [CrossRef]
- Gogas, A.; Laliotis, G.P.; Ladoukakis, E.; Trachana, V. Chemical Composition and Antioxidant Profile of Snails (Cornu Aspersum Aspersum) Fed Diets with Different Protein Sources under Intensive Rearing Conditions. J. Anim. Feed Sci. 2021, 30, 391–398. [Google Scholar] [CrossRef]
- Anand, T.; Chinnachamy, C.; Kumaran, S.; Shanthini, C. Biochemical Composition and Antioxidant Activity of Pleuroploca Trapezium Mean. J. Chem. Pharm. Res. 2010, 2, 526–535. [Google Scholar]
- Subhapradha, N.; Ramasamy, P.; Sadhasivam, S.; Seedevi, P.; Moovendhan, M.; Srinivasan, A.; Shanmugam, V.; Shanmugam, A. Preparation of Phosphorylated Chitosan from Gladius of the Squid Sepioteuthis Lessoniana (Lesson, 1830) and Its in Vitro Antioxidant Activity. Bioact. Carbohydr. Diet. Fibre 2013, 1, 145–158. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant Activity of a Flavonoid-Rich Extract of Hypericum Perforatum L. in Vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Jeong, Y.-K.; Wang, M.-H.; Lee, W.-Y.; Rhee, H.-I. Inhibitory Effect of Pine Extract on Alpha-Glucosidase Activity and Postprandial Hyperglycemia—PubMed. Natl. Med. 2005, 21, 756–761. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, J.R. In Vitro Antioxidant Activities of Methanol Extracts of Five Phyllanthus Species from India. LWT-Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Subavathy, P. Janet SMB: Molecular characterization of protein and antioxidant capacity of Turbo brunneus R. Cypraea annulus L. and Babylonia spirata L. Int. J. Pharm. Sci. Res. 2020, 11, 3285–3293. [Google Scholar] [CrossRef]
- Sivaperumal, P.; Kamala, K.; Natarajan, E.; Dilipan, E. Antimicrobial peptide from crab haemolymph of Ocypoda macrocera (Milne Edwards 1852) with reference to antioxidant: A case study. Int. J. Pharm. Pharm. Sci. 2013, 5, 719–727. [Google Scholar]
- OECD. Guidance Document on Acute Oral Toxicity Testing; OECD Series on Testing and Assessment; OECD: Paris, France, 2001; ISBN 978-92-64-07841-3. [Google Scholar]
- Hodge, H.C.; Sterner, J.H. Determination of substance acute toxicity by LD50. Am. Ind. Hyg. Assoc. 1943, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Danilova, I.S. Study of Toxicness of Snails Meat on Biological Model. Sci. Messenger LNU Vet. Med. Biotechnol. Ser. Vet. Sci. 2019, 21, 57–60. [Google Scholar] [CrossRef]
- Toader-Williams, A.; Golubkina, N. Investigation upon the edible snail’s potential as source of selenium for human health and nutrition observing its food chemical contaminant risk factor with heavy metals. Bull. Uasvm Agric. 2009, 66, 495–499. [Google Scholar] [CrossRef]
- Benahmed-Djilali, A.; Chemoul, T.; Kal, S.; Nabiev, M.; Besombes, C. Propriétés d’une pommade antibactérienne formulée à base de saponines extraites des feuilles de noyer. Phytothérapie 2017, 16, 1–9. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists, 18th ed.; AOAC: Washington, DC, USA, 2010. [Google Scholar]
- Brunetti, G.; Traversa, A.; De Mastro, F.; Cocozza, C. Short Term Effects of Synergistic Inorganic and Organic Fertilization on Soil Properties and Yield and Quality of Plum Tomato. Sci. Hortic. 2019, 252, 342–347. [Google Scholar] [CrossRef]
- ISO 936:1998; Meat and Meat Products—Determination of Total Ash. ISO: Geneva, Switzerland, 1998.
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Nannu, S.; Krishnamoorthy, M. Nutritional Quality in Freshwater Mussels, Parreysia Spp. of Periyar River, Kerala, India. Res. J. Recent Sci. 2014, 3, 267–270. [Google Scholar]
- AOAC. Official Method of Analysis, 18th ed.; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Zargoosh, Z.; Ghavam, M.; Bacchetta, G.; Tavili, A. Effects of Ecological Factors on the Antioxidant Potential and Total Phenol Content of Scrophularia Striata Boiss. Sci. Rep. 2019, 9, 16021. [Google Scholar] [CrossRef] [PubMed]
- Albu, A.; Radu-Rusu, R.-M.; Simeanu, D.; Radu-Rusu, C.-G.; Pop, I.M. Phenolic and Total Flavonoid Contents and Physicochemical Traits of Romanian Monofloral Honeys. Agriculture 2022, 12, 1378. [Google Scholar] [CrossRef]
- Negrulescu, A.; Patrulea, V.; Mincea, M.M.; Ionascu, C.; Vlad-Oros, B.A.; Ostafe, V. Adapting the Reducing Sugars Method with Dinitrosalicylic Acid to Microtiter Plates and Microwave Heating. J. Braz. Chem. Soc 2012, 23, 2176–2182. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Jawhari, F.Z.; Almehdi, A.M.; Elmsellem, H.; Fikri Benbrahim, K.; Bousta, D.; Bari, A. Antibacterial, Antifungal and Antioxidant Activity of Total Polyphenols of Withania Frutescens. L. Bioorg. Chem. 2019, 93, 103337. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ke, H.; He, J.; Ban, X.; Zeng, H.; Wang, Y. Extracts of Halenia elliptica exhibit antioxidant properties in vitro and in vivo. Food Chem. Toxicol. 2011, 49, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activites of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]


| Parameters | % |
|---|---|
| Moisture | 84.24 ± 1.21 |
| Dry matter | 17.62 ± 0.74 |
| Acidity | 0.45 ± 0.01 |
| Protein | 10.33 ± 0.21 |
| Carbohydrate | 2.88 ± 0.04 |
| Fat | 2.55 ± 0.00 |
| Ash | 1.89 ± 0.05 |
| Minerals | mg·100 g−1 |
|---|---|
| Calcium (Ca) | 432.47 ± 18.43 |
| Potassium (K) | 597.11 ± 30.37 |
| Magnesium (Mg) | 187.79 ± 0.50 |
| Sodium (Na) | 83.10 ± 2.52 |
| Cobalt (Co) | 0.06 ± 0.05 |
| Copper (Cu) | 0.59 ± 0.09 |
| Iron (Fe) | 9.59 ± 0.18 |
| Manganese (Mn) | 1.08 ± 0.16 |
| Zinc (Zn) | 7.70 ± 0.99 |
| Phosphorus (P) | 577.28 ± 7.19 |
| Energy/Nutrient | Reference Intake (per Day) |
|---|---|
| Energy | 8400 kJ (2000 kcal) |
| Fat | 70 g |
| Carbohydrates | 260 g |
| Sugars | 90 g |
| Proteins | 50 g |
| Calcium (Ca) | 800 mg |
| Magnesium (Mg) | 375 mg |
| Copper (Cu) | 1 mg |
| Iron (Fe) | 14 mg |
| Manganese (Mn) | 2 mg |
| Zinc (Zn) | 10 mg |
| Phosphorus (P) | 700 mg |
| Fatty Acids | % |
|---|---|
| C16:0 Palmitic acid | 7.11 |
| C17:0 Margaric acid | 2.58 |
| C17:0 Heptadecanoic acid | 1.94 |
| C18:0 Stearic acid | 16.86 |
| C20:0 Arachidic acid | 1.05 |
| C22:0 Behenic acid | 1.71 |
| Octanoic acid | 0.75 |
| C14:0 Myristic acid | 0.56 |
| C15:0 Pentadecanoic acid | 0.46 |
| C23:0 Tricosanoic acid | 0.38 |
| Saturated fatty acid SFA | 34.00 |
| C16:1 Palmetoleic acid | 0.60 |
| C18:1 Oleic acid (ω9) | 7.03 |
| C20:1 Eicosanoic acid (ω9) | 3.30 |
| C24:1 Nervonic acid (ω9) | 2.19 |
| Monunsaturated fatty acid MUFA | 10.33 |
| C18:2 Linoleic acid (ω6) | 14.16 |
| C18:2 Linoelaidic acid (ω6) | 1.70 |
| C18:3 Linolenic acid (ω3) | 5.56 |
| C20:2 11.14-Eicosadienoic acid (ω11) | 5.42 |
| C20:4 Arachidonic acid (ω6) | 10.67 |
| 5.8.11.14.17-Eicosatetraenoic (ω3) | 2.94 |
| Polyunsaturated fatty acid PUFA | 40.45 |
| Total ω3 | 8.50 |
| Total ω6 | 26.53 |
| PUFA/SFA | 1.18 |
| PUFA/MUFA | 3.92 |
| Not identified | 13.03 |
| Dose | Number of Rats | Signs of Toxicity | Mortality | LD50 | Category |
|---|---|---|---|---|---|
| Control | 5 | - | 0 | >2000 mg·kg−1 | 5 |
| 500 mg·kg−1 | 5 | - | 0 | ||
| 1000 mg·kg−1 | 5 | - | 0 | ||
| 2000 mg·kg−1 | 5 | - | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aouji, M.; Imtara, H.; Rkhaila, A.; Bouhaddioui, B.; Alahdab, A.; Parvez, M.K.; Saleh Alzahrani, M.; Aicha Lrhorfi, L.; Bengueddour, R. Nutritional Composition, Fatty Acids Profile, Mineral Content, Antioxidant Activity and Acute Toxicity of the Flesh of Helix aspersa Müller. Molecules 2023, 28, 6323. https://doi.org/10.3390/molecules28176323
Aouji M, Imtara H, Rkhaila A, Bouhaddioui B, Alahdab A, Parvez MK, Saleh Alzahrani M, Aicha Lrhorfi L, Bengueddour R. Nutritional Composition, Fatty Acids Profile, Mineral Content, Antioxidant Activity and Acute Toxicity of the Flesh of Helix aspersa Müller. Molecules. 2023; 28(17):6323. https://doi.org/10.3390/molecules28176323
Chicago/Turabian StyleAouji, Marouane, Hamada Imtara, Amine Rkhaila, Bouchra Bouhaddioui, Ahmad Alahdab, Mohammad Khalid Parvez, Mohamed Saleh Alzahrani, Lalla Aicha Lrhorfi, and Rachid Bengueddour. 2023. "Nutritional Composition, Fatty Acids Profile, Mineral Content, Antioxidant Activity and Acute Toxicity of the Flesh of Helix aspersa Müller" Molecules 28, no. 17: 6323. https://doi.org/10.3390/molecules28176323
APA StyleAouji, M., Imtara, H., Rkhaila, A., Bouhaddioui, B., Alahdab, A., Parvez, M. K., Saleh Alzahrani, M., Aicha Lrhorfi, L., & Bengueddour, R. (2023). Nutritional Composition, Fatty Acids Profile, Mineral Content, Antioxidant Activity and Acute Toxicity of the Flesh of Helix aspersa Müller. Molecules, 28(17), 6323. https://doi.org/10.3390/molecules28176323