Rapid Chemical Profiling of Filipendula ulmaria Using CPC Fractionation, 2-D Mapping of 13C NMR Data, and High-Resolution LC–MS
Abstract
:1. Introduction
2. Results and Discussion
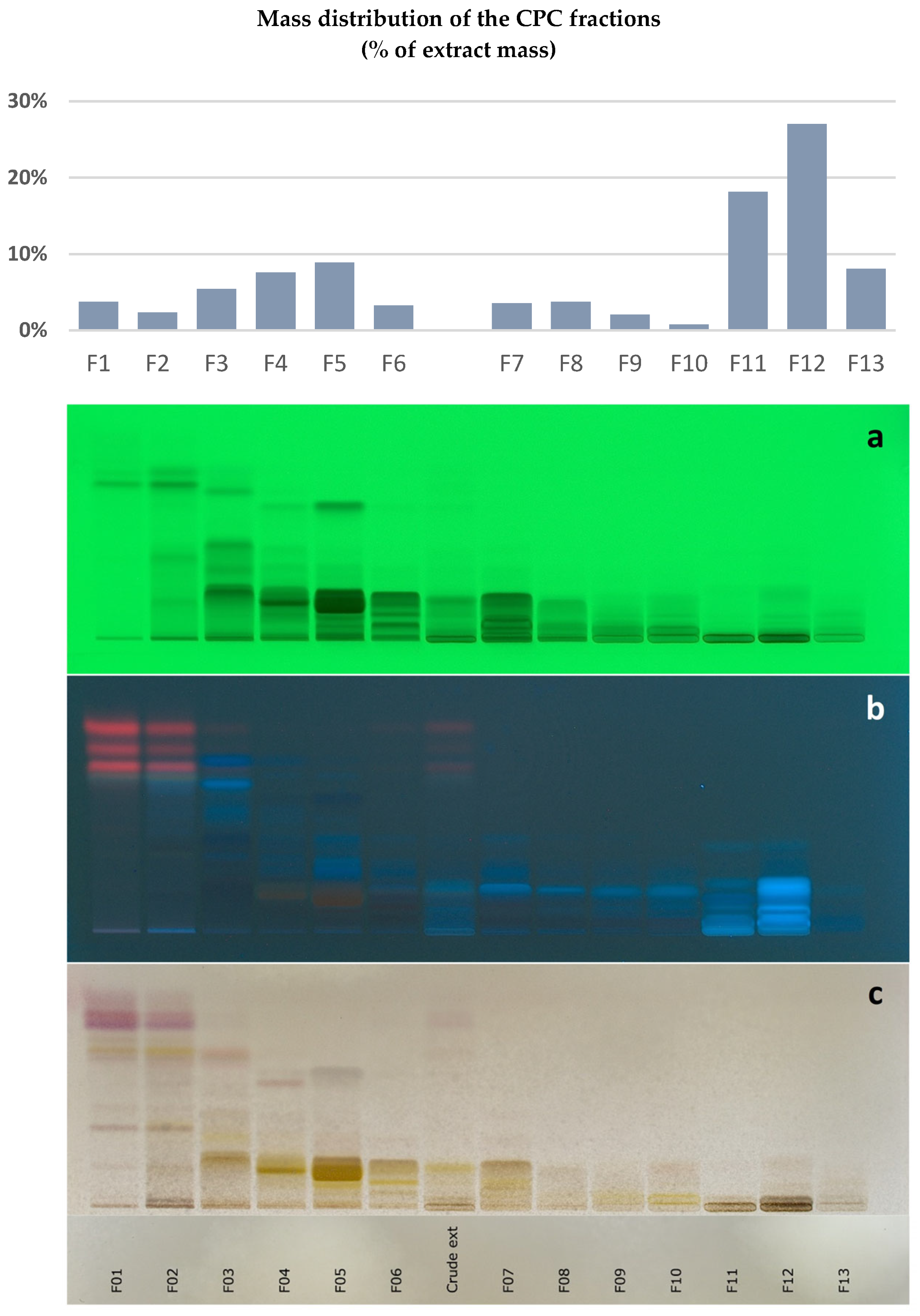
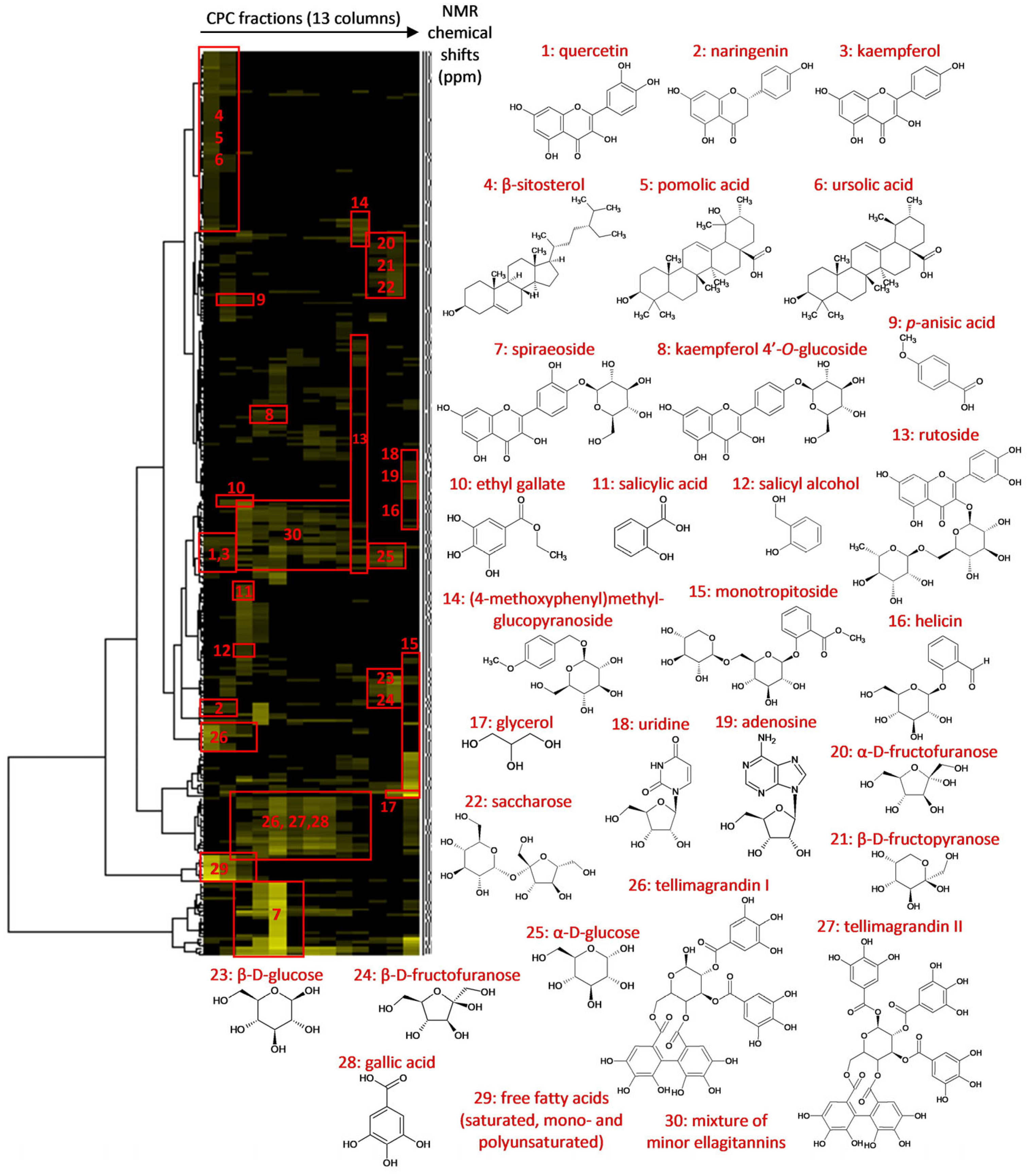
2.1. Chemical Profiling of the Filipendula ulmaria Extract
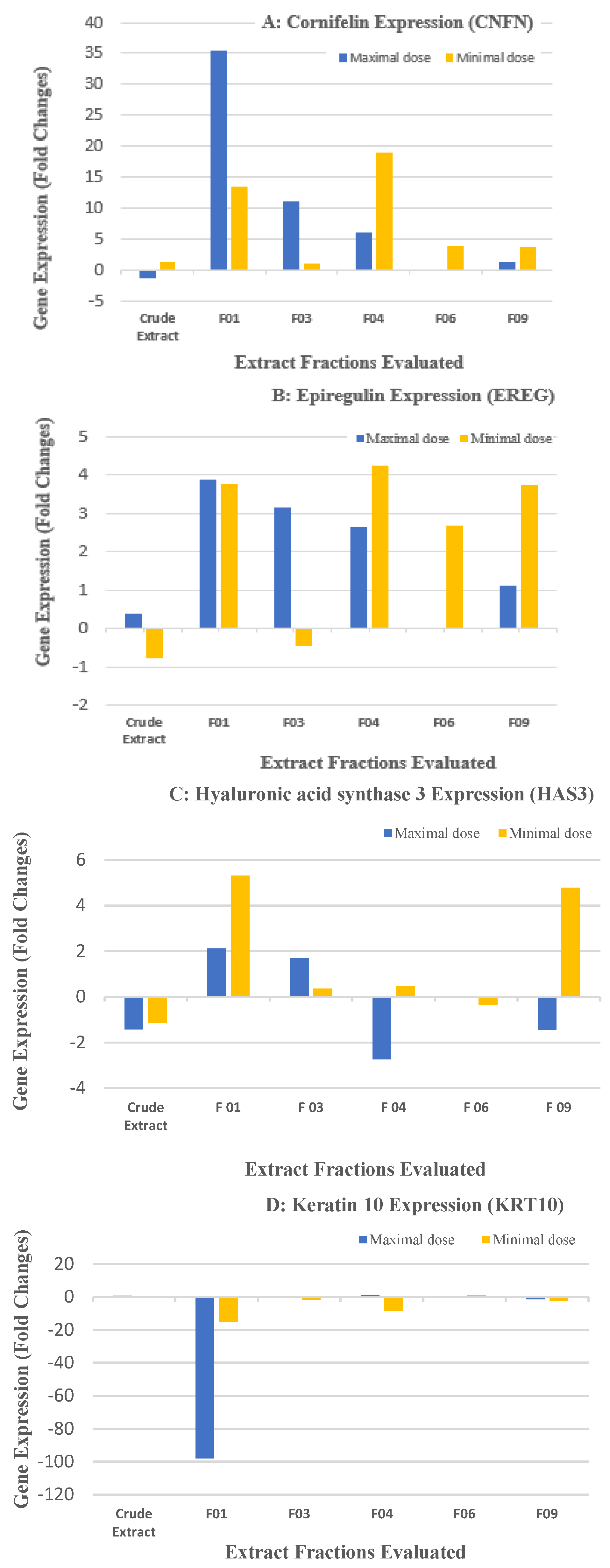
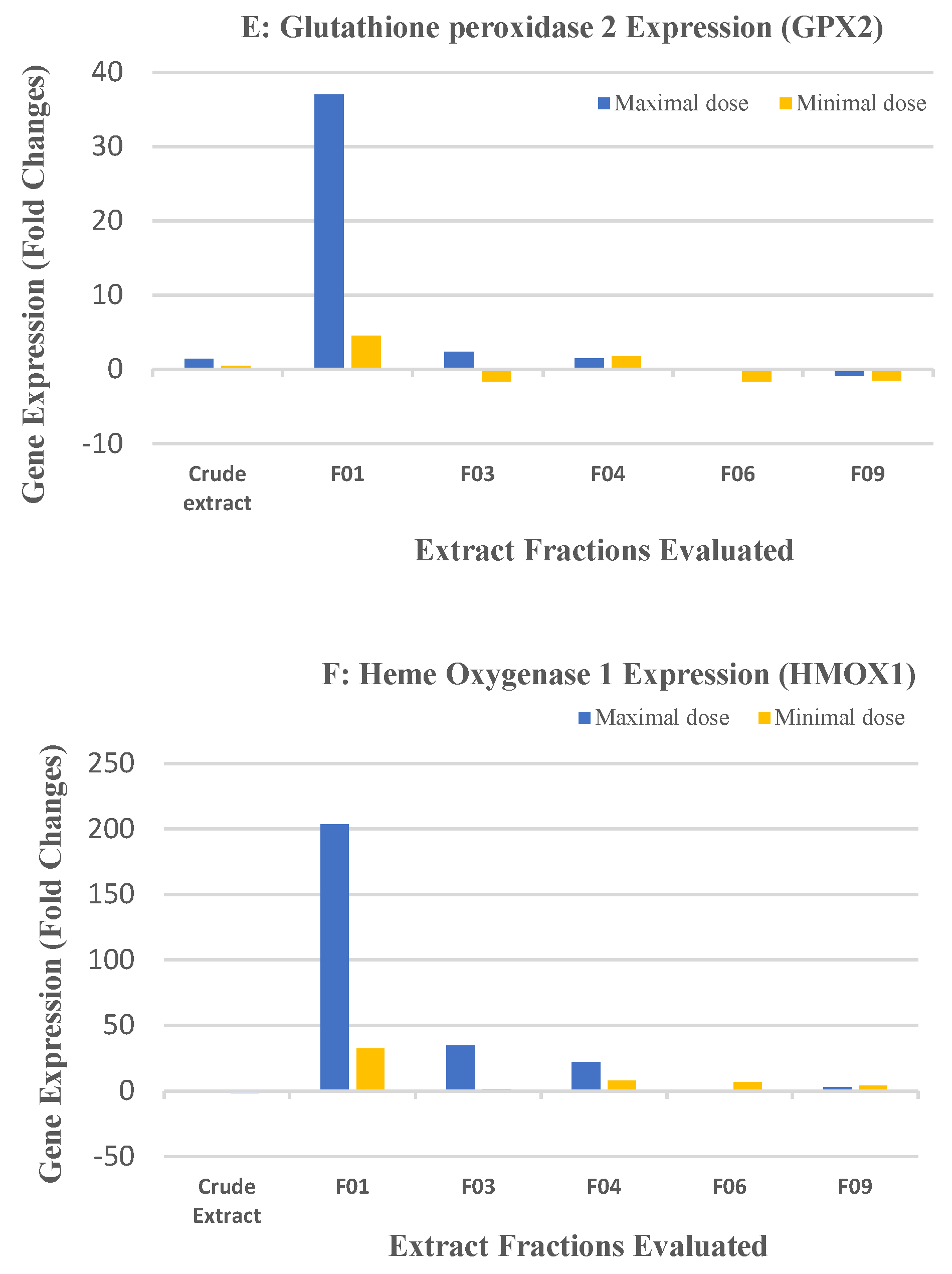
2.2. Biological Results for the Crude Extract and CPC Fractions and Assignment of the Metabolites Responsible for Skin Barrier Function Improvement
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction Procedure
3.3. Centrifugal Partition Chromatography (CPC)
3.4. NMR Analyses and Metabolite Identification
3.5. Liquid Chromatography—Mass Spectrometry Analyses of the Extract (LC/MS)
3.6. Biological Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| CPC | Centrifugal Partition Chromatography |
| NMR | Nuclear Magnetic Resonance |
| HCA | Hierarchical Clustering Analysis |
| BV | Bed Volume |
| TLC | Thin Layer Chromatography |
| HSQC | Heteronuclear single quantum coherence |
| HMBC | Heteronuclear multiple-bond coherence |
| COSY | Correlated Spectroscopy |
| LC-MS | Liquid Chromatography Mass Spectra |
| FC | Fold Changes |
| CLDN1 | Claudin 1 |
| CNFN | Cornifelin |
| DSG1 | Desmoglein 1 |
| KLK7 | Kallikrein-related peptidase 7 |
| TJP1 | Tight junction protein 1 |
| EREG | Epiregulin |
| HAS3 | Hyaluronic acid synthase 3 |
| HBEGF | Heparin-binding EGF-like growth factor |
| KRT19 | Keratin 19 |
| KRT10 | Keratin 10 |
| SPRR1A | Small prolin-rich protein A1 |
| TGM1 | Transglutaminase 1 |
| GPX2 | Glutathione peroxidase 2 |
| HMOX1 | Heme oxygenase 1 |
| PPAR | Peroxisome proliferator-activated receptor-α |
References
- Ball, P.W.; Miller, I.N.; Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Roseaceae to Umbelliferae; Cambridge University Press: London, UK, 1969; Volume 2, pp. 6–7. [Google Scholar]
- Šarić-Kundalić, B.; Dobeš, C.; Klatte-Asselmeyer, V.; Saukel, J. Ethnobotanical survey of traditionally used plants in human therapy of east, north and north-east Bosnia and Herzegovina. J. Ethnopharmacol. 2011, 133, 1051–1076. [Google Scholar] [CrossRef] [PubMed]
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J.; et al. Ethnopharmacological in vitro studies on Austria’s folk medicine—An unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J. Ethnopharmacol. 2013, 149, 750–771. [Google Scholar] [CrossRef]
- Halkes, S.B.A. Filipendula ulmaria—A Study on the Immunomodulatory Activity of Extracts and Constituents. Ph.D. Thesis, University of Utrecht, Utrecht, The Netherlands, 1998. [Google Scholar]
- British Herbal Pharmacopoeia; British Herbal Medicine Association: Aughton, UK, 1983; pp. 91–92.
- British Herbal Pharmacopoeia; British Herbal Medicine Association: Aughton, UK, 1990; Volume 1, pp. 65–66.
- Bradley, P.R. (Ed.) British Herbal Compendium; British Herbal Medicine Association: Aughton, UK, 1992; Volume 1, pp. 158–160. [Google Scholar]
- Bespalov, V.G.; Alexandrov, V.A.; Semenov, A.L.; Kovan’ko, E.G.; Ivanov, S.D.; Vysochina, G.I.; Kostikova, V.A.; Baranenko, D.A. The inhibitory effect of meadowsweet (Filipendula ulmaria) on radiation-induced carcinogenesis in rats. Int. J. Radiat. Biol. 2016, 93, 394–401. [Google Scholar] [CrossRef]
- Zeylstra, H. Filipendula ulmaria. Br. J. Phytother. 1998, 5, 8–12. [Google Scholar]
- Filipendulae ulmaria Herba—Meadowsweet, ESCOP Monographs, European Scientific Cooperative on Phytotherapy, Editor, Thieme. 2003, Volume 2, pp. 157–161. Available online: https://escop.com/downloads/meadowsweet/ (accessed on 15 August 2023).
- Berardesca, E.; Distante, F.; Vignoli, G.; Oresajo, C.; Green, B. Alpha hydroxyacids modulate stratum corneum barrier function. Br. J. Dermatol. 1997, 137, 934–938. [Google Scholar] [CrossRef]
- Bashir, S.; Dreher, F.; Chew, A.; Zhai, H.; Levin, C.; Stern, R.; Maibach, H. Cutaneous bioassay of salicylic acid as a keratolytic. Int. J. Pharm. 2005, 292, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Ademola, J.; Frazier, C.; Kim, S.J.; Theaux, C.; Saudez, X. Clinical evaluation of 40% urea and 12% ammonium lactate in the treatment of xerosis. Am. J. Clin. Dermatol. 2002, 3, 217–222. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Marti, G.; Thomas, A.; Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef] [PubMed]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Y.; Zhou, H.; Cao, X.; Jiang, X.; Wang, K.; Wu, S. A novel strategy for screening new natural products by a combination of reversed-phase liquid chromatography fractionation and 13C NMR pattern recognition: The discovery of new anti-cancer flavone dimers from Dysosma versipellis (Hance). RSC Adv. 2015, 5, 77553–77564. [Google Scholar] [CrossRef]
- Hubert, J.; Nuzillard, J.-M.; Renault, J.-H. Dereplication strategies in natural product research: How many tools and methodologies behind the same concept? Phytochem. Rev. 2017, 16, 55–95. [Google Scholar] [CrossRef]
- Yang, J.Y.; Sanchez, L.M.; Rath, C.M.; Liu, X.; Boudreau, P.D.; Bruns, N.; Glukhov, E.; Wodtke, A.; de Felicio, R.; Fenner, A.; et al. Molecular Networking as a Dereplication Strategy. J. Nat. Prod. 2013, 76, 1686–1699. [Google Scholar] [CrossRef]
- Chao, R.; Hou, X.-M.; Xu, W.-F.; Hai, Y.; Wei, M.-Y.; Wang, C.-Y.; Gu, Y.-C.; Shao, C.-L. Targeted Isolation of Asperheptatides from a Coral-Derived Fungus Using LC-MS/MS-Based Molecular Networking and Antitubercular Activities of Modified Cinnamate Derivatives. J. Nat. Prod. 2020, 84, 11–19. [Google Scholar] [CrossRef]
- Ding, W.; Tian, D.; Chen, M.; Xia, Z.; Tang, X.; Zhang, S.; Wei, J.; Li, X.; Yao, X.; Wu, B.; et al. Molecular Networking-Guided Isolation of Cyclopentapeptides from the Hydrothermal Vent Sediment Derived Fungus Aspergillus pseudoviridinutans TW58-5 and Their Anti-inflammatory Effects. J. Nat. Prod. 2023, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Bruguière, A.; Derbré, S.; Dietsch, J.; Leguy, J.; Rahier, V.; Pottier, Q.; Bréard, D.; Suor-Cherer, S.; Viault, G.; Le Ray, A.-M.; et al. MixONat, a Software for the Dereplication of Mixtures Based on 13C NMR Spectroscopy. Anal. Chem. 2020, 92, 8793–8801. [Google Scholar] [CrossRef] [PubMed]
- Pannakal, S.T.; Eilstein, J.; Prasad, A.; Ekhar, P.; Shetty, S.; Peng, Z.; Bordier, E.; Boudah, S.; Paillat, L.; Marrot, L.; et al. Comprehensive characterization of naturally occurring antioxidants from the twigs of mulberry (Morus alba) using on-line high-performance liquid chromatography coupled with chemical detection and high-resolution mass spectrometry. Phytochem. Anal. 2021, 33, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.; Nuzillard, J.-M.; Purson, S.; Hamzaoui, M.; Borie, N.; Reynaud, R.; Renault, J.-H. Identification of Natural Metabolites in Mixture: A Pattern Recognition Strategy Based on 13C NMR. Anal. Chem. 2014, 86, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Colin, M.; Hubert, J.; Charpentier, E.; Angelis, A.; Bounasri, H.; Bertaux, B.; Kotland, A.; Reffuveille, F.; Nuzillard, J.-M.; et al. Abundant Extractable Metabolites from Temperate Tree Barks: The Specific Antimicrobial Activity of Prunus avium Extracts. Antibiotics 2020, 9, 111. [Google Scholar] [CrossRef]
- Mahdi, D.H.; Hubert, J.; Renault, J.-H.; Martinez, A.; Schubert, A.; Engel, K.M.; Koudogbo, B.; Vissiennon, Z.; Ahyi, V.; Nieber, K.; et al. Chemical Profile and Antimicrobial Activity of the Fungus-Growing Termite Strain Macrotermes bellicosus Used in Traditional Medicine in the Republic of Benin. Molecules 2020, 25, 5015. [Google Scholar] [CrossRef]
- Angelis, A.; Hubert, J.; Aligiannis, N.; Michalea, R.; Abedini, A.; Nuzillard, J.-M.; Gangloff, S.C.; Skaltsounis, A.-L.; Renault, J.-H. Bio-Guided Isolation of Methanol-Soluble Metabolites of Common Spruce (Picea abies) Bark by-Products and Investigation of Their Dermo-Cosmetic Properties. Molecules 2016, 21, 1586. [Google Scholar] [CrossRef]
- Darme, P.; Spalenka, J.; Hubert, J.; Escotte-Binet, S.; Debelle, L.; Villena, I.; Sayagh, C.; Borie, N.; Martinez, A.; Bertaux, B.; et al. Investigation of Antiparasitic Activity of 10 European Tree Bark Extracts on Toxoplasma gondii and Bioguided Identification of Triterpenes in Alnus glutinosa Barks. Antimicrob. Agents Chemother. 2022, 66, e0109821. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.; Kotland, A.; Henes, B.; Poigny, S.; Wandrey, F. Deciphering the Phytochemical Profile of an Alpine Rose (Rhododendron ferrugineum L.) Leaf Extract for a Better Understanding of Its Senolytic and Skin-Rejuvenation Effects. Cosmetics 2022, 9, 37. [Google Scholar] [CrossRef]
- Favre-Godal, Q.; Hubert, J.; Kotland, A.; Garnier, D.; Beaugendre, C.; Gourguillon, L.; Urbain, A.; Lordel-Madeleine, S.; Choisy, P. Extensive Phytochemical Assessment of Dendrobium fimbriatum Hook (Orchidaceae). Nat. Prod. Commun. 2022, 17, 1934578X2210745. [Google Scholar] [CrossRef]
- Grigolon, G.; Nowak, K.; Poigny, S.; Hubert, J.; Kotland, A.; Waldschütz, L.; Wandrey, F. From Coffee Waste to Active Ingredient for Cosmetic Applications. Int. J. Mol. Sci. 2023, 24, 8516. [Google Scholar] [CrossRef] [PubMed]
- Bijttebier, S.; Van der Auwera, A.; Voorspoels, S.; Noten, B.; Hermans, N.; Pieters, L.; Apers, S. A First Step in the Quest for the Active Constituents in Filipendula ulmaria (Meadowsweet): Comprehensive Phytochemical Identification by Liquid Chromatography Coupled to Quadrupole-Orbitrap Mass Spectrometry. Planta Medica 2016, 82, 559–572. [Google Scholar] [CrossRef]
- Gainche, M.; Ogeron, C.; Ripoche, I.; Senejoux, F.; Cholet, J.; Decombat, C.; Delort, L.; Berthon, J.-Y.; Saunier, E.; Chezet, F.C.; et al. Xanthine Oxidase Inhibitors from Filipendula ulmaria (L.) Maxim. and Their Efficient Detections by HPTLC and HPLC Analyses. Molecules 2021, 26, 1939. [Google Scholar] [CrossRef]
- Farzaneh, A.; Hadjiakhoondi, A.; Khanavi, M.; Manayi, A.; Bahramsoltani, R.; Kalkhorani, M. Filipendula ulmaria (L.) Maxim. (Meadowsweet): A Review of Traditional Uses, Phytochemistry and Pharmacology. Res. J. Pharmacogn. 2022, 9, 85–106. [Google Scholar] [CrossRef]
- Samardžić, S.; Arsenijević, J.; Božić, D.; Milenković, M.; Tešević, V.; Maksimović, Z. Antioxidant, anti-inflammatory and gastroprotective activity of Filipendula ulmaria (L.) Maxim. and Filipendula vulgaris Moench. J. Ethnopharmacol. 2018, 213, 132–137. [Google Scholar] [CrossRef]
- Katanić, J.; Boroja, T.; Mihailović, V.; Nikles, S.; Pan, S.-P.; Rosić, G.; Selaković, D.; Joksimović, J.; Mitrović, S.; Bauer, R. In vitro and in vivo assessment of meadowsweet (Filipendula ulmaria) as anti-inflammatory agent. J. Ethnopharmacol. 2016, 193, 627–636. [Google Scholar] [CrossRef]
- Harbourne, N.; Jacquier, J.C.; O’Riordan, D. Optimisation of the aqueous extraction conditions of phenols from meadowsweet (Filipendula ulmaria L.) for incorporation into beverages. Food Chem. 2009, 116, 722–727. [Google Scholar] [CrossRef]
- Nitta, Y.; Kikuzaki, H.; Azuma, T.; Ye, Y.; Sakaue, M.; Higuchi, Y.; Komori, H.; Ueno, H. Inhibitory activity of Filipendula ulmaria constituents on recombinant human histidine decarboxylase. Food Chem. 2013, 138, 1551–1556. [Google Scholar] [CrossRef]
- Pukalskienė, M.; Venskutonis, R.; Pukalskas, A. Phytochemical Characterization of Filipendula ulmaria by UPLC/Q-TOF-MS and Evaluation of Antioxidant Activity. Rec. Nat. Prod. 2015, 9, 451–455. [Google Scholar]
- Rockwell, G.; Johnson, G.; Sibatani, A. In vitro senescence of human keratinocyte cultures. Cell Struct. Funct. 1987, 12, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Skoog, T.; Jouhilahti, E.-M.; Siitonen, H.A.; Nuutila, K.; Tervaniemi, M.H.; Vuola, J.; Johnsson, A.; Lönnerberg, P.; Linnarsson, S.; et al. Gene expression analysis of skin grafts and cultured keratinocytes using synthetic RNA normalization reveals insights into differentiation and growth control. BMC Genom. 2015, 16, 476. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Lee, J.O.; Kim, J.H.; Lee, S.K.; You, G.Y.; Park, S.H.; Park, J.M.; Kim, E.-K.; Suh, P.-G.; An, J.K.; et al. Quercetin suppresses HeLa cell viability via AMPK-induced HSP70 and EGFR down-regulation. J. Cell. Physiol. 2010, 223, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Hwang, J.J. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br. J. Pharmacol. 1999, 128, 999–1010. [Google Scholar] [CrossRef]
- Cuevas, M.J.; Tieppo, J.; Marroni, N.P.; Tuñón, M.J.; González-Gallego, J. Suppression of Amphiregulin/Epidermal Growth Factor Receptor Signals Contributes to the Protective Effects of Quercetin in Cirrhotic Rats. J. Nutr. 2011, 141, 1299–1305. [Google Scholar] [CrossRef]
- Kim, J.; Cho, N.; Kim, E.-M.; Park, K.-S.; Kang, Y.W.; Nam, J.H.; Nam, M.S.; Kim, K.K. Cudrania tricuspidata leaf extracts and its components, chlorogenic acid, kaempferol, and quercetin, increase claudin 1 expression in human keratinocytes, enhancing intercellular tight junction capacity. Appl. Biol. Chem. 2020, 63, 23. [Google Scholar] [CrossRef]
- Al-Roujayee, A.S. Naringenin improves the healing process of thermally-induced skin damage in rats. J. Int. Med Res. 2017, 45, 570–582. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Lobo, J.M.S.; Almeida, I.F. Sensitive skin: Active ingredients on the spotlight. Int. J. Cosmet. Sci. 2021, 44, 56–73. [Google Scholar] [CrossRef]
- Lim, S.W.; Hong, S.P.; Jeong, S.W.; Kim, B.; Bak, H.; Ryoo, H.C.; Lee, S.H.; Ahn, S.K. Simultaneous effect of ursolic acid and oleanolic acid on epidermal permeability barrier function and epidermal keratinocyte differentiation via peroxisome proliferator-activated receptor-α. J. Dermatol. 2007, 34, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Sonam, T.; Shimizu, K. The Potential of Triterpenoids from Loquat Leaves (Eriobotrya japonica) for Prevention and Treatment of Skin Disorder. Int. J. Mol. Sci. 2017, 18, 1030. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Hwang, I.H.; Lee, M.W. Anti-acne vulgaris effect including skin barrier improvement and 5α-reductase inhibition by tellimagrandin I from Carpinus tschonoskii. BMC Complement. Altern. Med. 2019, 19, 323. [Google Scholar] [CrossRef] [PubMed]

| Retention Time (rt in min) | Observed m/z | Molecular Formula | Δppm | Tentative Identification |
|---|---|---|---|---|
| 1.6 | 285.0815 | C9H17O10 | −2.5 | Not assigned |
| 1.8 | 195.0505 [M − H]− | C6H11O7 | 0.0 | Hexonic acid |
| 1.9 | 191.0555 [M − H]− | C7H11O6 | −0.5 | Quinic acid |
| 2.2 | 341.1089 [M − H]− | C12H21O11 | 1.5 | Saccharose * |
| 4.6 | 331.0664 [M − H]− | C13H15O10 | 0.0 | Mono-O-galloyl-hexoside isomer 1 |
| 4.9 | 331.0665 [M − H]− | C13H15O10 | −0.3 | Mono-O-galloyl-hexoside isomer 2 |
| 5.1 | 339.1292 | C13H23O10 | 0.3 | Not assigned |
| 5.2 | 169.0137 [M − H]− | C7H5O5 | 0.0 | Gallic acid * |
| 5.4 | 483.0783 [M − H]− | C20H19O14 | 1.7 | Di-O-galloyl-hexoside isomer 1 |
| 5.8 | 331.0667 [M − H]− | C13H15O10 | 0.6 | Mono-O-galloyl-hexoside isomer 3 |
| 6.1 | 483.0775 [M − H]− | C20H19O14 | 0.2 | Di-O-galloyl-hexoside isomer 2 |
| 6.3 | 315.0715 [M − H]− | C13H15O9 | −0.3 | Dihydroxybenzoic acid O-hexoside |
| 7.7 | 319.0423 [M − H]− | C15H11O8 | −9.7 | Dihydromyricetin |
| 7.8 | 483.0775 [M − H]− | C20H19O14 | 0.0 | Di-O-galloyl-hexoside isomer 3 |
| 8.0 | 785.0839 [M − H]− | C34H25O22 | 0.3 | Tellimagrandin I * or isomer |
| 8.2 | 635.0889 [M − H]− | C27H23O18 | 0.8 | Tri-O-galloyl-hexoside |
| 8.3 | 451.1010 [M − H]− | C24H19O9 | −4.2 | Coumaroylepigallocatechin |
| 8.4 | 375.0694 191.0556 quinic acid fragment | C18H15O9 C7H11O6 | −5.9 0.0 | Not assigned |
| 8.5 | 289.0714 909.0999, 785.0842, 454.0461 | C15H13O6 | 0.7 | Catechin |
| 8.8 | 953.0895 [M − H]− 909.0999 [M − COOH]− 785.0837, 465.0367, 454.0460 | C41H29O27 C40H29O25 C34H25O22 | −0.1 0.0 | Chebulagic acid or isomer |
| 8.9 | 785.0840 [M − H]− | C34H25O22 | 0.4 | Tellimagrandin I * or isomer |
| 9.3 | 319.0431 | C15H11O8 | Not assigned | |
| 9.7 | 339.0718 | C15H15O9 | 0.6 | Not assigned |
| 9.8 | 359.0745 337.0925 coumaroylquinic acid 191.0556 quinic acid fragment | C18H15O8 C16H17O8 C7H11O6 | Not assigned | |
| 9.9 | 785.0845 [M − H]− 481.1118, 491.1403, 625.1407 | C34H25O22 | 1.0 | Minor isomer of Tellimagrandin I |
| 10.1 | 935.0803 [M − H]− 467.0357 [M − H-3galloyl]− | C41H27O26 | 1.3 | Casuarinin or Casuarictin |
| 10.2 | 1105.1012 [M − H]− 1061.1110 fragment of Rugosin D 936.0874 [M − 2H]2− 530.0513 fragment of rugosin A 541.0423 | C48H33O31 C47H33O29 | 0.5 0.2 | Rugosin A Rugosin D |
| 10.4 | 937.0955 [M − H]− 959.0774, 479.0345, 468.0435 | C41H29O26 | 0.9 | Tellimagrandin II * |
| 10.8 | 935.0800 [M − H]− 787.1003 [M − H-galloyl]− 467.0357 [M − H-3galloyl]− 303.0485 [M − H-4galloyl]− | C41H27O26 | 1.0 | Casuarinin or Casuarictin |
| 10.9 | 687.3029 [M − H]− | xx | xx | Not assigned |
| 11.0 | 609.1450 [M − H]− | C27H29O16 | −1.0 | Rutoside * |
| 11.3 | 197.0454 [M − H]− | C9H9O5 | 2.0 | Syringic acid |
| 11.4 | 463.0877 [M − H]− | C21H19O12 | 0.0 | Quercetin O-hexoside isomer 1 |
| 11.5 | 463.0876 [M − H]− 301.0348 quercetin fragment | C21H19O12 | −0.2 | Quercetin O-hexoside isomer 2 |
| 11.9 | 593.1505 [M − H]− 1087.0900 [2M − H]− 285.0396 kaempferol fragment | C27H29O15 | −0.2 | Kaempferol-O-hexoside-rhamnoside |
| 11.9 | 433.0771 [M − H]− | C20H17O11 | 0.0 | Quercetin-O-pentoside |
| 12.1 | 447.0930 [M − H]− | C21H19O11 | 0.0 | Quercetin-O-rhamnoside |
| 12.2 | 477.1034 [M − H]− | C22H21O12 | 0.2 | Methyl-quercetin O-hexoside |
| 12.3 | 433.0772 [M − H]− 301.0353 quercetin fragment | C20H17O11 | 0.2 | Quercetin-O-pentoside |
| 12.4 | 447.0927 [M − H]− | C21H19O11 | 0.0 | Quercetin-O-rhamnoside |
| 12.5 | 477.1031 [M − H]− | C22H21O12 | −0.4 | Methylquercetin-O-hexoside |
| 12.7 | 463.0882 301.0353 quercetin fragment | C21H19O12 | 1.1 | Spiraeoside * (Quercetin-O-hexoside isomer 3) |
| 12.8 | 601.0827 [M − H]− 301.0347 quercetin fragment | C27H21O16 | −0.5 | Quercetin-O-galloyl-pentoside |
| 13.0 | 447.0922 [M − H]− 285.0389 kaempferol fragment | C21H19O11 | −1.1 | Kaempferol-4′-O-glucoside * |
| 13.2 | 519.1136 465.1031 | C24H23O13 C21H21O12 | −0.6 −0.4 | Not assigned |
| 13.4 | 615.0984 [M − H]− | C28H23O16 | −0.3 | Quercetin-O-galloyl-hexoside |
| 13.8 | 585.0880 [M − H]− 301.0350 quercetin fragment | C27H21O15 C15H9O7 | 0.9 0.7 | Quercetin-O-galloyl-arabinoside |
| 14.2 | 297.0399 [M − H]− | C16H9O6 | −7.1 | Not assigned |
| 14.9 | 301.0348 [M − H]− | C15H9O7 | 1.0 | Quercetin * |
| 16.1 | 271.0606 [M − H]− | C15H11O5 | 0.4 | Naringenin * |
| 16.2 | 285.0399 [M − H]− | C15H9O6 | 1.1 | Kaempferol * |
| 16.5 | 329.2329 | C18H33O5 | 0.3 | Tri-HOME (trihydroxyyoctadecenoic acid) |
| 16.9 | 287.222 | C16H31O4 | 1.5 | Dihydroxypalmitic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pannakal, S.T.; Eilstein, J.; Hubert, J.; Kotland, A.; Prasad, A.; Gueguiniat-Prevot, A.; Juchaux, F.; Beaumard, F.; Seru, G.; John, S.; et al. Rapid Chemical Profiling of Filipendula ulmaria Using CPC Fractionation, 2-D Mapping of 13C NMR Data, and High-Resolution LC–MS. Molecules 2023, 28, 6349. https://doi.org/10.3390/molecules28176349
Pannakal ST, Eilstein J, Hubert J, Kotland A, Prasad A, Gueguiniat-Prevot A, Juchaux F, Beaumard F, Seru G, John S, et al. Rapid Chemical Profiling of Filipendula ulmaria Using CPC Fractionation, 2-D Mapping of 13C NMR Data, and High-Resolution LC–MS. Molecules. 2023; 28(17):6349. https://doi.org/10.3390/molecules28176349
Chicago/Turabian StylePannakal, Steve Thomas, Joan Eilstein, Jane Hubert, Alexis Kotland, Arpita Prasad, Amelie Gueguiniat-Prevot, Franck Juchaux, Floriane Beaumard, Ganapaty Seru, Sherluck John, and et al. 2023. "Rapid Chemical Profiling of Filipendula ulmaria Using CPC Fractionation, 2-D Mapping of 13C NMR Data, and High-Resolution LC–MS" Molecules 28, no. 17: 6349. https://doi.org/10.3390/molecules28176349
APA StylePannakal, S. T., Eilstein, J., Hubert, J., Kotland, A., Prasad, A., Gueguiniat-Prevot, A., Juchaux, F., Beaumard, F., Seru, G., John, S., & Roy, D. (2023). Rapid Chemical Profiling of Filipendula ulmaria Using CPC Fractionation, 2-D Mapping of 13C NMR Data, and High-Resolution LC–MS. Molecules, 28(17), 6349. https://doi.org/10.3390/molecules28176349








