A Series of Novel 3D Coordination Polymers Based on the Quinoline-2,4-dicarboxylate Building Block and Lanthanide(III) Ions—Temperature Dependence Investigations
Abstract
:1. Introduction
2. Results and Discussion
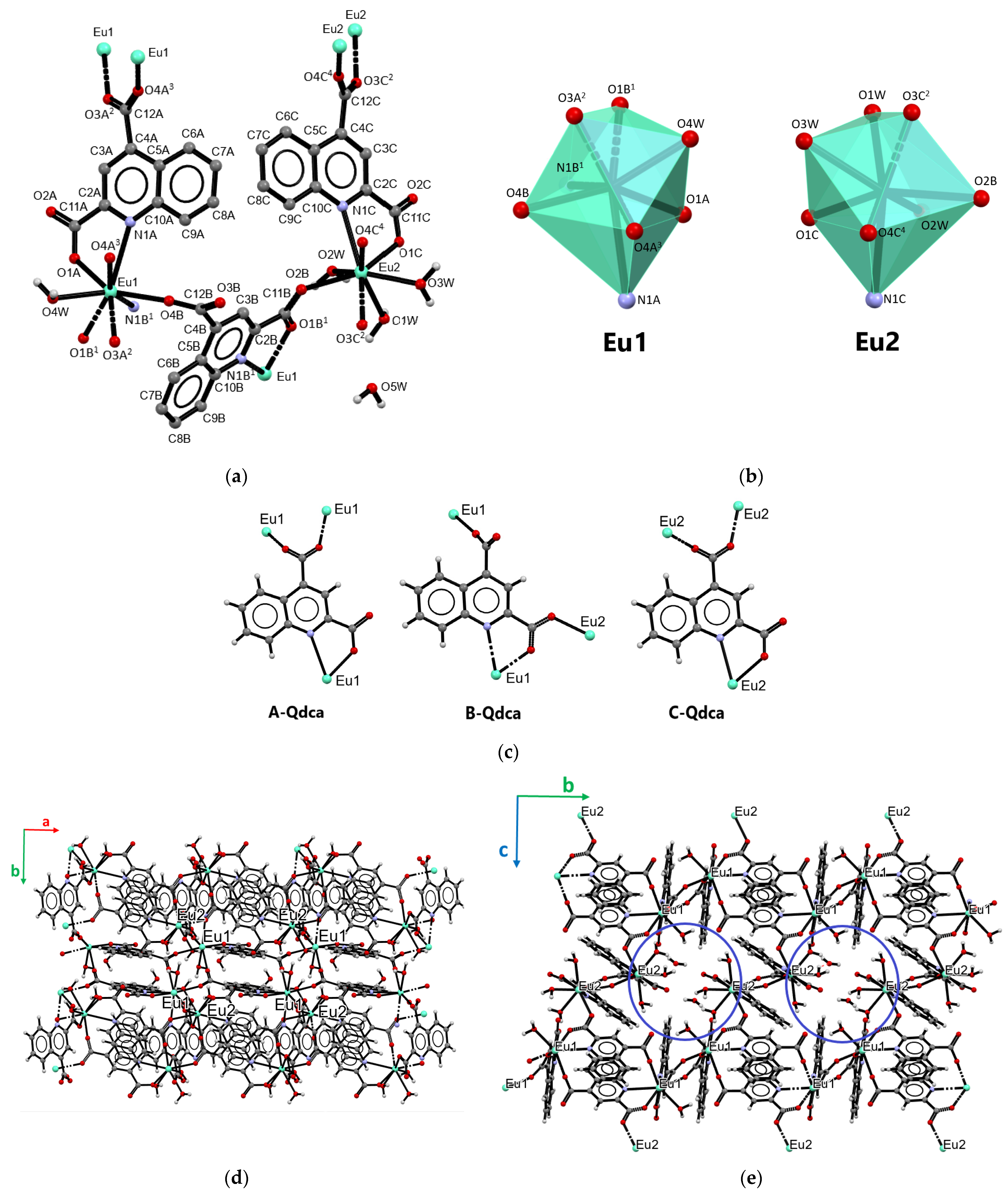
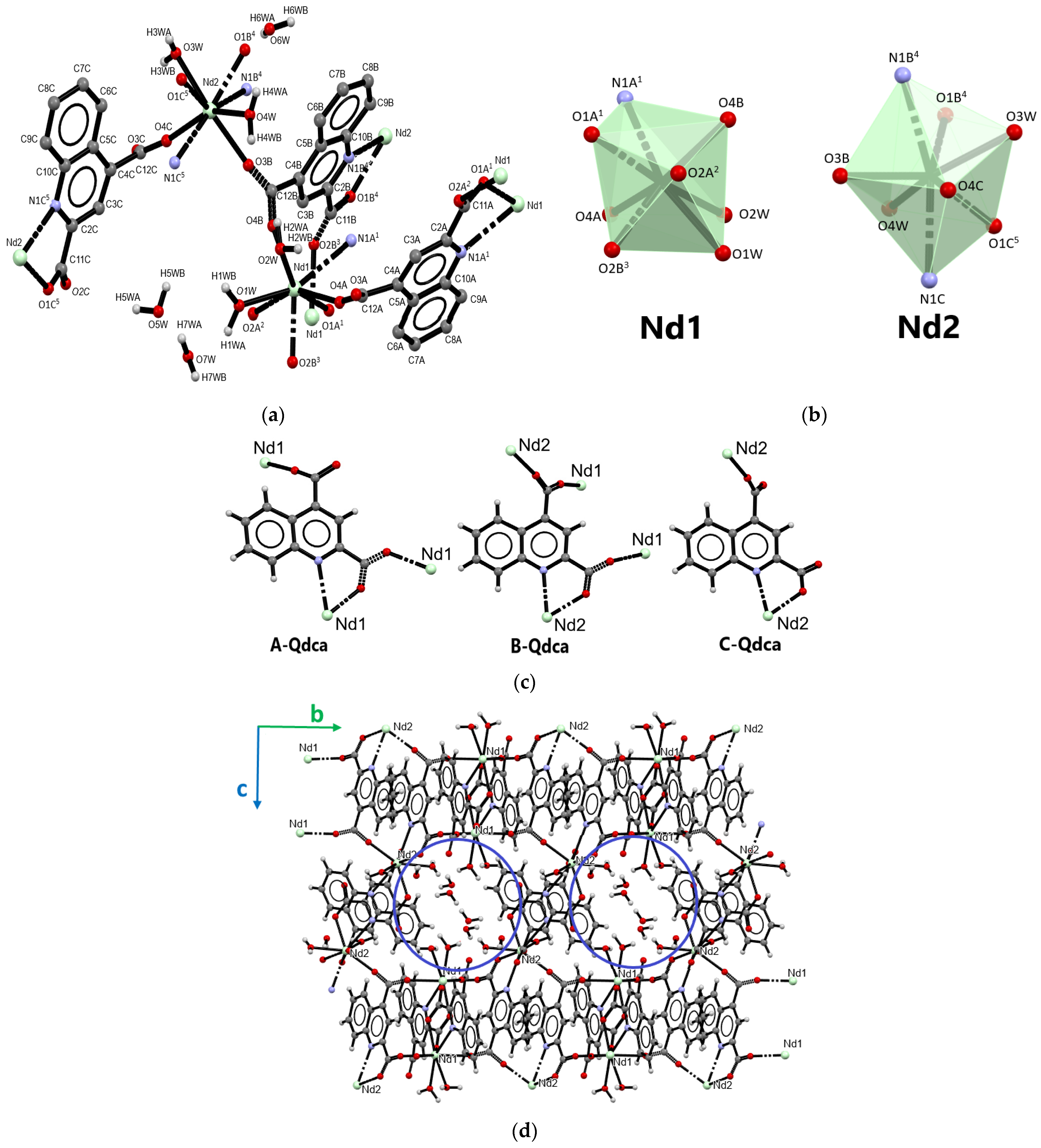
2.1. Structural Characterization
2.2. PXRD Analysis and Structural Transformations
2.3. Infrared and Electronic Spectra
2.4. Thermal Analysis
2.5. Luminescence Investigations
3. Materials and Methods
3.1. Synthetic Procedures
3.2. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seidi, F.; Jouyandeh, M.; Taghizadeh, M.; Taghizadeh, A.; Vahabi, H.; Habibzadeh, S.; Formela, K.; Saeb, M.R. Metal-Organic Framework (MOF)/Epoxy Coatings: A Review. Materials 2020, 13, 2881. [Google Scholar] [CrossRef] [PubMed]
- Łyszczek, R.; Rusinek, I.; Sienkiewicz-Gromiuk, J.; Iwan, M.; Pavlyuk, O. 3-D Lanthanide Coordination Polymers with the Flexible 1,3-Phenylenediacetate Linker: Spectroscopic, Structural and Thermal Investigations. Polyhedron 2019, 159, 93–101. [Google Scholar] [CrossRef]
- Gustafsson, M.; Bartoszewicz, A.; Martín-Matute, B.; Sun, J.; Grins, J.; Zhao, T.; Li, Z.; Zhu, G.; Zou, X. A Family of Highly Stable Lanthanide Metal−Organic Frameworks: Structural Evolution and Catalytic Activity. Chem. Mater. 2010, 22, 3316–3322. [Google Scholar] [CrossRef]
- Gao, Y.; Gong, S.-Y.; Chen, B.; Xing, W.-H.; Fei, Y.-F.; Hu, Z.-T.; Pan, Z. Progress in Metal-Organic Framework Catalysts for Selective Catalytic Reduction of NOx: A Mini-Review. Atmosphere 2022, 13, 793. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Luo, Z.-D.; Pan, Y.; Kumar Singh, A.; Trivedi, M.; Kumar, A. Recent Developments in Luminescent Coordination Polymers: Designing Strategies, Sensing Application and Theoretical Evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Kapteijn, F. Water and Metal–Organic Frameworks: From Interaction toward Utilization. Chem. Rev. 2020, 120, 8303–8377. [Google Scholar] [CrossRef]
- Wu, D.; Navrotsky, A. Thermodynamics of Metal-Organic Frameworks. J. Solid State Chem. 2015, 223, 53–58. [Google Scholar] [CrossRef]
- Seetharaj, R.; Vandana, P.V.; Arya, P.; Mathew, S. Dependence of Solvents, PH, Molar Ratio and Temperature in Tuning Metal Organic Framework Architecture. Arab. J. Chem. 2019, 12, 295–315. [Google Scholar] [CrossRef]
- Pan, L.; Frydel, T.; Sander, M.B.; Huang, X.; Li, J. The Effect of PH on the Dimensionality of Coordination Polymers. Inorg. Chem. 2001, 40, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.; Kim, J.; Ahn, W.-S. Synthesis of Metal-Organic Frameworks: A Mini Review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New Synthetic Routes towards MOF Production at Scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef]
- Zhang, P.; Kang, X.; Tao, L.; Zheng, L.; Xiang, J.; Duan, R.; Li, J.; Chen, P.; Xing, X.; Mo, G.; et al. A New Route for the Rapid Synthesis of Metal–Organic Frameworks at Room Temperature. CCS Chem. 2022, 5, 1462–1469. [Google Scholar] [CrossRef]
- Ehrling, S.; Senkovska, I.; Efimova, A.; Bon, V.; Abylgazina, L.; Petkov, P.; Evans, J.D.; Gamal Attallah, A.; Wharmby, M.T.; Roslova, M.; et al. Temperature Driven Transformation of the Flexible Metal–Organic Framework DUT-8(Ni). Chem. Eur. J. 2022, 28, e202201281. [Google Scholar] [CrossRef]
- Khoshhal, S.; Ghoreyshi, A.A.; Jahanshahi, M.; Mohammadi, M. Study of the Temperature and Solvent Content Effects on the Structure of Cu–BTC Metal Organic Framework for Hydrogen Storage. RSC Adv. 2015, 5, 24758–24768. [Google Scholar] [CrossRef]
- Krause, S.; Bon, V.; Du, H.; Dunin-Borkowski, R.E.; Stoeck, U.; Senkovska, I.; Kaskel, S. The Impact of Crystal Size and Temperature on the Adsorption-Induced Flexibility of the Zr-Based Metal–Organic Framework DUT-98. Beilstein J. Nanotechnol. 2019, 10, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Ohsaki, S.; Hiraide, S.; Yamamoto, D.; Watanabe, S.; Miyahara, M.T. Adsorption-Induced Structural Transition of ZIF-8: A Combined Experimental and Simulation Study. J. Phys. Chem. C 2014, 118, 8445–8454. [Google Scholar] [CrossRef]
- Gallaba, D.H.; Albesa, A.G.; Migone, A.D. Evidence of Gate-Opening on Xenon Adsorption on ZIF-8: An Adsorption and Computer Simulation Study. J. Phys. Chem. C 2016, 120, 16649–16657. [Google Scholar] [CrossRef]
- Zhang, C.; Gee, J.A.; Sholl, D.S.; Lively, R.P. Crystal-Size-Dependent Structural Transitions in Nanoporous Crystals: Adsorption-Induced Transitions in ZIF-8. J. Phys. Chem. C 2014, 118, 20727–20733. [Google Scholar] [CrossRef]
- Yang, F.; Mu, H.; Wang, C.; Xiang, L.; Yao, K.X.; Liu, L.; Yang, Y.; Han, Y.; Li, Y.; Pan, Y. Morphological Map of ZIF-8 Crystals with Five Distinctive Shapes: Feature of Filler in Mixed-Matrix Membranes on C3H6/C3H8 Separation. Chem. Mater. 2018, 30, 3467–3473. [Google Scholar] [CrossRef]
- Kavoosi, N.; Bon, V.; Senkovska, I.; Krause, S.; Atzori, C.; Bonino, F.; Pallmann, J.; Paasch, S.; Brunner, E.; Kaskel, S. Tailoring Adsorption Induced Phase Transitions in the Pillared-Layer Type Metal–Organic Framework DUT-8(Ni). Dalton Trans. 2017, 46, 4685–4695. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Bon, V.; Senkovska, I.; Ehrling, S.; Watanabe, S.; Ohba, M.; Kaskel, S. Tuning the Gate-Opening Pressure and Particle Size Distribution of the Switchable Metal–Organic Framework DUT-8(Ni) by Controlled Nucleation in a Micromixer. Dalton Trans. 2017, 46, 14002–14011. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.-H.; Guo, Z.-F.; Liu, L.; Wang, Z.; Li, B. Catena-Poly[[[Diaquacopper(II)]-μ-Quinoline-2,3-Dicarboxylato-κ3N,O2:O3] Monohydrate]. Acta Crystallogr. E Struct. Rep. Online 2012, 68, m1395. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Liu, G.-Z. Crystal Structure of Bis(Hydrogen 2,3-Quinolinedicarboxylato)Copper(II), Cu(C11H6NO4)2. Z. Für Krist. New Cryst. Struct. 2010, 225, 761–762. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, M.M.; Qin, J.B.; Li, J.; Meng, J.P.; Lin, J.H. Metal(II) Complexes Synthesized Based on Quinoline-2,3-Dicarboxylate as Electrocatalysts for the Degradation of Methyl Orange. Dalton Trans. 2014, 43, 8454–8460. [Google Scholar] [CrossRef]
- Hu, M.-Y.; He, Q.; Fan, S.-J.; Wang, Z.-C.; Liu, L.-Y.; Mu, Y.-J.; Peng, Q.; Zhu, S.-F. Ligands with 1,10-Phenanthroline Scaffold for Highly Regioselective Iron-Catalyzed Alkene Hydrosilylation. Nat. Commun. 2018, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Awad, D.J.; Conrad, F.; Koch, A.; Schilde, U.; Pöppl, A.; Strauch, P. 1,10-Phenanthroline-Dithiolate Mixed Ligand Transition Metal Complexes. Synthesis, Characterization and EPR Spectroscopy. Inorganica Chim. Acta 2010, 363, 1488–1494. [Google Scholar] [CrossRef]
- Biradha, K.; Sarkar, M.; Rajput, L. Crystal Engineering of Coordination Polymers Using 4,4′-Bipyridine as a Bond between Transition Metal Atoms. Chem. Commun. 2006, 40, 4169–4179. [Google Scholar] [CrossRef]
- Jia, J.; Blake, A.J.; Champness, N.R.; Hubberstey, P.; Wilson, C.; Schröder, M. Multi-Dimensional Transition-Metal Coordination Polymers of 4,4′-Bipyridine-N,N′-Dioxide: 1D Chains and 2D Sheets. Inorg. Chem. 2008, 47, 8652–8664. [Google Scholar] [CrossRef]
- Seidel, R.W.; Goddard, R.; Zibrowius, B.; Oppel, I.M. A Molecular Antenna Coordination Polymer from Cadmium(II) and 4,4’-Bipyridine Featuring Three Distinct Polymer Strands in the Crystal. Polymers 2011, 3, 1458–1474. [Google Scholar] [CrossRef]
- Irwin, M.; Doyle, L.R.; Krämer, T.; Herchel, R.; McGrady, J.E.; Goicoechea, J.M. A Homologous Series of First-Row Transition-Metal Complexes of 2,2′-Bipyridine and Their Ligand Radical Derivatives: Trends in Structure, Magnetism, and Bonding. Inorg. Chem. 2012, 51, 12301–12312. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Housecroft, C.E. The Early Years of 2,2’-Bipyridine—A Ligand in Its Own Lifetime. Molecules 2019, 24, 3951. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.C.G.; Choppin, G.R. Lanthanide Probes in Life, Chemical and Earth Sciences; Theory and Practice; Elsevier: Amsterdam, The Netherlands, 1989; Volume 7, pp. 219–293. [Google Scholar]
- Yu, X.; Ryadun, A.A.; Pavlov, D.I.; Guselnikova, T.Y.; Potapov, A.S.; Fedin, V.P. Highly Luminescent Lanthanide Metal-Organic Frameworks with Tunable Color for Nanomolar Detection of Iron(III), Ofloxacin and Gossypol and Anti-counterfeiting Applications. Angew. Chem. Int. Ed. 2023, 62, e202306680. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ryadun, A.A.; Kovalenko, K.A.; Guselnikova, T.Y.; Ponomareva, V.G.; Potapov, A.S.; Fedin, V.P. 4 in 1: Multifunctional Europium–Organic Frameworks with Luminescence Sensing Properties, White Light Emission, Proton Conductivity and Reverse Acetylene–Carbon Dioxide Adsorption Selectivity. Dalton Trans. 2023, 52, 8695–8703. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Smirnova, K.S.; Pozdnyakov, I.P.; Potapov, A.S.; Lider, E.V. Synthesis, Crystal Structures, and Luminescence Properties of Lanthanide(III) Complexes with 1-(1H-Benzimidazol-1yl-Methyl)-1H-Benzotriazole. Inorganica Chim. Acta 2023, 557, 121697. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Smirnova, K.S.; Pozdnyakov, I.P.; Potapov, A.S.; Lider, E.V. Photoluminescent Lanthanide(III) Coordination Polymers with Bis(1,2,4-Triazol-1-Yl)Methane Linker. Inorganics 2023, 11, 317. [Google Scholar] [CrossRef]
- Lis, S.; Elbanowski, M.; Mąkowska, B.; Hnatejko, Z. Energy Transfer in Solution of Lanthanide Complexes. J. Photochem. Photobiol. A Chem. 2002, 150, 233–247. [Google Scholar] [CrossRef]
- Kłonkowski, A.M.; Lis, S.; Pietraszkiewicz, M.; Hnatejko, Z.; Czarnobaj, K.; Elbanowski, M. Luminescence Properties of Materials with Eu(III) Complexes: Role of Ligand, Coligand, Anion, and Matrix. Chem. Mater. 2003, 15, 656–663. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide Luminescence for Functional Materials and Bio-Sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Weissleder, R.; Ntziachristos, V. Shedding Light onto Live Molecular Targets. Nat Med 2003, 9, 123–128. [Google Scholar] [CrossRef]
- Lenaerts, P.; Driesen, K.; Van Deun, R.; Binnemans, K. Covalent Coupling of Luminescent Tris(2-Thenoyltrifluoroacetonato)Lanthanide(III) Complexes on a Merrifield Resin. Chem. Mater. 2005, 17, 2148–2154. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Wada, Y.; Yanagida, S. Strategies for the Design of Luminescent Lanthanide(III) Complexes and Their Photonic Applications. J. Photochem. Photobiol. C: Photochem. Rev. 2004, 5, 183–202. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.; Bai, G.; Yang, X.; Xie, H.; Xu, S. Near-Infrared Excitation and Emitting Thermometer Based on Nd3+ Doped Ytterbium Molybdate with Thermally Enhanced Emissions. J. Lumin. 2020, 228, 117655. [Google Scholar] [CrossRef]
- Bart, S.C. What Is the “Lanthanide Contraction”? Inorg. Chem. 2023, 62, 3713–3714. [Google Scholar] [CrossRef]
- Sharma, S.K. Comparative Vibrational Spectroscopic Studies of 7-Chloro-4-Hydroxy-3-Quinolinecarboxylic Acid Based on Density Functional Theory. IOSRJAP 2012, 1, 27–37. [Google Scholar] [CrossRef]
- Özel, A.E.; Büyükmurat, Y.; Akyüz, S. Infrared-Spectra and Normal-Coordinate Analysis of Quinoline and Quinoline Complexes. J. Mol. Struct. 2001, 565–566, 455–462. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. On the Design of Highly Luminescent Lanthanide Complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, Thermal and Mechanical Stabilities of Metal–Organic Frameworks. Nat. Rev. Mater 2016, 1, 15018. [Google Scholar] [CrossRef]
- Vlasyuk, D.; Łyszczek, R. Effect of Different Synthesis Approaches on Structural and Thermal Properties of Lanthanide(III) Metal–Organic Frameworks Based on the 1H-Pyrazole-3,5-Dicarboxylate Linker. J. Inorg. Organomet. Polym. 2021, 31, 3534–3548. [Google Scholar] [CrossRef]
- Łyszczek, R.; Vlasyuk, D.; Podkościelna, B.; Głuchowska, H.; Piramidowicz, R.; Jusza, A. A Top-Down Approach and Thermal Characterization of Luminescent Hybrid BPA.DA-MMA@Ln2L3 Materials Based on Lanthanide(III) 1H-Pyrazole-3,5-Dicarboxylates. Materials 2022, 15, 8826. [Google Scholar] [CrossRef]
- Głuchowska, H.; Łyszczek, R.; Jusza, A.; Piramidowicz, R. Effect of N,N′-Dimethylformamide Solvent on Structure and Thermal Properties of Lanthanide(III) Complexes with Flexible Biphenyl-4,4′-Dioxydiacetic Acid. J. Therm. Anal. Calorim. 2022, 147, 1187–1200. [Google Scholar] [CrossRef]
- Keene, F.R.; Szalda, D.J.; Wilson, T.A. Mode of Coordination of Tris(2-Pyridyl)Methanol to Ruthenium(II): Synthetic, Spectral, and Structural Studies of the Bis(Ligand) Species. Inorg. Chem. 1987, 26, 2211–2216. [Google Scholar] [CrossRef]
- Salaam, J.; N’Dala-Louika, I.; Balogh, C.; Suleimanov, I.; Pilet, G.; Veyre, L.; Camp, C.; Thieuleux, C.; Riobé, F.; Maury, O. Tris-dipicolinate Lanthanide Complexes: Influence of the Second Hydration Sphere on the Solid-State Luminescence Properties. Eur. J. Inorg. Chem. 2022, 2022, e202200412. [Google Scholar] [CrossRef]
- Chen, S.; Fan, R.-Q.; Sun, C.-F.; Wang, P.; Yang, Y.-L.; Su, Q.; Mu, Y. Synthesis, Structure, and Luminescent Properties of Lanthanide-Based Two-Dimensional and Three-Dimensional Metal–Organic Frameworks with 2,4′-Biphenyldicarboxylic Acid. Cryst. Growth Des. 2012, 12, 1337–1346. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, C.-Y.; Gao, W.-H.; Wu, Y.; Xie, Y.-B.; Sun, J.-H. Two Binuclear Lanthanide Complexes with 4-Quinoline Carboxylic Acid: Crystal Structures and Luminescent Properties. J. Coord. Chem. 2009, 62, 2689–2697. [Google Scholar] [CrossRef]
- Yan, B.; Zhou, B. Photophysical Properties of Dysprosium Complexes with Aromatic Carboxylic Acids by Molecular Spectroscopy. J. Photochem. Photobiol. A Chem. 2005, 171, 181–186. [Google Scholar] [CrossRef]
- Latva, M.; Takalo, H.; Mukkala, V.-M.; Matachescu, C.; Rodríguez-Ubis, J.C.; Kankare, J. Correlation between the Lowest Triplet State Energy Level of the Ligand and Lanthanide(III) Luminescence Quantum Yield. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]
- Huskowska, E.; Turowska-Tyrk, I.; Legendziewicz, J.; Riehl, J.P. The Structure and Spectroscopy of Lanthanide(iii) Complexes with 2,2′-Bipyridine-1,1′-Dioxide in Solution and in the Solid State: Effects of Ionic Size and Solvent on Photophysics, Ligand Structure and Coordination. New J. Chem. 2002, 26, 1461–1467. [Google Scholar] [CrossRef]
- Aebischer, A.; Gumy, F.; Bünzli, J.-C.G. Intrinsic Quantum Yields and Radiative Lifetimes of Lanthanide Tris(Dipicolinates). Phys. Chem. Chem. Phys. 2009, 11, 1346. [Google Scholar] [CrossRef]
- Kwiatek, D.; Kubicki, M.; Toliński, T.; Ferenc, W.; Lis, S.; Hnatejko, Z. A Series of New Pyridine Carboxamide Complexes and Self-Assemblies with Tb(III), Eu(III), Zn(II), Cu(II) Ions and Their Luminescent and Magnetic Properties. J. Coord. Chem. 2019, 72, 727–748. [Google Scholar] [CrossRef]
- Kimura, T.; Kato, Y. Luminescence Study on the Inner-Sphere Hydration Number of Lanthanide(III) Ions in Concentrated Aqueous Salt Solutions in Fluid and Frozen States. J. Alloys Compd. 1998, 278, 92–97. [Google Scholar] [CrossRef]
- Wong, K.-L.; Bünzli, J.-C.G.; Tanner, P.A. Quantum Yield and Brightness. J. Lumin. 2020, 224, 117256. [Google Scholar] [CrossRef]
- Woźny, P.; Soler-Carracedo, K.; Stopikowska, N.; Martín, I.R.; Runowski, M. Structure-Dependent Luminescence of Eu3+-Doped Strontium Vanadates Synthesized with Different V: Sr Ratios—Application in WLEDs and Ultra-Sensitive Optical Thermometry. J. Mater. Chem. C 2023, 11, 4792–4807. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Kalinichev, A.A.; Kurochkin, M.A.; Golyeva, E.V.; Kolesnikov, E.Y.; Kurochkin, A.V.; Lähderanta, E.; Mikhailov, M.D. YVO4:Nd3+ Nanophosphors as NIR-to-NIR Thermal Sensors in Wide Temperature Range. Sci. Rep. 2017, 7, 18002. [Google Scholar] [CrossRef]
- Manyum, P.; Rittisut, W.; Mool-am-kha, P.; Ekwongsa, C.; Wantana, N.; Ruangtaweep, Y.; Popanao, M.; Rujirawat, S.; Yimnirun, R.; Kidkhunthod, P.; et al. Structural and Luminescence Investigations of Gd3+-Er3+ Doped in Lithium Aluminum Borate Glasses Using XANES and EXAFS Techniques. Radiat. Phys. Chem. 2023, 206, 110801. [Google Scholar] [CrossRef]
- CrysAlisPRO Software System; Rigaku: Oxford, UK, 2016.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Faruggia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]











| Complex/ Temperature | 100 °C | 120 °C | 150 °C | |
|---|---|---|---|---|
| Crystal Structure Type x; y (Number of Water Molecules from Inner and Outer Coordination Sphere) Crystal System, Space Group a/b/c [Å] α/β/ƴ [°] | ||||
| [Nd2(Qdca)3(H2O)x]·yH2O | Type-I x = 3; y = 0 (1) orthorhombic, Pna21 14.985/30.366/6.672 90/90/90 | Type-II x = 4; y = 1 (2) triclinic, P-1 9.932/12.315/14.211 89.697/82.258/86.568 | Type-II x = 4; y = 1 (3) triclinic, P-1 9.946/12.323/14.184 89.901/81.851/86.24 | Type-III x = 4; y = 3 (4) triclinic, P-1 10.593/11.806/15.040 97.070/101.073/103.862 |
| [Eu2(Qdca)3(H2O)x]·yH2O | Type-III x = 4; y = 3 (5) triclinic, P-1 10.531/11.698/14.9681 96.498/101.195/104.43 | Type-II x = 4; y = 1 (6) triclinic, P-1 9.859/12.339/14.144 89.89/97.53/93.55 | Type-II x = 4; y = 1 (7) triclinic, P-1 9.853/12.305/14.075 90.02/98.182/93.877 | Type-II x = 4; y = 1 (8) triclinic, P-1 9.858/12.3124/14.055 90.133/98.245/94.016 |
| [Tb2(Qdca)3(H2O)x]·yH2O | Type-II x = 4; y = 1 (9) triclinic, P-1 9.802/12.309/13.988 90.212/98.326/94.157 | Type-III x = 4; y = 3 (10) triclinic, P-1 10.492/11.608/14.911 96.144/101.267/104.977 | Type-III x = 4; y = 3 (11) triclinic, P-1 10.492/11.632/14.969 96.000/101.459/104.980 | |
| [Er2(Qdca)3(H2O)x]·yH2O | Type-IV x = 4; y = 4 (12) triclinic, P-1 9.937/12.285/15.121 84.246/86.472/89.165 | Type-IV x = 4; y = 4 (13) triclinic, P-1 9.936/12.269/15.093 84.151/86.415/89.199 | Type-V x + y = 14 (14) triclinic, P-1 11.75/13.39/15.37 91.27/105.56/114.09 | |
| Compound (Sample Number) | [Nd2(Qdca)3(H2O)3] (1) | [Nd2(Qdca)3(H2O)4]·3H2O (4) | [Eu2(Qdca)3(H2O)4]·H2O (8) | [Tb2(Qdca)3(H2O)4]·H2O (9) | [Er2(Qdca)3(H2O)4]·4H2O (13) |
|---|---|---|---|---|---|
| Empirical formula | C33H21N3O15Nd2 | C33H29N3O19Nd2 | C33H25N3O17Eu2 | C33H25N3O17Tb2 | C33H31N3O20Er2 |
| Formula weight | 988.01 | 1060.07 | 1039.48 | 1053.40 | 1124.13 |
| T/K | 295(2) | 295(2) | 295(2) | 295(2) | 295(2) |
| Crystal system | orthorhombic | triclinic | triclinic | triclinic | triclinic |
| Space group | Pna21 | P-1 | P-1 | P-1 | P-1 |
| a/Å | 14.9850(1) | 10.5934(4) | 9.8575(4) | 9.8018(4) | 9.9361(2) |
| b/Å | 30.3661(2) | 11.8063(4) | 12.3124(4) | 12.3088(5) | 12.2695(2) |
| c/Å | 6.6721(1) | 15.0403(3) | 14.0546(4) | 13.9881(5) | 15.0930(3) |
| α/° | 90 | 97.070(2) | 90.133(2) | 90.212(3) | 84.151(1) |
| β/° | 90 | 101.073(3) | 98.245(3) | 98.326(3) | 86.415(1) |
| γ/° | 90 | 103.862(3) | 94.016(3) | 94.157(3) | 89.199(2) |
| Volume/Å3 | 3036.05(5) | 1763.8(1) | 1683.9(1) | 1665.29(1) | 1826.79(6) |
| Z | 4 | 2 | 2 | 2 | 2 |
| dcalc/g∙cm3 | 2.162 | 1.996 | 2.050 | 2.101 | 2.044 |
| μ/mm−1 | 26.57 | 23.00 | 27.16 | 21.39 | 9.09 |
| 2Θ range/° | 8.29–136.85 | 7.84–136.94 | 7.20–138.06 | 7.20–136.83 | 8.87–136.03 |
| Ref. collected | 18,174 | 26,149 | 23,719 | 20,895 | 20,077 |
| Independent reflections | 5154 [Rint = 0.0326] | 6384 [Rint = 0.0539] | 6084 [Rint = 0.0437] | 6004 [Rint = 0.0446] | 6525 [Rint = 0.0228] |
| Data/restr./ parameters | 5154/10/494 | 6384/21/518 | 6084/15/526 | 6004/13/526 | 6525/23/568 |
| GooF on F2 | 1.020 | 1.110 | 1.044 | 1.100 | 1.064 |
| Final R1, wR2 indices [I > 2σ(I)] | 0.0301, 0.0789 | 0.0504, 0.1381 | 0.0395, 0.1079 | 0.0477, 0.1316 | 0.0315, 0.0874 |
| Final R1, wR2 indices [all data] | 0.0308, 0.0794 | 0.0574, 0.1428 | 0.0458, 0.1119 | 0.0541, 0.1364 | 0.0322, 0.0879 |
| Largest diff. peak/hole/e Å−3 | 1.40/−0.91 | 0.84/−1.70 | 1.35/−1.43 | 0.91/−2.26 | 1.39/−1.45 |
| Flack parameter | −0.016(4) | - | - | - | - |
| CCDC number | 2281669 | 2281670 | 2281671 | 2281672 | 2281673 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasyuk, D.; Łyszczek, R.; Mazur, L.; Pladzyk, A.; Hnatejko, Z.; Woźny, P. A Series of Novel 3D Coordination Polymers Based on the Quinoline-2,4-dicarboxylate Building Block and Lanthanide(III) Ions—Temperature Dependence Investigations. Molecules 2023, 28, 6360. https://doi.org/10.3390/molecules28176360
Vlasyuk D, Łyszczek R, Mazur L, Pladzyk A, Hnatejko Z, Woźny P. A Series of Novel 3D Coordination Polymers Based on the Quinoline-2,4-dicarboxylate Building Block and Lanthanide(III) Ions—Temperature Dependence Investigations. Molecules. 2023; 28(17):6360. https://doi.org/10.3390/molecules28176360
Chicago/Turabian StyleVlasyuk, Dmytro, Renata Łyszczek, Liliana Mazur, Agnieszka Pladzyk, Zbigniew Hnatejko, and Przemysław Woźny. 2023. "A Series of Novel 3D Coordination Polymers Based on the Quinoline-2,4-dicarboxylate Building Block and Lanthanide(III) Ions—Temperature Dependence Investigations" Molecules 28, no. 17: 6360. https://doi.org/10.3390/molecules28176360
APA StyleVlasyuk, D., Łyszczek, R., Mazur, L., Pladzyk, A., Hnatejko, Z., & Woźny, P. (2023). A Series of Novel 3D Coordination Polymers Based on the Quinoline-2,4-dicarboxylate Building Block and Lanthanide(III) Ions—Temperature Dependence Investigations. Molecules, 28(17), 6360. https://doi.org/10.3390/molecules28176360







