Synthesis and Biological Activities of Luminescent 5,6-Membered Bis(Metallacyclic) Platinum(II) Complexes
Abstract
:1. Introduction
2. Results and Discussion
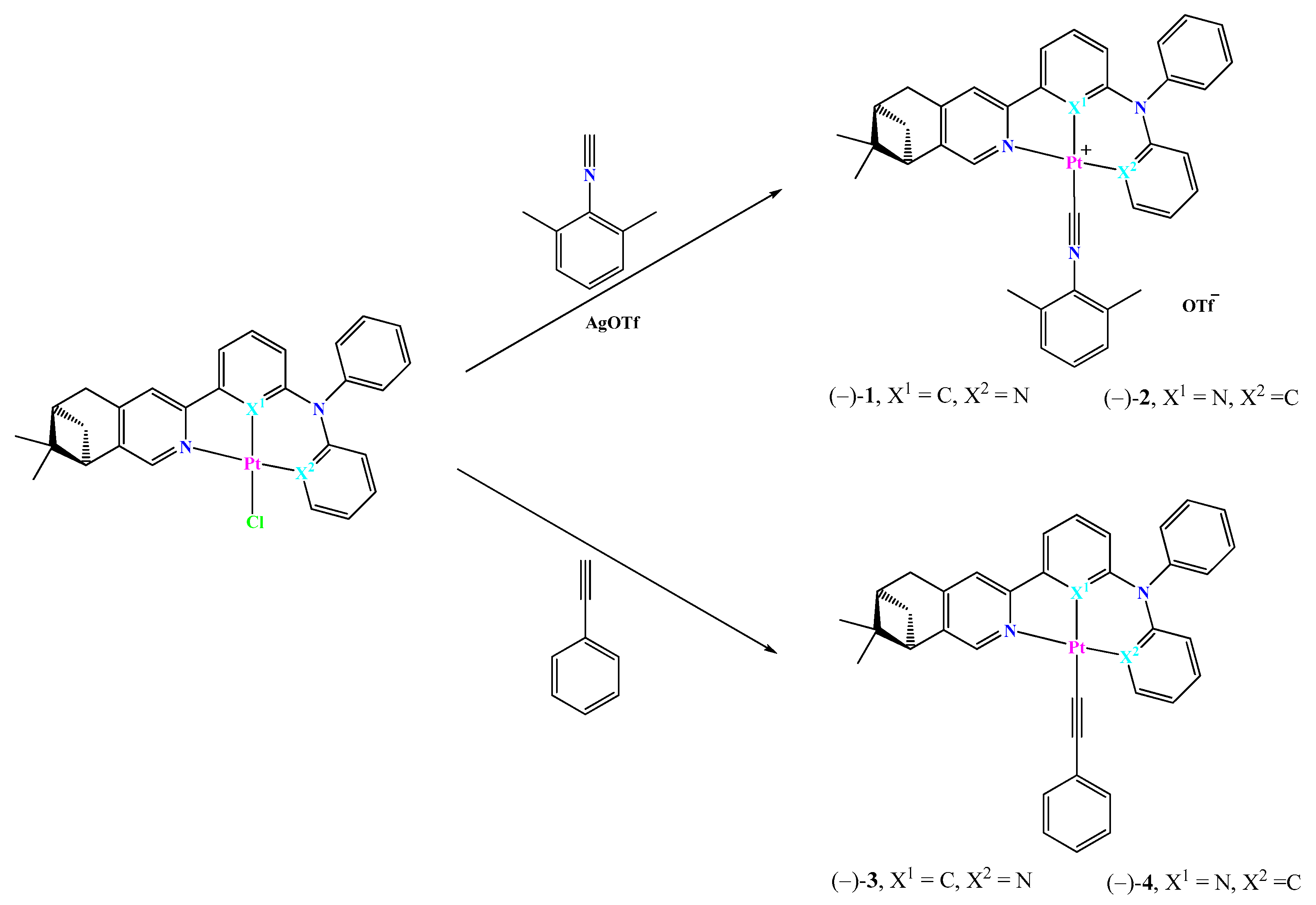
2.1. Synthesis and Characterization
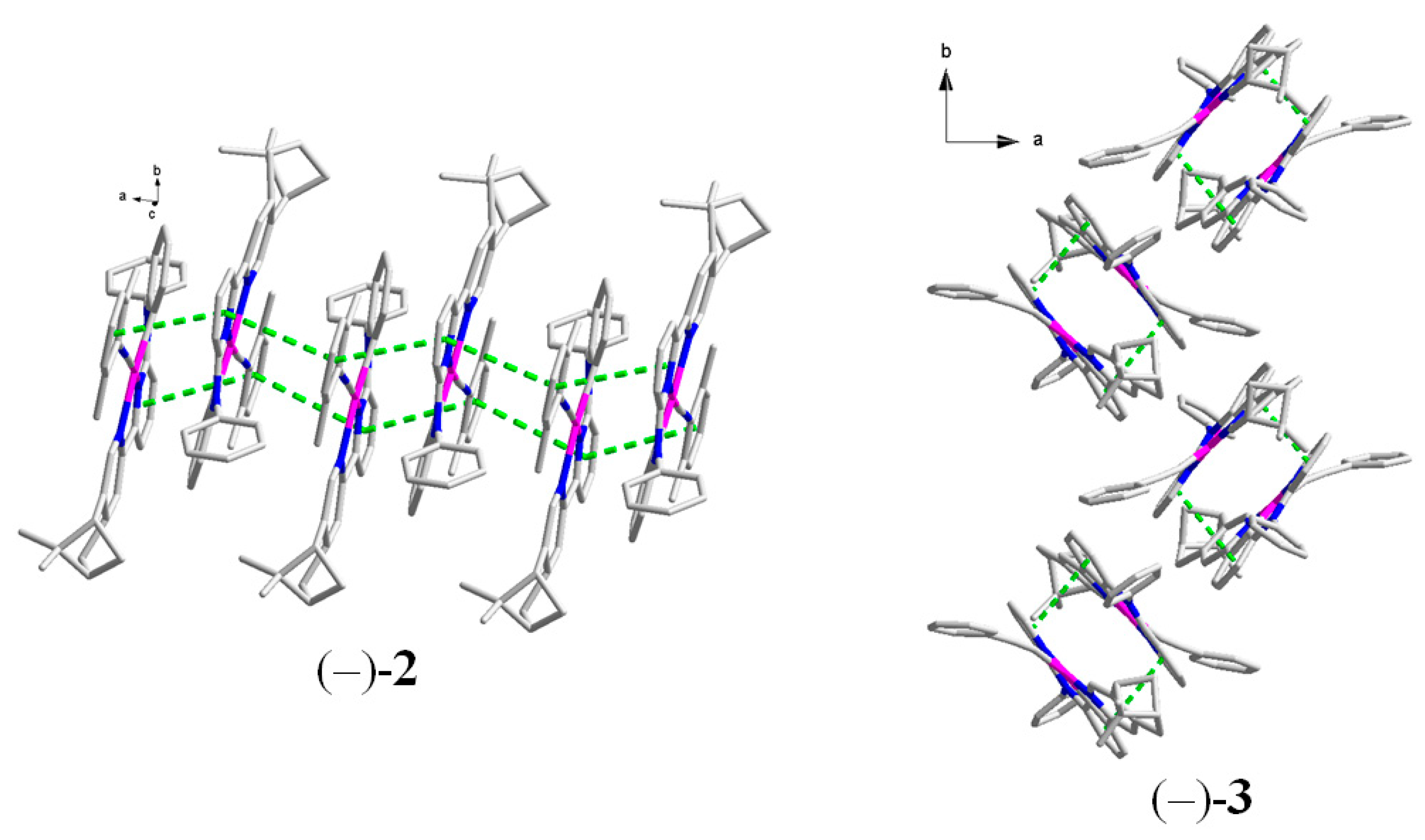
2.2. Crystal Structures
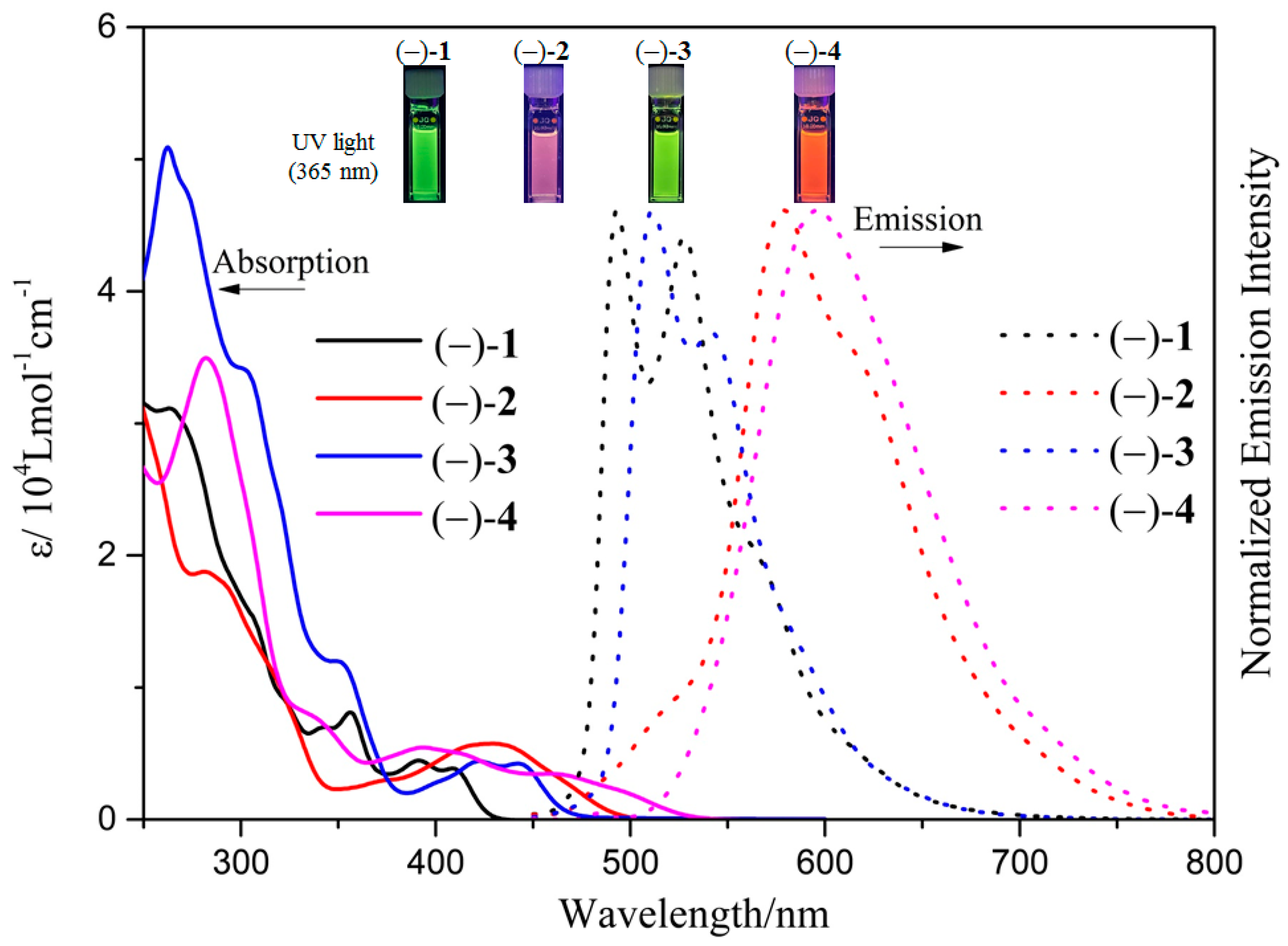
2.3. Spectroscopic Properties
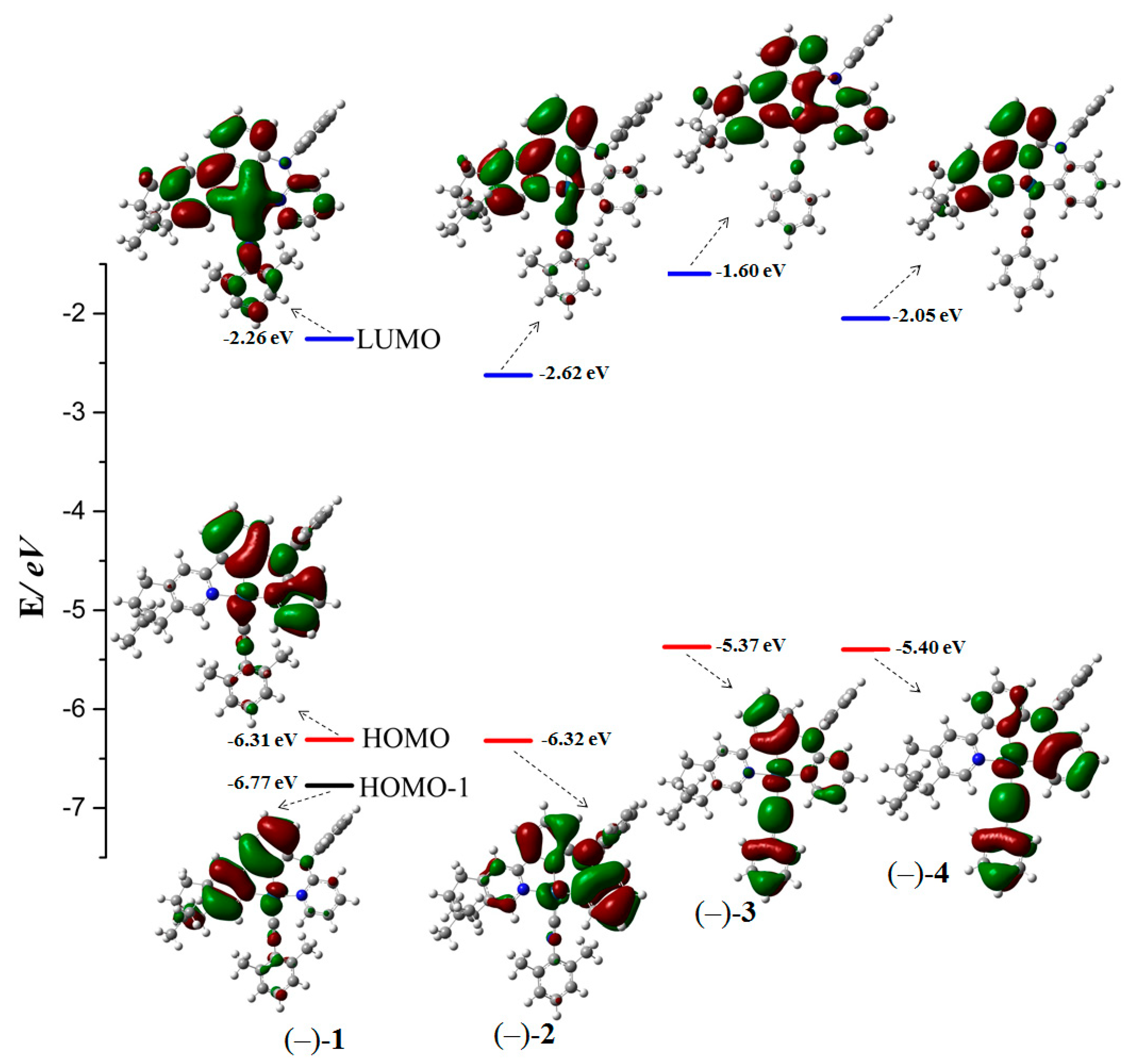
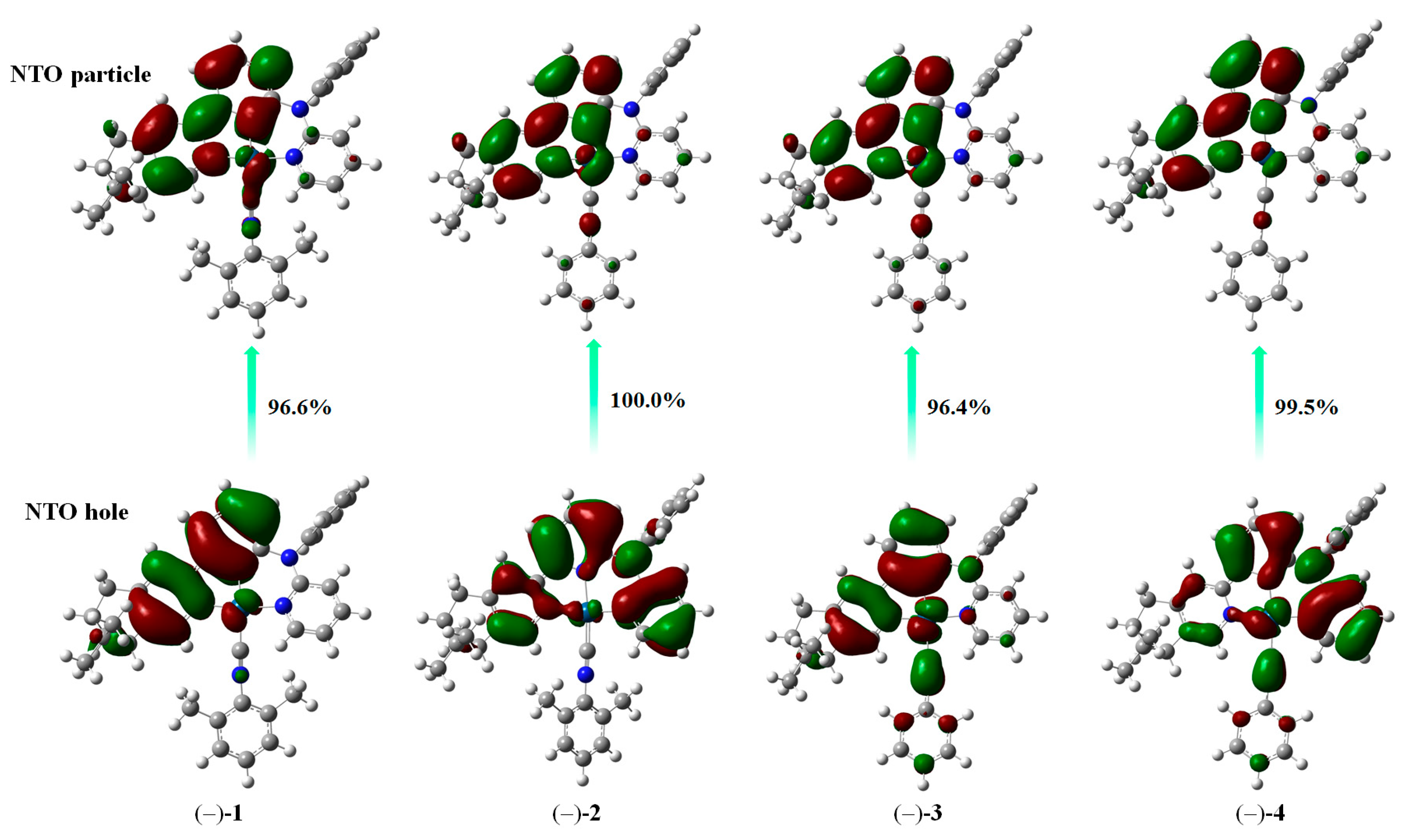
2.4. Theoretical Investigation
2.5. Cytotoxicity
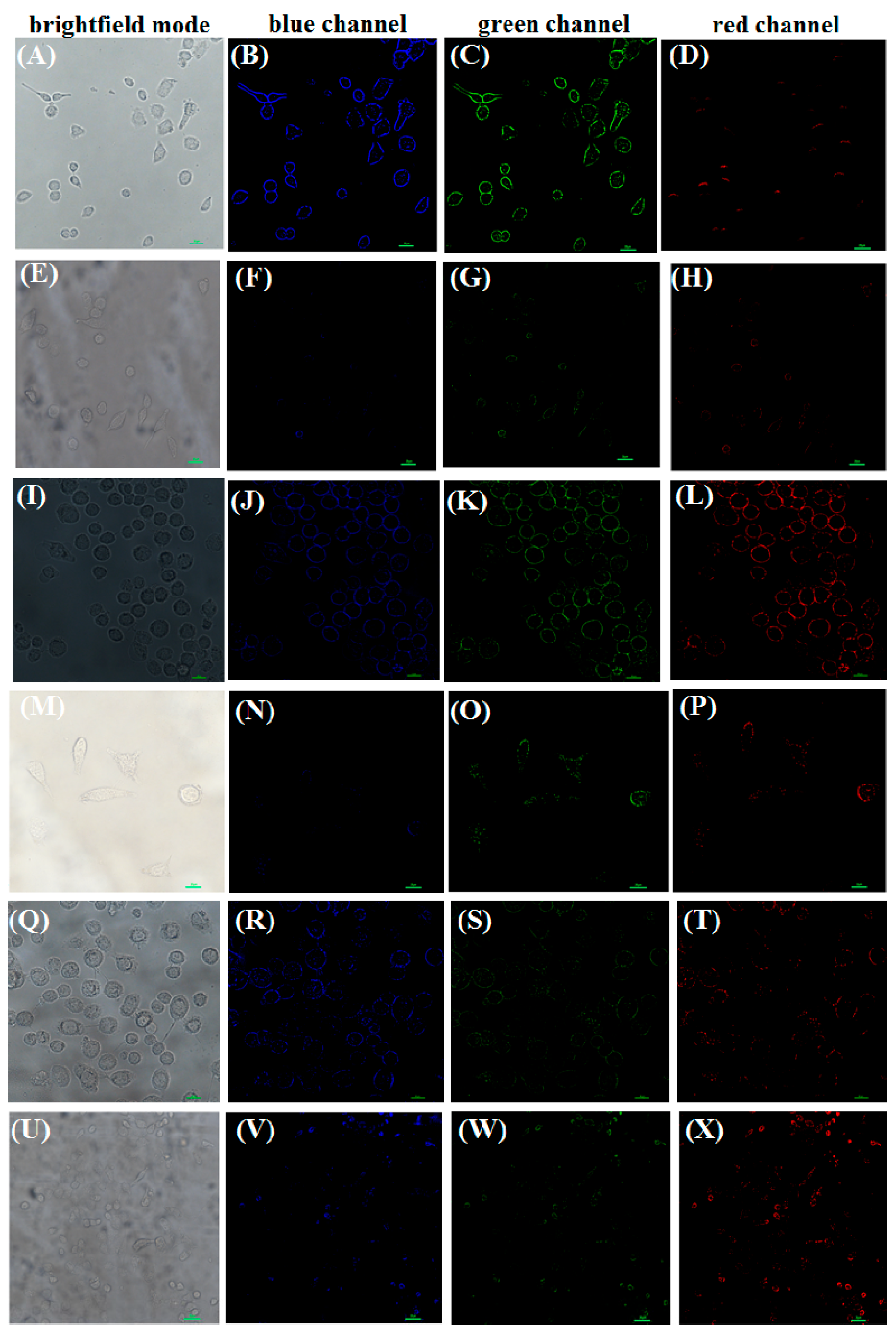
2.6. Cell Imaging
3. Experimental
3.1. General Methods
3.2. Synthetic Procedures
3.2.1. Preparation of Chloride Precursors
3.2.2. Preparation of (−)-1
3.2.3. Preparation of (−)-2
3.2.4. Preparation of (−)-3
3.2.5. Preparation of (−)-4
3.3. Single-Crystal X-ray Structure Determination
3.4. Calculation Methods
3.5. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zou, T.-T.; Liu, J.; Lum, C.T.; Ma, C.-S.; Chan, R.C.T.; Lok, C.-N.; Kwok, W.M.; Che, C.-M. Luminescent Cyclometalated Platinum(II) Complex Forms Emissive Intercalating Adducts with Double-Stranded DNA and RNA: Differential Emissions and Anticancer Activities. Angew. Chem. Int. Ed. 2014, 53, 10119–10123. [Google Scholar] [CrossRef]
- Li, B.-N.; Li, Y.-G.; Chan, M.H.-Y.; Yam, V.W.-W. Phosphorescent cyclometalated platinum(II) enantiomers with circularly polarized luminescence properties and their assembly behaviors. J. Am. Chem. Soc. 2021, 143, 21676–21684. [Google Scholar] [CrossRef] [PubMed]
- Hagui, W.; Cordier, M.; Boixel, J.; Soulé, J.-F. Access to functionalized luminescent Pt(II) complexes by photoredox-catalyzed Minisci alkylation of 6-aryl-2,2′-bipyridines. Chem. Commun. 2021, 57, 038–1041. [Google Scholar] [CrossRef]
- Roberto, D.; Colombo, A.; Dragonetti, C.; Fagnani, F.; Cocchi, M.; Marinotto, D. A Novel Class of Cyclometalated Platinum(II) Complexes for Solution-Processable OLEDs. Molecules 2022, 27, 5171. [Google Scholar] [CrossRef]
- Hruzd, M.; Le Poul, N.; Cordier, M.; Kahlal, S.; Saillard, J.-Y.; Achelle, S.; Gauthier, S.; Guen, F.R. Luminescent cyclometalated alkynylplatinum(II) complexes with 1,3-di(pyrimidin-2-yl)benzene ligands: Synthesis, electrochemistry, photophysics and computational studies. Dalton Trans. 2022, 51, 5546–5560. [Google Scholar] [CrossRef] [PubMed]
- Theiss, T.; Buss, S.; Maisuls, I.; López-Arteaga, R.; Brünink, D.; Kösters, J.; Hepp, A.; Doltsinis, N.L.; Weiss, E.A.; Strassert, C.A. Room-Temperature Phosphorescence from Pd(II) and Pt(II) Complexes as Supramolecular Luminophores: The Role of Self-Assembly, Metal-Metal Interactions, Spin-Orbit Coupling, and Ligand-Field Splitting. J. Am. Chem. Soc. 2023, 145, 3937–3951. [Google Scholar] [CrossRef] [PubMed]
- Ravindranathan, D.; Vezzu, D.A.; Bartolotti, L.; Boyle, P.D.; Huo, S.-Q. Improvement in phosphorescence efficiency through tuning of coordination geometry of tridentate cyclometalated platinum(II) complexes. Inorg. Chem. 2010, 49, 8922–8928. [Google Scholar] [CrossRef]
- Vezzu, D.A.; Ravindranathan, D.; Garner, A.W.; Bartolotti, L.; Smith, M.E.; Boyle, P.D.; Huo, S.-Q. Highly Luminescent Tridentate N^C*N Platinum(II) Complexes Featured in Fused Five-Six-Membered Metallacycle and Diminishing Concentration Quenching. Inorg. Chem. 2011, 50, 8261–8273. [Google Scholar] [CrossRef]
- Harris, C.F.; Vezzu, D.A.; Bartolotti, L.; Boyle, P.D.; Huo, S.-Q. Synthesis, structure, photophysics, and a DFT study of phosphorescent C*N^N- and C^N^N-coordinated platinum complexes. Inorg. Chem. 2013, 52, 11711–11722. [Google Scholar] [CrossRef]
- Wu, W.-T.; Wu, X.-Y.; Zhao, J.-H.; Wu, M.-B. Synergetic effect of C*N^N/C^N^N coordination and the arylacetylide ligands on the photophysical properties of cyclometalated platinum complexes. J. Mater. Chem. C 2015, 3, 2291–2301. [Google Scholar] [CrossRef]
- Knedel, T.O.; Buss, S.; Maisuls, I.; Daniliuc, C.G.; Schlüsener, C.; Brandt, P.; Strassert, C.A. Encapsulation of phosphorescent Pt(II) complexes in Zn-based metal-organic frameworks toward oxygen-sensing porous materials. Inorg. Chem. 2020, 59, 7252–7264. [Google Scholar] [CrossRef] [PubMed]
- Garner, A.W.; Harris, C.F.; Vezzu, D.A.; Pike, R.D.; Huo, S.-Q. Solvent-controlled switch of selectivity between sp2 and sp3 C-H bond activation by platinum(II). Chem. Commun. 2011, 47, 1902–1904. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.; Gagnier, J.P.; Garner, A.W.; Moots, J.G.; Pike, R.D.; Li, Y.-M.; Huo, S.-Q. Reaction of N-Isopropyl-N-phenyl-2,2′-bipyridin-6-amine with K2PtCl4: Selective C-H Bond Activation, C-N Bond Cleavage, and Selective Acylation. Organometallics 2013, 32, 4828–4836. [Google Scholar] [CrossRef]
- Carroll, J.; Woolard, H.G.; Mroz, R.; Nason, C.A.; Huo, S.-Q. Regiospecific acylation of cycloplatinated complexes: Scope, limitations, and mechanistic implications. Organometallics 2016, 35, 1313–1322. [Google Scholar] [CrossRef]
- Chen, Y.; Li, K.; Lu, W.; Chui, S.S.-Y.; Ma, C.-W.; Che, C.-M. Photoresponsive Supramolecular Organometallic Nanosheets Induced by PtII⋅⋅⋅PtII and C-H⋅⋅⋅π Interactions. Angew. Chem. Int. Ed. 2009, 48, 9909–9913. [Google Scholar] [CrossRef]
- Lu, W.; Chen, Y.; Roy, V.A.L.; Chui, S.S.-Y.; Che, C.-M. Supramolecular Polymers and Chromonic Mesophases Self-Organized from Phosphorescent Cationic Organoplatinum(II) Complexes in Water. Angew. Chem. Int. Ed. 2009, 48, 7621–7625. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-Y.; Zhang, H.-H.; Qi, X.-W.; Sun, S.-S.; Zhang, D.-S.; Han, L.-Z.; Zhang, X.-P.; Shi, Z.-F. Mechanochromic luminescence properties of fluoro-substituted pinene-containing cyclometalated platinum(II) complexes with multiple triplet excited states. Dalton Trans. 2021, 50, 8938–8946. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Jing, J.; Wu, S.-X.; Luo, Y.-P.; Sun, S.-S.; Zhang, D.-S.; Shi, Z.-F.; Zhang, X.-P. Tunable circularly polarized luminescence of sol-gels based on chiral cyclometalated platinum(II) complexes. Dyes Pigments 2022, 201, 110228. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Wu, S.-X.; Wang, Y.-Q.; Xie, T.-G.; Sun, S.-S.; Liu, Y.-L.; Han, L.-Z.; Zhang, X.-P.; Shi, Z.-F. Mechanochromic luminescent property and anti-counterfeiting application of AIE-active cyclometalated platinum(II) complexes featuring a fused five-six-membered metallacycle. Dyes Pigments 2022, 197, 109857. [Google Scholar] [CrossRef]
- Baik, C.; Han, W.-S.; Kang, Y.; Kang, S.O.; Ko, J. Synthesis and photophysical properties of luminescent platinum(II) complexes with terdentate polypyridine ligands: [Pt(bpqb)X] and [Pt(tbbpqpy)X](PF6)(bpqb-H=1,3-bis(4′-phenyl-2′-quinolinyl) benzene; tbbpqpy=4-tert-butyl-1,3-bis (4′-phenyl-2′-quinolinyl) pyridine; X=Cl, CCC6H5, C≡CC6H4NMe2, C≡CC6H4NO2). J. Organomet. Chem. 2006, 691, 5900–5910. [Google Scholar]
- Lai, S.-W.; Lam, H.-W.; Lu, W.; Cheung, K.-K.; Che, C.-M. Observation of low-energy metal−metal-to-ligand charge transfer absorption and emission: Electronic spectroscopy of cyclometalated platinum(II) complexes with isocyanide ligands. Organometallics 2002, 21, 226–234. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, W.; Che, C.-M. Luminescent pincer-type cyclometalated platinum(II) complexes with auxiliary isocyanide ligands: Phase-transfer preparation, solvatomorphism, and self-aggregation. Organometallics 2013, 32, 350–353. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Chang, V.Y.; Liu, J.; Yang, X.-L.; Huang, W.; Li, Y.-Z.; Li, C.-H.; Muller, G.; You, X.-Z. Potential switchable circularly polarized luminescence from chiral cyclometalated platinum(II) complexes. Inorg. Chem. 2015, 54, 143–152. [Google Scholar] [CrossRef]
- Maidich, L.; Pilo, M.I.; Rourke, J.P.; Clarkson, G.J.; Canu, P.; Stoccoro, S.; Zucca, A. Classical vs. Non-classical Cyclometalated Pt(II) Complexes. Molecules 2022, 27, 7249. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.-W.; Mei, Z.-B.; Li, C.-X.; Li, J.-Y.; Liu, J.-C.; Liu, W.-Q.; Huo, J.-S.; Wang, Q.-M. Realization of an optical thermometer via structural confinement and energy transfer. Inorg. Chem. 2021, 60, 19315–19327. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Pan, Y.; Han, H.-Y.; Gu, Z.; Chen, H.-J.; Wu, Z.-R.; Liu, H.-T.; Peng, S.; Zhang, X.-P.; Zhang, R.; et al. Molecular engineering of π-extended viologens consisting of thiophene-based bridges for electrochromic devices. J. Mol. Struct. 2023, 1288, 135769. [Google Scholar] [CrossRef]
- Li, W.-Q.; Wang, J.; Yan, X.; Zhang, H.-Z.; Shen, W. Electronic structures and photophysical properties of phosphorescent platinum (II) complexes with tridentate C^N*N cyclometalated ligands. Appl. Organomet. Chem. 2018, 32, e3929. [Google Scholar] [CrossRef]
- Yang, H.; Li, H.; Yue, L.; Chen, X.; Song, D.; Yang, X.; Sun, Y.; Zhou, G.; Wu, Z. Aggregation-induced phosphorescence emission (AIPE) behaviors in PtII(C^N)(N-donor ligand)Cl-type complexes through restrained D2d deformation of the coordinating skeleton and their optoelectronic properties. J. Mater. Chem. C 2021, 9, 2334–2349. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.-Q.; Cai, C.-H.; Yuan, J.-Z.; Gai, C.-J.; Liu, S.-B.; Mei, W.-L.; Dai, H.-F. Seven new 2-(2-phenethyl) chromone derivatives from agarwood of Aquilaria walla. Fitoterapia 2023, 165, 105421. [Google Scholar] [CrossRef]
- Wu, S.; Wu, Z.; Ge, Q.; Zheng, X.; Yang, Z. Antitumor activity of tridentate pincer and related metal complexes. Org. Biomol. Chem. 2021, 19, 5254–5273. [Google Scholar] [CrossRef] [PubMed]
- Vezzu, D.A.; Lu, Q.; Chen, Y.-H.; Huo, S.-Q. Cytotoxicity of cyclometalated platinum complexes based on tridentate NCN and CNN-coordinating ligands: Remarkable coordination dependence. J. Inorg. Biochem. 2014, 134, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Leung, C.-H.; Ma, D.-L.; Lu, W.; Che, C.-M. Organoplatinum(II) Complexes with Nucleobase Motifs as Inhibitors of Human Topoisomerase II Catalytic Activity. Chem. Asian J. 2010, 5, 2271–2280. [Google Scholar] [CrossRef]
- Wang, P.; Leung, C.-H.; Ma, D.-L.; Sun, R.W.-Y.; Yan, S.-C.; Chen, Q.-S.; Che, C.-M. Specific Blocking of CREB/DNA Binding by Cyclometalated Platinum(II) Complexes. Angew. Chem. Int. Ed. 2011, 50, 2554–2558. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zou, T.; Chen, Y.; Guan, X.; Che, C.-M. Pincer-Type Platinum(II) Complexes Containing N-Heterocyclic Carbene (NHC) Ligand: Structures, Photophysical and Anion-Binding Properties, and Anticancer Activities. Chem. Eur. J. 2015, 21, 7441–7453. [Google Scholar] [CrossRef]
- Doherty, R.E.; Sazanovich, I.V.; McKenzie, L.K.; Stasheuski, A.S.; Coyle, R.; Baggaley, E.; Bottomley, S.; Weinstein, J.A.; Bryant, H.E. Photodynamic killing of cancer cells by a Platinum(II) complex with cyclometallating ligand. Sci. Rep. 2016, 6, 22668. [Google Scholar] [CrossRef]
- Sawamura, R.; Sato, M.; Masuya-Suzuki, A.; Iki, N. Photostable near-infrared-absorbing diradicalplatinum(II) complex solubilized by albumin toward a cancer photothermal therapy agent. RSC Adv. 2020, 10, 6460–6463. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Q.; Wang, E.; Niu, Z. Wavelength-tunable AIEgens based on 6-methoxy-2-naphthaldehyde: AIE behavior and bioimaging performance. Spectrochim. Acta A 2022, 281, 121621. [Google Scholar] [CrossRef]
- Chen, P.; Zhao, Y.; Niu, Z.; Wang, E. Substituent Effects on Fluorescence Properties of AIEgens Based on Coumarin-3-formylhydrazone and their Application in Cell Imaging. J. Fluoresc. 2023, 33, 663–669. [Google Scholar] [CrossRef]
- Borah, S.T.; Das, B.; Biswas, P.; Mallick, A.I.; Gupta, P. Aqua-friendly organometallic Ir-Pt complexes: PH-responsive AIPE-guided imaging of bacterial cells. Dalton Trans. 2023, 52, 2282–2292. [Google Scholar] [CrossRef]
- He, L.; Meng, Z.; Guo, Q.; Wu, X.; Teulade-Fichou, M.-P.; Yeow, E.K.L.; Shao, F. Fluorogenic Pt complexes distinguish the quantity and folding behavior of RNA G-quadruplexes between live cancerous and healthy cells. Chem. Commun. 2020, 56, 14459–14462. [Google Scholar] [CrossRef] [PubMed]
- SAINT-Plus, Version 6.02; Bruker Analytical X-ray System: Madison, WI, USA, 1999.
- Sheldrick, G.M. SADABS, an Empirical Absorption Correction Program; Bruker Analytical X-ray Systems: Madison, WI, USA, 1996. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Frisch, M.J. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhu, L.; Sha, C.; Lv, A.; Xie, W.; Shen, K.; Chen, Y.; Xie, G.; Ma, H.; Li, H.; Hang, X.-C. Tetradentate Pt(II) Complexes with Peripheral Hindrances for Highly Efficient Solution-Processed Blue Phosphorescent OLEDs. Inorg. Chem. 2022, 61, 10402–10409. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]


| (−)-2 | (−)-3 | |
|---|---|---|
| Formula | C77H70F3N8O3Pt2S | C37H31N3Pt |
| Mr/g mol−1 | 1634.65 | 712.74 |
| Crystal system | Triclinic | Monoclinic |
| Space group | P1 | P21 |
| a/Å | 7.2605(2) | 11.8935(3) |
| b/Å | 14.3218(3) | 13.8385(4) |
| c/Å | 18.0040(3) | 17.6968(5) |
| α/° | 70.891(2) | 90.00 |
| β/° | 86.709(2) | 91.2660(10) |
| γ/° | 84.755(2) | 90.00 |
| V/Å3 | 1760.80(7) | 2911.97(14) |
| Z | 1 | 4 |
| T/K | 100.00(10) | 193(2) |
| Radiation, λ/Å | 0.71073 | 0.71073 |
| Dcalcd, g/cm−3 | 1.542 | 1.626 |
| μ/mm−1 | 4.059 | 4.849 |
| F(000) | 811 | 1408 |
| θ range/° | 2.210 to 24.998 | 1.71 to 25.00 |
| Reflections measured | 22,952 | 17,628 |
| Unique reflections | 11,281 | 9510 |
| Rint | 0.0292 | 0.0453 |
| Reflections with F2 > 2σ(F2) | 10450 | 8293 |
| Number of parameters | 795 | 743 |
| Goodness-of-fit on F2 | 1.053 | 1.045 |
| R1 [F2 > 2σ(F2)] | 0.0323 | 0.0346 |
| wR2 (all data) | 0.0740 | 0.0970 |
| Δρmax, Δρmin/eÅ−3 | 0.939, −0.935 | 1.133, −1.956 |
| Flack parameter | 0.185(12) | 0.153(11) |
| CCDC number | 2,279,412 | 2,279,411 |
| Complexes | Medium | λem (nm) | τem (μs) | Φem (%) |
|---|---|---|---|---|
| (−)-1 | CH2Cl2 solution (RT) | 493 | 0.98 | 2.49 |
| Rigid glass (77 K) | 488 | |||
| Solid state (RT) | 531 | 1.98 | 4.76 | |
| (−)-2 | CH2Cl2 solution (RT) | 578 | 1.03 | 0.73 |
| Rigid glass (77 K) | 556 | |||
| Solid state (RT) | 597 | 2.82 | 3.33 | |
| (−)-3 | CH2Cl2 solution (RT) | 511 | 1.81 | 1.72 |
| Rigid glass (77 K) | 503 | |||
| Solid state (RT) | 555 | 3.01 | 7.47 | |
| (−)-4 | CH2Cl2 solution (RT) | 595 | 1.07 | 0.80 |
| Rigid glass (77 K) | 561 | |||
| Solid state (RT) | 628 | 3.17 | 2.61 |
| IC50 (μM) | K562 | SGC-7901 | BEL-7402 | A549 | Hela |
|---|---|---|---|---|---|
| Pt(N^C*N′)Cl | >50 | >50 | >50 | >50 | >50 |
| Pt(N^N′*C)Cl | 1.17 ± 0.02 | 8.85 ± 0.48 | 4.32 ± 0.07 | 3.25 ± 0.04 | 3.83 ± 0.09 |
| (−)-1 | 2.87 ± 0.18 | 6.58 ± 0.30 | 4.23 ± 0.03 | 3.14 ± 0.01 | 3.76 ± 0.06 |
| (−)-2 | 0.47 ± 0.04 | 1.58 ± 0.25 | 0.64 ± 0.04 | 0.98 ± 0.02 | 0.76 ± 0.03 |
| (−)-3 | >50 | >50 | >50 | >50 | >50 |
| (−)-4 | 1.54 ± 0.04 | 1.36 ± 0.41 | 3.57 ± 0.14 | 3.30 ± 0.06 | 3.72 ± 0.06 |
| Cisplatin a | 3.08 ± 0.05 | 4.11 ± 0.02 | 4.02 ± 0.06 | 1.93 ± 0.02 | 11.29 ± 0.15 |
| IC50 (μM) | NCI-H522 | RV1 | NCI-H1299 | HCC827 | |
| PtII(NCN′)Cl [32] | 21.5 ± 2.1 | 37.7 ± 3.2 | 19.4 ± 2.2 | 22.4 ± 2.07 | |
| PtII(CNN′)Cl [32] | >236 | >236 | >236 | >236 | |
| Cisplatin a | 49.8 ± 1.6 | >333 | 163.0 ± 4.1 | >666 | |
| IC50 (μM) | HeLa | HepG2 | CNE1 | ||
| (CNN′)PtII(C≡CCH2R) [33] | 0.5–8.9 | 2.0–13.7 | 0.8–5.8 | ||
| Cisplatin a | 11.6 ± 0.4 | 16.7 ± 0.8 | 1.93 ± 0.4 | ||
| IC50 (μM) | HeLa | HepG2 | SUNE1 | ||
| (CNN′)PtII(C≡NL)+ [34] | 0.86–19.7 | 4.23–24.8 | 5.04–100 | ||
| Cisplatin a | 10.7 ± 0.3 | 9.56 ± 0.27 | 2.13 ± 0.17 | ||
| IC50 (μM) | HeLa | ||||
| [Pt(trpy)(NHC)]2+ [35] | 0.46–100 | ||||
| Cisplatin a | 10 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, J.; Yu, M.; Pan, L.; Zhao, Y.; Xu, G.; Zhang, H.-H.; Li, C.; Zhang, X.-P. Synthesis and Biological Activities of Luminescent 5,6-Membered Bis(Metallacyclic) Platinum(II) Complexes. Molecules 2023, 28, 6369. https://doi.org/10.3390/molecules28176369
Jing J, Yu M, Pan L, Zhao Y, Xu G, Zhang H-H, Li C, Zhang X-P. Synthesis and Biological Activities of Luminescent 5,6-Membered Bis(Metallacyclic) Platinum(II) Complexes. Molecules. 2023; 28(17):6369. https://doi.org/10.3390/molecules28176369
Chicago/Turabian StyleJing, Jing, Miao Yu, Lei Pan, Yang Zhao, Guo Xu, Hua-Hong Zhang, Chen Li, and Xiao-Peng Zhang. 2023. "Synthesis and Biological Activities of Luminescent 5,6-Membered Bis(Metallacyclic) Platinum(II) Complexes" Molecules 28, no. 17: 6369. https://doi.org/10.3390/molecules28176369
APA StyleJing, J., Yu, M., Pan, L., Zhao, Y., Xu, G., Zhang, H.-H., Li, C., & Zhang, X.-P. (2023). Synthesis and Biological Activities of Luminescent 5,6-Membered Bis(Metallacyclic) Platinum(II) Complexes. Molecules, 28(17), 6369. https://doi.org/10.3390/molecules28176369







