Herbal Medicine Nanocrystals: A Potential Novel Therapeutic Strategy
Abstract
:1. Introduction
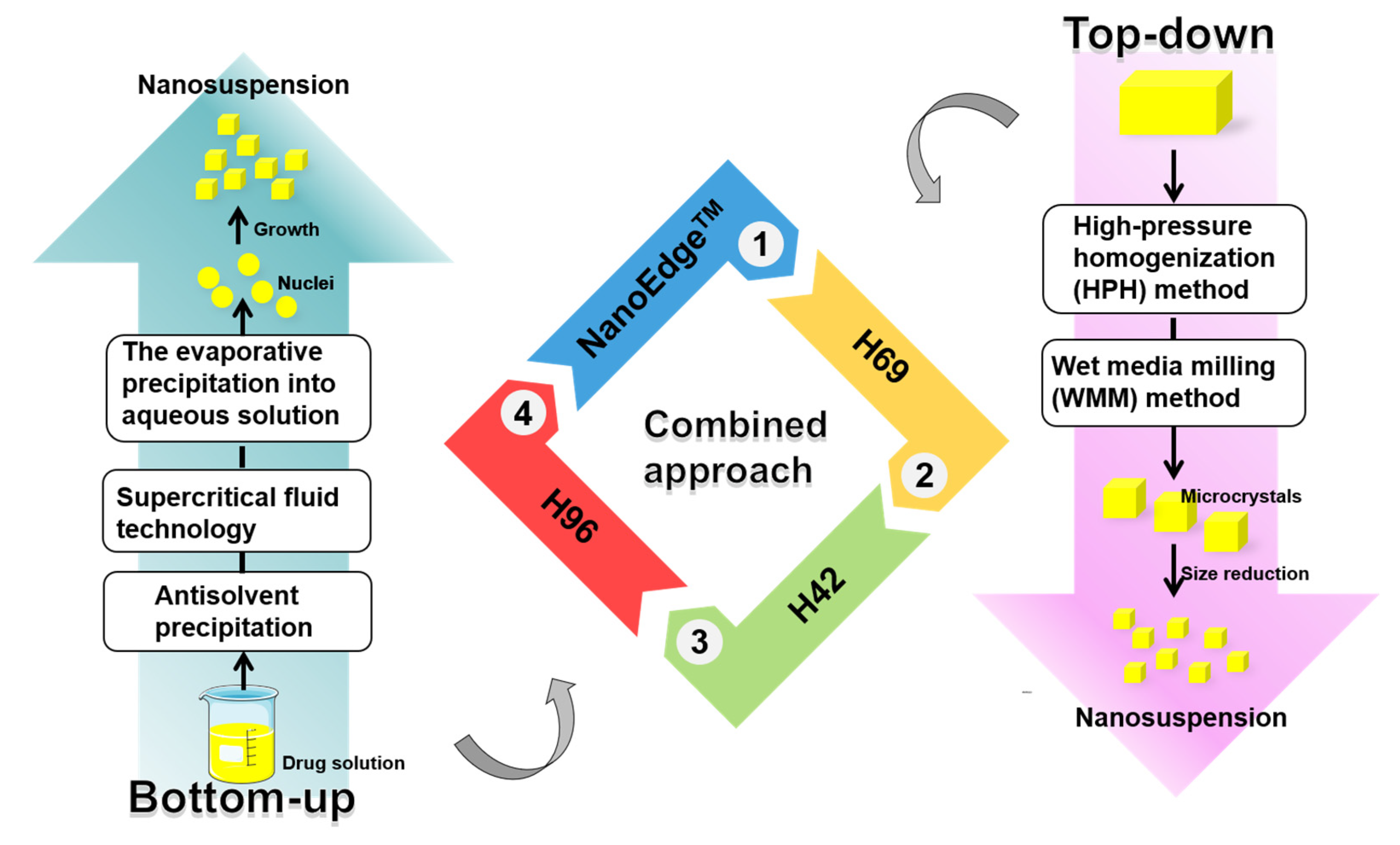
2. Preparation Methods of Herbal Medicine Nanocrystals
2.1. Bottom-Up Methods
2.1.1. Antisolvent Precipitation
2.1.2. Supercritical Fluid Technology
2.1.3. Evaporative Precipitation into Aqueous Solution Method
2.2. Top-Down Methods
2.2.1. Wet Media Milling
2.2.2. High-Pressure Homogenization
2.3. Combinative Technology
2.3.1. NanoEdge™
2.3.2. H69 Technology
2.3.3. H42 Technology
2.3.4. H96 Technology
2.4. Key Factors to Control Nucleation and Polymorphism
2.4.1. Polymorphism Transformation in the Top-Down Method
2.4.2. Nucleation and Polymorphism Transformation in the Bottom-Up Method
3. Characterization of Nanocrystals
3.1. Particle Size
3.2. Solid State
3.3. Morphology
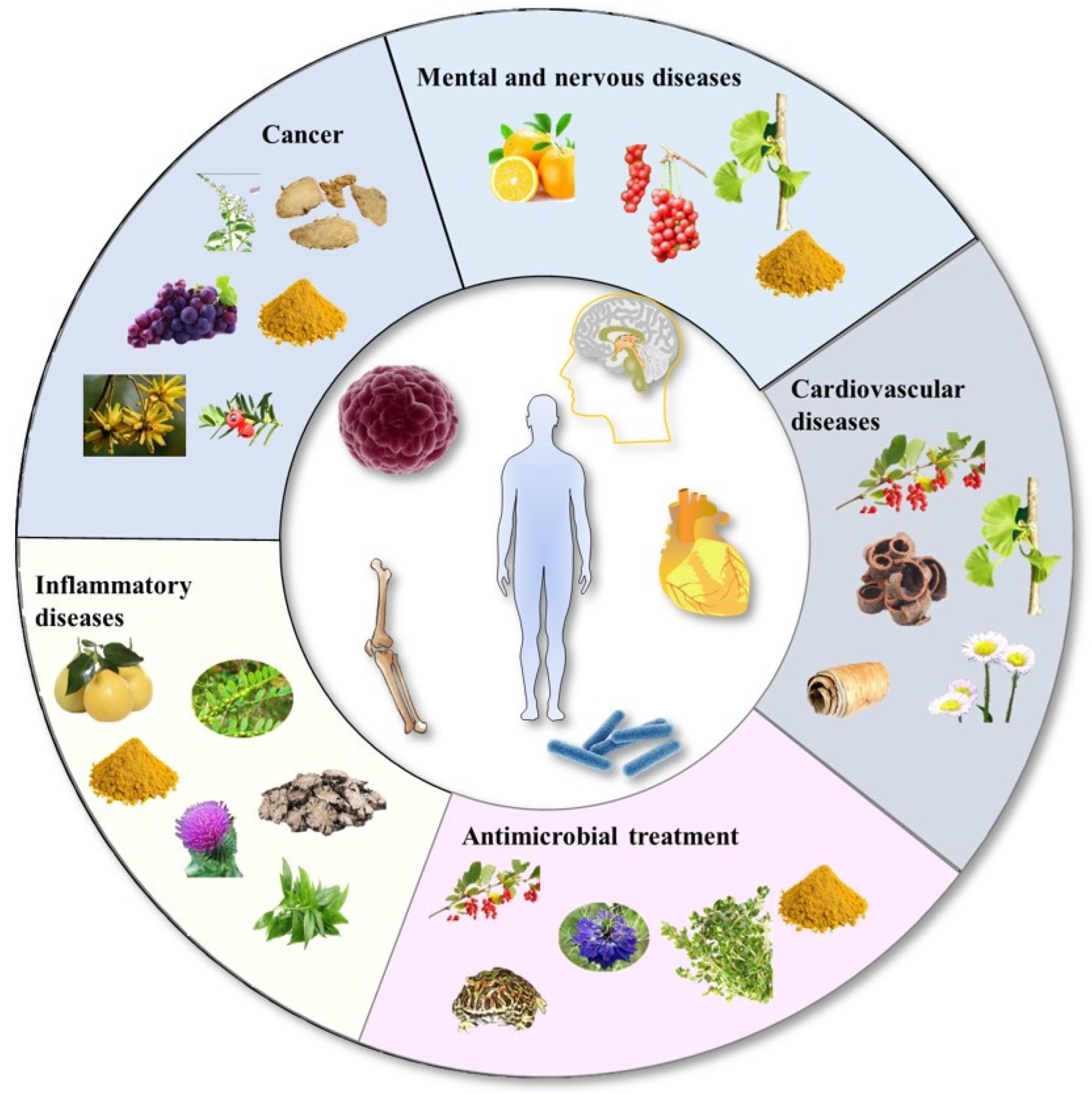
4. Application of Nanocrystals in Herbal Medicines
4.1. Cancer Therapy
| Extract/Compounds | Stabilizers | Preparation Methods | Particle Size | Bioavailability | Advantages | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Annonaceous acetogenins | mPEG2000–PCL2000 | Antisolvent sonoprecipitation | 123.2 ± 3.54 nm | / | Nanocrystal achieved much better therapeutic efficacy than the traditional dosage form (oil solution) | Antitumor | [50] |
| Oridonin | Lecithin, HPMC, and PVP | High pressure homogenization | 912.5 ± 17.6 nm | / | Significantly inhibited the proliferation of PC-3 cells; enhanced growth suppression; and induced apoptosis of PC-3 cells, higher antitumor efficacy, lower toxicity | Prostatic carcinoma | [28,69] |
| Gambogenic acid | PVPK30 and PEG2000 | Antisolvent precipitation | 183.7 nm | AUC and t1/2 of GNA-NS were increased 2.63- and 1.77-fold than that of the reference formulation | Exhibited superior cytotoxicity compared with GNA solution toward HepG2 cells | Antitumor | [61,70] |
| Isoliquiritigenin | HPC SSL and PVP K30 | Wet media milling | 238.1 ± 4.9 nm and 354.1 ± 9.1 nm | / | Improved the solubility; enhanced the cytotoxicity | Antitumor | [71] |
| Oleanolic acid | Sucrose monolaurate and sucrose monopalmitate | O/W emulsion and organic solvent evaporation methods | ~100 nm | Oral bioavailability of the oleanolic acid nanocrystal was 6–7-times higher than that of the oleanolic acid coarse suspension | NS group had significantly higher bioavailability (6- to 7-fold) than the suspension group | Anticancer | [72] |
| Silibinin | Lecithin and poloxamer 188 | High pressure homogenization | 641.8 ± 14.7 nm 127 ± 1.9 nm | / | Showed better apoptosis effect on PC-3 cells | Prostatic carcinoma | [43] |
| Celastrol | P 188 | Antisolvent precipitation | 147.9 nm | / | Displayed a significantly enhanced tumor inhibition rate and therapeutic efficacy in comparison with that of the CSL suspension. | Breast cancer | [73] |
| 10-hydroxycamptot-hecin | P 188 | A modified acid–base microprecipitation combined with a high-pressure homogenization technique | ~130 nm | The AUC0–24 value was 2.81-fold, as high as the injections group. Meanwhile, the mean residence time of the 10-HCPT nanocrystal was significantly higher than that of the 10-HCPT injections group. | 10-HCPT nanocrystals exhibited much higher drug levels in the plasma and tissues of the test mice than the marketed 10-HCPT injections, and significantly improved the antitumor therapeutic effect. | Antitumor | [29,74] |
| Quercetin | Polysorbate 80 | Nanoprecipitation and high-pressure homogenization | 393.5 nm | Exhibited a significant reduction in clearance rate and increase in AUC compared with the control suspension. | The solubility of QT in nanocrystals was approximately 70-fold that of crude QT, and the dissolution of QT from QT-NS was increased as compared to that of the original QT powder. | Antitumor | [75] |
| Andrographolide analogue (3A.1) | Chitosan derivatives | Antisolvent precipitation | 220–270 nm | / | Increased solubility and pharmacological effectiveness with the induction of apoptosis; had the strongest anticancer effect compared to the drug solution | Colorectal cancer | [76] |
| Resveratrol | TPGS and DSPE-PEG-FA | Antisolvent precipitation | 100–200 nm | / | Higher antitumor efficacy due to reduced tumor volume and weight | Antitumor | [67] |
| Curcumin | mPEG2000-DSPE and soybean lecithin | Antisolvent sonoprecipitation | 186.33 ± 2.73 nm | After i.v. administration, the AUC0–24 of CUR-NSps was 4.50 times that of the CUR injections; t1/2 of CUR-NSps was approximately 35.95 times that of CUR injections. The mean residence time of CUR-NSps was 18.90-fold longer than that of CUR solution. | CUR-NSps exhibited a significantly greater AUC0–24 and prolonged MRT compared to CUR injections after i.v. administration. Higher biodistribution in the liver, kidney, brain, and tumor for CUR-NSps compared to CUR injections. | Antitumor | [77] |
| Curcumin | TPGS | Trituration followed by ultraturrax homogenization and high-pressure homogenization | 210.2 nm | AUC0–∞ of CUR-NS was approximately 3.8-fold greater than CUR solution; the mean residence time was 11.2-fold longer. | CUR-NS showed greater AUC0–∞ and prolonged MRT compared to CUR solution in rabbits after i.v. administration. | Antitumor | [78] |
4.2. Inflammatory Diseases
| Extract/Compounds | Stabilizers | Preparation Methods | Particle Size | Bioavailability | Advantages | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Curcumin | P188 | Wet media milling | 200–240 nm | / | / | Bronchial Asthma | [87] |
| Naringenin | PVP K90 | Antisolvent sonoprecipitation | 117 ± 5 nm | / | NRG-NS showed a decrease in the levels of acid phosphatase and inorganic phosphorus compared to plain NRG | Anti-osteoporotic | [83] |
| Naringenin | TPGS | Wet media milling | ~182.2 nm | / | Nanocrystal showed an excellent antitussive effect. The cough frequency decreased by threefold compared with the blank model, with an inhibition rate of 66.7%. Showed a good cough-relieving effect, decreasing IL-6 and MDA, increasing SOD, and reducing lung damage | Post-infectious cough | [84] |
| lipophilic aglycone icaritin | HPMC E3 | Antisolvent precipitation | 216.6 ± 12.4 nm | 2.0-fold in AUC0–12 and 4.7-fold in Cmax | ICTN exhibited a faster dissolution rate, significantly faster absorption. Enhanced proliferation and differentiation activities. | Treats impotence and prevent osteoporosis | [85] |
| Phyllanthus amarus extract | 1.5% PVA | Nanoprecipitation method | 243 ± 9.7 nm | / | The levels of the serum enzymes and bile salt were significantly lower. | Hepatic disorders | [88] |
| Silymarin | PVA | Antisolvent sonoprecipitation | 277.3 ± 10.4 nm | / | Exhibited faster dissolution rate, higher drug content, and pronounced enhancement of saturation solubility. | Liver disorders, for instance, acute and chronic viral hepatitis, cirrhosis, toxic hepatitis and fatty liver | [89] |
| Tetramethylpyrazine | 0.2% PVP K30 and 0.8% HPMC | Wet media milling | 588 nm | The t1/2 of TMP-NS was approximately three times longer than in the TMP group. The average retention time of TMP-NS was approximately five times longer than TMP. | After intra-articular injection in rats, NS had a longer retention time in the articular cavity, higher TMP concentrations in the joints, and greater anti-osteoarthritic efficacy than the TMP solution. | Osteoarthritis | [86] |
| Andrographolide | TPGS | Wet media milling | 244.6 ± 3.0 nm | The AUC0–t and Cmax of the freeze-dried ADG-NS were threefold and twofold higher than the ADG coarse powder. The AUC0–t of the freeze-dried ADG-NS was 54.3% higher than the freeze-dried ADG-NS without TPGS. The AUC0–t of the freeze-dried ADG-NS (with or without TPGS) was 1.38- or 2.14-fold higher than that of the ADG dripping pills. | ADG-NS showed higher permeability and plasma exposure. ADG-NS were more effective in reducing the rate of paw swelling and producing a greater increase in the serum levels of nitric oxide (NO), interleukin-1 (IL-1), and tumor necrosis factor-α (TNF-α), as well as an increase in superoxide dismutase activity | Anti-inflammatory | [90] |
4.3. Cardiovascular Diseases
4.4. Mental and Nervous Diseases
| Extract/Compounds | Stabilizers | Preparation Methods | Particle Size | Bioavailability | Advantages | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Quercetin | / | Evaporative precipitation | 120 nm | / | Increased antioxidant enzyme activities | Parkinson’s disease | [109,110] |
| Schisantherin A | 0.1% HPMC E3 | Antisolvent precipitation | ∼160 nm | In plasma, SA-NC increased 7.88-fold in Cmax and 6.37-fold in AUC0–t. In the brain, SA-NC increased by 5.47-fold in Cmax and 6.32-fold in AUC0–t. | A fast dissolution rate in vitro, Higher concentration in plasma and brain; higher efficiency in reversing MPTP-induced dopaminergic neuronal loss and locomotion deficiency in zebrafish, as well as the MPP+-induced damage of neuronal cell culture model; stronger neuroprotective effect | Parkinson’s disease | [111] |
| Ginkgolide B | 0.05% HPMC E5 | Antisolvent precipitation | 83.48 ± 1.77 nm | In plasma, GB-NCs increased by 13-fold in Cmax and by 5-fold in AUC0–t. GBNCs had a shorter Tmax and t1/2. In the brain, GB-NCs increased by threefold in Cmax, and by 2.5-fold in AUC0–t. GBNC increased Tmax and delayed t1/2. The MRT0–t of the GB-NCs in the brain were 2.11-fold longer than those of the plasma. | GB-NCs have high rates of dissolution, enhanced cellular uptake, and permeability; higher concentrations in the plasma and brain; remain for longer times in the brain; and possess higher efficiency in protecting neurons against cytotoxicity induced by MPP+, The GB-NCs can protect neurons against cytotoxicity induced by MPP+, improve behavior, reduce dopamine deficiency, and elevate dopamine metabolite levels. | Parkinson’s disease | [107] |
| Hesperetin | Plantacare 2000 | High-pressure homogenization and wet media milling | Between 200 and 800 nm | / | Increased dissolution rate and kinetic solubility, higher antioxidant capacity | Alzheimer’s disease | [108,112] |
| Curcumin didecanoate | 5% F68 | Wet media milling | 517 ± 9 nm | 3.73-fold in Cmax, 4.7-fold in AUC0–t. | Higher concentration in brain | Antidepressant | [113] |
4.5. Antimicrobial Treatment
| Extract/Compounds | Stabilizers | Preparation Methods | Particle Size | Bioavailability | Advantages | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Berberine | 1% HPMC and 1% PG | Evaporative precipitation of nanocrystal (EPN) and anti-solvent precipitation with a syringe pump (APSP) | 71.53 nm for EPN method, 102.62 nm for APSP method | / | BBR NPs prepared by the EPN method showed higher solubility and dissolution rate BBR NPs produced by both APSP and EPN methods showed promising activities against gram-positive and gram-negative bacteria, as well as yeasts, with NPs prepared by the EPN method showing superior results compared to those made with the APSP method. | Antimicrobial | [116] |
| Herpetrione | 0.2% SLS and 0.3% PVP K30 | High-pressure homogenization | 286 ± 1.3 nm | 2.45-fold in Cmax and 2.49-fold in AUC0–t, and decrease in Tmax and MRT | HPE NS increased dissolution velocity markedly; was more effective in reducing the replication and expression of HBsAg and HBeAg; and had a more significant inhibitory effect on HBV-DNA | Antiviral | [117] |
| Tretinoin | Bean lecithin | Antisolvent precipitation | 324 nm | / | Improved drug permeation and UV irradiation stability | Acne vulgaris | [120] |
| Curcumin | F127 and CTAB | Wet media milling | ~ 150 nm | / | Solubility and dissolution rate of CUR were significantly enhanced CUR NCs displayed low cytotoxicity against normal human kidney-2 cells CUR NCs with high positive surface charge exhibited excellent antibacterial activity compared to free CUR against E. coli and S. aureus. | Antimicrobial | [121] |
| Nigella sativa L. extract | 1.5% PVA | Evaporative precipitation | / | / | Nanocrystals showed higher antioxidant activity than the extract Higher biofilm inhibition activity against Escherichia coli than the extract and ciprofloxacin | Antimicrobial | [122] |
| Thymol | Caseinate | A modified acid–base microprecipitation | 79.4 nm | / | No enhancement in antimicrobial activity against four common pathogenic bacteria (Salmomella enterca, Staphylococcus aureus, Escherichia coli, and Listeria monocytogenes) | Antimicrobial | [119] |
5. Challenges and Future Perspectives
5.1. Nanotoxicity of Nanocrystals
5.2. In Vivo Fate of Herbal Medicine Nanocrystals
5.3. Potential Advantages of Overcoming Drug Resistance
5.4. Herbal Ingredients as Stabilizers of Nanocrystals
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 10-HCPT | 10-hydroxycamptothecin |
| ADG | Andrographolide |
| ANDA | Abbreviated New Drug Application |
| ART | Artemisinin |
| BCS | Biopharmaceutics classification system |
| BSA | Bovine serum albumin |
| CUR | Curcumin |
| DSPE | 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine |
| EPAS | Evaporative precipitation into aqueous solution |
| GNA | Gambogenic acid |
| HPC | Hydroxypropyl cellulose |
| HPH | High-pressure homogenization |
| HPMC | Hydroxypropyl Methyl Cellulose |
| ICT | Icaritin |
| IND | Investigational New Drug |
| MDA | Malondialdehyde |
| NDA | New Drug Application |
| NRG | Naringenin |
| P188 | Poloxamer 188 |
| PG | Propylene glycol |
| PVA | Polyvinyl acetate |
| PVP | Polyvinylpyrrolidone |
| PVP/VA | Polyvinylpyrrolidone/vinyl acetate |
| QT | Quercetin |
| SCF | Supercritical fluid technology |
| SDS | Sodium dodecyl sulfate |
| SOD | Superoxide dismutase |
| TPGS | D-α-Tocopherol polyethylene glycol succinate |
| WMM | Wet media milling |
References
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Teja, P.K.; Mithiya, J.; Kate, A.S.; Bairwa, K.; Chauthe, S.K. Herbal nanomedicines: Recent advancements, challenges, opportunities and regulatory overview. Phytomedicine 2022, 96, 153890. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Sharma, S.; Xu, S.; Tewari, D.; Fang, J. Curcumin as a Natural Remedy for Atherosclerosis: A Pharmacological Review. Molecules 2021, 26, 4036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, K.; Di, L.; Wang, P.; Liu, Z.; Zhang, J.; Yue, P.; Song, W.; Zhang, J.; Chen, T.; et al. Traditional herbal medicine and nanomedicine: Converging disciplines to improve therapeutic efficacy and human health. Adv. Drug Deliv. Rev. 2021, 178, 113964. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Singh, S.K.; Vaidya, Y.; Gulati, M.; Bhattacharya, S.; Garg, V.; Pandey, N.K. Nanosuspension: Principles, Perspectives and Practices. Curr. Drug Deliv. 2016, 13, 1222–1246. [Google Scholar] [CrossRef]
- Keck, C.M.; Muller, R.H. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur. J. Pharm. Biopharm. 2006, 62, 3–16. [Google Scholar] [CrossRef]
- Guo, M.; Wei, M.; Li, W.; Guo, M.; Guo, C.; Ma, M.; Wang, Y.; Yang, Z.; Li, M.; Fu, Q.; et al. Impacts of particle shapes on the oral delivery of drug nanocrystals: Mucus permeation, transepithelial transport and bioavailability. J. Control. Release 2019, 307, 64–75. [Google Scholar] [CrossRef]
- Guo, M.R.; Chen, H.N.; Zhang, C.; Zhang, G.S.; Wang, Y.Z.; Li, P.C.; Fu, Q. Probing the particle shape effects on the biodistribution and antihyperlipidemic efficiency for oral lovastatin nanocrystals. J. Mol. Liq. 2021, 324, 114700. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Zhang, Z.; He, Z.; Liu, Y.; Fu, Q. FRET imaging revealed that nanocrystals enhanced drug oral absorption by dissolution rather than endocytosis: A case study of coumarin 6. J. Control. Release 2021, 332, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Sun, J.; Ai, X.; Zhang, P.; Li, M.; Wang, Y.; Liu, X.; Sun, Y.; Sui, X.; Sun, L.; et al. Nimodipine nanocrystals for oral bioavailability improvement: Role of mesenteric lymph transport in the oral absorption. Int. J. Pharm. 2013, 448, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.H.; Ma, Y.Y.; Ding, S.J.; Liu, Y.C.; Song, Z.M.; Liu, X.; Tang, L.L.; Wang, Y.C. The technology for improving stability of nanosuspensions in drug delivery. J. Nanopart. Res. 2022, 24, 14. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of nanosuspensions in drug delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef]
- Chen, M.L.; John, M.; Lee, S.L.; Tyner, K.M. Development Considerations for Nanocrystal Drug Products. AAPS J. 2017, 19, 642–651. [Google Scholar] [CrossRef]
- Fan, M.; Geng, S.; Liu, Y.; Wang, J.; Wang, Y.; Zhong, J.; Yan, Z.; Yu, L. Nanocrystal Technology as a Strategy to Improve Drug Bioavailability and Antitumor Efficacy for the Cancer Treatment. Curr. Pharm. Des. 2018, 24, 2416–2424. [Google Scholar] [CrossRef]
- Lee, H.; Bang, J.B.; Na, Y.G.; Lee, J.Y.; Cho, C.W.; Baek, J.S.; Lee, H.K. Development and Evaluation of Tannic Acid-Coated Nanosuspension for Enhancing Oral Bioavailability of Curcumin. Pharmaceutics 2021, 13, 1460. [Google Scholar] [CrossRef]
- Murakami, Y.; Shimoyama, Y. Production of nanosuspension functionalized by chitosan using supercritical fluid extraction of emulsion. J. Supercrit. Fluids 2017, 128, 121–127. [Google Scholar] [CrossRef]
- Sarkari, M.; Brown, J.; Chen, X.; Swinnea, S.; Williams, R.O., III; Johnston, K.P. Enhanced drug dissolution using evaporative precipitation into aqueous solution. Int. J. Pharm. 2002, 243, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, G.; Kang, J.; Niu, M.; Wang, Z.; Wang, H.; Ma, J.; Wang, X. Paclitaxel nanosuspensions coated with P-gp inhibitory surfactants: I. Acute toxicity and pharmacokinetics studies. Colloids Surf. B Biointerfaces 2013, 111, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Bosselmann, S.; Nagao, M.; Chow, K.T.; Williams, R.O., III. Influence of formulation and processing variables on properties of itraconazole nanoparticles made by advanced evaporative precipitation into aqueous solution. AAPS PharmSciTech 2012, 13, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Kakran, M.; Sahoo, N.G.; Li, L.; Judeh, Z. Dissolution of artemisinin/polymer composite nanoparticles fabricated by evaporative precipitation of nanosuspension. J. Pharm. Pharmacol. 2010, 62, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, Y.; Gemeinhart, R.A.; Wu, W.; Li, T. Developing nanocrystals for cancer treatment. Nanomedicine 2015, 10, 2537–2552. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef]
- Lizonova, D.; Hladek, F.; Chvila, S.; Balaz, A.; Stankova, S.; Stepanek, F. Surface stabilization determines macrophage uptake, cytotoxicity, and bioactivity of curcumin nanocrystals. Int. J. Pharm. 2022, 626, 122133. [Google Scholar] [CrossRef]
- Lou, H.; Gao, L.; Wei, X.; Zhang, Z.; Zheng, D.; Zhang, D.; Zhang, X.; Li, Y.; Zhang, Q. Oridonin nanosuspension enhances anti-tumor efficacy in SMMC-7721 cells and H22 tumor bearing mice. Colloids Surf. B Biointerfaces 2011, 87, 319–325. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Zhao, Y.; Han, M.; Guo, Y.; Kuang, H.; Wang, X. A stabilizer-free and organic solvent-free method to prepare 10-hydroxycamptothecin nanocrystals: In vitro and in vivo evaluation. Int. J. Nanomed. 2016, 11, 2979–2994. [Google Scholar] [CrossRef]
- Meng, T.T.; Qiao, F.X.; Ma, S.J.; Gao, T.; Li, L.; Hou, Y.H.; Yang, J.H. Exploring the influence factors and improvement strategies of drug polymorphic transformation combined kinetic and thermodynamic perspectives during the formation of nanosuspensions. Drug Dev. Ind. Pharm. 2021, 47, 1867–1880. [Google Scholar] [CrossRef]
- Dupont, A.; Guerain, M.; Danede, F.; Paccou, L.; Guinet, Y.; Hedoux, A.; Willart, J.F. Kinetics and mechanism of polymorphic transformation of sorbitol under mechanical milling. Int. J. Pharm. 2020, 590, 119902. [Google Scholar] [CrossRef] [PubMed]
- Mazel, V.; Delplace, C.; Busignies, V.; Faivre, V.; Tchoreloff, P.; Yagoubi, N. Polymorphic transformation of anhydrous caffeine under compression and grinding: A re-evaluation. Drug Dev. Ind. Pharm. 2011, 37, 832–840. [Google Scholar] [CrossRef]
- Descamps, M.; Willart, J.F. Perspectives on the amorphisation/milling relationship in pharmaceutical materials. Adv. Drug Deliv. Rev. 2016, 100, 51–66. [Google Scholar] [CrossRef]
- Aleandri, S.; Schonenberger, M.; Niederquell, A.; Kuentz, M. Temperature-Induced Surface Effects on Drug Nanosuspensions. Pharm. Res. 2018, 35, 69. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Padrela, L.; Tajber, L.; Collas, A. Nucleation kinetics-based solvent selection for the liquid antisolvent crystallization of a lipophilic intermediate. J. Mol. Liq. 2023, 375, 121306. [Google Scholar] [CrossRef]
- Shariare, M.H.; Sharmin, S.; Jahan, I.; Reza, H.M.; Mohsin, K. The impact of process parameters on carrier free paracetamol nanosuspension prepared using different stabilizers by antisolvent precipitation method. J. Drug Deliv. Sci. Technol. 2018, 43, 122–128. [Google Scholar] [CrossRef]
- Chen, Z.J.; Wu, W.; Lu, Y. What is the future for nanocrystal-based drug-delivery systems? Ther. Deliv. 2020, 11, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Tehrani, A.; Omranpoor, M.M.; Vatanara, A.; Seyedabadi, M.; Ramezani, V. Formation of nanosuspensions in bottom-up approach: Theories and optimization. Daru 2019, 27, 451–473. [Google Scholar] [CrossRef]
- Yang, X.; Ong, T.C.; Michaelis, V.K.; Heng, S.; Griffin, R.G.; Myerson, A.S. Formation of Organic Molecular Nanocrystals under Soft Confinement. CrystEngComm 2015, 17, 6044–6052. [Google Scholar] [CrossRef]
- Lindfors, L.; Forssen, S.; Westergren, J.; Olsson, U. Nucleation and crystal growth in supersaturated solutions of a model drug. J. Colloid Interface Sci. 2008, 325, 404–413. [Google Scholar] [CrossRef]
- Wang, X.H.; Liu, Y.; Shen, C.Y.; Shen, B.D.; Zhong, R.N.; Yuan, H.L. Effect of particle size on in vitro and in vivo behavior of astilbin nanosuspensions. J. Drug Deliv. Sci. Technol. 2019, 52, 778–783. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Liu, Z.; Zhang, D.; Zhang, Q. In vivo evaluation of silybin nanosuspensions targeting liver. J. Biomed. Nanotechnol. 2012, 8, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Wang, Y.; Zhang, D.; Liu, Z.; Duan, C.; Jia, L.; Wang, F.; Liu, Y.; Liu, G.; Hao, L.; et al. In vitro antitumor activity of silybin nanosuspension in PC-3 cells. Cancer Lett. 2011, 307, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Shelar, D.B.; Pawar, S.K.; Vavia, P.R. Fabrication of isradipine nanosuspension by anti-solvent microprecipitation-high-pressure homogenization method for enhancing dissolution rate and oral bioavailability. Drug Deliv. Transl. Res. 2013, 3, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Muller, R.H.; Moschwitzer, J.P. Effect of drug physico-chemical properties on the efficiency of top-down process and characterization of nanosuspension. Expert Opin. Drug Deliv. 2015, 12, 1741–1754. [Google Scholar] [CrossRef]
- Shin, G.H.; Li, J.; Cho, J.H.; Kim, J.T.; Park, H.J. Enhancement of Curcumin Solubility by Phase Change from Crystalline to Amorphous in Cur-TPGS Nanosuspension. J. Food Sci. 2016, 81, N494–N501. [Google Scholar] [CrossRef]
- Liu, T.; Muller, R.H.; Moschwitzer, J.P. Consideration of the solid state for resveratrol nanocrystal production. Powder Technol. 2018, 332, 63–69. [Google Scholar] [CrossRef]
- Bellucci, M.A.; Marx, A.; Wang, B.; Fang, L.; Zhou, Y.; Greenwell, C.; Li, Z.; Becker, A.; Sun, G.; Brandenburg, J.G.; et al. Effect of Polymer Additives on the Crystal Habit of Metformin HCl. Small Methods 2023, 7, e2201692. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Chang, F.R.; Liaw, C.C.; Wu, Y.C.; Bastow, K.F.; Lee, K.H. Acetogenins as selective inhibitors of the human ovarian 1A9 tumor cell line. J. Med. Chem. 2003, 46, 3185–3188. [Google Scholar] [CrossRef]
- Hong, J.; Li, Y.; Li, Y.; Xiao, Y.; Kuang, H.; Wang, X. Annonaceous acetogenins nanosuspensions stabilized by PCL-PEG block polymer: Significantly improved antitumor efficacy. Int. J. Nanomed. 2016, 11, 3239–3253. [Google Scholar] [CrossRef]
- Hopp, D.C.; Alali, F.Q.; Gu, Z.M.; McLaughlin, J.L. Three new bioactive bis-adjacent THF-ring acetogenins from the bark of Annona squamosa. Bioorg. Med. Chem. 1998, 6, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Ahammadsahib, K.I.; Hollingworth, R.M.; Mcgovren, J.P.; Hui, Y.H.; Mclaughlin, J.L. Mode of Action of Bullatacin—A Potent Antitumor and Pesticidal Annonaceous Acetogenin. Life Sci. 1993, 53, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.W.; Li, X. Cytotoxic Bistetrahydrofuran Annonaceous Acetogenins from the Seeds of Annona squamosa. J. Nat. Prod. 2011, 74, 2477–2481. [Google Scholar] [CrossRef] [PubMed]
- Oberlies, N.H.; Croy, V.L.; Harrison, M.L.; McLaughlin, J.L. The Annonaceous acetogenin bullatacin is cytotoxic against multidrug-resistant human mammary adenocarcinoma cells. Cancer Lett. 1997, 115, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.W.; Zhai, J.H.; Wang, Y.; Wang, S.L.; Li, X. Antitumor activity and toxicity relationship of annonaceous acetogenins. Food Chem. Toxicol. 2013, 58, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Peng, A.H.; He, S.C.; Shao, X.M.; Nie, C.L.; Chen, L.J. Isogambogenic acid inhibits tumour angiogenesis by suppressing Rho GTPases and vascular endothelial growth factor receptor 2 signalling pathway. J. Chemother. 2013, 25, 298–308. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, Y.H.; Wang, J.R.; Lu, B.B.; Wang, K.M.; Tian, Y. Gambogenic Acid Induction of Apoptosis in a Breast Cancer Cell Line. Asian Pac. J. Cancer Prev. 2013, 14, 7601–7605. [Google Scholar] [CrossRef]
- Wang, M.; Chen, D.; Cheng, H.; Li, B.; Yan, F.G.; Su, J.J.; Peng, C.; Sun, M.L.; Hu, Y.W.; Wang, X.S.; et al. Gambogenic Acid Kills Lung Cancer Cells through Aberrant Autophagy. PLoS ONE 2014, 9, e83604. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, H.; Liu, P.; Wu, X.; Chen, B. Gambogenic acid synergistically potentiates bortezomib-induced apoptosis in multiple myeloma. J. Cancer 2017, 8, 839–851. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, J.; Chen, W. Gambogenic acid reverses P-glycoprotein mediated multidrug resistance in HepG2/Adr cells and its underlying mechanism. Biochem. Biophys. Res. Commun. 2019, 508, 882–888. [Google Scholar] [CrossRef]
- Yuan, H.; Li, X.; Zhang, C.; Pan, W.; Liang, Y.; Chen, Y.; Chen, W.; Liu, L.; Wang, X. Nanosuspensions as delivery system for gambogenic acid: Characterization and in vitro/in vivo evaluation. Drug Deliv. 2016, 23, 2772–2779. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Zu, Y.G.; Shi, R.Z.; Yao, L.P. Review Camptothecin: Current Perspectives. Curr. Med. Chem. 2006, 13, 2021–2039. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Wang, W.; Ning, J.; Zhang, X.; Zheng, W.; Qian, Y.; Fan, C. Hydroxycamptothecin Inhibits Peritendinous Adhesion via the Endoplasmic Reticulum Stress-Dependent Apoptosis. Front. Pharmacol. 2019, 10, 967. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, X.; Yu, C.; Nan, X.; Chen, X.; Zhang, X. Shape regulated anticancer activities and systematic toxicities of drug nanocrystals in vivo. Nanomedicine 2016, 12, 181–189. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, H.L. Resveratrol and its analogues: Promising antitumor agents. Anti-Cancer Agents Med. Chem. 2011, 11, 479–490. [Google Scholar] [CrossRef]
- Bian, P.; Hu, W.; Liu, C.; Li, L. Resveratrol potentiates the anti-tumor effects of rapamycin in papillary thyroid cancer: PI3K/AKT/mTOR pathway involved. Arch. Biochem. Biophys. 2020, 689, 108461. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Zhao, J.; Li, C.; Zhou, Y.; Du, J.; Wang, Y. In vitro and in vivo evaluation of targeting tumor with folate-based amphiphilic multifunctional stabilizer for resveratrol nanosuspensions. Colloids Surf. B Biointerfaces 2017, 160, 462–472. [Google Scholar] [CrossRef]
- Ancic, D.; Orsolic, N.; Odeh, D.; Tomasevic, M.; Pepic, I.; Ramic, S. Resveratrol and its nanocrystals: A promising approach for cancer therapy? Toxicol. Appl. Pharmacol. 2022, 435, 115851. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Xue, W.; Yangyang, Y.; Xu, D.; Zhao, Y.; Lou, H. Effects of oridonin nanosuspension on cell proliferation and apoptosis of human prostatic carcinoma PC-3 cell line. Int. J. Nanomed. 2010, 5, 735–742. [Google Scholar] [CrossRef]
- Tang, X.; Sun, J.; Ge, T.; Zhang, K.; Gui, Q.; Zhang, S.; Chen, W. PEGylated liposomes as delivery systems for Gambogenic acid: Characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2018, 172, 26–36. [Google Scholar] [CrossRef]
- Qiao, F.; Zhao, Y.; Mai, Y.; Guo, J.; Dong, L.; Zhang, W.; Yang, J. Isoliquiritigenin Nanosuspension Enhances Cytostatic Effects in A549 Lung Cancer Cells. Planta Med. 2020, 86, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Das, S.; Ng, K.Y.; Heng, P.W. Formulation, biological and pharmacokinetic studies of sucrose ester-stabilized nanosuspensions of oleanolic Acid. Pharm. Res. 2011, 28, 2020–2033. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wang, Y.; Shen, Y.; Ao, H.; Guo, Y.; Han, M.; Wang, X. Preparation of high drug-loading celastrol nanosuspensions and their anti-breast cancer activities in vitro and in vivo. Sci. Rep 2020, 10, 8851. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hong, J.; Di, J.; Guo, Y.; Han, M.; Liu, M.; Wang, X. 10-Hydroxycamptothecin (HCPT) nanosuspensions stabilized by mPEG(1000)-HCPT conjugate: High stabilizing efficiency and improved antitumor efficacy. Int. J. Nanomed. 2017, 12, 3681–3695. [Google Scholar] [CrossRef]
- Sun, M.; Gao, Y.; Pei, Y.; Guo, C.; Li, H.; Cao, F.; Yu, A.; Zhai, G. Development of nanosuspension formulation for oral delivery of quercetin. J. Biomed. Nanotechnol. 2010, 6, 325–332. [Google Scholar] [CrossRef]
- Pengnam, S.; Charoensuksai, P.; Yingyongnarongkul, B.E.; Saeeng, R.; Uludag, H.; Patrojanasophon, P.; Opanasopit, P.; Plianwong, S. siRNA Targeting Mcl-1 Potentiates the Anticancer Activity of Andrographolide Nanosuspensions via Apoptosis in Breast Cancer Cells. Pharmaceutics 2022, 14, 1196. [Google Scholar] [CrossRef]
- Hong, J.Y.; Liu, Y.Y.; Xiao, Y.; Yang, X.F.; Su, W.J.; Zhang, M.Z.; Liao, Y.H.; Kuang, H.X.; Wang, X.T. High drug payload curcumin nanosuspensions stabilized by mPEG-DSPE and SPC: In vitro and in vivo evaluation. Drug Deliv. 2017, 24, 109–120. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.G.; Sun, M.; Li, H.L.; Guo, C.Y.; Cui, J.; Li, A.G.; Cao, F.L.; Xi, Y.W.; Lou, H.X.; et al. Preparation, characterization, pharmacokinetics, and tissue distribution of curcumin nanosuspension with TPGS as stabilizer. Drug Dev. Ind. Pharm. 2010, 36, 1225–1234. [Google Scholar] [CrossRef]
- Jin, L.; Zeng, W.; Zhang, F.; Zhang, C.; Liang, W. Naringenin Ameliorates Acute Inflammation by Regulating Intracellular Cytokine Degradation. J. Immunol. 2017, 199, 3466–3477. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Eid, B.G. Icariin modulates carrageenan-induced acute inflammation through HO-1/Nrf2 and NF-kB signaling pathways. Biomed. Pharmacother. 2019, 120, 109567. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Wang, Y.; Zhu, T. Silymarin improved diet-induced liver damage and insulin resistance by decreasing inflammation in mice. Pharm. Biol. 2016, 54, 2995–3000. [Google Scholar] [CrossRef] [PubMed]
- Yardim, A.; Kucukler, S.; Ozdemir, S.; Comakli, S.; Caglayan, C.; Kandemir, F.M.; Celik, H. Silymarin alleviates docetaxel-induced central and peripheral neurotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Gene 2021, 769, 145239. [Google Scholar] [CrossRef] [PubMed]
- Gera, S.; Sampathi, S.; Maddukuri, S.; Dodoala, S.; Junnuthula, V.; Dyawanapelly, S. Therapeutic Potential of Naringenin Nanosuspension: In Vitro and In Vivo Anti-Osteoporotic Studies. Pharmaceutics 2022, 14, 1449. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Q.; Wang, X.T.; Wang, M.Y.; Wang, R.; Meng, Z.; Wang, X.T.; Yu, B.; Han, M.H.; Guo, Y.F. Optimization of Naringenin Nanoparticles to Improve the Antitussive Effects on Post-Infectious Cough. Molecules 2022, 27, 3736. [Google Scholar] [CrossRef]
- Li, Y.; Sun, S.P.; Chang, Q.; Zhang, L.; Wang, G.N.; Chen, W.X.; Miao, X.Q.; Zheng, Y. A Strategy for the Improvement of the Bioavailability and Antiosteoporosis Activity of BCS IV Flavonoid Glycosides through the Formulation of Their Lipophilic Aglycone into Nanocrystals. Mol. Pharm. 2013, 10, 2534–2542. [Google Scholar] [CrossRef]
- Li, H.M.; Zhuo, H.Y.; Yin, D.; Li, W.; Zhang, Y.M.; Li, P.; Zou, L. Intra-Articular Injection of a Nanosuspension of Tetramethylpyrazine Dihydroxynaphthalenate for Stronger and Longer-Lasting Effects Against Osteoarthritis. J. Biomed. Nanotechnol. 2021, 17, 1199–1207. [Google Scholar] [CrossRef]
- Casula, L.; Lai, F.; Pini, E.; Valenti, D.; Sinico, C.; Cardia, M.C.; Marceddu, S.; Ailuno, G.; Fadda, A.M. Pulmonary Delivery of Curcumin and Beclomethasone Dipropionate in a Multicomponent Nanosuspension for the Treatment of Bronchial Asthma. Pharmaceutics 2021, 13, 1300. [Google Scholar] [CrossRef]
- Mishra, S.B.; Pandey, H.; Pandey, A.C. Nanosuspension of Phyllanthus amarus extract for improving oral bioavailability and prevention of paracetamol induced hepatotoxicity in Sprague-Dawley rats. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 035007. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; Rosqvist, E.; Smatt, J.H.; Ibrahim, H.M.; Ismael, H.R.; Afouna, M.I.; Samy, A.M.; Rosenholm, J.M. Formulation and optimization of lyophilized nanosuspension tablets to improve the physicochemical properties and provide immediate release of silymarin. Int. J. Pharm. 2019, 563, 217–227. [Google Scholar] [CrossRef]
- Qiao, H.Z.; Chen, L.H.; Rui, T.Q.; Wang, J.X.; Chen, T.; Fu, T.M.; Li, J.S.; Di, L.Q. Fabrication and in vitro/in vivo evaluation of amorphous andrographolide nanosuspensions stabilized by D-alpha-tocopheryl polyethylene glycol 1000 succinate/sodium lauryl sulfate. Int. J. Nanomed. 2017, 12, 1033–1046. [Google Scholar] [CrossRef]
- Koonrungsesomboon, N.; Karbwang, J. Ethical considerations in clinical research on herbal medicine for prevention of cardiovascular disease in the ageing. Phytomedicine 2016, 23, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Thuan, D.T.B.; Phu, H.T.; Nguyen, T.H.D.; Hasan, H.; Halabi, S.; Abdelhady, S.; Nasrallah, G.K.; Eid, A.H.; Pintus, G. Herbal Medicine for Cardiovascular Diseases: Efficacy, Mechanisms, and Safety. Front. Pharmacol. 2020, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Q.; Wang, S.Z.; Goel, V.; Ling, L.; Shan, J. Anti-Oxidant Activity of Different Herbal Extracts Used in Traditional Chinese Medicine for the Treatment of Cardiovascular Disease. Pharm. Biol. 2010, 48, 29–30. [Google Scholar]
- Lee, T.Y.; Chang, C.C.; Lu, W.J.; Yen, T.L.; Lin, K.H.; Geraldine, P.; Li, J.Y.; Sheu, J.R. Honokiol as a specific collagen receptor glycoprotein VI antagonist on human platelets: Functional ex vivo and in vivo studies. Sci. Rep. 2017, 7, 40002. [Google Scholar] [CrossRef] [PubMed]
- Jayakumari, N.R.; Rajendran, R.S.; Sivasailam, A.; Parambil, S.T.; Reghuvaran, A.C.; Sreelatha, H.V.; Gopala, S. Honokiol regulates mitochondrial substrate utilization and cellular fatty acid metabolism in diabetic mice heart. Eur. J. Pharmacol. 2021, 896, 173918. [Google Scholar] [CrossRef] [PubMed]
- Elbarbry, F.; Moshirian, N. The Modulation of Arachidonic Acid Metabolism and Blood Pressure-Lowering Effect of Honokiol in Spontaneously Hypertensive Rats. Molecules 2022, 27, 3396. [Google Scholar] [CrossRef]
- Han, M.H.; Yu, X.; Guo, Y.F.; Wang, Y.H.; Kuang, H.X.; Wang, X.T. Honokiol nanosuspensions: Preparation, increased oral bioavailability and dramatically enhanced biodistribution in the cardio-cerebro-vascular system. Colloids Surf. B Biointerfaces 2014, 116, 114–120. [Google Scholar] [CrossRef]
- Wang, Z.P.; Wu, J.B.; Zhou, Q.; Wang, Y.F.; Chen, T.S. Berberine Nanosuspension Enhances Hypoglycemic Efficacy on Streptozotocin Induced Diabetic C57BL/6 Mice. Evid.-Based Complement. Altern. Med. 2015, 2015, 239749. [Google Scholar] [CrossRef]
- Rui, T.Q.; Zhang, L.; Qiao, H.Z.; Huang, P.; Qian, S.; Li, J.S.; Chen, Z.P.; Fu, T.M.; Di, L.Q.; Cai, B.C. Preparation and Physicochemical and Pharmacokinetic Characterization of Ginkgo Lactone Nanosuspensions for Antiplatelet Aggregation. J. Pharm. Sci. 2016, 105, 242–249. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Zu, Y.; Zhang, Y.; Li, Y.; Sun, W.; Shan, C.; Ge, Y. Preparation and characterization of betulin nanoparticles for oral hypoglycemic drug by antisolvent precipitation. Drug Deliv. 2014, 21, 467–479. [Google Scholar] [CrossRef]
- Singh, A.K.; Pandey, H.; Ramteke, P.W.; Mishra, S.B. Nano-suspension of ursolic acid for improving oral bioavailability and attenuation of type II diabetes: A histopathological investigation. Biocatal. Agric. Biotechnol. 2019, 22, 101433. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.X.; Xu, J.W.; Shao, J.B.; Ding, M.H.; Shi, S.L. Preparation, Characterization, and Evaluation of Breviscapine Nanosuspension and Its Freeze-Dried Powder. Pharmaceutics 2022, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Li, R.L.; Wang, L.Y.; Duan, H.X.; Zhang, Q.; Guo, X.; Wu, C.; Peng, W. Regulation of mitochondrial dysfunction induced cell apoptosis is a potential therapeutic strategy for herbal medicine to treat neurodegenerative diseases. Front. Pharmacol. 2022, 13, 937289. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Xue, J.; Tan, Y.; Fang, C.; Hu, C.; Yang, Q.; Mei, X.; Qi, D. The Positive Role and Mechanism of Herbal Medicine in Parkinson’s Disease. Oxidative Med. Cell. Longev. 2021, 2021, 9923331. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Q.G.; Lai, L.Y.; Wen, X.J.; Zheng, T.; Cheung, C.W.; Zhou, S.Q.; Xu, S.Y. Neuroprotective Effect of Ginkgolide B on Bupivacaine-Induced Apoptosis in SH-SY5Y Cells. Oxidative Med. Cell. Longev. 2013, 2013, 159864. [Google Scholar] [CrossRef]
- Hua, J.; Yin, N.; Yang, B.B.; Zhang, J.; Ding, J.H.; Fan, Y.; Hu, G. Ginkgolide B and bilobalide ameliorate neural cell apoptosis in alpha-synuclein aggregates. Biomed. Pharmacother. 2017, 96, 792–797. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Xiong, S.; Luo, J.S.; Li, Y.; Zhao, Y.Y.; Wang, Q.; Zhang, Z.X.; Chen, X.J.; Chen, T.K. Highly stabilized nanocrystals delivering Ginkgolide B in protecting against the Parkinson’s disease. Int. J. Pharm. 2020, 577, 119053. [Google Scholar] [CrossRef]
- Stahr, P.L.; Grewal, R.; Eckert, G.P.; Keck, C.M. Investigating hesperetin nanocrystals with tailor-made sizes for the prevention and treatment of Alzheimer’s disease. Drug Deliv. Transl. Res. 2021, 11, 659–674. [Google Scholar] [CrossRef]
- Kakran, M.; Sahoo, N.G.; Li, L. Dissolution enhancement of quercetin through nanofabrication, complexation, and solid dispersion. Colloids Surf. B Biointerfaces 2011, 88, 121–130. [Google Scholar] [CrossRef]
- Ghaffafi, F.; Moghaddam, A.H.; Zare, M. Neuroprotective Effect of Quercetin Nanocrystal in a 6-Hydroxydopamine Model of Parkinson Disease: Biochemical and Behavioral Evidence. Basic Clin. Neurosci. 2018, 9, 317–324. [Google Scholar] [CrossRef]
- Chen, T.K.; Li, C.W.; Li, Y.; Yi, X.; Lee, S.M.Y.; Zheng, Y. Oral Delivery of a Nanocrystal Formulation of Schisantherin A with Improved Bioavailability and Brain Delivery for the Treatment of Parkinson’s Disease. Mol. Pharm. 2016, 13, 3864–3875. [Google Scholar] [CrossRef] [PubMed]
- Babylon, L.; Grewal, R.; Stahr, P.L.; Eckert, R.W.; Keck, C.M.; Eckert, G.P. Hesperetin Nanocrystals Improve Mitochondrial Function in a Cell Model of Early Alzheimer Disease. Antioxidants 2021, 10, 1003. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.L.; Han, Y.R.; Quan, L.H.; Liu, C.Y.; Liao, Y.H. Oily nanosuspension for long-acting intramuscular delivery of curcumin didecanoate prodrug: Preparation, characterization and in vivo evaluation. Eur. J. Pharm. Sci. 2013, 49, 286–293. [Google Scholar] [CrossRef]
- Ang, L.; Song, E.; Zhang, J.; Lee, H.W.; Lee, M.S. Herbal medicine for COVID-19: An overview of systematic reviews and meta-analysis. Phytomedicine 2022, 102, 154136. [Google Scholar] [CrossRef]
- Yu, M.; Jin, X.; Liang, C.; Bu, F.; Pan, D.; He, Q.; Ming, Y.; Little, P.; Du, H.; Liang, S.; et al. Berberine for diarrhea in children and adults: A systematic review and meta-analysis. Ther. Adv. Gastroenterol. 2020, 13, 1756284820961299. [Google Scholar] [CrossRef] [PubMed]
- Sahibzada, M.U.K.; Sadiq, A.; Faidah, H.S.; Khurram, M.; Amin, M.U.; Haseeb, A.; Kakar, M. Berberine nanoparticles with enhanced in vitro bioavailability: Characterization and antimicrobial activity. Drug Des. Dev. Ther. 2018, 12, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Yue, P.F.; Lv, J.L.; Han, J.; Fu, S.S.; Jin, S.X.; Jin, S.Y.; Yuan, H.L. Development and in vivo/in vitro evaluation of novel herpetrione nanosuspension. Int. J. Pharm. 2013, 441, 227–233. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Y.; Li, R.Y.; Peng, S.F.; Ruan, R.; Li, J.H.; Liu, W. Fabrication of Caseinate Stabilized Thymol Nanosuspensions via the pH-Driven Method: Enhancement in Water Solubility of Thymol. Foods 2021, 10, 1074. [Google Scholar] [CrossRef]
- Lai, F.; Pireddu, R.; Corrias, F.; Fadda, A.M.; Valenti, D.; Pini, E.; Sinico, C. Nanosuspension improves tretinoin photostability and delivery to the skin. Int. J. Pharm. 2013, 458, 104–109. [Google Scholar] [CrossRef]
- Zong, R.; Ruan, H.A.; Zhu, W.Z.; Zhang, P.; Feng, Z.J.; Liu, C.M.; Fan, S.H.; Liang, H.M.; Li, J. Curcumin nanocrystals with tunable surface zeta potential: Preparation, characterization and antibacterial study. J. Drug Deliv. Sci. Technol. 2022, 76, 103771. [Google Scholar] [CrossRef]
- Sahar, P.; Ali, T.; Naeem, M.; Hussain, F. Nanotechnology approach for exploring the enhanced bioactivities, biochemical characterisation and phytochemistry of freshly prepared Mentha arvensis L. nanosuspensions. Phytochem. Anal. 2022. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, H.; Komasaka, T.; Tomari, T.; Kitano, Y.; Takekawa, K. Nanosuspension formulations of poorly water-soluble compounds for intravenous administration in exploratory toxicity studies: In vitro and in vivo evaluation. J. Appl. Toxicol. 2016, 36, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Bidgoli, S.A.; Zanjani, M.S.; Arabshahi, P.; Gazori, T.; Hamidi, M. Pharmacokinetics and repeated dose 28-day oral toxicity studies of acetaminophen nanosuspension. J. Biomed. Mater. Res. Part B 2023, 111, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Ong, C.; Bay, B.H.; Baeg, G.H. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.T.; Yu, L.E.; Ong, C.N.; Bay, B.H.; Baeg, G.H. The use of Drosophila melanogaster as a model organism to study immune-nanotoxicity. Nanotoxicology 2019, 13, 429–446. [Google Scholar] [CrossRef]
- Muller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals—Special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef]
- Doshi, N.; Mitragotri, S. Needle-shaped polymeric particles induce transient disruption of cell membranes. J. R. Soc. Interface 2010, 7, S403–S410. [Google Scholar] [CrossRef]
- Zhang, B.K.; Lung, P.S.; Zhao, S.S.; Chu, Z.Q.; Chrzanowski, W.; Li, Q. Shape dependent cytotoxicity of PLGA-PEG nanoparticles on human cells. Sci. Rep. 2017, 7, 7315. [Google Scholar] [CrossRef]
- Wang, T.; Qi, J.; Ding, N.; Dong, X.; Zhao, W.; Lu, Y.; Wang, C.; Wu, W. Tracking translocation of self-discriminating curcumin hybrid nanocrystals following intravenous delivery. Int. J. Pharm. 2018, 546, 10–19. [Google Scholar] [CrossRef]
- Shen, C.; Yang, Y.; Shen, B.; Xie, Y.; Qi, J.; Dong, X.; Zhao, W.; Zhu, W.; Wu, W.; Yuan, H.; et al. Self-discriminating fluorescent hybrid nanocrystals: Efficient and accurate tracking of translocation via oral delivery. Nanoscale 2017, 10, 436–450. [Google Scholar] [CrossRef]
- Qiao, Y.H.; Wei, Z.H.; Qin, T.T.; Song, R.F.; Yu, Z.Q.; Yuan, Q.; Du, J.; Zeng, Q.B.; Zong, L.L.; Duan, S.F.; et al. Combined nanosuspensions from two natural active ingredients for cancer therapy with reduced side effects. Chin. Chem. Lett. 2021, 32, 2877–2881. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Herbal Products and Their Active Constituents Used Alone and in Combination with Antibiotics against Multidrug-Resistant Bacteria. Planta Med. 2022, 89, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Kathpalia, H.; Juvekar, S.; Mohanraj, K.; Apsingekar, M.; Shidhaye, S. Investigation of pre-clinical pharmacokinetic parameters of atovaquone nanosuspension prepared using a pH-based precipitation method and its pharmacodynamic properties in a novel artemisinin combination. J. Glob. Antimicrob. Resist. 2020, 22, 248–256. [Google Scholar] [CrossRef]
- Siram, K.; Raghavan, C.V.; Marslin, G.; Rahman, H.; Selvaraj, D.; Balakumar, K.; Franklin, G. Quillaja saponin: A prospective emulsifier for the preparation of solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2016, 147, 274–280. [Google Scholar] [CrossRef]
- Chen, Y.C.; Liu, Y.; Xu, J.N.; Xie, Y.B.; Zheng, Q.; Yue, P.F.; Yang, M. A Natural Triterpenoid Saponin as Multifunctional Stabilizer for Drug Nanosuspension Powder. Aaps Pharmscitech 2017, 18, 2744–2753. [Google Scholar] [CrossRef]
- Long, J.Y.; Song, J.W.; Zhang, X.M.; Deng, M.; Xie, L.; Zhang, L.L.; Li, X.F. Tea saponins as natural stabilizers for the production of hesperidin nanosuspensions. Int. J. Pharm. 2020, 583, 119406. [Google Scholar] [CrossRef]
- Chen, H.J.; Deng, M.; Xie, L.; Liu, K.; Zhang, X.M.; Li, X.F. Preparation and characterization of quercetin nanosuspensions using gypenosides as novel stabilizers. J. Drug Deliv. Sci. Technol. 2022, 67, 102962. [Google Scholar] [CrossRef]
- Suo, Z.L.; Sun, Q.M.; Peng, X.; Zhang, S.S.; Gan, N.; Zhao, L.D.; Yuan, N.; Zhang, Y.K.; Li, H. Lentinan as a natural stabilizer with bioactivities for preparation of drug-drug nanosuspensions. Int. J. Biol. Macromol. 2021, 184, 101–108. [Google Scholar] [CrossRef] [PubMed]

| Preparation Method | Advantages | Limitations | Subdivide Method |
|---|---|---|---|
| Bottom-up |
|
|
|
| Top-down |
|
|
|
| Combinative methods |
|
|
|
| Extract/Compounds | Stabilizers | Preparation Methods | Particle Size | Bioavailability | Advantages | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Berberine | TPGS | High-pressure homogenization | 73.1 ± 3.7 nm | / | Exhibited superior hypoglycemic, total cholesterol (TC), and body weight reduction effects compared to bulk Ber and metformin (Met, 300 mg/kg). | Antidiabetic effect | [98] |
| Ginkgo Lactone/ginkgolide | 5% P 188 and 5% HPMC | High-pressure homogenization | 254 ± 2.8 nm | Twofold higher in Cmax and AUC0–t for three ginkgolides | Exhibited a significantly higher antiplatelet aggregation effect | Antiplatelet aggregation | [99] |
| Honokiol | Bovine serum albumin (BSA) and PVP | Antisolvent sonoprecipitation | 116.2 ± 2 nm | Honokiol nanocrystals improved the oral bioavailability in rats by 3.94-fold in Cmax and 2.2-fold in AUC0–t | Honokiol was released more quickly in vitro from nanocrystals, with no burst release. Honokiol nanocrystals improved the oral bioavailability. After intraperitoneal administration, Honokiol nanocrystals could dramatically alter the biodistribution, resulting in much higher drug levels and tissue bioavailability in the blood, heart, and brain. | Cardio-cerebro-vascular system | [97] |
| Betulin | 0.5% Tween 80 | Antisolvent precipitation | ~110 nm | The oral bioavailability of the betulin nanocrystal was 2.21 times that of raw betulin. | Higher dissolution rate, solubility, and bioavailability of botulin nanocrystal compared with raw botulin. Excellent hypoglycemic effect compared with raw betulin | Diabetes mellitus | [100] |
| Ursolic acid | 2% of PVA | Nanoprecipitation | 246.4 ± 4.21 nm | / | Ursolic acid nanocrystal showed a significant reduction in elevated blood glucose level in a dose-dependent manner with prominent lipid-lowering and antioxidant effects. | Type II diabetes | [101] |
| Breviscapine | Soybean phospholipid | Antisolvent sonoprecipitation | 303.7 ± 7.3 nm | / | Breviscapine nanocrystal displayed good stability, increased solubility, and better in vitro release | Cardiovascular and cerebrovascular diseases | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Qin, S.; Wang, S.; Sun, M.; Yang, H.; Wang, X.; Fan, P.; Jin, Z. Herbal Medicine Nanocrystals: A Potential Novel Therapeutic Strategy. Molecules 2023, 28, 6370. https://doi.org/10.3390/molecules28176370
Guo M, Qin S, Wang S, Sun M, Yang H, Wang X, Fan P, Jin Z. Herbal Medicine Nanocrystals: A Potential Novel Therapeutic Strategy. Molecules. 2023; 28(17):6370. https://doi.org/10.3390/molecules28176370
Chicago/Turabian StyleGuo, Mengran, Shugang Qin, Shiyan Wang, Min Sun, Huiling Yang, Xinchun Wang, Ping Fan, and Zhaohui Jin. 2023. "Herbal Medicine Nanocrystals: A Potential Novel Therapeutic Strategy" Molecules 28, no. 17: 6370. https://doi.org/10.3390/molecules28176370






