Electrochemical Determination of 17-β-Estradiol Using a Glassy Carbon Electrode Modified with α-Fe2O3 Nanoparticles Supported on Carbon Nanotubes
Abstract
:1. Introduction
2. Results
2.1. Chemical and Morphological Characterization of α-Fe2O3-CNT/GCE
2.2. Electrochemical Behavior of E2 at α-Fe2O3-CNT/GCE
2.3. Determination of E2 at α-Fe2O3-CNT/GCE
3. Materials and Methods
3.1. Chemical Reagents and Solutions
3.2. Apparatus
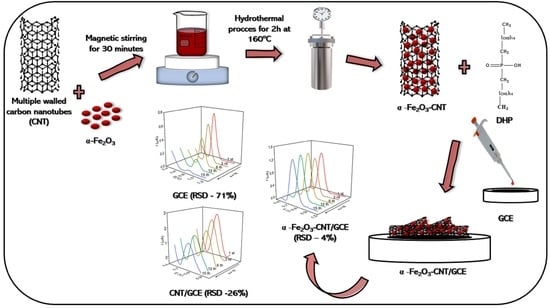
3.3. Synthesis of α-Fe2O3-CNT and Preparation of Electrochemical Sensors
3.4. Preparation of Lake Water, Synthetic Urine, and Pharmaceutical Samples
3.5. Measurement Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kavetskyy, T.; Khalilov, R.; Smutok, O.; Demkiv, O. Construction of Amperometric Laccase-Based Biosensors Using the Ureasil and Photocross-Linked Polymers. AB&ES 2019, 4, 137–149. [Google Scholar]
- Eftekhari, A.; Dalili, M.; Karimi, Z.; Rouhani, S.; Hasanzadeh, A.; Rostamnia, S.; Khaksar, S.; Idris, A.O.; Karimi-Maleh, H.; Yola, M.L.; et al. Sensitive and Selective Electrochemical Detection of Bisphenol a Based on SBA-15 like Cu-PMO Modified Glassy Carbon Electrode. Food Chem. 2021, 358, 129763. [Google Scholar] [CrossRef]
- Smajdor, J.; Piech, R.; Ławrywianiec, M.; Paczosa-Bator, B. Glassy Carbon Electrode Modified with Carbon Black for Sensitive Estradiol Determination by Means of Voltammetry and Flow Injection Analysis with Amperometric Detection. Anal. Biochem. 2018, 544, 7–12. [Google Scholar] [CrossRef]
- Nazari, E.; Suja, F. Effects of 17β-Estradiol (E2) on Aqueous Organisms and Its Treatment Problem: A Review. Rev. Environ. Health 2016, 31, 465–491. [Google Scholar] [CrossRef]
- Starling, M.C.V.M.; Amorim, C.C.; Leão, M.M.D. Occurrence, Control and Fate of Contaminants of Emerging Concern in Environmental Compartments in Brazil. J. Hazard. Mater. 2019, 372, 17–36. [Google Scholar] [CrossRef]
- Moraes, F.C.; Rossi, B.; Donatoni, M.C.; de Oliveira, K.T.; Pereira, E.C. Sensitive Determination of 17β-Estradiol in River Water Using a Graphene Based Electrochemical Sensor. Anal. Chim. Acta 2015, 881, 37–43. [Google Scholar] [CrossRef]
- Tseng, T.T.C.; Gusviputri, A.; Hoa, L.N.Q. A Simple, Sensitive and Compact Electrochemical ELISA for Estradiol Based on Chitosan Deposited Platinum Wire Microelectrodes. J. Electroanal. Chem. 2015, 758, 59–67. [Google Scholar] [CrossRef]
- Song, X.; Wen, Y.; Wang, Y.; Adeel, M.; Yang, Y. Environmental Risk Assessment of the Emerging EDCs Contaminants from Rural Soil and Aqueous Sources: Analytical and Modelling Approaches. Chemosphere 2018, 198, 546–555. [Google Scholar] [CrossRef]
- Mafra, G.; Spudeit, D.; Brognoli, R.; Merib, J.; Carasek, E. Expanding the Applicability of Cork as Extraction Phase for Disposable Pipette Extraction in Multiresidue Analysis of Pharmaceuticals in Urine Samples. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2018, 1102–1103, 159–166. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, M.; Jia, N.; Zhai, C.; Han, Y.; Yan, H. Graphene/Multi-Walled Carbon Nanotubes as an Adsorbent for Pipette-Tip Solid-Phase Extraction for the Determination of 17β-Estradiol in Milk Products. J. Chromatogr. A 2019, 1600, 73–79. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, L.; Liu, L.; Kuang, H.; Xu, C. Colloidal Gold-Based Immunochromatographic Strip Assay for the Rapid Detection of Three Natural Estrogens in Milk. Food Chem. 2018, 259, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Kellermann, G. Simultaneous Determination of Three Estrogens in Human Saliva without Derivatization or Liquid-Liquid Extraction for Routine Testing via Miniaturized Solid Phase Extraction with LC-MS/MS Detection. Talanta 2018, 178, 464–472. [Google Scholar] [CrossRef]
- Xie, P.; Liu, Z.; Huang, S.; Chen, J.; Yan, Y.; Li, N.; Zhang, M.; Jin, M.; Shui, L. A Sensitive Electrochemical Sensor Based on Wrinkled Mesoporous Carbon Nanomaterials for Rapid and Reliable Assay of 17β-Estradiol. Electrochim. Acta 2022, 408, 139960. [Google Scholar] [CrossRef]
- Nunes da Silva, D.; Leijoto de Oliveira, H.; Borges, K.B.; Pereira, A.C. Sensitive Determination of 17β-Estradiol Using a Magneto Sensor Based on Magnetic Molecularly Imprinted Polymer. Electroanalysis 2021, 33, 506–514. [Google Scholar] [CrossRef]
- Bergamin, B.; Pupin, R.R.; Wong, A.; Sotomayor, M.D.P.T. A New Electrochemical Platform Based on a Polyurethane Composite Electrode Modified with Magnetic Nanoparticles Coated with Molecularly Imprinted Polymer for the Determination of Estradiol Valerate in Different Matrices. J. Braz. Chem. Soc. 2019, 30, 2344–2354. [Google Scholar] [CrossRef]
- Masikini, M.; Ghica, M.E.; Baker, P.G.L.; Iwuoha, E.I.; Brett, C.M.A. Electrochemical Sensor Based on Multi-Walled Carbon Nanotube/Gold Nanoparticle Modified Glassy Carbon Electrode for Detection of Estradiol in Environmental Samples. Electroanalysis 2019, 31, 1925–1933. [Google Scholar] [CrossRef]
- Musa, A.M.; Kiely, J.; Luxton, R.; Honeychurch, K.C. An Electrochemical Screen-Printed Sensor Based on Gold-Nanoparticle-Decorated Reduced Graphene Oxide–Carbon Nanotubes Composites for the Determination of 17-β Estradiol. Biosensors 2023, 13, 491. [Google Scholar] [CrossRef]
- Scala-Benuzzi, M.L.; Soler-Illia, G.J.A.A.; Raba, J.; Battaglini, F.; Schneider, R.J.; Pereira, S.V.; Messina, G.A. Immunosensor Based on Porous Gold and Reduced Graphene Platform for the Determination of EE2 by Electrochemical Impedance Spectroscopy. J. Electroanal. Chem. 2021, 897, 115604. [Google Scholar] [CrossRef]
- Spychalska, K.; Baluta, S.; Świst, A.; Cabaj, J. Biosensors for Β17-Estradiol Detection Based on Graphene Quantum Dots (GQDs)/Conducting Polymer and Laccase Modified Platinum/Gold Electrodes. Int. J. Electrochem. Sci. 2020, 15, 3127–3142. [Google Scholar] [CrossRef]
- Chen, X.; Shi, Z.; Hu, Y.; Xiao, X.; Li, G. A Novel Electrochemical Sensor Based on Fe3O4-Doped Nanoporous Carbon for Simultaneous Determination of Diethylstilbestrol and 17β-Estradiol in Toner. Talanta 2018, 188, 81–90. [Google Scholar] [CrossRef]
- Moreira, F.; Santana, E.R.; Spinelli, A. Ionic Liquid-Supported Magnetite Nanoparticles as Electrode Modifier Materials for Estrogens Sensing. Sci. Rep. 2020, 10, 1955. [Google Scholar] [CrossRef] [PubMed]
- Lahcen, A.A.; Baleg, A.A.; Baker, P.; Iwuoha, E.; Amine, A. Synthesis and Electrochemical Characterization of Nanostructured Magnetic Molecularly Imprinted Polymers for 17-β-Estradiol Determination. Sens. Actuators B Chem. 2017, 241, 698–705. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Li, P.; Huang, Y.; Wang, J.; Zhang, J. Highly Sensitive Fe3O4 Nanobeads/Graphene-Based Molecularly Imprinted Electrochemical Sensor for 17β-Estradiol in Water. Anal. Chim. Acta 2015, 884, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Adarakatti, P.S.; Kempahanumakkagari, S.K. Modified Electrodes for Sensing. SPR Electrochemistry 2019, 15, 58–95. [Google Scholar] [CrossRef]
- Zaib, M.; Athar, M.M.; Saeed, A.; Farooq, U. Electrochemical Determination of Inorganic Mercury and Arsenic-A Review. Biosens. Bioelectron. 2015, 74, 895–908. [Google Scholar] [CrossRef]
- Suherman, A.L.; Ngamchuea, K.; Tanner, E.E.L.; Sokolov, S.V.; Holter, J.; Young, N.P.; Compton, R.G. Electrochemical Detection of Ultratrace (Picomolar) Levels of Hg2+ Using a Silver Nanoparticle-Modified Glassy Carbon Electrode. Anal. Chem. 2017, 89, 7166–7173. [Google Scholar] [CrossRef]
- Šišoláková, I.; Hovancová, J.; Oriňaková, R.; Oriňak, A.; Trnková, L.; Třísková, I.; Farka, Z.; Pastucha, M.; Radoňák, J. Electrochemical Determination of Insulin at CuNPs/Chitosan-MWCNTs and CoNPs/Chitosan-MWCNTs Modified Screen Printed Carbon Electrodes. J. Electroanal. Chem. 2020, 860, 113881. [Google Scholar] [CrossRef]
- Wong, A.; Silva, T.A.; Vicentini, F.C.; Fatibello-Filho, O. Electrochemical Sensor Based on Graphene Oxide and Ionic Liquid for Ofloxacin Determination at Nanomolar Levels. Talanta 2016, 161, 333–341. [Google Scholar] [CrossRef]
- Üğe, A.; Koyuncu Zeybek, D.; Zeybek, B. An Electrochemical Sensor for Sensitive Detection of Dopamine Based on MWCNTs/CeO 2 -PEDOT Composite. J. Electroanal. Chem. 2018, 813, 134–142. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.; Wang, H.; Wu, Y.; Chan, K.C.; Lu, Z. Sandwich Nanoporous Framework Decorated with Vertical CuO Nanowire Arrays for Electrochemical Glucose Sensing. Electrochim. Acta 2019, 299, 470–478. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Adekunle, A.S.; Kumara Swamy, B.E.; Ebenso, E.E. Electrochemical Sensor for the Detection of Dopamine in Real Samples Using Polyaniline/NiO, ZnO, and Fe3O4 Nanocomposites on Glassy Carbon Electrode. J. Electroanal. Chem. 2018, 818, 236–249. [Google Scholar] [CrossRef]
- Rajaji, U.; Muthumariyappan, A.; Chen, S.M.; Chen, T.W.; Ramalingam, R.J. A Novel Electrochemical Sensor for the Detection of Oxidative Stress and Cancer Biomarker (4-Nitroquinoline N-Oxide) Based on Iron Nitride Nanoparticles with Multilayer Reduced Graphene Nanosheets Modified Electrode. Sens. Actuators B Chem. 2019, 291, 120–129. [Google Scholar] [CrossRef]
- Mao, Y.; Fan, Q.; Li, J.; Yu, L.; Qu, L.B. A Novel and Green CTAB-Functionalized Graphene Nanosheets Electrochemical Sensor for Sudan i Determination. Sens. Actuators B Chem. 2014, 203, 759–765. [Google Scholar] [CrossRef]
- Zahirifar, F.; Rahimnejad, M.; Abdulkareem, R.A.; Najafpour, G. Determination of Diazinon in Fruit Samples Using Electrochemical Sensor Based on Carbon Nanotubes Modified Carbon Paste Electrode. Biocatal. Agric. Biotechnol. 2019, 20, 101245. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Zhu, Z. A Novel Electrochemical Sensor Based on Carbon Nanotubes Array for Selective Detection of Dopamine or Uric Acid. Talanta 2019, 201, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Sgobba, V.; Guldi, D.M. Carbon Nanotubes—Electronic/Electrochemical Properties and Application for Nanoelectronics and Photonics. Chem. Soc. Rev. 2009, 38, 165–184. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Zheng, Y.; Huang, R.; Yao, J. Facile and Efficient Synthesis of A-Fe2O3 Nanocrystals by Glucose-Assisted Thermal Decomposition Method and Its Application in Lithium Ion Batteries. J. Power Sources 2019, 416, 62–71. [Google Scholar] [CrossRef]
- Quddus, M.; Rahman, M.; Khanam, J.; Biswas, B.; Sharmin, N.; Ahmed, S.; Neger, A. Synthesis and Characterization of Pigment Grade Red Iron Oxide from Mill Scale. Int. Res. J. Pure Appl. Chem. 2018, 16, 1–9. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Meng, D.; Qin, X.; Diao, G. Hydrothermal Synthesis of Hematite Nanoparticles and Their Electrochemical Properties. J. Phys. Chem. C 2012, 116, 16276–16285. [Google Scholar] [CrossRef]
- Campos, E.A.; Pinto, D.V.B.S.; de Oliveira, J.I.S.; da Costa Mattos, E.; Dutra, R.d.C.L. Synthesis, Characterization and Applications of Iron Oxide Nanoparticles—A Short Review. J. Aerosp. Technol. Manag. 2015, 7, 267–276. [Google Scholar] [CrossRef]
- Wasag, J.; Grabarczyk, M. Copper Film Modified Glassy Carbon Electrode and Copper Film with Carbon Nanotubes Modified Screen-Printed Electrode for the Cd(Ii) Determination. Materials 2021, 14, 5148. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; El Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.G.; Barhoum, A. T-Butyl Calixarene/Fe2O3@MWCNTs Composite-Based Potentiometric Sensor for Determination of Ivabradine Hydrochloride in Pharmaceutical Formulations. Mater. Sci. Eng. C 2020, 116, 111110. [Google Scholar] [CrossRef]
- Cai, X.-Q.; Zhu, K.; Liu, B.-T.; Zhang, Q.-Y.; Luo, Y.-H.; Zhang, D.-E. γ-Fe2O3/CNTs Composites for Electrochemical Detection of Paracetamol: Synthesis, Phase Transition and Enhanced Properties. J. Electrochem. Soc. 2021, 168, 057511. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Zhang, Y.; Mu, X.; Huang, B.; Du, J.; Zhou, J.; Pan, X.; Xie, E. Carbon Nanotube/Hematite Core/Shell Nanowires on Carbon Cloth for Supercapacitor Anode with Ultrahigh Specific Capacitance and Superb Cycling Stability. Chem. Eng. J. 2017, 325, 221–228. [Google Scholar] [CrossRef]
- Nate, Z.; Gill, A.A.S.; Shinde, S.; Chauhan, R.; Inamdar, S.N.; Karpoormath, R. A Simple In-Situ Flame Synthesis of Nanocomposite (MWCNTs-Fe2O3) for Electrochemical Sensing of Proguanil in Pharmaceutical Formulation. Diam. Relat. Mater. 2021, 111, 108178. [Google Scholar] [CrossRef]
- Özcan, A.; Topçuoğulları, D. Voltammetric Determination of 17-Β-Estradiol by Cysteamine Self-Assembled Gold Nanoparticle Modified Fumed Silica Decorated Graphene Nanoribbon Nanocomposite. Sens. Actuators B Chem. 2017, 250, 85–90. [Google Scholar] [CrossRef]
- Antoniazzi, C.; de Lima, C.A.; Marangoni, R.; Spinelli, A.; de Castro, E.G. Voltammetric Determination of 17β-Estradiol in Human Urine and Buttermilk Samples Using a Simple Copper(II) Oxide-Modified Carbon Paste Electrode. J. Solid. State Electrochem. 2018, 22, 1373–1383. [Google Scholar] [CrossRef]
- Liu, X.; Deng, K.; Wang, H.; Li, C.; Zhang, S.; Huang, H. Aptamer Based Ratiometric Electrochemical Sensing of 17β-Estradiol Using an Electrode Modified with Gold Nanoparticles, Thionine, and Multiwalled Carbon Nanotubes. Microchimica Acta 2019, 186, 347. [Google Scholar] [CrossRef]
- Mustafa, S.; Tasleem, S.; Naeem, A. Surface Charge Properties of Fe2O3 in Aqueous and Alcoholic Mixed Solvents. J. Colloid. Interface Sci. 2004, 275, 523–529. [Google Scholar] [CrossRef]
- Vega, D.; Agüí, L.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical Detection of Phenolic Estrogenic Compounds at Carbon Nanotube-Modified Electrodes. Talanta 2007, 71, 1031–1038. [Google Scholar] [CrossRef]
- Ngundi, M.M.; Sadik, O.A.; Yamaguchi, T.; Suye, S.-I. First Comparative Reaction Mechanisms of B-Estradiol and Selected Environmental Hormones in a Redox Environment. Electrochem. Commun 2003, 5, 61–67. [Google Scholar] [CrossRef]
- Gosser, D.K. Cyclic Voltammetry: Simulation and Analysis of Reaction Mechanisms, 1st ed.; Wiley–Blackwell: New York, NY, USA, 1993; ISBN 9781560810261. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001; ISBN 9781119524113. [Google Scholar]
- Spychalska, K.; Zając, D.; Cabaj, J. Electrochemical Biosensor for Detection of 17β-Estradiol Using Semi-Conducting Polymer and Horseradish Peroxidase. RSC Adv. 2020, 10, 9079–9087. [Google Scholar] [CrossRef] [PubMed]
- Terui, N.; Fugetsu, B.; Tanaka, S. Voltammetric Behavior and Determination of 17b-Estradiol at Multi-Wall Carbon Nanotube-Nafion Modified Glassy Carbon Electrode. Anal. Sci. 2006, 22, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Santos, A.M.; Fava, E.L.; Fatibello-Filho, O.; Sotomayor, M.D.P.T. Voltammetric Determination of 17β-Estradiol in Different Matrices Using a Screen-Printed Sensor Modified with CuPc, Printex 6L Carbon and Nafion Film. Microchem. J. 2019, 147, 365–373. [Google Scholar] [CrossRef]
- Brocenschi, R.F.; Rocha-Filho, R.C.; Duran, B.; Swain, G.M. The Analysis of Estrogenic Compounds by Flow Injection Analysis with Amperometric Detection Using a Boron-Doped Diamond Electrode. Talanta 2014, 126, 12–19. [Google Scholar] [CrossRef]
- Yilmaz, B.; Kadioglu, Y. Determination of 17 β-Estradiol in Pharmaceutical Preparation by UV Spectrophotometry and High Performance Liquid Chromatography Methods. Arab. J. Chem. 2017, 10, S1422–S1428. [Google Scholar] [CrossRef]
- Neto, V. Synthesis of Iron-Containing Composites: Characterization and Thin Films for Application in Photoelectrocatalysis. Ph.D Thesis, State University of Londrina, Londrina, Brazil, 2020. [Google Scholar]
- Janegitz, B.C.; Pauliukaite, R.; Ghica, M.E.; Brett, C.M.A.; Fatibello-Filho, O. Direct Electron Transfer of Glucose Oxidase at Glassy Carbon Electrode Modified with Functionalized Carbon Nanotubes within a Dihexadecylphosphate Film. Sens. Actuators B Chem. 2011, 158, 411–417. [Google Scholar] [CrossRef]
- Laube, N.; Mohr, B.; Hesse, A. Laser-Probe-Based Investigation of the Evolution of Particle Size Distributions of Calcium Oxalate Particles Formed in Artificial Urines. J. Cryst. Growth 2001, 233, 367–374. [Google Scholar] [CrossRef]
- ICH Expert Working Group. Validation Of Analytical Procedures: Text and Methodology Q2(R1). Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 16 July 2023).







| Sensor | Technique | Linear Range (mol L−1) | LOD (mol L−1) | Reference |
|---|---|---|---|---|
| RGO-CuTthP/GCE a | DPV | 1.0 × 10−7–1.0 × 10−6 | 5.3 × 10−9 | [6] |
| wMC/GCE b | DPV | 5.0 × 10−8–1.0 × 10−5 | 8.3 × 10−9 | [13] |
| MMIP/MCPEc c | DPAdSV | 6.0 × 10−8–1.8 × 10−4 | 2.0 × 10−8 | [14] |
| mag-MIP/GEC d | SWV | 5.0 × 10−8–7.5 × 10−4 | 1.0 × 10−8 | [15] |
| AuNP-MWCNT/GCE e | LSV | 1.0 × 10−6–2.0 × 10−5 | 7.0 × 10−9 | [16] |
| rGO-AuNPs/CNT/SPE f | DPV | 5.0 × 10−8–1.0 × 10−6 | 3.0 × 10−9 | [17] |
| Ab-Au-RG-SPCE g | EIS j | 0.0–1.2 × 10−7 | 1.5 × 10−9 | [18] |
| LAC-CP/Pt h | CV | 1.0 × 10−7–1.2 × 10−4 | 9.9 × 10−7 | [19] |
| LAC-GQDs/Au i | CV | 5.0 × 10−6–5.0 × 10−5 | 1.5 × 10−6 | |
| CuO/CPE j | SWV | 6.0 × 10−8–8.0 × 10−7 | 2.1 × 10−8 | [47] |
| AuNP-Thi-CNTs/GCE k | DPV | 1.2 × 10−11–6.0 × 10−8 | 1.5 × 10−12 | [48] |
| Fe3O4-NC/GCE l | DPV | 1.0 × 10−8–2.0 × 10−5 | 4.9 × 10−9 | [20] |
| Fe3O4 NPs-BMI.PF6/CPE m | SWV | 1.0 × 10−7–1.0 × 10−6 | 5.0 × 10−8 | [21] |
| HRP-Pol/Pt n | DPV | 1.0 × 10−7–2.0 × 10−4 | 1.0 × 10−7 | [54] |
| Fe3O4-MIP/SPCE o | SWV | 5.0 × 10−8–1.0 × 10−5 | 2.0 × 10−8 | [22] |
| Fe3O4-MIP@RGO/GCE p | DPV | 5.0 × 10−8–1.0 × 10−5 | 8.2 × 10−10 | [23] |
| MWCNT/GCE q | SWV | 2.5 × 10−7–1.0 × 10−5 | 1.0 × 10−8 | [55] |
| CuPc-P6LC-Nafion/SPEF r | DPV | 8.0 × 10−8–7.3 × 10−6 | 5.0 × 10−9 | [56] |
| Fe2O3-CNT/GCE | SWV | 5.0 × 10−9–1.0 × 10−7 | 4.4 × 10−9 | This work |
| Sample | Added (µmol L−1) | Found (µmol L−1) | Recovery (%) a |
|---|---|---|---|
| Lake water | 0.05 | 0.052 ± 0.005 | 105 |
| Synthetic urine | 0.05 | 0.050 ± 0.007 | 100 |
| E2 Concentration (mg/Tablet) | ||||
|---|---|---|---|---|
| Sample | Labeled | HPLC Method | Proposed Method | Relative Error (%) a |
| 1 | 1.0 | 0.96 ± 0.02 | 0.95 ± 0.05 | 1.0 |
| 2 | 2.0 | 1.83 ± 0.03 | 1.81 ± 0.02 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvão, J.C.R.; Araujo, M.d.S.; Prete, M.C.; Neto, V.L.; Dall’Antonia, L.H.; Matos, R.; Tarley, C.R.T.; Medeiros, R.A. Electrochemical Determination of 17-β-Estradiol Using a Glassy Carbon Electrode Modified with α-Fe2O3 Nanoparticles Supported on Carbon Nanotubes. Molecules 2023, 28, 6372. https://doi.org/10.3390/molecules28176372
Galvão JCR, Araujo MdS, Prete MC, Neto VL, Dall’Antonia LH, Matos R, Tarley CRT, Medeiros RA. Electrochemical Determination of 17-β-Estradiol Using a Glassy Carbon Electrode Modified with α-Fe2O3 Nanoparticles Supported on Carbon Nanotubes. Molecules. 2023; 28(17):6372. https://doi.org/10.3390/molecules28176372
Chicago/Turabian StyleGalvão, Juliana Costa Rolim, Mayara da Silva Araujo, Maiyara Carolyne Prete, Vanildo Leão Neto, Luiz Henrique Dall’Antonia, Roberto Matos, Cesar Ricardo Texeira Tarley, and Roberta Antigo Medeiros. 2023. "Electrochemical Determination of 17-β-Estradiol Using a Glassy Carbon Electrode Modified with α-Fe2O3 Nanoparticles Supported on Carbon Nanotubes" Molecules 28, no. 17: 6372. https://doi.org/10.3390/molecules28176372
APA StyleGalvão, J. C. R., Araujo, M. d. S., Prete, M. C., Neto, V. L., Dall’Antonia, L. H., Matos, R., Tarley, C. R. T., & Medeiros, R. A. (2023). Electrochemical Determination of 17-β-Estradiol Using a Glassy Carbon Electrode Modified with α-Fe2O3 Nanoparticles Supported on Carbon Nanotubes. Molecules, 28(17), 6372. https://doi.org/10.3390/molecules28176372