The Chemical Composition and Health-Promoting Benefits of Vegetable Oils—A Review
Abstract
:1. Introduction
2. Chemical Composition
2.1. Fatty Acid Composition
2.2. Tocols
2.3. Phytosterol
2.4. Squalene
2.5. Carotenoids
2.6. Total Phenolics
2.7. Phospholipids
3. Health-promoting Benefits of Vegetable Oil
3.1. Antioxidant Activity
3.2. Prevention of Cardiovascular Disease (CVD)
3.3. Anti-Inflammatory
3.4. Anti-Obesity
3.5. Anti-Cancer
3.6. Diabetes Treatment
3.7. Kidney and Liver Protection
3.8. Other Health Benefits
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Oils | Lauric C12:0 | Myristic C14:0 | Pentadecanoic C15:0 | Palmitic C16:0 | Palmitoleic C16:1 | Margaric C17:0 | Stearic C18:0 | Oleic C18:1n-9 | Linoleic C18:2n-6 | Linolenic C18:3n-3 | Arachidic C20:0 | Gadoleic C20:1 | Behenic C22:0 | Lignoceric C24:0 | Others | SFA | MUFA | PUFA | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soybean oil | - | 0.12 | - | 12.20 | 0.16 | 0.04 | 6.50 | 29.10 | 53.33 | 7.65 | 0.32 | 0.22 | 0.27 | 0.13 | - | 16.18 | 23.88 | 60.98 | [15] |
| Rapeseed oil | - | 0.04 | 0.02 | 4.60 | 0.23 | 0.07 | 1.70 | 63.44 | 20.65 | 8.71 | 0.87 | 9.10 | 0.27 | 0.04 | - | 7.52 | 72.77 | 29.36 | [15] |
| Palm oil | - | 0.88 | 0.03 | 38.08 | 0.14 | - | 4.50 | 45.10 | 9.01 | 0.14 | 0.39 | 0.17 | 0.06 | 0.07 | 0.89 | 44.15 | 46.30 | 9.38 | [24] |
| Peanut oil | - | 0.04 | - | 7.50 | 0.07 | 0.07 | 2.10 | 71.10 | 18.20 | 0.12 | 1.01 | - | - | - | - | 10.70 | 71.10 | 18.20 | [16] |
| Sunflower oil | - | 0.09 | 0.02 | 6.35 | 0.12 | 0.02 | 3.92 | 28.00 | 67.58 | 0.17 | 0.22 | 0.18 | 0.66 | 0.26 | - | 11.54 | 28.30 | 67.75 | [15] |
| Cottonseed oil | - | 0.80 | - | 24.20 | 0.70 | - | 2.30 | 17.40 | 53.20 | 0.20 | 0.20 | 0.10 | 0.10 | 0.10 | - | 27.80 | 18.20 | 53.40 | [17] |
| Corn oil | - | - | - | 12.94 | 1.70 | - | 2.12 | 31.97 | 48.97 | 0.76 | 0.68 | - | 0.39 | 0.47 | - | 16.60 | 33.67 | 49.74 | [12] |
| Camellia oil | - | - | - | 13.80 | 0.06 | - | 3.10 | 72.50 | 9.50 | 0.60 | 0.36 | - | - | - | - | 17.26 | 72.50 | 10.10 | [9] |
| Coconut oil | 47.70 | 19.90 | - | - | - | - | 2.70 | 6.20 | 1.60 | - | - | - | - | - | 13.62 | 92.10 | 6.20 | 1.60 | [16] |
| Olive oil | - | 0.08 | - | 16.50 | 1.80 | 0.07 | 2.79 | 74.52 | 16.40 | 1.60 | 0.49 | 0.30 | 0.16 | 0.17 | - | 20.19 | 76.62 | 18.00 | [15] |
| Flaxseed oil | - | 0.05 | 0.01 | 6.42 | 0.20 | 0.04 | 5.96 | 22.56 | 15.88 | 61.06 | 0.20 | 0.21 | 0.14 | 0.13 | 0.12 | 12.90 | 23.00 | 76.94 | [15] |
| Jackfruit seed oil | - | - | - | 36.20 | - | - | 3.54 | 4.15 | 35.11 | 2.82 | - | - | - | - | 49.13 | 4.15 | 46.72 | [123] | |
| Papaya seed oil | - | 0.21 | - | 13.70 | 0.11 | - | 5.63 | 75.89 | 3.62 | 0.34 | 0.38 | 0.10 | - | - | 19.92 | 76.10 | 3.96 | [14] | |
| Avocado seed oil | - | - | - | 11.62 | 5.91 | - | 0.12 | 67.80 | 13.67 | 0.11 | - | - | - | - | 11.74 | 73.71 | 13.78 | [124] | |
| Pomegranate seed oil | - | - | - | 2.41 | - | - | 2.42 | 6.18 | 5.54 | 46.27 | 0.52 | - | - | - | 36.66 | 5.35 | 6.79 | 87.87 | [13] |
| Cheery oil | - | - | - | 7.60 | 0.20 | - | 5.20 | 39.40 | 46.20 | 0.10 | - | - | - | - | 12.80 | 39.60 | 46.30 | [125] | |
| Sweet cherry seed oil | 0.13 | - | - | 9.05 | 0.47 | - | 3.02 | 35.05 | 41.45 | - | - | - | - | - | 12.20 | 39.49 | 44.32 | [126] | |
| Sour cherry seed oil | - | 0.02 | - | 4.92 | 0.29 | 0.05 | 1.60 | 37.89 | 42.42 | 0.11 | 0.64 | 0.31 | 0.15 | 0.08 | 7.46 | 38.49 | 54.05 | [127] | |
| Custard-apple seed oil | - | - | - | 14.86 | - | - | 8.18 | 51.04 | 23.06 | 1.90 | - | - | - | - | 23.04 | 51.04 | 24.96 | [128] | |
| Cress oil | - | 0.13 | - | 10.13 | 0.20 | - | 2.84 | 22.45 | 12.61 | 36.25 | 2.06 | 9.88 | 0.33 | 0.02 | 15.51 | 35.06 | 48.86 | [129] | |
| Mustard oil | - | - | - | 2.19 | 0.17 | - | 1.17 | 10.16 | 15.58 | 11.70 | 0.98 | 5.48 | 1.40 | 1.18 | 5.73 | 66.98 | 27.28 | [12] | |
| Walnut oil | - | 0.03 | - | 7.35 | 0.15 | 0.10 | 3.00 | 17.20 | 60.30 | 12.00 | 0.04 | 0.20 | 0.03 | 0.02 | 10.57 | 17.55 | 72.30 | [130] | |
| Wheat germ oil | 0.07 | - | 0.04 | 17.40 | 0.21 | 0.03 | 0.70 | 12.70 | 59.70 | 1.20 | 7.91 | - | - | - | - | 18.20 | 20.90 | 61.00 | [16] |
| Safflower oil | - | 0.10 | - | 6.70 | 0.08 | 0.04 | 2.40 | 11.5 | 79.00 | 0.15 | 0.43 | 0.13 | 0.75 | 0.12 | - | 9.30 | 11.60 | 79.10 | [16] |
| Grape seed oil | - | - | - | 7.20 | - | - | 4.80 | 19.90 | 68.10 | 0.10 | - | - | - | - | 12.00 | 19.90 | 68.20 | [131] | |
| Pumpkin seed oil | - | - | - | 17.58 | - | - | 7.62 | 25.54 | 47.45 | 0.69 | - | - | - | - | 25.20 | 25.54 | 48.14 | [126] | |
| Sesame oil | - | - | - | 9.70 | 0.11 | - | 6.50 | 41.50 | 40.90 | 0.21 | 0.63 | 0.40 | 0.14 | 0.32 | 16.90 | 42.00 | 41.20 | [16] | |
| Rice bran oil | - | 0.35 | - | 19.34 | 0.29 | - | 2.00 | 43.42 | 32.04 | 0.59 | 1.09 | - | 0.33 | 0.51 | 23.63 | 43.71 | 32.66 | [12] | |
| Almond oil | - | - | - | 6.58 | 0.48 | - | 1.64 | 76.59 | 21.86 | 0.73 | 0.13 | - | - | - | 8.35 | 77.07 | 22.59 | [132] | |
| Date Palm Seed Oil | 17.26 | 10.74 | 0.04 | 9.88 | 0.06 | 0.05 | 2.82 | 48.67 | 8.13 | 0.03 | 0.37 | 0.44 | 0.27 | 0.14 | 1.09 | 42.22 | 49.59 | 8.16 | [24] |
| Evening Primrose oil | - | - | 6.00 | 0.10 | 1.60 | 8.30 | 73.80 | 0.10 | 0.30 | 0.30 | 0.10 | - | 10.30 | 8.10 | 9.40 | 83.40 | [17] | ||
| Perilla seed oil | - | 0.02 | - | 6.33 | 0.12 | 0.03 | 1.70 | 12.61 | 18.30 | 59.87 | 0.11 | 0.13 | 0.02 | 0.01 | 8.22 | 12.89 | 76.25 | [133] | |
| Eucommia ulmoides Oliver seed oil | - | 0.09 | - | 9.82 | 0.3 | 0.11 | 2.59 | 16.86 | 12.66 | 56.79 | 0.3 | 0.18 | 0.19 | 0.11 | - | 13.21 | 17.34 | 69.45 | [9] |
| Peony seed oil | - | - | - | 5.80 | - | - | 1.97 | 21.76 | 24.60 | 44.38 | - | - | - | - | 8.17 | 22.37 | 69.46 | [134] | |
| Sea buckthorn seed oil | - | 0.11 | - | 8.73 | 0.61 | - | 2.58 | 22.33 | 31.6 | 28.24 | 0.45 | - | - | - | - | 11.87 | 22.94 | 59.84 | [9] |
| Acer truncatum Bunge seed oil | - | - | - | 4.19 | 0.18 | - | 2.4 | 25.8 | 37.35 | 1.85 | 0.25 | 8.23 | 0.78 | 0.32 | 18.60 | 7.94 | 52.81 | 39.20 | [9] |
| Torreya grandis seed oil | - | - | - | 9.18 | - | 0.07 | 2.35 | 22.89 | 45.39 | 0.81 | 0.14 | 1.54 | - | - | 11.74 | 24.43 | 63.44 | [135] | |
| Milk thistle seed oil | - | 0.07 | - | 6.25 | 18.98 | 0.08 | 4.03 | 15.78 | 48.70 | 0.11 | 2.33 | 0.72 | 1.86 | 0.40 | - | 15.02 | 35.94 | 48.81 | [136] |
| Tomato seed oil | - | - | - | 17.14 | - | - | 5.21 | 21.79 | 53.7 | - | 2.13 | - | - | - | - | 24.48 | 21.79 | 53.70 | [9] |
| Oils (mg/kg) | α-Tocopherol | β-Tocopherol | γ-Tocopherol | δ-Tocopherol | α-Tocotrienol | β-Tocotrienol | γ-Tocotrienol | δ-Tocotrienol | Total | α-TE (mg) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Soybean oil | 101.6 | 9.1 | 1639.7 | 304.7 | - | - | - | - | 1774.6 | 181.2 | [20,22] |
| Rapeseed oil | 267.0 | - | 398.0 | 23.0 | - | - | - | - | 718.0 | 235.4 | [35] |
| Palm oil | 151.2 | 4.8 | 19.6 | - | 203.6 | - | 303.2 | 163.9 | 843.6 | 297.0 | [24] |
| Palm kernel oil | 13.0 | - | - | - | 21.0 | - | - | - | 34.0 | 19.3 | [22] |
| Peanut oil | 36.9 | 21.8 | 188.0 | 13.0 | - | - | - | - | 259.6 | 178.9 | [20] |
| Sunflower oil | 570.5 | 95.8 | 56.2 | - | - | - | - | - | 722.5 | 645.9 | [20] |
| Cottonseed oil | 386.2 | 7.8 | 380.7 | 2.3 | - | - | - | - | 777.0 | 428.1 | [22] |
| Corn oil | 317.1 | 39.9 | 518.7 | 28.6 | - | - | - | - | 886.5 | - | [9,137] |
| Rice bran oil | 299.5 | 14.7 | 73.6 | 10.0 | 518.1 | - | 823.4 | 48.9 | 1556.8 | - | [9,20] |
| Coconut oil | 4.0 | - | 3.0 | 3.0 | 8.1 | 1.0 | 14.3 | 2.0 | 35.0 | 5.9 | [20,22] |
| Olive oil | 290.0 | 10.0 | 10.0 | 1.0 | - | - | - | - | 311.0 | 142.5 | [22,138] |
| Date Palm Seed Oil | 124.0 | 16.8 | 167.8 | 5.3 | 213.6 | - | 134.9 | 44.8 | 707.5 | - | [24] |
| Sesame oil | 6.1 | 27.1 | 724.1 | 10.4 | - | - | 13.5 | - | 775.0 | 52.5 | [20,22] |
| Camellia oil | 378.0 | - | 26.0 | 12.0 | - | - | - | - | 416.0 | - | [18] |
| Perilla seed oil | 23.0 | 2.0 | 831.0 | 14.0 | - | - | - | - | 870.0 | - | [133] |
| Safflower oil | 451.2 | 7.0 | 32.9 | 7.0 | - | - | - | - | 498.0 | - | [22] |
| Grape seed oil | 67.0 | 2.1 | 7.3 | 0.24 | 521.1 | 7.7 | 132.2 | 18.3 | 755.8 | - | [139] |
| Peony seed oil | 12.1 | - | 484.2 | 25.6 | - | - | - | - | 521.9 | - | [9] |
| Torreya grandis seed oil | 30.0 | 510.0 | 30.0 | - | - | - | - | - | 570.0 | - | [9] |
| Walnut oil | 13.9 | - | 169.2 | 26.3 | - | - | - | - | 209.4 | - | [9] |
| Sea buckthorn seed oil | 325.0 | 62.7 | 459.1 | 51.3 | - | - | - | - | 898.1 | - | [9] |
| Evening Primrose oil | 118.6 | - | 273.3 | - | - | - | - | - | 391.9 | - | [9] |
| Almond oil | 179.6 | 21.9 | 8.0 | - | 0.9 | - | - | - | 210.4 | - | [132] |
| Cress oil | 80.0 | 60.0 | 1550.0 | 190.0 | - | - | - | - | 1754.0 | - | [138] |
| Eucommia ulmoides Oliver seed oil | 8.2 | 6.3 | 906.2 | 146.9 | - | - | - | - | 1067.6 | - | [9] |
| Flaxseed oil | 21.1 | 1.7 | 407.0 | 14.6 | - | - | - | - | 553.0 | 44.7 | [9,22] |
| Pomegranate seed oil | 139.0 | - | 3829.0 | 96.0 | - | - | - | - | 5246.0 | - | [19] |
| Milk thistle seed oil | 286.2 | 3.6 | 14.2 | 14.2 | - | - | - | - | 318.3 | - | [136] |
| Tomato seed oil | 23.4 | 49.0 | 136.9 | 136.5 | - | - | - | - | 345.8 | - | [9] |
| Oils (mg/100 g) | Brassicasterol | Ergosterol | Campesterol | Campestanol | Stigmasterol | β-Sitosterol | ∆5-Avenasterol | Δ7-stigmastenol | Cycloartanol | Cycloartenol | Methylene-Cycloartanol | Sitostanol | Total | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soybean oil | 12.80 | 1.98 | 62.68 | 5.91 | 87.28 | 360.03 | 7.21 | 5.10 | 2.72 | 4.71 | 4.34 | - | 448.30 | [20,28] |
| Rapeseed oil | 136.64 | 2.54 | 267.50 | 2.83 | 25.67 | 394.11 | 40.92 | 18.46 | 1.10 | 17.26 | 5.28 | - | 893.84 | [28] |
| palm oil | - | - | 18.89 | - | 6.91 | 55.58 | 1.83 | - | - | - | - | - | 83.20 | [22] |
| Palm kernel oil | - | - | 12.05 | - | 14.02 | 91.96 | 7.99 | 1.05 | - | - | - | - | 131.00 | [22] |
| Peanut oil | - | 1.56 | 41.19 | 4.95 | 48.16 | 189.12 | 19.70 | - | 0.73 | 9.42 | 4.92 | - | 319.75 | [28] |
| Sunflower oil | 0.58 | 0.25 | 28.36 | 1.71 | 18.69 | 324.00 | 12.45 | 63.88 | 0.18 | 8.85 | 11.27 | 18.95 | 453.30 | [20,28] |
| Cottonseed oil | - | - | 34.01 | - | 5.20 | 412.79 | 19.84 | - | - | - | - | - | 472.30 | [22] |
| Corn oil | 4.13 | 0.87 | 197.32 | 74.53 | 45.53 | 661.70 | 97.92 | - | 1.89 | 15.85 | 12.97 | 112.44 | 1032.07 | [28,29] |
| Camellia oil | - | 2.62 | 16.52 | 0.22 | 22.11 | 50.09 | 1.81 | - | 29.11 | 17.84 | 2.33 | 116.97 | 142.64 | [28] |
| Coconut oil | - | - | 11.41 | - | 13.07 | 51.58 | 16.28 | - | - | - | - | - | 97.50 | [22] |
| Olive oil | - | 0.83 | 25.85 | 1.61 | 21.13 | 152.05 | 29.73 | - | 2.79 | 19.44 | 34.58 | - | 288.02 | [28] |
| Flaxseed oil | 1.66 | - | 175.9 | 4.17 | 53.50 | 237.5 | 56.01 | - | 0.99 | 78.67 | 39.29 | 29.59 | 775.20 | [9,28] |
| Sesame oil | - | 2.05 | 90.30 | 7.48 | 86.89 | 555.0 | 98.79 | 5.98 | 0.83 | 23.79 | 4.75 | - | 637.60 | [20,28] |
| Pumpkin seed oil | - | - | 2.30 | - | 1.30 | 182.30 | - | - | - | - | - | - | 185.90 | [140] |
| Perilla seed oil | - | - | 18.70 | - | 10.50 | 318.60 | - | - | - | - | - | - | 347.80 | [9] |
| Safflower oil | - | - | 50.0 | 3.39 | 25.40 | 168.30 | 9.07 | 73.01 | - | - | - | 23.29 | 412.50 | [9,22] |
| Grape seed oil | - | - | 30.40 | - | 47.92 | 230.64 | 10.33 | - | - | - | - | 19.53 | 338.83 | [139] |
| Peony seed oil | 1.77 | 2.65 | 21.32 | 4.25 | 2.57 | 258.71 | 3.64 | - | - | 6.37 | 65.90 | - | 367.30 | [28] |
| Torreya grandis seed oil | - | - | 20.00 | - | 5.90 | 109.30 | - | - | - | - | - | - | 135.20 | [141] |
| Walnut oil | 2.14 | 0.67 | 31.53 | 6.94 | 32.80 | 165.23 | 5.99 | - | 0.44 | 15.33 | 10.97 | - | 272.04 | [28] |
| Sea buckthorn seed oil | - | - | - | - | - | 104.70 | - | - | - | - | - | - | 104.70 | [9] |
| Evening Primrose oil | - | - | 18.20 | - | 29.2 | 44.00 | - | - | - | - | - | - | 91.40 | [9] |
| Almond oil | - | - | 22.61 | - | 19.67 | 171.68 | 20.58 | - | - | - | - | 258.12 | [132] | |
| Rice bran oil | 6.33 | 2.17 | 226.43 | 221.20 | 132.90 | 735.17 | 157.41 | - | 31.08 | 156.25 | 222.88 | - | 1891.82 | [28] |
| Sour cherry seed oil | - | - | 18.70 | 0.40 | 1.00 | 601.83 | 17.74 | - | - | - | - | 18.50 | 678.54 | [127] |
| Milk thistle seed oil | - | - | 24.27 | 2.75 | 30.07 | 166.80 | 15.42 | - | - | 8.50 | 12.21 | - | 508.85 | [136] |
| Pomegranate seed oil | - | - | 36.30 | - | 16.30 | 354.20 | - | - | - | - | - | 23.60 | 552.70 | [19] |
References
- Xu, H.; Zhu, L.; Dong, J.; Wei, Q.; Lei, M. Composition of Catalpa ovata Seed Oil and Flavonoids in Seed Meal as Well as Their Antioxidant Activities. J. Am. Oil Chem. Soc. 2015, 92, 361–369. [Google Scholar] [CrossRef]
- Li, X.; Kong, W.; Shi, W.; Shen, Q. A combination of chemometrics methods and GC-MS for the classification of edible vegetable oils. Chemom. Intell. Lab. Syst. 2016, 155, 145–150. [Google Scholar] [CrossRef]
- Parcell, J. Global Edible Vegetable Oil Market Trends. Biomed. J. Sci. Technol. Res. 2018, 2, 2282–2291. [Google Scholar]
- Zhang, M.; Wang, O.; Cai, S.; Zhao, L.; Zhao, L. Composition, functional properties, health benefits and applications of oilseed proteins: A systematic review. Food Res. Int. 2023, 171, 113061. [Google Scholar] [PubMed]
- Sun, Y.; Neelakantan, N.; Wu, Y.; Lote-Oke, R.; Pan, A.; van Dam, R.M. Palm Oil Consumption Increases LDL Cholesterol Compared with Vegetable Oils Low in Saturated Fat in a Meta-Analysis of Clinical Trials. J. Nutr. 2015, 145, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Usda, E. Oil crops yearbook: Dataset. In Fats and Oils: Production, Consumption and Stocks; USDA Economics, Statistics and Market Information System (ESMIS): Washington, DC, USA, 2023; Volume 2023. [Google Scholar]
- National Bureau of Statistics. Refined Edible Vegetable Oil Production. Available online: https://data.stats.gov.cn/index.htm (accessed on 6 July 2023).
- Sharma, K.; Kumar, M.; Lorenzo, J.M.; Guleria, S.; Saxena, S. Manoeuvring the physicochemical and nutritional properties of vegetable oils through blending. J. Am. Oil Chem. Soc. 2023, 100, 5–24. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, L.; Li, P.; Yu, L.; Mao, J.; Wang, X.; Zhang, Q. A review of chemical composition and nutritional properties of minor vegetable oils in China. Trends Food Sci. Technol. 2018, 74, 26–32. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, B. New insights into chemical compositions and health promoting effects of edible oils from new resources. Food Chem. 2021, 364, 130363. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.; Williamson, C.S.; Lunn, J. Culinary oils and their health effects. Nutr. Bull. 2009, 34, 4–47. [Google Scholar] [CrossRef]
- Dorni, C.; Sharma, P.; Saikia, G.; Longvah, T. Fatty acid profile of edible oils and fats consumed in India. Food Chem. 2018, 238, 9–15. [Google Scholar] [CrossRef]
- Drinic, Z.; Mudric, J.; Zdunic, G.; Bigovic, D.; Menkovic, N.; Savikin, K. Effect of pomegranate peel extract on the oxidative stability of pomegranate seed oil. Food Chem. 2020, 333, 127501. [Google Scholar] [CrossRef]
- Senrayan, J.; Venkatachalam, S. A short extraction time of vegetable oil from Carica papaya L. seeds using continuous ultrasound acoustic cavitation: Analysis of fatty acid profile and thermal behavior. J. Food Process Eng. 2019, 42, e12950. [Google Scholar] [CrossRef]
- Yang, J.; Wen, C.T.; Duan, Y.Q.; Deng, Q.C.; Peng, D.F.; Zhang, H.H.; Ma, H.L. The composition, extraction, analysis, bioactivities, bioavailability and applications in food system of flaxseed (Linum usitatissimum L.) oil: A review. Trends Food Sci. Technol. 2021, 118, 252–260. [Google Scholar]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Dubois, V.; Breton, S.; Linder, M.; Fanni, J.; Parmentier, M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur. J. Lipid Sci. Technol. 2007, 109, 710–732. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Yang, R.; Mao, J.; Jiang, J.; Wang, X.; Zhang, W.; Zhang, Q.; Li, P. Simultaneous determination of tocopherols, carotenoids and phytosterols in edible vegetable oil by ultrasound-assisted saponification, LLE and LC-MS/MS. Food Chem. 2019, 289, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Pereira, J.A.; Lopez-Cortes, I.; Salazar, D.M.; Ramalhosa, E.; Casal, S. Fatty acid, vitamin E and sterols composition of seed oils from nine different pomegranate (Punica granatum L.) cultivars grown in Spain. J. Food Compos. Anal. 2015, 39, 13–22. [Google Scholar] [CrossRef]
- Pokkanta, P.; Sookwong, P.; Tanang, M.; Setchaiyan, S.; Boontakham, P.; Mahatheeranont, S. Simultaneous determination of tocols, gamma-oryzanols, phytosterols, squalene, cholecalciferol and phylloquinone in rice bran and vegetable oil samples. Food Chem. 2019, 271, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Wu, X.; Zhang, Y. Analysis on fatty acid and unsaponifiable matter in Torreya grandis var. Merriii seed oil. J. Chin. Cereals Oils Assoc. 2011, 26, 52–55. [Google Scholar]
- Velasco, L.; Ruiz-Méndez, M.V. Sunflower oil minor constituents. In Sunflower; Elsevier: Amsterdam, The Netherlands, 2015; pp. 297–329. [Google Scholar]
- Tanska, M.; Mikolajczak, N.; Konopka, I. Comparison of the effect of sinapic and ferulic acids derivatives (4-vinylsyringol vs. 4-vinylguaiacol) as antioxidants of rapeseed, flaxseed, and extra virgin olive oils. Food Chem. 2018, 240, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Nehdi, I.A.; Sbihi, H.M.; Tan, C.P.; Rashid, U.; Al-Resayes, S.I. Chemical Composition of Date Palm (Phoenix dactylifera L.) Seed Oil from Six Saudi Arabian Cultivars. J. Food Sci. 2018, 83, 624–630. [Google Scholar] [PubMed]
- Miras-Moreno, B.; Belen Sabater-Jara, A.; Pedreno, M.A.; Almagro, L. Bioactivity of Phytosterols and Their Production in Plant in Vitro Cultures. J. Agric. Food Chem. 2016, 64, 7049–7058. [Google Scholar]
- Bishop, G.J.; Yokota, T. Plants steroid hormones, brassinosteroids: Current highlights of molecular aspects on their synthesis/metabolism, transport, perception and response. Plant Cell Physiol. 2001, 42, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Nes, W.D. Biosynthesis of cholesterol and other sterols. Chem. Rev. 2011, 111, 6423–6451. [Google Scholar]
- Yang, R.; Xue, L.; Zhang, L.; Wang, X.; Qi, X.; Jiang, J.; Yu, L.; Wang, X.; Zhang, W.; Zhang, Q.; et al. Phytosterol Contents of Edible Oils and Their Contributions to Estimated Phytosterol Intake in the Chinese Diet. Foods 2019, 8, 334. [Google Scholar] [PubMed]
- Han, J.; Yang, Y.; Feng, M.; Wang, G. Analysis of phytosterol contents in Chinese plant food and primary estimation of its intake of people. J. Hyg. Res. 2007, 36, 301–305. [Google Scholar]
- Salvo, A.; La Torre, G.L.; Rotondo, A.; Mangano, V.; Casale, K.E.; Pellizzeri, V.; Clodoveo, M.L.; Corbo, F.; Cicero, N.; Dugo, G. Determination of Squalene in Organic Extra Virgin Olive Oils (EVOOs) by UPLC/PDA Using a Single-Step SPE Sample Preparation. Food Anal. Method. 2017, 10, 1377–1385. [Google Scholar] [CrossRef]
- Shen, M.; Zhao, S.; Zhang, F.; Huang, M.; Xie, J. Characterization and authentication of olive, camellia and other vegetable oils by combination of chromatographic and chemometric techniques: Role of fatty acids, tocopherols, sterols and squalene. Eur. Food Res. Technol. 2021, 247, 411–426. [Google Scholar] [CrossRef]
- Feng, S.; Xu, X.; Tao, S.; Chen, T.; Zhou, L.; Huang, Y.; Yang, H.; Yuan, M.; Ding, C. Comprehensive evaluation of chemical composition and health-promoting effects with chemometrics analysis of plant derived edible oils. Food Chem. X 2022, 14, 100341. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Xie, Y.; Jin, R.; Ren, L.; Zhou, L.; Zhu, M.; Ju, Y. Simultaneous Analysis of Tocopherols, Phytosterols, and Squalene in Vegetable Oils by High-Performance Liquid Chromatography. Food Anal. Method. 2017, 10, 3716–3722. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, C.; Zhou, Q.; Huang, F.; Liu, C.; Wang, H. Minor components and oxidative stability of cold-pressed oil from rapeseed cultivars in China. J. Food Compos. Anal. 2013, 29, 1–9. [Google Scholar] [CrossRef]
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [PubMed]
- Diwakar, B.T.; Dutta, P.K.; Lokesh, B.R.; Naidu, K.A. Physicochemical Properties of Garden Cress (Lepidium sativum L.) Seed Oil. J. Am. Oil Chem. Soc. 2010, 87, 539–548. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [PubMed]
- Fine, F.; Brochet, C.; Gaud, M.; Carre, P.; Simon, N.; Ramli, F.; Joffre, F. Micronutrients in vegetable oils: The impact of crushing and refining processes on vitamins and antioxidants in sunflower, rapeseed, and soybean oils. Eur. J. Lipid Sci. Technol. 2016, 118, 680–697. [Google Scholar] [CrossRef]
- Siger, A.; Nogala-Kalucka, M.; Lampart-Szczapa, E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- Jayathilaka, N.; Seneviratne, K.N. Phenolic antioxidants in coconut oil: Factors affecting the quantity and quality. A review. Grasas Aceites 2022, 73, e466. [Google Scholar] [CrossRef]
- Cicerale, S.; Conlan, X.A.; Sinclair, A.J.; Keast, R.S.J. Chemistry and Health of Olive Oil Phenolics. Crit. Rev. Food Sci. Nutr. 2009, 49, 218–236. [Google Scholar] [CrossRef]
- Kraljic, K.; Skevin, D.; Barisic, L.; Kovacevic, M.; Obranovic, M.; Jurcevic, I. Changes in 4-vinylsyringol and other phenolics during rapeseed oil refining. Food Chem. 2015, 187, 236–242. [Google Scholar]
- Wang, X.Q.; Zeng, Q.M.; Contreras, M.D.; Wang, L.J. Profiling and quantification of phenolic compounds in Camellia seed oils: Natural tea polyphenols in vegetable oil. Food Res. Int. 2017, 102, 184–194. [Google Scholar] [CrossRef]
- Appaiah, P.; Sunil, L.; Kumar, P.K.P.; Krishna, A.G.G. Composition of Coconut Testa, Coconut Kernel and its Oil. J. Am. Oil Chem. Soc. 2014, 91, 917–924. [Google Scholar]
- Mikolajczak, N.; Tanska, M.; Ogrodowska, D. Phenolic compounds in plant oils: A review of composition, analytical methods, and effect on oxidative stability. Trends Food Sci. Technol. 2021, 113, 110–138. [Google Scholar]
- Zhao, X.; Xiang, X.; Huang, J.; Ma, Y.; Sun, J.; Zhu, D. Studying the Evaluation Model of the Nutritional Quality of Edible Vegetable Oil Based on Dietary Nutrient Reference Intake. Acs Omega 2021, 6, 6691–6698. [Google Scholar] [CrossRef]
- Ojeda-Amador, R.M.; Desamparados Salvador, M.; Gomez-Alonso, S.; Fregapane, G. Characterization of virgin walnut oils and their residual cakes produced from different varieties. Food Res. Int. 2018, 108, 396–404. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.; Duan, X.; Sun, H.; Cao, Y. Lipidomics reveals the changes in lipid profile of flaxseed oil affected by roasting. Food Chem. 2021, 364, 130431. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Oxidative stress and peritoneal endometriosis. Fertil. Steril. 2002, 77, 861–870. [Google Scholar]
- Sook Chin, C.; Kar Lin, N. Kenaf (Hibiscus cannabinus L.) seed oil. In Fruit Oils: Chemistry and Functionality; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Castelo-Branco, V.N.; Torres, A.G. Potential application of antioxidant capacity assays to assess the quality of edible vegetable oils. Lipid Technol. 2009, 21, 152–155. [Google Scholar]
- Oppedisano, F.; Macri, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Durdevic, S.; Savikin, K.; Zivkovic, J.; Boehm, V.; Stanojkovic, T.; Damjanovic, A.; Petrovic, S. Antioxidant and cytotoxic activity of fatty oil isolated by supercritical fluid extraction from microwave pretreated seeds of wild growing Punica granatum L. J. Supercrit. Fluids 2018, 133, 225–232. [Google Scholar] [CrossRef]
- Umesha, S.S.; Naidu, K.A. Vegetable oil blends with alpha-linolenic acid rich Garden cress oil modulate lipid metabolism in experimental rats. Food Chem. 2012, 135, 2845–2851. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, V.; Verma, S.K.; Shakya, A.K.; Chauhan, G.S. Tocopherol content and profile of soybean: Genotypic variability and correlation studies. J. Am. Oil Chem. Soc. 2007, 84, 377–383. [Google Scholar]
- Wall-Medrano, A.; De la Rosa, L.A.; Vazquez-Flores, A.A.; Mercado-Mercado, G.; Gonzalez-Arellanes, R.; Lopez-Diaz, J.A.; Gonzalez-Cordova, A.F.; Gonzalez-Aguilar, G.A.; Vallejo-Cordoba, B.; Molina-Corral, F.J. Lipidomic and Antioxidant Response to Grape Seed, Corn and Coconut Oils in Healthy Wistar Rats. Nutrients 2017, 9, 82. [Google Scholar] [PubMed]
- Jeyakumar, N.; Narayanasamy, B. Effect of natural antioxidants on oxidation stability of jackfruit seed oil (Artocarpus heterophyllus) biodiesel. Energy Source Part A 2020, 1–17. [Google Scholar] [CrossRef]
- Widomska, J.; Welc, R.; Gruszecki, W.I. The effect of carotenoids on the concentration of singlet oxygen in lipid membranes. BBA-Biomembr. 2019, 1861, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Eller, F.J.; Moser, J.K.; Kenar, J.A.; Taylor, S.L. Extraction and Analysis of Tomato Seed Oil. J. Am. Oil Chem. Soc. 2010, 87, 755–762. [Google Scholar] [CrossRef]
- Sanwal, N.; Mishra, S.; Sahu, J.K.; Naik, S.N. Effect of ultrasound-assisted extraction on efficiency, antioxidant activity, and physicochemical properties of sea buckthorn (Hippophae salicipholia) seed oil. LWT-Food Sci. Technol. 2022, 153, 112386. [Google Scholar] [CrossRef]
- Divya, P.M.; Roopa, B.S.; Manusha, C.; Balannara, P. A concise review on oil extraction methods, nutritional and therapeutic role of coconut products. J. Food Sci. Tech. Mys. 2023, 60, 441–452. [Google Scholar] [CrossRef]
- Ni, Q.; Gao, Q.; Yu, W.; Liu, X.; Xu, G.; Zhang, Y. Supercritical carbon dioxide extraction of oils from two Torreya grandis varieties seeds and their physicochemical and antioxidant properties. LWT-Food Sci. Technol. 2015, 60, 1226–1234. [Google Scholar] [CrossRef]
- Kaithwas, G.; Majumdar, D.K. In vitro antioxidant and in vivo antidiabetic, antihyperlipidemic activity of linseed oil against streptozotocin-induced toxicity in albino rats. Eur. J. Lipid Sci. Technol. 2012, 114, 1237–1245. [Google Scholar] [CrossRef]
- Milian-Linares, M.C.; Bermudez, B.; Martin, M.E.; Munoz, E.; Abia, R.; Milian, F.; Muriana, F.J.G.; Montserrat-de la Paz, S. Unsaponifiable fraction isolated from grape (Vitis vinifera L.) seed oil attenuates oxidative and inflammatory responses in human primary monocytes. Food Funct. 2018, 9, 2517–2523. [Google Scholar]
- Tang, Z.; Ying, R.; Lv, B.; Yang, L.; Xu, Z.; Yan, L.; Bu, J.; Wei, Y. Flaxseed oil: Extraction, Health benefits and products. Qual. Assur. Saf. Crops Foods 2021, 13, 1–19. [Google Scholar] [CrossRef]
- Ferguson, J.J.A.; Stojanovski, E.; MacDonald-Wicks, L.; Garg, M.L. Curcumin potentiates cholesterol-lowering effects of phytosterols in hypercholesterolaemic individuals. A randomised controlled trial. Metabolism 2018, 82, 22–33. [Google Scholar] [PubMed]
- Huth, P.J.; Fulgoni, V.L., III; Larson, B.T. A Systematic Review of High-Oleic Vegetable Oil Substitutions for Other Fats and Oils on Cardiovascular Disease Risk Factors: Implications for Novel High-Oleic Soybean Oils. Adv. Nutr. 2015, 6, 674–693. [Google Scholar] [PubMed]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [PubMed]
- Gawron-Skarbek, A.; Guligowska, A.; Prymont-Przyminska, A.; Nowak, D.; Kostka, T. The Anti-Inflammatory and Antioxidant Impact of Dietary Fatty Acids in Cardiovascular Protection in Older Adults May Be Related to Vitamin C Intake. Antioxidants 2023, 12, 267. [Google Scholar] [CrossRef]
- Antonia, T.; Tina, C.; Christina, B.; Dimitrios, T. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 1–15. [Google Scholar]
- Bhardwaj, R.; Dod, H.; Sandhu, M.S.; Bedi, R.; Dod, S.; Konat, G.; Chopra, H.K.; Sharma, R.; Jain, A.C.; Nanda, N. Acute effects of diets rich in almonds and walnuts on endothelial function. Indian Heart J. 2018, 70, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Grosshagauer, S.; Steinschaden, R.; Pignitter, M. Strategies to increase the oxidative stability of cold pressed oils. LWT-Food Sci. Technol. 2019, 106, 72–77. [Google Scholar] [CrossRef]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar]
- Siri-Tarino, P.W.; Chiu, S.; Bergeron, N.; Krauss, R.M. Saturated Fats Versus Polyunsaturated Fats Versus Carbohydrates for Cardiovascular Disease Prevention and Treatment. Annu. Rev. Nutr. 2015, 35, 517–543. [Google Scholar] [PubMed]
- Han, H.; Qiu, F.B.; Zhao, H.F.; Tang, H.Y.; Li, X.H.; Shi, D.X. Dietary flaxseed oil improved western-type diet-induced atherosclerosis in apolipoprotein-E knockout mice. J. Funct. Foods 2018, 40, 417–425. [Google Scholar] [CrossRef]
- Han, H.; Yan, P.; Chen, L.; Luo, C.; Gao, H.; Deng, Q.; Zheng, M.; Shi, Y.; Liu, L. Flaxseed Oil Containing alpha-Linolenic Acid Ester of Plant Sterol Improved Atherosclerosis in ApoE Deficient Mice. Oxid. Med. Cell. Longev. 2015, 2015, 958217. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Chen, X.; Tu, Y.; Zhou, Y. Vegetable oils rich in polyunsaturated fatty acids and their health-beneficial effects: A review. Food Sci. China 2014, 35, 350–354. [Google Scholar]
- Teh, H.E.; Yokoyama, W.H.; German, J.B.; McHugh, T.H.; Pan, Z. Hypocholesterolemic Effects of Expeller-Pressed and Solvent-Extracted Fruit Seed Oils and Defatted Pomegranate Seed Meals. J. Agric. Food Chem. 2019, 67, 6150–6159. [Google Scholar] [CrossRef] [PubMed]
- Demonty, I.; Ras, R.T.; van der Kniap, H.C.M.; Duchateau, G.S.M.J.F.; Meijer, L.; Zock, P.L.; Geleijnse, J.M.; Trautwein, E.A. Continuous Dose-Response Relationship of the LDL-Cholesterol-Lowering Effect of Phytosterol Intake. J. Nutr. 2009, 139, 271–284. [Google Scholar] [PubMed]
- Ghaedi, E.; Foshati, S.; Ziaei, R.; Beigrezaei, S.; Kord-Varkaneh, H.; Ghavami, A.; Miraghajani, M. Effects of phytosterols supplementation on blood pressure: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 2702–2710. [Google Scholar] [PubMed]
- Mazzocchi, A.; De Cosmi, V.; Rise, P.; Milani, G.P.; Turolo, S.; Syren, M.-L.; Sala, A.; Agostoni, C. Bioactive Compounds in Edible Oils and Their Role in Oxidative Stress and Inflammation. Front. Physiol. 2021, 12, 659551. [Google Scholar]
- Zhang, J.; Wen, C.; Duan, Y.; Zhang, H.; Ma, H. Advance in Cordyceps militaris (Linn) Link polysaccharides: Isolation, structure, and bioactivities: A review. Int. J. Biol. Macromol. 2019, 132, 906–914. [Google Scholar]
- Dipasquale, D.; Basirico, L.; Morera, P.; Primi, R.; Troescher, A.; Bernabucci, U. Anti-inflammatory effects of conjugated linoleic acid isomers and essential fatty acids in bovine mammary epithelial cells. Animal 2018, 12, 2108–2114. [Google Scholar] [CrossRef]
- Morgan, L.V.; Petry, F.; Scatolin, M.; de Oliveira, P.V.; Alves, B.O.; Lisboa Zilli, G.A.; Bueno Volfe, C.R.; Oltramari, A.R.; de Oliveira, D.; Scapinello, J.; et al. Investigation of the anti-inflammatory effects of stigmasterol in mice: Insight into its mechanism of action. Behav. Pharmacol. 2021, 32, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, S.M.; Vajreswari, A. Pharmaconutrition strategy to resolve SARS-CoV-2-induced inflammatory cytokine storm in non-alcoholic fatty liver disease: Omega-3 long -chain polyunsaturated fatty acids. World J. Clin. Cases 2021, 9, 9333–9349. [Google Scholar] [PubMed]
- Mosavat, S.H.; Masoudi, N.; Hajimehdipoor, H.; Meybodi, M.K.E.; Niktabe, Z.; Tabarrai, M.; Ghorat, F.; Khodadoost, M. Efficacy of topical Linum usitatissimum L. (flaxseed) oil in knee osteoarthritis: A double-blind, randomized, placebo-controlled clinical trial. Complement. Ther. Clin. Pract. 2018, 31, 302–307. [Google Scholar] [PubMed]
- Akrami, A.; Makiabadi, E.; Askarpour, M.; Zamani, K.; Hadi, A.; Mokari-Yamchi, A.; Babajafari, S.; Faghih, S.; Hojhabrimanesh, A. A Comparative Study of the Effect of Flaxseed Oil and Sunflower Oil on the Coagulation Score, Selected Oxidative and Inflammatory Parameters in Metabolic Syndrome Patients. Clin Nutr. 2020, 9, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Timoszuk, M.; Bielawska, K.; Skrzydlewska, E. Evening Primrose (Oenothera biennis) Biological Activity Dependent on Chemical Composition. Antioxidants 2018, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yu, S.; Kim, W. Rice Bran Oil Attenuates Chronic Inflammation by Inducing M2 Macrophage Switching in High-Fat Diet-Fed Obese Mice. Foods 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Shang, K.; Lin, C.C.; Wang, C.; Shi, X.Q.; Wang, H.; Li, H. Processing technologies, phytochemical constituents, and biological activities of grape seed oil (GSO): A review. Trends Food Sci. Technol. 2021, 116, 1074–1083. [Google Scholar]
- Misawa, E.; Tanaka, M.; Nomaguchi, K.; Nabeshima, K.; Yamada, M.; Toida, T.; Iwatsuki, K. Oral Ingestion of Aloe vera Phytosterols Alters Hepatic Gene Expression Profiles and Ameliorates Obesity-Associated Metabolic Disorders in Zucker Diabetic Fatty Rats. J. Agric. Food Chem. 2012, 60, 2799–2806. [Google Scholar] [CrossRef]
- Thomas, S.S.; Cha, Y.-S.; Kim, K.-A. Effect of vegetable oils with different fatty acid composition on high-fat diet-induced obesity and colon inflammation. Nutr. Res. Pract. 2020, 14, 425–437. [Google Scholar] [CrossRef]
- Akrami, A.; Nikaein, F.; Babajafari, S.; Faghih, S.; Yarmohammadi, H. Comparison of the effects of flaxseed oil and sunflower seed oil consumption on serum glucose, lipid profile, blood pressure, and lipid peroxidation in patients with metabolic syndrome. J. Clin. Lipidol. 2018, 12, 70–77. [Google Scholar] [CrossRef]
- World Health Organization-Fact sheets-Detail-Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 6 July 2023).
- Shahzad, N.; Khan, W.; Shadab, M.D.; Ali, A.; Saluja, S.S.; Sharma, S.; Al-Allaf, F.A.; Abduljaleel, Z.; Ibrahim, I.A.A.; Abdel-Wahab, A.F.; et al. Phytosterols as a natural anticancer agent: Current status and future perspective. Biomed. Pharmacother. 2017, 88, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, J.; Wang, J.; Sun, B. The anti-cancer activity and potential clinical application of rice bran extracts and fermentation products. RSC Adv. 2019, 9, 18060–18069. [Google Scholar] [CrossRef]
- Wiggins, A.K.A.; Mason, J.K.; Thompson, L.U. Growth and gene expression differ over time in alpha-linolenic acid treated breast cancer cells. Exp. Cell Res. 2015, 333, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.Y.; Akanda, J.M.H.; Nyam, K.L. Kenaf seed oil: A potential new source of edible oil. Trends Food Sci. Technol. 2016, 52, 57–65. [Google Scholar] [CrossRef]
- Miura, D.; Kida, Y.; Nojima, H. Camellia oil and its distillate fractions effectively inhibit the spontaneous metastasis of mouse melanoma BL6 cells. FEBS Lett. 2007, 581, 2541–2548. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Kowalczyk, A.; Sarritzu, E.; Cabras, P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007, 103, 1494–1501. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, Y.; Yang, F.; Zeng, A.; Yang, S.; Luo, Y.; Zhang, Y.; Xie, Y.; Ye, T.; Xia, Y.; et al. The extract from Punica granatum (pomegranate) peel induces apoptosis and impairs metastasis in prostate cancer cells. Biomed. Pharmacother. 2017, 93, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Shirode, A.B.; Kovvuru, P.; Chittur, S.V.; Henning, S.M.; Heber, D.; Reliene, R. Antiproliferative effects of pomegranate extract in MCF-7 breast cancer cells are associated with reduced DNA repair gene expression and induction of double strand breaks. Mol. Carcinog. 2014, 53, 458–470. [Google Scholar]
- Smith, T.J. Squalene: Potential chemopreventive agent. Expert Opin. Investig. Drugs 2000, 9, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Ditano-Vazquez, P.; Torres-Pena, J.D.; Galeano-Valle, F.; Perez-Caballero, A.I.; Demelo-Rodriguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, C.; Filesi, C.; Vari, R.; Scazzocchio, B.; Filardi, T.; Fogliano, V.; D’Archivio, M.; Giovannini, C.; Lenzi, A.; Morano, S.; et al. Consumption of extra-virgin olive oil rich in phenolic compounds improves metabolic control in patients with type 2 diabetes mellitus: A possible involvement of reduced levels of circulating visfatin. J. Endocrinol. Investig. 2016, 39, 1295–1301. [Google Scholar]
- Deen, A.; Visvanathan, R.; Wickramarachchi, D.; Marikkar, N.; Nammi, S.; Jayawardana, B.C.; Liyanage, R. Chemical composition and health benefits of coconut oil: An overview. J. Sci. Food Agric. 2021, 101, 2182–2193. [Google Scholar] [CrossRef]
- Aslam, F.; Iqbal, S.; Nasir, M.; Anjum, A.A. White Sesame Seed Oil Mitigates Blood Glucose Level, Reduces Oxidative Stress, and Improves Biomarkers of Hepatic and Renal Function in Participants with Type 2 Diabetes Mellitus. J. Am. Coll. Nutr. 2019, 38, 235–246. [Google Scholar] [CrossRef]
- Khajebishak, Y.; Payahoo, L.; Alivand, M.; Alipour, B. Punicic acid: A potential compound of pomegranate seed oil in Type 2 diabetes mellitus management. J. Cell. Physiol. 2019, 234, 2112–2120. [Google Scholar]
- Wang, L.; Tu, Z.; Wang, H.; Wang, S.; Wang, X.; Zhu, H.; Hu, C.-A.A.; Liu, Y. Flaxseed oil improves liver injury and inhibits necroptotic and inflammatory signaling pathways following lipopolysaccharide challenge in a piglet model. J. Funct. Foods 2018, 46, 482–489. [Google Scholar] [CrossRef]
- Kheira, H.S.; El-Sayed, S.A.E.-S.; Elsayed, G.R.; Rizk, M.A. Dietary flaxseed oil inhibits kidney NF-kappa B activation and pro-inflammatory cytokine expression in cisplatin-treated rats. Comp. Clin. Pathol. 2018, 28, 349–357. [Google Scholar] [CrossRef]
- Omar, A.M.S. The potential protective influence of flaxseed oil against renal toxicity induced by thioacetamide in rats. Saudi J. Biol. Sci. 2018, 25, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Aquino, L.B.B.; Barbaza, M.Y.U.; Hsieh, C.-L.; De Castro-Cruz, K.A.; Yang, L.-L.; Tsai, P.-W. Anti-Inflammatory and Anticancer Properties of Bioactive Compounds from Sesamum indicum L.-A Review. Molecules 2019, 24, 4426. [Google Scholar] [CrossRef]
- Opyd, P.M.; Jurgonski, A. Intestinal, liver and lipid disorders in genetically obese rats are more efficiently reduced by dietary milk thistle seeds than their oil. Sci. Rep. 2021, 11, 20895. [Google Scholar] [CrossRef] [PubMed]
- Berahmand, F.; Anoush, G.; Hosseini, M.-J.; Anoush, M. Grape Seed Oil as a Natural Therapy in Male Rats with Alzheimer’s Diseases. Adv. Pharm. Bull. 2020, 10, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Tech. Mys. 2014, 51, 1633–1653. [Google Scholar]
- Straccia, M.C.; Siano, F.; Coppola, R.; La Cara, F.; Volpe, M.G. In Extraction and Characterization of Vegetable Oils from Cherry Seed by Different Extraction Processes. In Proceedings of the 3rd International Conference on Industrial Biotechnology (IBIC), Palermo, Italy, 24–27 June 2012; pp. 391–396. [Google Scholar]
- Sangeetha, K.; Ramyaa, R.B.; Khaneghah, A.M.; Radhakrishnan, M. Extraction, characterization, and application of tomato seed oil in the food industry: An updated review. J. Agric. Food Res. 2023, 11, 100529. [Google Scholar]
- Petrella, C.; Di Certo, M.G.; Gabanella, F.; Barbato, C.; Ceci, F.M.; Greco, A.; Ralli, M.; Polimeni, A.; Angeloni, A.; Severini, C.; et al. Mediterranean Diet, Brain and Nuscle: Olive Polyphenols and Resveratrol Protection in Neurodegenerative and Neuromuscular Disorders. Curr. Med. Chem. 2021, 28, 7595–7613. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Y.; Rayman, M.P.; Zhang, J. Prospective Selective Mechanism of Emerging Senolytic Agents Derived from Flavonoids. J. Agric. Food Chem. 2021, 69, 12418–12423. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.S.T.; Quintana, L.E.A.; Suarez, P.A.; Cabrera, M.A.R.; Lagunes, A.G. Physicochemical Properties, Antioxidant Capacity, Prebiotic Activity and Anticancer Potential in Human Cells of Jackfruit (Artocarpus heterophyllus) Seed Flour. Molecules 2021, 26, 4854. [Google Scholar] [CrossRef] [PubMed]
- Gidigbi, J.A.; Ngoshe, A.; Martins, A. Industrial viability study of the avocado seed oil. Int. J. Rcent. Innov. Acad. Res. 2019, 3, 48–57. [Google Scholar]
- Fratianni, F.; D’Acierno, A.; Ombra, M.N.; Amato, G.; De Feo, V.; Ayala-Zavala, J.F.; Coppola, R.; Nazzaro, F. Fatty Acid Composition, Antioxidant, and in vitro Anti-inflammatory Activity of Five Cold-Pressed Prunus Seed Oils, and Their Anti-biofilm Effect Against Pathogenic Bacteria. Front. Nutr. 2021, 8, 775751. [Google Scholar] [PubMed]
- Siano, F.; Straccia, M.C.; Paolucci, M.; Fasulo, G.; Boscaino, F.; Volpe, M.G. Physico-chemical properties and fatty acid composition of pomegranate, cherry and pumpkin seed oils. J. Sci. Food Agric. 2016, 96, 1730–1735. [Google Scholar] [CrossRef]
- Atik, I.; Karasu, S.; Sevik, R. Physicochemical and bioactive properties of cold press wild plum (Prunus spinosa) and sour cherry (Prunus cerasus) kernel oils: Fatty acid, sterol and phenolic profile. Riv. Ital. Sostanze Gr. 2022, 99, 13–20. [Google Scholar]
- Panadare, D.C.; Gondaliya, A.; Rathod, V.K. Comparative study of ultrasonic pretreatment and ultrasound assisted three phase partitioning for extraction of custard apple seed oil. Ultrason. Sonochem. 2020, 61, 104821. [Google Scholar] [CrossRef] [PubMed]
- Dhara, O.; Azmeera, T.; Eanti, A.; Chakrabarti, P.P. Garden cress oil as a vegan source of PUFA: Achieving through optimized supercritical carbon dioxide extraction. Innovative Food Sci. Emerg. Technol. 2023, 84, 103283. [Google Scholar] [CrossRef]
- Sena-Moreno, E.; Pardo, J.E.; Pardo-Gimenez, A.; Gomez, R.; Alvarez-Orti, M. Differences in Oils from Nuts Extracted by Means of Two Pressure Systems. Int. J. Food Prop. 2016, 19, 2750–2760. [Google Scholar] [CrossRef]
- Alves, A.Q.; da Silva, V.A.; Silva Goes, A.J.; Silva, M.S.; de Oliveira, G.G.; Gomes Alves Bastos, I.V.; de Castro Neto, A.G.; Alves, A.J. The Fatty Acid Composition of Vegetable Oils and Their Potential Use in Wound Care. Adv. Skin Wound Care. 2019, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, M.M.; Al Juhaimi, F.; Ghafoor, K.; Babiker, E.E.; Ozcan, M.M. Characterization of physico-chemical and bioactive properties of oils of some important almond cultivars by cold press and soxhlet extraction. J. Food Sci. Tech. Mys. 2020, 57, 955–961. [Google Scholar] [CrossRef]
- Bondioli, P.; Folegatti, L.; Rovellini, P. Oils rich in alpha linolenic acid: Chemical composition of perilla (Perilla frutescens) seed oil. OCL 2020, 27, 67. [Google Scholar] [CrossRef]
- Deng, R.; Gao, J.; Yi, J.; Liu, P. Peony seeds oil by-products: Chemistry and bioactivity. Ind. Crops Prod. 2022, 187, 115333. [Google Scholar]
- Huang, Z.; Du, M.; Qian, X.; Cui, H.; Tong, P.; Jin, H.; Feng, Y.; Zhang, J.; Wu, Y.; Zhou, S.; et al. Oxidative stability, shelf-life and stir-frying application of Torreya grandis seed oil. Int. J. Food Sci. Technol. 2022, 57, 1836–1845. [Google Scholar] [CrossRef]
- Meddeb, W.; Rezig, L.; Zarrouk, A.; Nury, T.; Vejux, A.; Prost, M.; Bretillon, L.; Mejri, M.; Lizard, G. Cytoprotective Activities of Milk Thistle Seed Oil Used in Traditional Tunisian Medicine on 7-Ketocholesterol and 24S-Hydroxycholesterol-Induced Toxicity on 158N Murine Oligodendrocytes. Antioxidants 2018, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Wu, G.; Jin, Q.; Wang, X. Camellia oil adulteration detection using fatty acid ratios and tocopherol compositions with chemometrics. Food Control 2022, 133, 108565. [Google Scholar] [CrossRef]
- Nehdi, I.A.; Hadj-Kali, M.K.; Sbihi, H.M.; Tan, C.P.; Al-Resayes, S.I. Characterization of Ternary Blends of Vegetable Oils with Optimal omega-6/omega-3 Fatty Acid Ratios. J. Oleo Sci. 2019, 68, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Gornas, P.; Rudzinska, M.; Grygier, A.; Lacis, G. Diversity of oil yield, fatty acids, tocopherols, tocotrienols, and sterols in the seeds of 19 interspecific grapes crosses. J. Sci. Food Agric. 2019, 99, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xue, Y.; Zhang, D.; Xu, R.; Chai, J. Analysis of sterols content and composition in special vegetable oils. Sci. Technol. Cereals Oils Foods 2015, 23, 49–52. [Google Scholar]
- He, Z.; Zhu, H.; Li, W.; Zeng, M.; Wu, S.; Chen, S.; Qin, F.; Chen, J. Chemical components of cold pressed kernel oils from different Torreya grandis cultivars. Food Chem. 2016, 209, 196–202. [Google Scholar] [CrossRef] [PubMed]
| Oils (mg/100 g) | Squalene | References |
|---|---|---|
| Olive oil | 153.4–747.4 | [30] |
| Rice bran oil | 24.0–318.9 | [9,18] |
| Corn oil | 6.8–256.8 | [31,33] |
| Pumpkin seed oil | 66.7–198.0 | [9] |
| Camellia oil | 1.5–159.8 | [31,33] |
| Peanut oil | 5.3–132.9 | [18,31] |
| Soybean oil | 1.6–92.1 | [31,33] |
| Flaxseed oil | 2.4–83.0 | [23,32] |
| Sesame oil | 8.8–60.7 | [18,32] |
| Coconut oil | 51.5 | [32] |
| Palm oil | 6.8–48.3 | [31,32] |
| Perilla seed oil | 27.9 | [32] |
| Sunflower oil | 5.3–27.1 | [31] |
| Peony seed oil | 4.0–18.7 | [9,32] |
| Cottonseed oil | 15.8 | [32] |
| Grape seed oil | 11.8–13.0 | [9,32] |
| Rapeseed oil | 2.1–12.5 | [31] |
| Walnut oil | 9.0–12.0 | [32] |
| Safflower oil | 5.6 | [32] |
| Torreya grandis seed oil | 3.0 | [9] |
| Evening Primrose oil | - | [9] |
| Oils (mg/kg) | β-Carotene | Lutein | Zeaxanthin | Lycopene | α-Carotene | Total | References |
|---|---|---|---|---|---|---|---|
| Tomato seed oil | 765.7 | - | - | - | - | 765.7 | [9] |
| Flaxseed oil | 34.9–76.9 | 11.6 | 1.1 | - | - | 76.9 | [9] |
| Sea buckthorn seed oil | 55.3 | - | - | - | - | 55.3 | [9] |
| Rapeseed oil | 6.0–18.8 | 32.6–95.0 | 1.2 | - | - | 52.6–358.7 | [23,35] |
| Olive oil | 36 | 0.8–4.4 | 0.8 | 0.8 | 3.6 | 50.3 | [35] |
| Sunflower oil | - | 11.6–12.4 | - | - | - | 3.1–15.3 | [22,35] |
| Camellia oil | 21.0 | 1.6 | - | 10.0 | - | 32.6 | [35] |
| Corn oil | 0.1 | - | - | - | - | 0.1 | [9] |
| Rice bran oil | - | - | - | - | - | 0 | [9] |
| Peanut oil | - | - | - | - | - | 1.8 | [35] |
| Cress oil | 4.3 | 1.0 | 0.2 | - | 5.3 | [36] |
| Oils (mg/kg) | Phenolic Acids | Total Phenolic | References |
|---|---|---|---|
| Coconut oil | 4.250 | 21.0–59,300.0 | [40,44] |
| Torreya grandis seed oil | - | 12,630.0 | [9] |
| Perilla seed oil | - | 38.6–11,090.0 | [9] |
| Rice bran oil | 0.004 | 14.4–10,220.0 | [45] |
| Pomegranate seed oil | - | 90.0–9000.0 | [13] |
| Flaxseed oil | 2.570 | 4.0–3073.0 | [23,45] |
| Pumpkin seed oil | 164.800 | 3.9–2360.0 | [45] |
| Olive oil | 0.841 | 23.0–2180.0 | [45] |
| Sunflower oil | 0.178 | 4.8–1920.0 | [22,45] |
| Rapeseed oil | 3.710 | 10.3–1654.5 | [23,35,45] |
| Sesame oil | - | 0.4–1337.5 | [45] |
| Avocado seed oil | - | 11.6–1301.7 | [45] |
| Grape seed oil | 92.490 | 5.1–1155.0 | [45] |
| Safflower oil | - | 26.2–711.0 | [45] |
| Evening Primrose oil | - | 48.6–679.0 | [9,45] |
| Almond oil | - | 644.5 | [9] |
| Soybean oil | - | 4.2–643.7 | [45] |
| Peanut oil | 0.016 | 5.7–501.3 | [45] |
| Palm oil | - | 18.2–403.0 | [45] |
| Camellia oil | 31.243 | 19.8–400.0 | [9,45] |
| Cottonseed oil | - | 98.6 | [46] |
| Corn oil | 8.850 | 12.6–53.6 | [45] |
| Peony seed oil | - | 49.4 | [46] |
| Palm kernel oil | - | 3.2–27.2 | [45] |
| Walnut oil | 0.022 | 14.0–26.0 | [45,47] |
| Functional Components | Oil Types | Mechanisms | References |
|---|---|---|---|
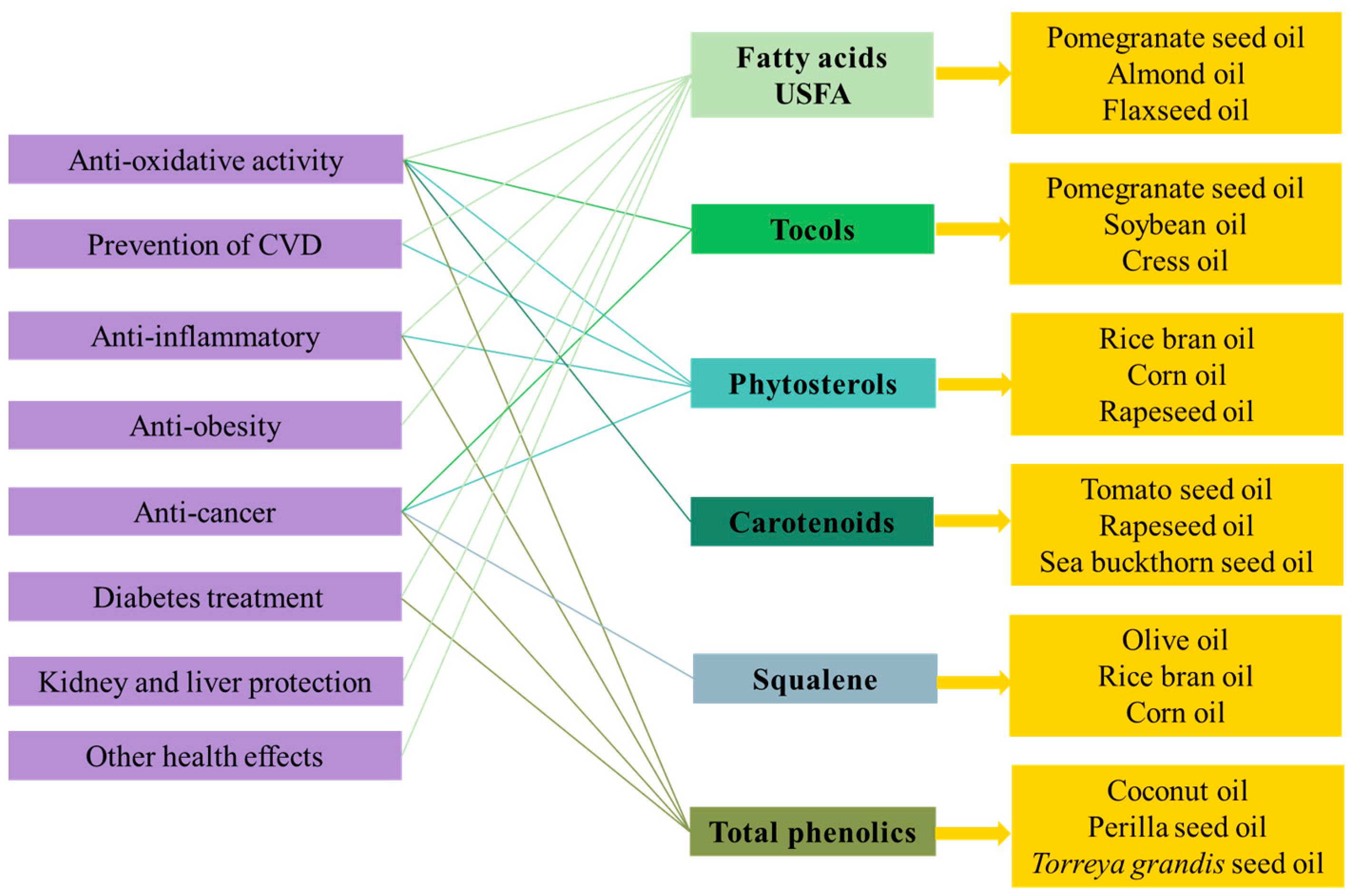
| Tocols | Pomegranate seed oil Soybean oil Cress oil | Primary or chain breaking antioxidants; tocopherols can provide electrons to FR to make them become inactive compounds with the exchange of becoming tocopherol FR, which is easy excrete in feces and urine after metabolism. | [9,52] |
| Phytosterols | Rice bran oil Corn oil | Possible primary or chain breaking antioxidant. | [52] |
| Phenolics | Coconut oil Torreya grandis seed oil | Primary or chain breaking antioxidants. Secondary or preventive antioxidants act as chelators of metal ions. Stabilize and prevent decomposition of hydroperoxides. Phenolic compounds can prevent the generation of FR in the body and block the oxidation reaction of PUFAs or LDLs induced by FR. | [52] |
| Carotenoids | Tomato seed oil Sea buckthorn seed oil | Secondary or preventive antioxidants act as singlet oxygen quenchers. Primary or chain breaking antioxidants. Carotenoids are able to donate an electron and neutralize FR, resulting in the suppression of excess FR production to inhibit the deterioration of internal redox balance and terminate some chain reactions. | [52] |
| Fatty acids | Flaxseed oil | n-3 PUFAs can reduce mitochondrial dysfunction and endothelial cell apoptosis associated with oxidative stress by increasing the activity of endogenous antioxidant enzymes. | [53] |
| Functional Components | Oil types | Mechanisms | References |
|---|---|---|---|
| MUFA | Almond oil Olive oil | Oleic acid can decrease plasma triacylglycerol and cholesterol concentrations. | [68] |
| PUFA | Flaxseed oil Pomegranate seed oil | LA helps to break down cholesterol by promoting cholesterol 7α-hydroxylase (CYP7) activity. LA enhances transcription of the liver X receptor (LXRα) gene via peroxisome proliferator-activated receptors (PPARs). In turn, LXRα upregulates the expression of the CYP7 gene; EPA and DHA protect the blood vessels and heart by regulating membrane phospholipids, improving cardiac mitochondrial function and energy production, and lowering triglyceride concentrations. | [69,70] |
| Phytosterols | Rice bran oil Corn oil Rapeseed oil | Inhibits the absorption of intestinal cholesterol. | [10] |
| Functional Components | Oil Types | Mechanisms | References |
|---|---|---|---|
| USFA | Flaxseed oil Perilla seed oil Almond oil | ALA exerts an anti-inflammatory effect by upregulating the expression of Peroxisome proliferator-activated receptor γ to inhibit the transcription of pro-inflammatory cytokines. EPA and DHA reduce the production of AA-derived eicosanoids by competing with AA for incorporation into cell membrane phospholipids, reduce AA release from the membrane, inhibit the action of the enzymes COX-2 and 5-lipoxygenase (5-LOX) on AA, or compete with AA for metabolism by COX and LOX enzymes. | [69,85] |
| Phytosterols | Rice bran oil | Phytosterols can suppress the transcription of inflammatory genes in macrophages. | [86] |
| FA | SFA | MUFA | PUFA | SFA: MUFA: PUFA | TPUFA/TSFA |
|---|---|---|---|---|---|
| Soybean oil | 16.18 | 23.88 | 60.98 | 1:1.5:3.8 | 3.77 |
| Rapeseed oil | 7.52 | 72.77 | 29.36 | 1:9.7:3.9 | 3.90 |
| Palm oil | 44.15 | 46.30 | 9.38 | 4.7:4.9:1 | 0.21 |
| Peanut oil | 10.70 | 71.10 | 18.20 | 1:6.6:1.7 | 1.70 |
| Sunflower oil | 11.54 | 28.30 | 67.75 | 1:2.5:5.9 | 5.87 |
| Cottonseed oil | 7.11 | 14.43 | 40.23 | 1:2.0:5.7 | 5.66 |
| Corn oil | 16.60 | 33.67 | 49.74 | 1:2.0:3.0 | 3.00 |
| Camellia oil | 17.26 | 72.50 | 10.10 | 1.7:7.2:1 | 0.59 |
| Coconut oil | 92.10 | 6.20 | 1.60 | 57.6:3.9:1 | 0.02 |
| Olive oil | 20.19 | 76.62 | 18.00 | 1.1:4.3:1 | 0.89 |
| Flaxseed oil | 12.90 | 23.00 | 76.94 | 1:1.8:6.0 | 5.96 |
| Jackfruit seed oil | 49.13 | 4.15 | 46.72 | 11.8:1:11.3 | 0.95 |
| Papaya seed oil | 19.92 | 76.10 | 3.96 | 5.0:19.2:1 | 0.20 |
| Avocado seed oil | 11.74 | 73.71 | 13.78 | 1:6.3:1.2 | 1.17 |
| Pomegranate seed oil | 5.35 | 6.79 | 87.87 | 1:1.3:16.4 | 16.42 |
| Cheery oil | 12.80 | 39.60 | 46.30 | 1:3.1:3.6 | 3.62 |
| Sweet cherry seed oil | 12.20 | 39.49 | 44.32 | 1:3.2:3.6 | 3.63 |
| Sour cherry seed oil | 7.46 | 38.49 | 54.05 | 1:5.2:7.2 | 7.25 |
| Custard-apple seed oil | 23.04 | 51.04 | 24.96 | 1:2.2:1.1 | 1.08 |
| Cress oil | 16.90 | 37.30 | 45.80 | 1:2.2:2.7 | 2.71 |
| Pumpkin seed oil | 25.20 | 25.54 | 48.14 | 1:1.0:1.9 | 1.91 |
| Sesame oil | 16.90 | 42.00 | 41.20 | 1:2.5:2.41 | 2.44 |
| Rice bran oil | 23.63 | 43.71 | 32.66 | 1:1.8:1.4 | 1.38 |
| Almond oil | 8.35 | 77.07 | 22.59 | 1:9.2:2.7 | 2.71 |
| Evening Primrose oil | 8.10 | 9.40 | 83.40 | 1:1.2:10.3 | 10.30 |
| Perilla seed oil | 8.22 | 12.89 | 76.25 | 1:1.6:9.3 | 9.28 |
| Milk thistle seed oil | 15.02 | 35.94 | 48.81 | 1:2.4:3.2 | 3.25 |
| Tomato seed oil | 24.48 | 21.79 | 53.70 | 1.1:1:2.5 | 2.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, M.; Bai, Y.; Tian, H.; Zhao, X. The Chemical Composition and Health-Promoting Benefits of Vegetable Oils—A Review. Molecules 2023, 28, 6393. https://doi.org/10.3390/molecules28176393
Tian M, Bai Y, Tian H, Zhao X. The Chemical Composition and Health-Promoting Benefits of Vegetable Oils—A Review. Molecules. 2023; 28(17):6393. https://doi.org/10.3390/molecules28176393
Chicago/Turabian StyleTian, Mingke, Yuchen Bai, Hongyu Tian, and Xuebing Zhao. 2023. "The Chemical Composition and Health-Promoting Benefits of Vegetable Oils—A Review" Molecules 28, no. 17: 6393. https://doi.org/10.3390/molecules28176393
APA StyleTian, M., Bai, Y., Tian, H., & Zhao, X. (2023). The Chemical Composition and Health-Promoting Benefits of Vegetable Oils—A Review. Molecules, 28(17), 6393. https://doi.org/10.3390/molecules28176393








