The Anti-Breast Cancer Stem Cell Potency of Copper(I)-Non-Steroidal Anti-Inflammatory Drug Complexes
Abstract
:1. Introduction
2. Results and Discussion
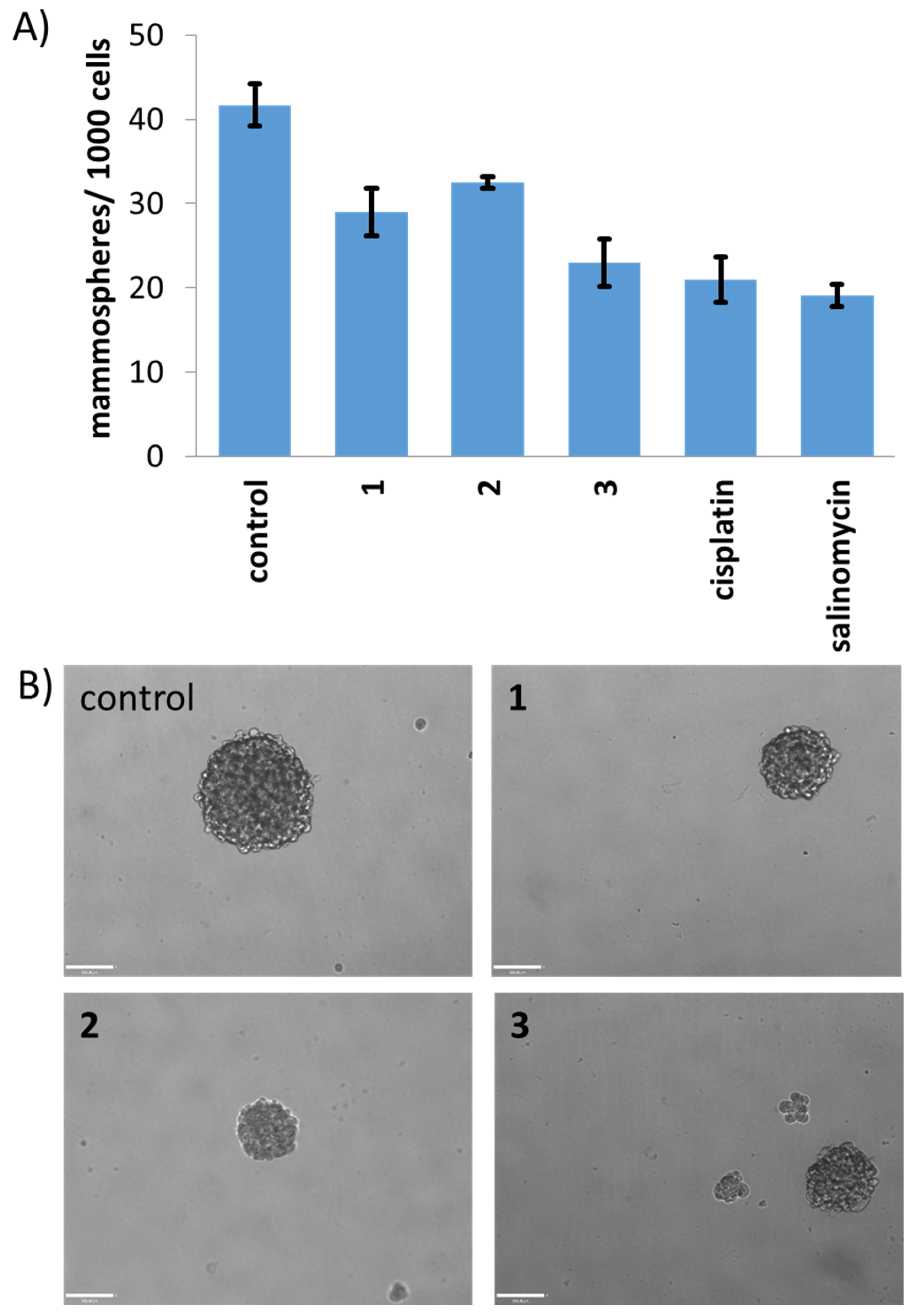
| Compound | HMLER IC50 [μM] | HMLER-shEcad IC50 [μM] | Mammosphere IC50 [μM] |
|---|---|---|---|
| 1 | 2.42 ± 0.10 | 4.61 ± 0.18 | 18.17 ± 2.02 |
| 2 | 2.36 ± 0.03 | 4.46 ± 0.08 | 11.80 ± 0.70 |
| 3 | 2.13 ± 0.002 | 4.55 ± 0.001 | 13.58 ± 0.74 |
| cisplatin 1 | 2.57 ± 0.02 | 5.65 ± 0.30 | 13.50 ± 2.34 |
| salinomycin 1 | 11.43 ± 0.42 | 4.23 ± 0.35 | 18.50 ± 1.50 |
3. Materials and Methods
3.1. Spectroscopic and Analytical Methods
3.2. Synthesis of [CuI(diclofenac)(PPh3)2] (1)
3.3. Synthesis of [CuI(naproxen)(PPh3)2] (2)
3.4. Synthesis of [CuI(salicylate)(PPh3)2] (3)
3.5. X-ray Crystallography
3.6. Measurement of Water–Octanol Partition Coefficient (LogP)
3.7. Cell Culture
3.8. Antiproliferative Studies: MTT Assay
3.9. Mammosphere Formation and Viability Assay
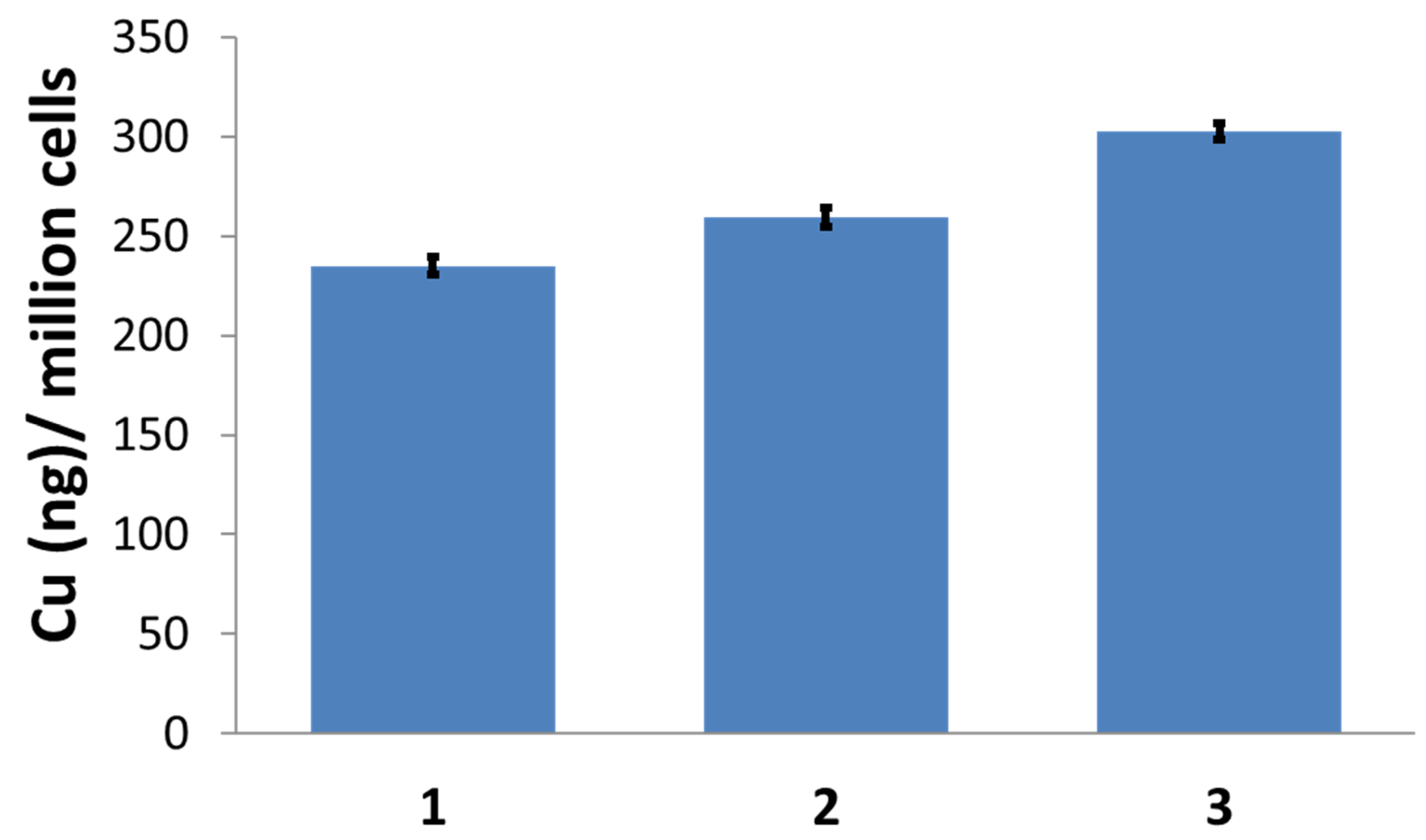
3.10. Cellular Uptake
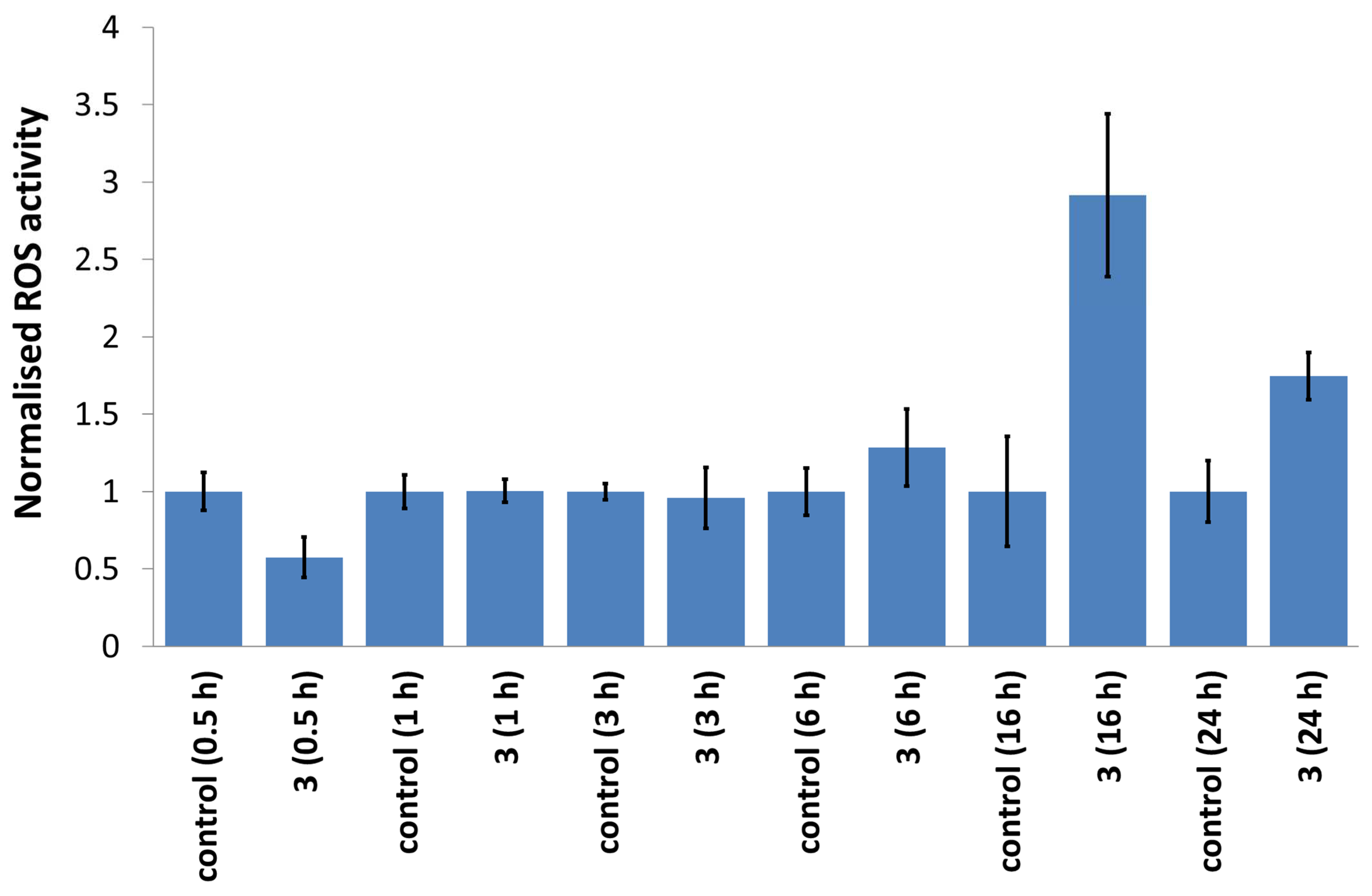
3.11. Intracellular ROS Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ferlay, J.E.M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 15 February 2021).
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C. Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine 2016, 95, S20–S25. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Won, M.; Kim, J.H.; Jung, E.; Min, K.; Jangili, P.; Kim, J.S. Cancer stem cell-targeted bio-imaging and chemotherapeutic perspective. Chem. Soc. Rev. 2020, 49, 7856–7878. [Google Scholar] [CrossRef]
- Lathia, J.; Liu, H.; Matei, D. The Clinical Impact of Cancer Stem Cells. Oncologist 2020, 25, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Saygin, C.; Matei, D.; Majeti, R.; Reizes, O.; Lathia, J.D. Targeting Cancer Stemness in the Clinic: From Hype to Hope. Cell Stem Cell 2019, 24, 25–40. [Google Scholar] [CrossRef]
- Nguyen, L.V.; Vanner, R.; Dirks, P.; Eaves, C.J. Cancer stem cells: An evolving concept. Nat. Rev. Cancer 2012, 12, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Du, F.Y.; Zhou, Q.F.; Sun, W.J.; Chen, G.L. Targeting cancer stem cells in drug discovery: Current state and future perspectives. World J. Stem Cells 2019, 11, 398–420. [Google Scholar] [CrossRef]
- Kaiser, J. The cancer stem cell gamble. Science 2015, 347, 226–229. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.G.; Lee, S.H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target 2020, 5, 8. [Google Scholar] [CrossRef]
- Laws, K.; Suntharalingam, K. The Next Generation of Anticancer Metallopharmaceuticals: Cancer Stem Cell-Active Inorganics. ChemBioChem 2018, 19, 2246–2253. [Google Scholar] [CrossRef] [PubMed]
- Northcote-Smith, J.; Suntharalingam, K. Targeting chemotherapy-resistant tumour sub-populations using inorganic chemistry: Anti-cancer stem cell metal complexes. Curr. Opin. Chem. Biol. 2023, 72, 102237. [Google Scholar] [CrossRef]
- Boodram, J.N.; McGregor, I.J.; Bruno, P.M.; Cressey, P.B.; Hemann, M.T.; Suntharalingam, K. Breast Cancer Stem Cell Potent Copper(II)-Non-Steroidal Anti-Inflammatory Drug Complexes. Angew. Chem. Int. Ed. 2016, 55, 2845–2850. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, A.; Boodram, J.N.; Cressey, P.B.; Lu, C.; Bruno, P.M.; Hemann, M.T.; Suntharalingam, K. The breast cancer stem cell potency of copper(II) complexes bearing nonsteroidal anti-inflammatory drugs and their encapsulation using polymeric nanoparticles. Dalton Trans. 2016, 45, 17867–17873. [Google Scholar] [CrossRef]
- Kaur, P.; Johnson, A.; Northcote-Smith, J.; Lu, C.; Suntharalingam, K. Immunogenic Cell Death of Breast Cancer Stem Cells Induced by an Endoplasmic Reticulum-Targeting Copper(II) Complex. ChemBioChem 2020, 21, 3618–3624. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Eskandari, A.; Cressey, P.B.; Suntharalingam, K. Cancer Stem Cell and Bulk Cancer Cell Active Copper(II) Complexes with Vanillin Schiff Base Derivatives and Naproxen. Chem. Eur. J. 2017, 23, 11366–11374. [Google Scholar] [CrossRef]
- Johnson, A.; Iffland-Mühlhaus, L.; Northcote-Smith, J.; Singh, K.; Ortu, F.; Apfel, U.-P.; Suntharalingam, K. A bioinspired redox-modulating copper(II)–macrocyclic complex bearing non-steroidal anti-inflammatory drugs with anti-cancer stem cell activity. Dalton Trans. 2022, 51, 5904–5912. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Zheng, J.; Pan, J. Reactive oxygen species in cancer stem cells. Antioxid. Redox Signal. 2012, 16, 1215–1228. [Google Scholar] [CrossRef]
- Balsa, L.M.; Ruiz, M.C.; Santa Maria de la Parra, L.; Baran, E.J.; Leon, I.E. Anticancer and antimetastatic activity of copper(II)-tropolone complex against human breast cancer cells, breast multicellular spheroids and mammospheres. J. Inorg. Biochem. 2020, 204, 110975. [Google Scholar] [CrossRef]
- Zehra, S.; Gómez-Ruiz, S.; Siddique, H.R.; Tabassum, S.; Arjmand, F. Water soluble ionic Co(II), Cu(II) and Zn(II) diimine–glycinate complexes targeted to tRNA: Structural description, in vitro comparative binding, cleavage and cytotoxic studies towards chemoresistant prostate cancer cells. Dalton Trans. 2020, 49, 16830–16848. [Google Scholar] [CrossRef] [PubMed]
- Plotek, M.; Dudek, K.; Kyziol, A. Selected copper (I) complexes as potential anticancer agent. Chemik 2013, 67, 1181–1190. [Google Scholar]
- Berners-Price, S.J.; Johnson, R.K.; Mirabelli, C.K.; Faucette, L.F.; McCabe, F.L.; Sadler, P.J. Copper(I) complexes with bidentate tertiary phosphine ligands: Solution chemistry and antitumor activity. Inorg. Chem. 1987, 26, 3383–3387. [Google Scholar] [CrossRef]
- Marzano, C.; Gandin, V.; Pellei, M.; Colavito, D.; Papini, G.; Lobbia, G.G.; Del Giudice, E.; Porchia, M.; Tisato, F.; Santini, C. In Vitro Antitumor Activity of the Water Soluble Copper(I) Complexes Bearing the Tris(hydroxymethyl)phosphine Ligand. J. Med. Chem. 2008, 51, 798–808. [Google Scholar] [CrossRef]
- Gandin, V.; Pellei, M.; Tisato, F.; Porchia, M.; Santini, C.; Marzano, C. A novel copper complex induces paraptosis in colon cancer cells via the activation of ER stress signalling. J. Cell Mol. Med. 2012, 16, 142–151. [Google Scholar] [CrossRef]
- Marzano, C.; Pellei, M.; Colavito, D.; Alidori, S.; Lobbia, G.G.; Gandin, V.; Tisato, F.; Santini, C. Synthesis, Characterization, and in Vitro Antitumor Properties of Tris(hydroxymethyl)phosphine Copper(I) Complexes Containing the New Bis(1,2,4-triazol-1-yl)acetate Ligand. J. Med. Chem. 2006, 49, 7317–7324. [Google Scholar] [CrossRef]
- Zanella, A.; Gandin, V.; Porchia, M.; Refosco, F.; Tisato, F.; Sorrentino, F.; Scutari, G.; Rigobello, M.P.; Marzano, C. Cytotoxicity in human cancer cells and mitochondrial dysfunction induced by a series of new copper(I) complexes containing tris(2-cyanoethyl)phosphines. Investig. New Drugs 2011, 29, 1213–1223. [Google Scholar] [CrossRef]
- Balakrishna, M.S.; Suresh, D.; Rai, A.; Mague, J.T.; Panda, D. Dinuclear Copper(I) Complexes Containing Cyclodiphosphazane Derivatives and Pyridyl Ligands: Synthesis, Structural Studies, and Antiproliferative Activity toward Human Cervical and Breast Cancer Cells. Inorg. Chem. 2010, 49, 8790–8801. [Google Scholar] [CrossRef]
- Adwankar, M.K.; Wycliff, C.; Samuelson, A. In vitro cytotoxic effect of new diphenylphosphinoethane-copper(I) complexes on human ovarian carcinoma cells. Indian J. Exp. Biol. 1997, 35, 810–814. [Google Scholar]
- Sanghamitra, N.J.; Phatak, P.; Das, S.; Samuelson, A.G.; Somasundaram, K. Mechanism of Cytotoxicity of Copper(I) Complexes of 1,2-Bis(diphenylphosphino)ethane. J. Med. Chem. 2005, 48, 977–985. [Google Scholar] [CrossRef]
- Cotton, F.A.; Goodgame, D.M.L. Tetrakis(triphenylphosphine)-Silver(I) and (Triphenylphosphine)-Copper(I) Complexes. J. Chem. Soc. 1960, 5267–5269. [Google Scholar]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Martinez, D.; Motevalli, M.; Watkinson, M. Is there really a diagnostically useful relationship between the carbon-oxygen stretching frequencies in metal carboxylate complexes and their coordination mode? Dalton Trans. 2010, 39, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Messmer, G.G.; Palenik, G.J. Crystal structure of bis(triphenylphosphine)copper(I) nitrate. Inorg. Chem. 1969, 8, 2750–2754. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Holtcamp, M.W.; Longridge, E.M.; Klausmeyer, K.K.; Reibenspies, J.H. Structural characterization of bidentate carboxylate derivatives of copper(I) bistriphenylphosphine. Inorganica Chim. Acta 1994, 227, 223–232. [Google Scholar] [CrossRef]
- Eskandari, A.; Suntharalingam, K. A reactive oxygen species-generating, cancer stem cell-potent manganese(II) complex and its encapsulation into polymeric nanoparticles. Chem. Sci. 2019, 10, 7792–7800. [Google Scholar] [CrossRef]
- Dontu, G.; Abdallah, W.M.; Foley, J.M.; Jackson, K.W.; Clarke, M.F.; Kawamura, M.J.; Wicha, M.S. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003, 17, 1253–1270. [Google Scholar] [CrossRef]
- Lu, C.; Laws, K.; Eskandari, A.; Suntharalingam, K. A reactive oxygen species-generating, cyclooxygenase-2 inhibiting, cancer stem cell-potent tetranuclear copper(II) cluster. Dalton Trans. 2017, 46, 12785–12789. [Google Scholar] [CrossRef]
- Eskandari, A.; Kundu, A.; Ghosh, S.; Suntharalingam, K. A Triangular Platinum(II) Multinuclear Complex with Cytotoxicity Towards Breast Cancer Stem Cells. Angew. Chem. Int. Ed. 2019, 58, 12059–12064. [Google Scholar] [CrossRef]
- Komarnicka, U.K.; Kozieł, S.; Zabierowski, P.; Kruszyński, R.; Lesiów, M.K.; Tisato, F.; Porchia, M.; Kyzioł, A. Copper(I) complexes with phosphines P(p-OCH3-Ph)2CH2OH and P(p-OCH3-Ph)2CH2SarGly. Synthesis, multimodal DNA interactions, and prooxidative and in vitro antiproliferative activity. J. Inorg. Biochem. 2020, 203, 110926. [Google Scholar] [CrossRef]
- Pathaw, L.; Khamrang, T.; Selvakumaran, B.; Murali, M.; Arul Prakash, P.; Mohamed Jaabir, M.S.; Velusamy, M. Synthesis, structure, characterization and biological evaluation of 3-substituted 1-pyridin-2-ylimidazo[1,5-a]pyridine-based copper(I)–phosphine complexes for anticancer drug screening. Appl. Organomet. Chem. 2021, 35, e6025. [Google Scholar] [CrossRef]
- Sheldrick, G. SADABS: Program for Absorption Correction Using Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- CrysAlisPro, version 1.171.35.11; Multi-Scans Absorption Correction with SCALE3 ABSPACK Scaling Algorithm; Agilent Technologies: Santa Clara, CA, USA, 2012.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]

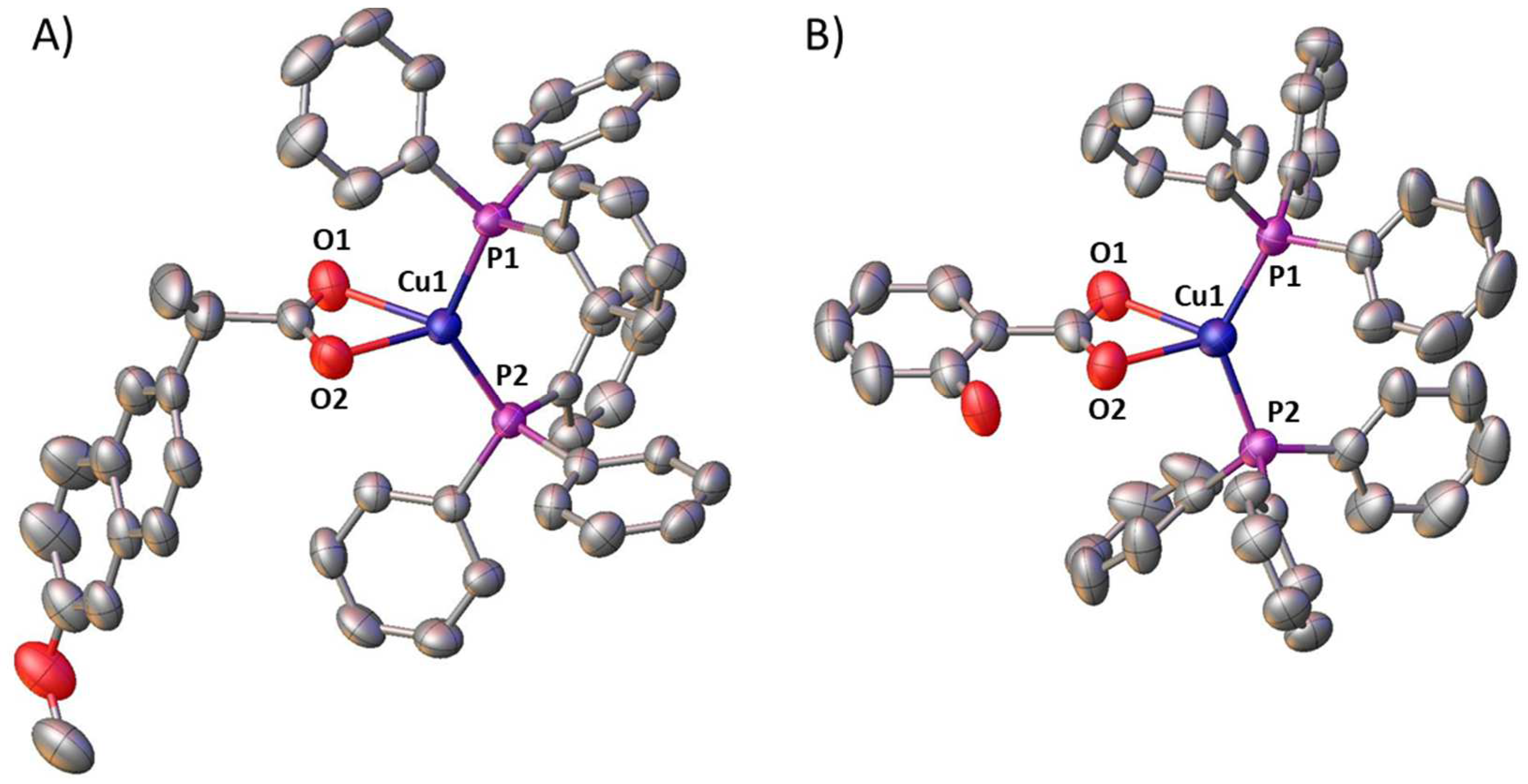
| Cu(1)-O(2) | 2.188(3) | Cu(1)-O(1) | 2.238(3) |
| Cu(1)-P(2) | 2.2305(13) | Cu(1)-P(1) | 2.2513(11) |
| O(2)-Cu(1)-P(2) | 118.00(8) | O(2)-Cu(1)-P(1) | 108.05(8) |
| O(2)-Cu(1)-O(1) | 58.99(10) | P(2)-Cu(1)-P(1) | 128.52(4) |
| P(2)-Cu(1)-O(1) | 117.57(8) | O(1)-Cu(1)-P(1) | 104.84(8) |
| Cu(1)-O(2) | 2.174(3) | Cu(1)-O(1) | 2.298(3) |
| Cu(1)-P(2) | 2.2318(13) | Cu(1)-P(1) | 2.2210(13) |
| O(2)-Cu(1)-P(2) | 102.56(7) | O(2)-Cu(1)-P(1) | 118.41(7) |
| O(2)-Cu(1)-O(1) | 58.73(9) | P(2)-Cu(1)-P(1) | 133.16(5) |
| P(2)-Cu(1)-O(1) | 114.54(7) | O(1)-Cu(1)-P(1) | 106.02(7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, A.; Feng, X.; Singh, K.; Ortu, F.; Suntharalingam, K. The Anti-Breast Cancer Stem Cell Potency of Copper(I)-Non-Steroidal Anti-Inflammatory Drug Complexes. Molecules 2023, 28, 6401. https://doi.org/10.3390/molecules28176401
Johnson A, Feng X, Singh K, Ortu F, Suntharalingam K. The Anti-Breast Cancer Stem Cell Potency of Copper(I)-Non-Steroidal Anti-Inflammatory Drug Complexes. Molecules. 2023; 28(17):6401. https://doi.org/10.3390/molecules28176401
Chicago/Turabian StyleJohnson, Alice, Xiao Feng, Kuldip Singh, Fabrizio Ortu, and Kogularamanan Suntharalingam. 2023. "The Anti-Breast Cancer Stem Cell Potency of Copper(I)-Non-Steroidal Anti-Inflammatory Drug Complexes" Molecules 28, no. 17: 6401. https://doi.org/10.3390/molecules28176401
APA StyleJohnson, A., Feng, X., Singh, K., Ortu, F., & Suntharalingam, K. (2023). The Anti-Breast Cancer Stem Cell Potency of Copper(I)-Non-Steroidal Anti-Inflammatory Drug Complexes. Molecules, 28(17), 6401. https://doi.org/10.3390/molecules28176401








