A Comparative Study of Ethanol and Citric Acid Solutions for Extracting Betalains and Total Phenolic Content from Freeze-Dried Beetroot Powder
Abstract
:1. Introduction
2. Results
2.1. Effect of Extraction Time on Betalains and Total Phenolic Content
2.2. Effect of Extraction Temperature on Betalains and Total Phenolic Content
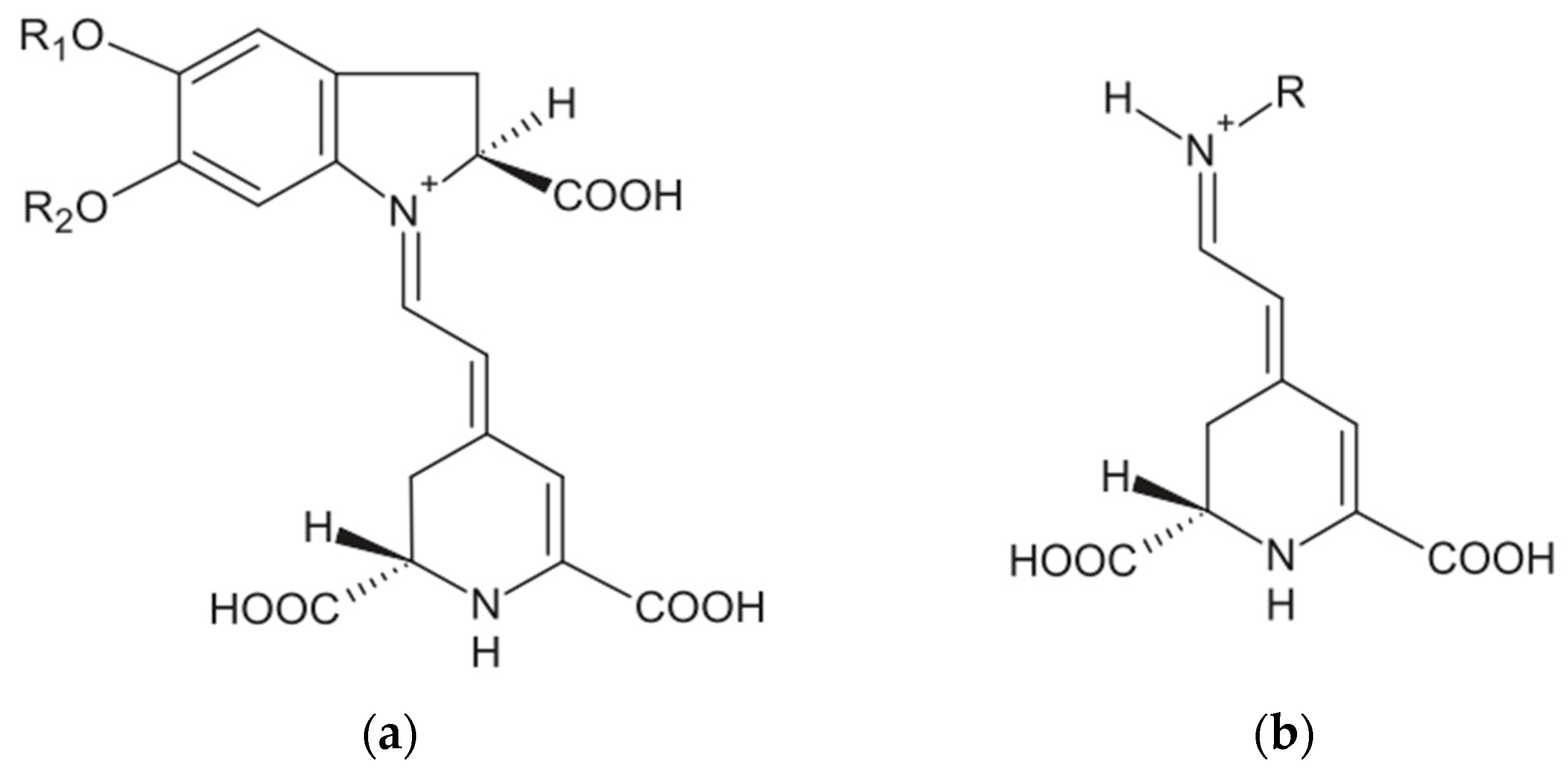
2.3. Effect of Solvent Type on Extraction of Betalains and Total Phenolic Content
2.4. Modelling, Prediction and Optimisation by RSM
2.4.1. Citric Acid Solution as an Extraction Solvent
2.4.2. Aqueous–Ethanol as an Extraction Solvent
2.5. ANN Modelling, Prediction and Comparison with RSM
Predictive Model Development with ANN
2.6. Prediction Performance Comparison for ANN with RSM
2.7. HPLC Analysis
3. Materials and Methods
3.1. Experimental Design
3.2. Chemicals
3.3. Sample Preparation
3.4. Extraction of Betalains
3.4.1. Ultrasound-Assisted Ethanolic Extraction
3.4.2. Preparation of Citric Acid Solution
3.4.3. Ultrasound-assisted Citric Acid Extraction
3.5. Analysis of Betalains
3.5.1. Spectrophotometric Analysis of Total Betalains
3.5.2. Identification and Quantification of Betacyanin and Betaxanthin by High Performance Liquid Chromatography (HPLC) to Validate Spectrophotometric Method
3.6. Total Phenolic Content
3.7. Predictive Modelling and Optimisation
3.7.1. Response Surface Methodology (RSM)
3.7.2. Artificial Neural Network
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nirmal, N.P.; Mereddy, R.; Maqsood, S. Recent developments in emerging technologies for beetroot pigment extraction and its food applications. Food Chem. 2021, 356, 129611. [Google Scholar] [CrossRef]
- Hobbs, D.A.; Kaffa, N.; George, T.W.; Methven, L.; Lovegrove, J.A. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br. J. Nutr. 2012, 108, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Verónica Fernández, M.; Jagus, R.J.; Agüero, M.V. Evaluation and Characterization of Nutritional, Microbiological and Sensory Properties of Beet Greens. Acta Sci. Nutr. Health 2017, 1, 37–45. [Google Scholar]
- Sawicki, T.; Bączek, N.; Wiczkowski, W. Betalain profile, content and antioxidant capacity of red beetroot dependent on the genotype and root part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef]
- Frankowska, A.; Jeswani, H.K.; Azapagic, A. Environmental impacts of vegetables consumption in the UK. Sci. Total Environ. 2019, 682, 80–105. [Google Scholar] [CrossRef]
- Celli, G.B.; Brooks, M.S.L. Impact of extraction and processing conditions on betalains and comparison of properties with anthocyanins—A current review. Food Res. Int. 2017, 100, 501–509. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Biological properties and applications of betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Yañez, J.; Mojica, L.; Luna-Vital, D.A. Technological applications of natural colorants in food systems: A review. Foods 2021, 10, 634. [Google Scholar] [CrossRef]
- Khan, M.I. Plant Betalains: Safety, Antioxidant Activity, Clinical Efficacy, and Bioavailability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.S.; Elbe, J.H.V. Effect of pH on the Degradation and Regeneration of Betanine. J. Food Sci. 1987, 52, 1689–1693. [Google Scholar] [CrossRef]
- Bengardino, M.B.; Fernandez, M.V.; Nutter, J.; Jagus, R.J.; Agüero, M.V. Recovery of bioactive compounds from beet leaves through simultaneous extraction: Modelling and process optimization. Food Bioprod. Process. 2019, 118, 227–236. [Google Scholar] [CrossRef]
- Singh, A.; Ganesapillai, M.; Gnanasundaram, N. Optimizaton of extraction of betalain pigments from beta vulgaris peels by microwave pretreatment. Proc. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032004. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.S.F. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Lazăr, S.; Constantin, O.E.; Stănciuc, N.; Aprodu, I.; Croitoru, C.; Râpeanu, G. Optimization of betalain pigments extraction using beetroot by-products as a valuable source. Inventions 2021, 6, 50. [Google Scholar] [CrossRef]
- Troter, D.; Zlatkovic, M.; Djokic-Stojanovic, D.; Konstantinovic, S.; Todorovic, Z. Citric acid-based deep eutectic solvents: Physical properties and their use as cosolvents in sulphuric acid-catalysed ethanolysis of oleic acid. Adv. Technol. 2016, 5, 53–65. [Google Scholar] [CrossRef]
- Wong, Y.M.; Siow, L.F. Effects of heat, pH, antioxidant, agitation and light on betacyanin stability using red-fleshed dragon fruit (Hylocereus polyrhizus) juice and concentrate as models. J. Food Sci. Technol. 2015, 52, 3086–3092. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Nutter, J.; Fernandez, M.V.; Jagus, R.J.; Agüero, M.V. Development of an aqueous ultrasound-assisted extraction process of bioactive compounds from beet leaves: A proposal for reducing losses and increasing biomass utilization. J. Sci. Food Agric. 2021, 101, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Gaete-Garretón, L.; Vargas-Hernández, Y.; Cares-Pacheco, M.G.; Sainz, J.; Alarcón, J. Ultrasonically enhanced extraction of bioactive principles from Quillaja Saponaria Molina. Ultrasonics 2011, 51, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Fernando, G.S.N.; Wood, K.; Papaioannou, E.H.; Marshall, L.J.; Sergeeva, N.N.; Boesch, C. Application of an Ultrasound-Assisted Extraction Method to Recover Betalains and Polyphenols from Red Beetroot Waste. ACS Sustain. Chem. Eng. 2021, 9, 8736–8747. [Google Scholar] [CrossRef]
- Silva, J.P.P.; Bolanho, B.C.; Stevanato, N.; Massa, T.B.; da Silva, C. Ultrasound-assisted extraction of red beet pigments (Beta vulgaris L.): Influence of operational parameters and kinetic modeling. J. Food Process. Preserv. 2020, 44, e14762. [Google Scholar] [CrossRef]
- Righi Pessoa da Silva, H.; da Silva, C.; Bolanho, B.C. Ultrasonic-assisted extraction of betalains from red beet (Beta vulgaris L.). J. Food Process Eng. 2018, 41, e12833. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Rybak, K.; Grzybowska, E.; Konopka, E.; Witrowa-Rajchert, D. The Influence of Different Pretreatment Methods on Color and Pigment Change in Beetroot Products. Molecules 2021, 26, 3683. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Evaluation of colour properties and chemical quality parameters of cactus juices. Eur. Food Res. Technol. 2003, 216, 303–311. [Google Scholar] [CrossRef]
- Castro-Enríquez, D.D.; Montaño-Leyva, B.; Del Toro-Sánchez, C.L.; Juaréz-Onofre, J.E.; Carvajal-Millan, E.; Burruel-Ibarra, S.E.; Tapia-Hernández, J.A.; Barreras-Urbina, C.G.; Rodríguez-Félix, F. Stabilization of betalains by encapsulation—A review. J. Food Sci. Technol. 2020, 57, 1587–1600. [Google Scholar] [CrossRef]
- Dumbravǎ, A.; Enache, I.; Oprea, C.I.; Georgescu, A.; Gîrţu, M.A. Toward a more efficient utilisation of betalains as pigments for Dye-Sensitized solar cells. Dig. J. Nanomater. Biostructures 2012, 7, 339–351. [Google Scholar]
- Skalicky, M.; Kubes, J.; Shokoofeh, H.; Tahjib-Ul-Arif, M.; Vachova, P.; Hejnak, V. Betacyanins and betaxanthins in cultivated varieties of beta vulgaris L. compared to weed beets. Molecules 2020, 25, 5395. [Google Scholar] [CrossRef]
- Kannan, V. Extraction of Bioactive Compounds from Whole Red Cabbage and Extraction of Bioactive Compounds from Whole Red Cabbage and Beetroot using Pulsed Electric Fields and Evaluation of Their Beetroot Using Pulsed Electric Fields and Evaluation of their Functionality Functionality. Master’s Thesis, University of Nebraska-Lincoln, Lincoln, NE, USA, 2011. [Google Scholar]
- Merin, U.; Gagel, S.; Popel, G.; Bernstein, S.; Rosenthal, I. Thermal Degradation Kinetics of Prickly-Pear-Fruit Red Pigment. J. Food Sci. 1987, 52, 485–486. [Google Scholar] [CrossRef]
- Cardoso-Ugarte, G.A.; Sosa-Morales, M.E.; Ballard, T.; Liceaga, A.; San Martín-González, M.F. Microwave-assisted extraction of betalains from red beet (Beta vulgaris). LWT 2014, 59, 276–282. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Prieto, M.A.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Modern extraction techniques optimized to extract betacyanins from Gomphrena globosa L. Ind. Crop. Prod. 2017, 105, 29–40. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, Y.; Yu, S. Optimisation of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chem. 2015, 172, 543–550. [Google Scholar] [CrossRef]
- Zafar, M.; Kumar, S.; Kumar, S.; Dhiman, A.K. Optimization of polyhydroxybutyrate (PHB) production by Azohydromonas lata MTCC 2311 by using genetic algorithm based on artificial neural network and response surface methodology. Biocatal. Agric. Biotechnol. 2012, 1, 70–79. [Google Scholar] [CrossRef]
- Desai, K.M.; Survase, S.A.; Saudagar, P.S.; Lele, S.S.; Singhal, R.S. Comparison of artificial neural network (ANN) and response surface methodology (RSM) in fermentation media optimization: Case study of fermentative production of scleroglucan. Biochem. Eng. J. 2008, 41, 266–273. [Google Scholar] [CrossRef]
- Bhagya Raj, G.V.S.; Dash, K.K. Microwave vacuum drying of dragon fruit slice: Artificial neural network modelling, genetic algorithm optimization, and kinetics study. Comput. Electron. Agric. 2020, 178, 105814. [Google Scholar] [CrossRef]
- Kumar, R.; Rao, P.S.; Rana, S.S.; Ghosh, P. Comparative performance analysis of enzyme inactivation of soy milk by using RSM and ANN. J. Food Process Eng. 2020, 43, e13530. [Google Scholar] [CrossRef]
- Pires dos Santos, R.; Dean, D.L.; Weaver, J.M.; Hovanski, Y. Identifying the relative importance of predictive variables in artificial neural networks based on data produced through a discrete event simulation of a manufacturing environment. Int. J. Model. Simul. 2019, 39, 234–245. [Google Scholar] [CrossRef]
- Chethana, S.; Nayak, C.A.; Raghavarao, K.S.M.S. Aqueous two phase extraction for purification and concentration of betalains. J. Food Eng. 2007, 81, 679–687. [Google Scholar] [CrossRef]
- Slavov, A.; Karagyozov, V.; Denev, P.; Kratchanova, M.; Kratchanov, C. Antioxidant Activity of Red Beet Juices Obtained after Microwave and Thermal Pretreatments. Czech J. Food Sci. 2013, 31, 139–147. [Google Scholar] [CrossRef]
- Nestora, S.; Merlier, F.; Prost, E.; Haupt, K.; Rossi, C.; Tse Sum Bui, B. Solid-phase extraction of betanin and isobetanin from beetroot extracts using a dipicolinic acid molecularly imprinted polymer. J. Chromatogr. A 2016, 1465, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Tumbas Šaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G.; Jakišić, M.; Djilas, S.; Vulić, J.; Stajčić, S.D.S. Encapsulation of beetroot pomace extract: RSM optimization, storage and gastrointestinal stability. Molecules 2016, 21, 584. [Google Scholar] [CrossRef] [PubMed]
- Bastos, E.L.; Gonçalves, L.C.P. Microwave-Assisted Extraction of Betalains. In Water Extraction of Bioactive Compounds: From Plants to Drug Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 245–268. [Google Scholar] [CrossRef]
- Kumar, R.; Oruna-Concha, M.J.; Methven, L.; Niranjan, K. Modelling extraction kinetics of betalains from freeze dried beetroot powder into aqueous ethanol solutions. J. Food Eng. 2023, 339, 111266. [Google Scholar] [CrossRef]
- Bhagya Raj, G.V.S.; Dash, K.K. Ultrasound-assisted extraction of phytocompounds from dragon fruit peel: Optimization, kinetics and thermodynamic studies. Ultrason. Sonochem. 2020, 68, 105180. [Google Scholar] [CrossRef]
- Malencić, D.; Popović, M.; Miladinović, J. Phenolic content and antioxidant properties of soybean (Glycine max (L.) Merr.) seeds. Molecules 2007, 12, 576–581. [Google Scholar] [CrossRef]
- Rani, P.; Kumar, A.; Purohit, S.R.; Rao, P.S. Impact of fermentation and extrusion processing on physicochemical, sensory and bioactive properties of rice-black gram mixed flour. LWT Food Sci. Technol. 2018, 89, 155–163. [Google Scholar] [CrossRef]
- Lawal, A.I.; Aladejare, A.E.; Onifade, M.; Bada, S.; Idris, M.A. Predictions of elemental composition of coal and biomass from their proximate analyses using ANFIS, ANN and MLR. Int. J. Coal Sci. Technol. 2021, 8, 124–140. [Google Scholar] [CrossRef]
- Elkatatny, S.; Tariq, Z.; Mahmoud, M. Real time prediction of drilling fluid rheological properties using Artificial Neural Networks visible mathematical model (white box). J. Pet. Sci. Eng. 2016, 146, 1202–1210. [Google Scholar] [CrossRef]
- Arab, M.M.; Yadollahi, A.; Shojaeiyan, A.; Ahmadi, H. Artificial neural network genetic algorithm as powerful tool to predict and optimize in vitro proliferation mineral medium for G × N15 rootstock. Front. Plant Sci. 2016, 7, 1526. [Google Scholar] [CrossRef]
- Ouma, Y.O.; Okuku, C.O.; Njau, E.N. Use of Artificial Neural Networks and Multiple Linear Regression Model for the Prediction of Dissolved Oxygen in Rivers: Case Study of Hydrographic Basin of River Nyando, Kenya. Complexity 2020, 2020, 9570789. [Google Scholar] [CrossRef]








| Sl. No. | Variable Name | Variable Coding * | Range |
|---|---|---|---|
| 1 | Extraction Time (min) | Ut | 5–30 |
| 2 | Extraction Temperature (°C) | UT | 20–40 |
| 3 | Ethanol (%) | EC | 10–30 |
| 1 | Extraction Time (min) | Ut | 5–30 |
| 2 | Extraction Temperature (°C) | UT | 20–40 |
| 3 | Citric Acid Solution (pH) | pH | 3–5 |
| Solvent: Aqueous–Ethanol Solutions | Solvent: Citric Acid | |||||
|---|---|---|---|---|---|---|
| Parameters | Betacyanin | Betaxanthin | TPC | Betacyanin | Betaxanthin | TPC |
| A-Extraction Time (min) (Ut) | 0.0233 | --- | 0.0028 | --- | --- | --- |
| B-Extraction Temperature (°C) (UT) | <0.0001 | 0.0033 | 0.0043 | <0.0001 | <0.0001 | <0.0001 |
| Ethanol Concentration (%) (EC)/pH | 0.0001 | 0.0364 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| AB | --- | --- | --- | --- | --- | --- |
| AC | 0.0010 | 0.0034 | --- | --- | --- | --- |
| BC | 0.0001 | --- | <0.0001 | 0.0153 | 0.0153 | 0.0153 |
| A2 | --- | --- | 0.0003 | --- | --- | --- |
| B2 | <0.0001 | <0.0001 | 0.0009 | 0.0222 | 0.0222 | 0.0222 |
| C2 | --- | --- | <0.0001 | --- | --- | --- |
| R2 | 0.887 | 0.799 | 0.863 | 0.782 | 0.892 | 0.794 |
| Sl. No. | Responses | Optimised Response | Average Real-Time experimental Value |
| 1. | BC | 3.95 | 3.91 ± 0.12 |
| 2. | BX | 3.54 | 3.59 ± 0.23 |
| 3. | TPC | 7.17 | 7.06 ± 0.36 |
| Sl. No. | Responses | Optimised Response | Average Real-Time experimental Value |
| 1. | BC | 4.15 | 4.07 ± 0.15 |
| 2. | BX | 3.52 | 3.68 ± 0.13 |
| 3. | TPC | 7.71 | 7.65 ± 0.41 |
| Experimental Responses | Predicted Responses by RSM | Predicted Responses by ANN | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sl. No. | Time (min) | Temperature (°C) | pH | TPC (mg of GA/g) | BC (mg/g) | BX (mg/g) | TPC (mg of GA/g) | BC (mg/g) | BX (mg/g) | TPC (mg of GA/g) | BC (mg/g) | BX (mg/g) |
| 1 | 5 | 40 | 5 | 5.26 | 2.44 | 2.42 | 5.51 | 2.88 | 3.13 | 5.31 | 2.39 | 2.41 |
| 2 | 10 | 40 | 5 | 5.30 | 2.34 | 2.33 | 5.32 | 2.67 | 2.99 | 5.34 | 2.43 | 2.34 |
| 3 | 15 | 40 | 5 | 5.30 | 2.28 | 2.29 | 5.08 | 2.56 | 2.91 | 5.25 | 2.35 | 2.28 |
| 4 | 20 | 40 | 5 | 4.82 | 2.17 | 2.23 | 4.82 | 2.55 | 2.86 | 4.76 | 2.20 | 2.23 |
| 5 | 25 | 40 | 5 | 4.83 | 2.15 | 2.20 | 4.53 | 2.64 | 2.83 | 4.74 | 2.11 | 2.20 |
| 6 | 30 | 40 | 5 | 4.66 | 2.13 | 2.17 | 4.22 | 2.83 | 2.83 | 4.74 | 2.11 | 2.17 |
| 7 | 5 | 40 | 4 | 5.17 | 1.18 | 1.70 | 5.68 | 1.71 | 2.4 | 5.23 | 1.08 | 1.64 |
| 8 | 10 | 40 | 4 | 4.86 | 0.78 | 1.56 | 5.52 | 1.61 | 2.27 | 4.84 | 0.80 | 1.45 |
| 9 | 15 | 40 | 4 | 4.53 | 0.67 | 1.32 | 5.33 | 1.61 | 2.16 | 4.48 | 0.79 | 1.36 |
| 10 | 20 | 40 | 4 | 4.67 | 0.62 | 1.27 | 5.12 | 1.71 | 2.08 | 4.69 | 0.71 | 1.30 |
| 11 | 25 | 40 | 4 | 4.65 | 0.61 | 1.23 | 4.87 | 1.89 | 2.02 | 4.66 | 0.59 | 1.26 |
| 12 | 30 | 40 | 4 | 4.43 | 0.56 | 1.22 | 4.62 | 2.18 | 1.99 | 4.40 | 0.58 | 1.22 |
| 13 | 5 | 40 | 3 | 5.20 | 0.44 | 1.10 | 4.94 | 0.33 | 1.22 | 5.11 | 0.48 | 1.07 |
| 14 | 10 | 40 | 3 | 5.05 | 0.33 | 1.01 | 4.83 | 0.33 | 1.05 | 4.93 | 0.31 | 1.05 |
| 15 | 15 | 40 | 3 | 4.85 | 0.25 | 0.87 | 4.68 | 0.43 | 0.92 | 4.88 | 0.26 | 0.97 |
| 16 | 20 | 40 | 3 | 5.33 | 0.24 | 0.96 | 4.51 | 0.63 | 0.80 | 5.21 | 0.23 | 0.94 |
| 17 | 25 | 40 | 3 | 4.54 | 2.32 | 0.85 | 4.31 | 0.93 | 0.72 | 4.65 | 2.34 | 0.90 |
| 18 | 30 | 40 | 3 | 4.47 | 2.23 | 0.84 | 4.07 | 1.32 | 0.66 | 4.35 | 2.02 | 0.88 |
| 19 | 5 | 30 | 3 | 5.23 | 0.68 | 1.23 | 5.22 | 1.21 | 1.79 | 5.14 | 0.69 | 1.13 |
| 20 | 10 | 30 | 3 | 4.26 | 0.44 | 0.78 | 5.14 | 1.13 | 1.65 | 4.42 | 0.50 | 0.82 |
| 21 | 15 | 30 | 3 | 4.62 | 0.33 | 0.85 | 5.03 | 1.14 | 1.53 | 4.43 | 0.39 | 0.77 |
| 22 | 20 | 30 | 3 | 3.90 | 0.26 | 0.66 | 4.89 | 1.26 | 1.44 | 4.08 | 0.37 | 0.68 |
| 23 | 25 | 30 | 3 | 3.86 | 0.25 | 0.65 | 4.72 | 1.47 | 1.38 | 4.06 | 0.26 | 0.65 |
| 24 | 30 | 30 | 3 | 3.80 | 1.00 | 0.64 | 4.52 | 1.77 | 1.34 | 3.85 | 0.89 | 0.63 |
| 25 | 5 | 30 | 4 | 7.08 | 2.77 | 2.49 | 6.73 | 2.84 | 2.95 | 6.99 | 2.70 | 2.48 |
| 26 | 10 | 30 | 4 | 7.12 | 2.65 | 2.46 | 6.61 | 2.65 | 2.84 | 7.09 | 2.56 | 2.42 |
| 27 | 15 | 30 | 4 | 7.14 | 2.53 | 2.38 | 6.46 | 2.56 | 2.75 | 7.03 | 2.41 | 2.40 |
| 28 | 20 | 30 | 4 | 7.26 | 2.58 | 2.44 | 6.28 | 2.57 | 2.69 | 7.15 | 2.46 | 2.42 |
| 29 | 25 | 30 | 4 | 7.17 | 2.51 | 2.38 | 6.07 | 2.67 | 2.66 | 7.30 | 2.46 | 2.40 |
| 30 | 30 | 30 | 4 | 7.12 | 2.47 | 2.38 | 5.83 | 2.88 | 2.65 | 7.23 | 2.47 | 2.34 |
| 31 | 5 | 30 | 5 | 8.13 | 3.35 | 2.81 | 7.34 | 4.24 | 3.63 | 8.13 | 3.20 | 2.75 |
| 32 | 10 | 30 | 5 | 8.28 | 3.33 | 2.80 | 7.18 | 3.95 | 3.54 | 8.17 | 3.20 | 2.79 |
| 33 | 15 | 30 | 5 | 8.20 | 3.30 | 2.77 | 6.98 | 3.76 | 3.48 | 7.96 | 3.22 | 2.80 |
| 34 | 20 | 30 | 5 | 5.23 | 3.28 | 2.78 | 6.76 | 3.66 | 3.45 | 5.35 | 3.36 | 2.78 |
| 35 | 25 | 30 | 5 | 5.08 | 3.20 | 2.73 | 6.51 | 3.66 | 3.44 | 5.11 | 3.36 | 2.75 |
| 36 | 30 | 30 | 5 | 5.03 | 3.07 | 2.73 | 6.23 | 3.76 | 3.46 | 4.97 | 3.18 | 2.72 |
| 37 | 5 | 20 | 3 | 5.42 | 1.34 | 1.71 | 4.56 | 1.29 | 2.01 | 5.56 | 1.42 | 1.63 |
| 38 | 10 | 20 | 3 | 5.21 | 1.07 | 1.55 | 4.52 | 1.12 | 1.89 | 5.37 | 1.28 | 1.57 |
| 39 | 15 | 20 | 3 | 4.52 | 0.90 | 1.43 | 4.44 | 1.05 | 1.79 | 4.56 | 1.05 | 1.51 |
| 40 | 20 | 20 | 3 | 4.28 | 0.91 | 1.48 | 4.34 | 1.07 | 1.72 | 4.26 | 0.88 | 1.47 |
| 41 | 25 | 20 | 3 | 4.22 | 0.88 | 1.42 | 4.21 | 1.23 | 1.68 | 4.30 | 0.84 | 1.43 |
| 42 | 30 | 20 | 3 | 4.22 | 0.85 | 1.41 | 4.05 | 1.42 | 1.66 | 4.16 | 0.80 | 1.41 |
| 43 | 5 | 20 | 4 | 5.81 | 2.42 | 2.40 | 6.86 | 3.15 | 3.15 | 5.72 | 2.39 | 2.43 |
| 44 | 10 | 20 | 4 | 6.12 | 2.31 | 2.32 | 6.77 | 2.88 | 3.06 | 6.01 | 2.37 | 2.37 |
| 45 | 15 | 20 | 4 | 6.60 | 2.28 | 2.33 | 6.66 | 2.75 | 2.99 | 6.44 | 2.34 | 2.32 |
| 46 | 20 | 20 | 4 | 6.28 | 2.23 | 2.27 | 6.51 | 2.63 | 2.95 | 6.22 | 2.30 | 2.28 |
| 47 | 25 | 20 | 4 | 6.18 | 2.22 | 2.23 | 6.34 | 2.65 | 2.94 | 6.13 | 2.24 | 2.29 |
| 48 | 30 | 20 | 4 | 6.19 | 2.21 | 2.21 | 6.13 | 2.76 | 2.95 | 6.19 | 2.21 | 2.30 |
| 49 | 5 | 20 | 5 | 7.26 | 3.31 | 2.75 | 8.25 | 4.80 | 3.8 | 7.23 | 3.37 | 2.74 |
| 50 | 10 | 20 | 5 | 8.13 | 3.37 | 2.80 | 8.12 | 4.42 | 3.74 | 7.99 | 3.34 | 2.73 |
| 51 | 15 | 20 | 5 | 8.28 | 3.36 | 2.77 | 7.96 | 4.14 | 3.7 | 8.42 | 3.32 | 2.74 |
| 52 | 20 | 20 | 5 | 8.05 | 3.36 | 2.79 | 7.78 | 3.96 | 3.69 | 8.16 | 3.32 | 2.78 |
| 53 | 25 | 20 | 5 | 7.95 | 3.29 | 2.77 | 7.56 | 3.88 | 3.75 | 7.73 | 3.37 | 2.78 |
| 54 | 30 | 20 | 5 | 7.67 | 3.29 | 2.74 | 7.32 | 3.89 | 3.75 | 7.59 | 3.38 | 2.75 |
| Experimental Data | Predicted Responses by RSM | Predicted Responses by ANN | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sl. No. | Time (min) | Temperature (°C) | EC (%) | BC (mg/g) | BX (mg/g) | TPC (mg of GA/g) | BC (mg/g) | BX (mg/g) | TPC (mg of GA/g) | BC (mg/g) | BX (mg/g) | TPC (mg of GA/g) |
| 1 | 5 | 40 | 30 | 4.04 | 3.61 | 7.92 | 3.95 | 3.43 | 8.09 | 3.96 | 3.57 | 7.93 |
| 2 | 10 | 40 | 30 | 3.81 | 3.43 | 8.16 | 3.91 | 3.39 | 8.30 | 3.91 | 3.51 | 8.15 |
| 3 | 15 | 40 | 30 | 3.98 | 3.45 | 8.31 | 3.87 | 3.35 | 8.43 | 3.88 | 3.43 | 8.30 |
| 4 | 20 | 40 | 30 | 3.81 | 3.38 | 8.32 | 3.83 | 3.31 | 8.49 | 3.84 | 3.37 | 8.33 |
| 5 | 25 | 40 | 30 | 3.77 | 3.30 | 8.30 | 3.78 | 3.27 | 8.47 | 3.79 | 3.31 | 8.31 |
| 6 | 30 | 40 | 30 | 3.72 | 3.23 | 8.25 | 3.73 | 3.23 | 8.37 | 3.68 | 3.26 | 8.27 |
| 7 | 5 | 40 | 20 | 3.65 | 3.16 | 7.85 | 3.80 | 3.33 | 7.83 | 3.68 | 3.17 | 7.88 |
| 8 | 10 | 40 | 20 | 3.69 | 3.19 | 8.02 | 3.80 | 3.33 | 8.04 | 3.72 | 3.20 | 8.09 |
| 9 | 15 | 40 | 20 | 3.77 | 3.29 | 8.18 | 3.79 | 3.33 | 8.17 | 3.74 | 3.24 | 8.17 |
| 10 | 20 | 40 | 20 | 3.71 | 3.29 | 8.21 | 3.78 | 3.32 | 8.22 | 3.74 | 3.29 | 8.18 |
| 11 | 25 | 40 | 20 | 3.76 | 3.35 | 8.17 | 3.77 | 3.32 | 8.19 | 3.77 | 3.34 | 8.17 |
| 12 | 30 | 40 | 20 | 3.79 | 3.40 | 8.13 | 3.75 | 3.31 | 8.09 | 3.79 | 3.42 | 8.15 |
| 13 | 5 | 40 | 10 | 3.88 | 3.34 | 7.34 | 3.75 | 3.27 | 7.07 | 3.88 | 3.41 | 7.31 |
| 14 | 10 | 40 | 10 | 3.89 | 3.34 | 7.39 | 3.78 | 3.31 | 7.27 | 3.90 | 3.35 | 7.43 |
| 15 | 15 | 40 | 10 | 3.82 | 3.28 | 7.44 | 3.81 | 3.34 | 7.40 | 3.91 | 3.33 | 7.46 |
| 16 | 20 | 40 | 10 | 3.89 | 3.34 | 7.51 | 3.84 | 3.37 | 7.44 | 3.91 | 3.33 | 7.47 |
| 17 | 25 | 40 | 10 | 3.86 | 3.31 | 7.50 | 3.86 | 3.46 | 7.41 | 3.87 | 3.32 | 7.47 |
| 18 | 30 | 40 | 10 | 3.86 | 3.31 | 7.49 | 3.88 | 3.43 | 7.3 | 3.81 | 3.31 | 7.46 |
| 19 | 5 | 30 | 30 | 4.03 | 3.39 | 7.56 | 4.10 | 3.58 | 7.28 | 4.04 | 3.40 | 7.56 |
| 20 | 10 | 30 | 30 | 4.03 | 3.39 | 7.76 | 4.08 | 3.56 | 7.49 | 4.06 | 3.38 | 7.75 |
| 21 | 15 | 30 | 30 | 4.17 | 3.51 | 7.87 | 4.05 | 3.53 | 7.62 | 4.11 | 3.38 | 7.89 |
| 22 | 20 | 30 | 30 | 4.04 | 3.39 | 7.89 | 4.03 | 3.5 | 7.68 | 4.13 | 3.41 | 7.90 |
| 23 | 25 | 30 | 30 | 4.12 | 3.46 | 7.85 | 4.00 | 3.47 | 7.66 | 4.12 | 3.45 | 7.84 |
| 24 | 30 | 30 | 30 | 4.14 | 3.47 | 7.80 | 3.96 | 3.43 | 7.56 | 4.10 | 3.50 | 7.76 |
| 25 | 5 | 30 | 20 | 4.12 | 3.57 | 7.49 | 4.03 | 3.53 | 7.41 | 4.14 | 3.63 | 7.48 |
| 26 | 10 | 30 | 20 | 4.20 | 3.69 | 7.70 | 4.05 | 3.54 | 7.62 | 4.09 | 3.60 | 7.69 |
| 27 | 15 | 30 | 20 | 4.06 | 3.51 | 7.78 | 4.06 | 3.55 | 7.75 | 4.02 | 3.54 | 7.78 |
| 28 | 20 | 30 | 20 | 3.97 | 3.44 | 7.79 | 4.07 | 3.55 | 7.87 | 3.98 | 3.47 | 7.79 |
| 29 | 25 | 30 | 20 | 3.94 | 3.41 | 7.77 | 4.07 | 3.55 | 7.77 | 3.98 | 3.40 | 7.78 |
| 30 | 30 | 30 | 20 | 3.88 | 3.35 | 7.74 | 4.07 | 3.55 | 7.67 | 3.99 | 3.35 | 7.77 |
| 31 | 5 | 30 | 10 | 3.95 | 3.54 | 6.96 | 4.07 | 3.51 | 7.04 | 3.98 | 3.53 | 6.97 |
| 32 | 10 | 30 | 10 | 4.00 | 3.58 | 7.03 | 4.12 | 3.56 | 7.24 | 4.07 | 3.60 | 7.09 |
| 33 | 15 | 30 | 10 | 4.21 | 3.78 | 7.12 | 4.17 | 3.62 | 7.37 | 4.16 | 3.67 | 7.11 |
| 34 | 20 | 30 | 10 | 4.16 | 3.74 | 7.13 | 4.22 | 3.64 | 7.41 | 4.24 | 3.76 | 7.11 |
| 35 | 25 | 30 | 10 | 4.29 | 3.87 | 7.11 | 4.26 | 3.68 | 7.38 | 4.29 | 3.87 | 7.10 |
| 36 | 30 | 30 | 10 | 4.38 | 3.95 | 7.10 | 4.29 | 3.72 | 7.27 | 4.35 | 3.95 | 7.10 |
| 37 | 5 | 20 | 10 | 3.84 | 3.24 | 7.53 | 4.01 | 3.41 | 7.38 | 3.98 | 3.31 | 7.53 |
| 38 | 10 | 20 | 10 | 4.22 | 3.52 | 7.71 | 4.08 | 3.46 | 7.58 | 4.14 | 3.44 | 7.68 |
| 39 | 15 | 20 | 10 | 3.99 | 3.33 | 7.78 | 4.14 | 3.52 | 7.71 | 4.07 | 3.43 | 7.75 |
| 40 | 20 | 20 | 10 | 4.22 | 3.48 | 7.78 | 4.26 | 3.57 | 7.75 | 4.16 | 3.48 | 7.78 |
| 41 | 25 | 20 | 10 | 4.26 | 3.52 | 7.76 | 4.26 | 3.62 | 7.72 | 4.24 | 3.54 | 7.76 |
| 42 | 30 | 20 | 10 | 4.32 | 3.58 | 7.72 | 4.31 | 3.66 | 7.62 | 4.31 | 3.62 | 7.72 |
| 43 | 5 | 20 | 20 | 3.96 | 3.42 | 7.49 | 3.88 | 3.38 | 7.36 | 4.01 | 3.46 | 7.48 |
| 44 | 10 | 20 | 20 | 4.08 | 3.57 | 7.60 | 3.91 | 3.44 | 7.57 | 4.06 | 3.50 | 7.62 |
| 45 | 15 | 20 | 20 | 4.05 | 3.51 | 7.67 | 3.94 | 3.42 | 7.74 | 4.08 | 3.53 | 7.67 |
| 46 | 20 | 20 | 20 | 4.02 | 3.49 | 7.69 | 3.97 | 3.44 | 7.75 | 4.07 | 3.55 | 7.69 |
| 47 | 25 | 20 | 20 | 4.05 | 3.53 | 7.68 | 3.99 | 3.45 | 7.72 | 4.06 | 3.56 | 7.69 |
| 48 | 30 | 20 | 20 | 4.06 | 3.55 | 7.66 | 4.01 | 3.46 | 7.62 | 4.05 | 3.55 | 7.66 |
| 49 | 5 | 20 | 30 | 3.88 | 3.47 | 6.86 | 3.85 | 3.4 | 6.84 | 3.88 | 3.43 | 6.85 |
| 50 | 10 | 20 | 30 | 3.75 | 3.37 | 6.91 | 3.85 | 3.38 | 7.05 | 3.82 | 3.41 | 6.92 |
| 51 | 15 | 20 | 30 | 3.76 | 3.36 | 6.96 | 3.85 | 3.37 | 7.18 | 3.78 | 3.38 | 6.99 |
| 52 | 20 | 20 | 30 | 3.78 | 3.37 | 6.99 | 3.84 | 3.35 | 7.24 | 3.75 | 3.35 | 6.99 |
| 53 | 25 | 20 | 30 | 3.74 | 3.31 | 7.00 | 3.82 | 3.32 | 7.21 | 3.73 | 3.33 | 6.99 |
| 54 | 30 | 20 | 30 | 3.73 | 3.28 | 6.99 | 3.81 | 3.34 | 7.12 | 3.72 | 3.30 | 7.01 |
| 1. Citric Acid solution as Solvent | ||||
| Sl. No. | Responses | RMSE | MSE | R2 |
| 1 | BC | 0.032 | 0.092 | 0.99 |
| 2 | BX | 0.052 | 0.002 | 0.99 |
| 3 | TPC | 0.023 | 0.055 | 0.99 |
| 2. Aqueous–ethanol as solvent | ||||
| 1 | BC | 0.051 | 0.003 | 0.99 |
| 2 | BX | 0.047 | 0.002 | 0.99 |
| 3 | TPC | 0.020 | 0.001 | 0.99 |
| 1. Citric acid solution as solvent | ||||
| Sl. No. | Responses | RMSE | MSE | R2 |
| 1 | BC | 0.600 | 0.101 | 0.78 |
| 2 | BX | 0.282 | 0.126 | 0.89 |
| 3 | TPC | 0.671 | 0.138 | 0.79 |
| 2. Aqueous–ethanol as solvent | ||||
| 1 | BC | 0.471 | 0.211 | 0.88 |
| 2 | BX | 0.206 | 0.193 | 0.79 |
| 3 | TPC | 0.262 | 0.164 | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Methven, L.; Oruna-Concha, M.J. A Comparative Study of Ethanol and Citric Acid Solutions for Extracting Betalains and Total Phenolic Content from Freeze-Dried Beetroot Powder. Molecules 2023, 28, 6405. https://doi.org/10.3390/molecules28176405
Kumar R, Methven L, Oruna-Concha MJ. A Comparative Study of Ethanol and Citric Acid Solutions for Extracting Betalains and Total Phenolic Content from Freeze-Dried Beetroot Powder. Molecules. 2023; 28(17):6405. https://doi.org/10.3390/molecules28176405
Chicago/Turabian StyleKumar, Rahul, Lisa Methven, and Maria Jose Oruna-Concha. 2023. "A Comparative Study of Ethanol and Citric Acid Solutions for Extracting Betalains and Total Phenolic Content from Freeze-Dried Beetroot Powder" Molecules 28, no. 17: 6405. https://doi.org/10.3390/molecules28176405
APA StyleKumar, R., Methven, L., & Oruna-Concha, M. J. (2023). A Comparative Study of Ethanol and Citric Acid Solutions for Extracting Betalains and Total Phenolic Content from Freeze-Dried Beetroot Powder. Molecules, 28(17), 6405. https://doi.org/10.3390/molecules28176405







