Influence of Backbone Ladderization and Side Chain Variation on the Orientation of Diketopyrrolopyrrole-Based Donor-Acceptor Copolymers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Choice of Polymers and Characterization
2.2. Molecular Orientation of Polymers in Thin Films Determined by PM-IRRAS
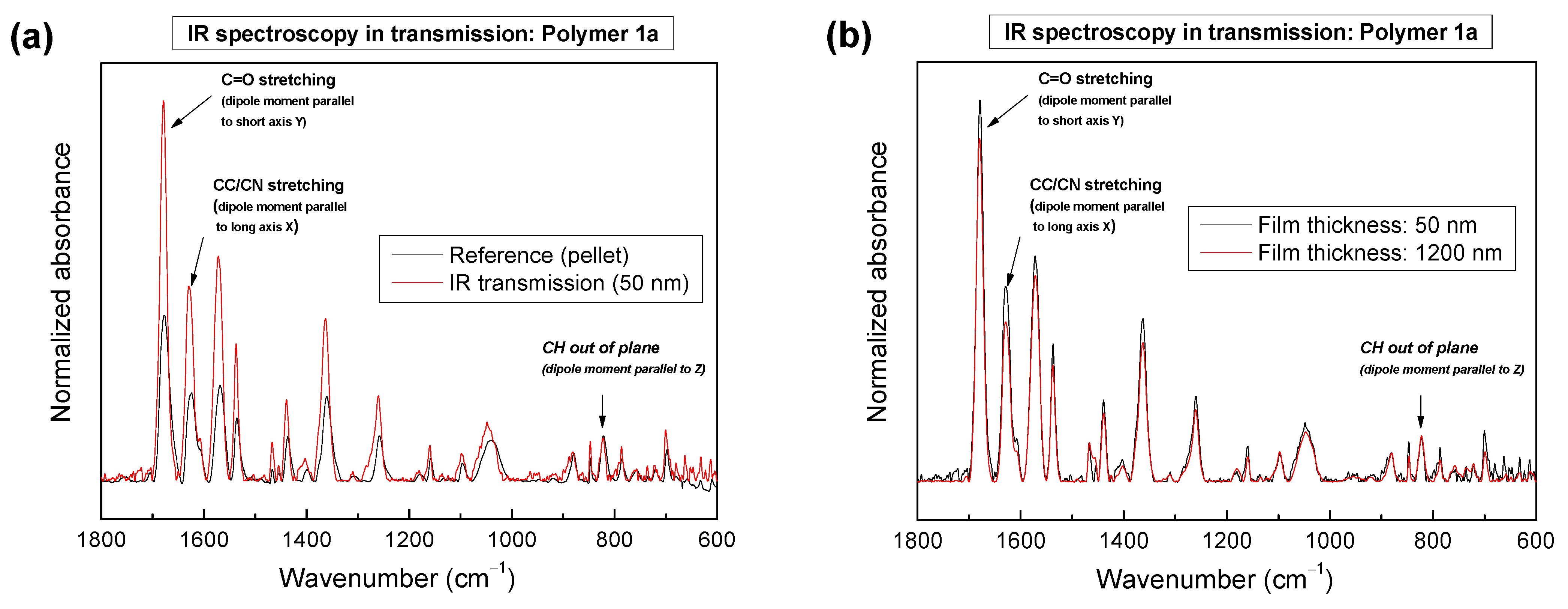
2.3. Azimuthal Orientation and Thickness-Dependence Studied Using IR Spectroscopy in Transmission
3. Materials and Methods
3.1. Materials and Sample Preparation
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, L.Y.; Lai, B.H.; Ran, X.G.; Tang, H.; Cao, D.R. Recent Advances of Diketopyrrolopyrrole Derivatives in Cancer Therapy and Imaging Applications. Molecules 2023, 28, 4097. [Google Scholar] [CrossRef] [PubMed]
- Bijleveld, J.C.; Zoombelt, A.P.; Mathijssen, S.G.J.; Wienk, M.M.; Turbiez, M.; de Leeuw, D.M.; Janssen, R.A.J. Poly(diketopyrrolopyrrole-terthiophene) for Ambipolar Logic and Photovoltaics. J. Am. Chem. Soc. 2009, 131, 16616. [Google Scholar] [CrossRef]
- Hendriks, K.H.; Li, W.W.; Wienk, M.M.; Janssen, R.A.J. Band Gap Control in Diketopyrrolopyrrole-Based Polymer Solar Cells Using Electron Donating Side Chains. Adv. Energy Mater. 2013, 3, 674–679. [Google Scholar] [CrossRef]
- Li, W.W.; Hendriks, K.H.; Wienk, M.M.; Janssen, R.A.J. Diketopyrrolopyrrole Polymers for Organic Solar Cells. Acc. Chem. Res. 2016, 49, 78–85. [Google Scholar] [CrossRef]
- He, Q.; Tam, T.L.D.; Koh, X.Q.; Tham, N.N.; Meng, H.; Huang, W.; Xu, J. P-and N-dopable ambipolar bulk heterojunction thermoelectrics based on ladder-type conjugated polymers. J. Mater. Chem. C 2023, 11, 204–210. [Google Scholar] [CrossRef]
- Otep, S.; Lin, Y.C.; Matsumoto, H.; Mori, T.; Wei, K.H.; Michinobu, T. Diketopyrrolopyrrole-thiophene-methoxythiophene based random copolymers for organic field effect transistor applications. Org. Electron. 2020, 87, 105986. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, C.H.; Tsai, B.S.; Hsueh, T.F.; Tsao, C.S.; Tan, S.; Chang, B.; Chang, Y.N.; Chu, T.Y.; Tsai, C.E.; et al. Alkoxy- and Alkyl-Side-Chain-Functionalized Terpolymer Acceptors for All-Polymer Photovoltaics Delivering High Open-Circuit Voltages and Efficiencies. Adv. Funct. Mater. 2023, 33, 2215095. [Google Scholar] [CrossRef]
- Wang, J.W.; Feng, K.; Jeong, S.Y.; Liu, B.; Wang, Y.M.; Wu, W.C.; Hou, Y.X.; Woo, H.Y.; Guo, X.G. Acceptor-acceptor type polymers based on cyano-substituted benzochalcogenadiazole and diketopyrrolopyrrole for high-efficiency n-type organic thermoelectrics. Polym. J. 2023, 55, 507–515. [Google Scholar] [CrossRef]
- Wang, M.; Fu, S.; Petkov, P.; Fu, Y.; Zhang, Z.; Liu, Y.; Ma, J.; Chen, G.; Gali, S.M.; Gao, L.; et al. Exceptionally high charge mobility in phthalocyanine-based poly(benzimidazobenzophenanthroline)-ladder-type two-dimensional conjugated polymers. Nat. Mater. 2023, 22, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.W.H.; Benedetti, F.M.; Ahn, J.M.; Robinson, A.M.; Wang, Y.; Pinnau, I.; Smith, Z.P.; Xia, Y. Hydrocarbon ladder polymers with ultrahigh permselectivity for membrane gas separations. Science 2022, 375, 1390–1392. [Google Scholar] [CrossRef]
- Zheng, W.; Oki, K.; Saha, R.; Hijikata, Y.; Yashima, E.; Ikai, T. One-Handed Helical Tubular Ladder Polymers for Chromatographic Enantioseparation**. Angew. Chem. 2023, 135, e202218297. [Google Scholar] [CrossRef]
- Lupton, J.M.; Scherf, U. Conjugated, Aromatic Ladder Polymers: From Precision Synthesis to Single Chain Spectroscopy and Strong Light-Matter Coupling. In Ladder Polymers; Wiley Online Library: Hoboken, NJ, USA, 2023; pp. 13–58. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, M.; Wan, S.; Yin, P.; Wang, P.; Cai, D.; Liu, F.; Zheng, Q. Efficient Organic Solar Cells from Molecular Orientation Control of M-Series Acceptors. Joule 2021, 5, 197–209. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, C.e.; Bi, Z.; Liu, Y.; Jiang, P.; Ming, S.; Xu, X.; Ma, W.; Bo, Z. Controlling Molecular Packing and Orientation via Constructing a Ladder-Type Electron Acceptor with Asymmetric Substituents for Thick-Film Nonfullerene Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 3098–3106. [Google Scholar] [CrossRef]
- Wang, P.; Ma, Y.; Yin, P.; Cai, D.; Wan, S.; Zheng, Q. Molecular orientation, anisotropic electron transport and photovoltaic properties of ladder-type heteroheptacene-based semiconductors. Chem. Eng. J. 2021, 418, 129497. [Google Scholar] [CrossRef]
- West, S.M.; Tran, D.K.; Guo, J.; Chen, S.E.; Ginger, D.S.; Jenekhe, S.A. Phenazine-Substituted Poly(benzimidazobenzophenanthrolinedione): Electronic Structure, Thin Film Morphology, Electron Transport, and Mechanical Properties of an n-Type Semiconducting Ladder Polymer. Macromolecules 2023, 56, 2081–2091. [Google Scholar] [CrossRef]
- Buffeteau, T.; Desbat, B.; Turlet, J.M. Polarization Modulation FT-IR Spectroscopy of Surfaces and Ultra-Thin Films-Experimental Procedure and Quantitative-Analysis. Appl. Spectrosc. 1991, 45, 380–389. [Google Scholar] [CrossRef]
- Pearce, H.A.; Sheppard, N. Possible Importance of a Metal-Surface Selection Rule in Interpretation of Infrared-Spectra of Molecules Adsorbed on Particulate Metals—Infrared-Spectra from Ethylene Chemisorbed on Silica-Supported Metal-Catalysts. Surf. Sci. 1976, 59, 205–217. [Google Scholar] [CrossRef]
- Greenler, R.G. Infrared Study of Adsorbed Molecules on Metal Surfaces by Reflection Techniques. J. Chem. Phys. 1966, 44, 310–315. [Google Scholar] [CrossRef]
- Umemura, J. Reflection–Absorption Spectroscopy of Thin Films on Metallic Substrates. In Handbook of Vibrational Spectroscopy; Wiley Online Library: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Früh, A.; Rutkowski, S.; Akimchenko, I.O.; Tverdokhlebov, S.I.; Frueh, J. Orientation analysis of polymer thin films on metal surfaces via IR absorbance of the relative transition dipole moments. Appl. Surf. Sci. 2022, 594, 153476. [Google Scholar] [CrossRef]
- Trilling, F.; Sachnik, O.; Scherf, U. pi-Expanded diketopyrrolopyrroles as acceptor building blocks for the formation of novel donor-acceptor copolymers. Polym. Chem. 2019, 10, 627–632. [Google Scholar] [CrossRef]
- Micaroni, L.; Nart, F.; Hümmelgen, I. Considerations about the electrochemical estimation of the ionization potential of conducting polymers. J. Solid State Electrochem. 2002, 7, 55–59. [Google Scholar] [CrossRef]
- Hwang, Y.-J.; Murari, N.M.; Jenekhe, S.A. New n-type polymer semiconductors based on naphthalene diimide and selenophene derivatives for organic field-effect transistors. Polym. Chem. 2013, 4, 3187–3195. [Google Scholar] [CrossRef]
- Zhu, P.; Fan, B.; Ying, L.; Huang, F.; Cao, Y. Recent Progress in All-Polymer Solar Cells Based on Wide-Bandgap p-Type Polymers. Chem. Asian J. 2019, 14, 3109–3118. [Google Scholar] [CrossRef]
- Braun, S.; Salaneck, W.R.; Fahlman, M. Energy-Level Alignment at Organic/Metal and Organic/Organic Interfaces. Adv. Mater. 2009, 21, 1450–1472. [Google Scholar] [CrossRef]
- Brocks, G.; Çakır, D.; Bokdam, M.; de Jong, M.P.; Fahlman, M. Charge equilibration and potential steps in organic semiconductor multilayers. Org. Electron. 2012, 13, 1793–1801. [Google Scholar] [CrossRef]
- Bao, Q.; Sandberg, O.; Dagnelund, D.; Sandén, S.; Braun, S.; Aarnio, H.; Liu, X.; Chen, W.M.; Österbacka, R.; Fahlman, M. Trap-Assisted Recombination via Integer Charge Transfer States in Organic Bulk Heterojunction Photovoltaics. Adv. Funct. Mater. 2014, 24, 6309–6316. [Google Scholar] [CrossRef]
- Gliboff, M.; Sang, L.; Knesting, K.M.; Schalnat, M.C.; Mudalige, A.; Ratcliff, E.L.; Li, H.; Sigdel, A.K.; Giordano, A.J.; Berry, J.J.; et al. Orientation of Phenylphosphonic Acid Self-Assembled Monolayers on a Transparent Conductive Oxide: A Combined NEXAFS, PM-IRRAS, and DFT Study. Langmuir 2013, 29, 2166–2174. [Google Scholar] [CrossRef]
- Ramin, M.A.; Le Bourdon, G.; Daugey, N.; Bennetau, B.; Vellutini, L.; Buffeteau, T. PM-IRRAS Investigation of Self-Assembled Monolayers Grafted onto SiO2/Au Substrates. Langmuir 2011, 27, 6076–6084. [Google Scholar] [CrossRef]
- Bölke, S.; Batchelor, D.; Früh, A.; Lassalle-Kaiser, B.; Keller, T.; Trilling, F.; Forster, M.; Scherf, U.; Chassé, T.; Peisert, H. Influence of the Side Chain Structure on the Electronic Structure and Self-Organization Properties of Low Band Gap Polymers. ACS Appl. Energy Mater. 2022, 5, 15290–15301. [Google Scholar] [CrossRef]
- Garcia-Araez, N.; Brosseau, C.L.; Rodriguez, P.; Lipkowski, J. Layer-by-layer PMIRRAS characterization of DMPC bilayers deposited on a Au(111) electrode surface. Langmuir 2006, 22, 10365–10371. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, R.; Mao, G.R.; Flach, C.R. Infrared reflection-absorption spectroscopy: Principles and applications to lipid-protein interaction in Langmuir films. Biochim. Et Biophys. Acta-Biomembr. 2010, 1798, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Takeda, S.; Kawaguchi, A.; Umemura, J. Quantitative analysis of uniaxial molecular orientation in Langmuir-Blodgett films by infrared reflection spectroscopy. Langmuir 1995, 11, 1236–1243. [Google Scholar] [CrossRef]
- Debe, M.K. Extracting physical structure information from thin organic films with reflection absorption infrared spectroscopy. J. Appl. Phys. 1984, 55, 3354–3366. [Google Scholar] [CrossRef]
- Ivanovic, M.; Aygül, U.; Dettinger, U.; Tournebize, A.; Polek, M.; Batchelor, D.; Mangold, S.; Forster, M.; Scherf, U.; Peisert, H.; et al. Electronic structure and self-organization properties of low band gap polymers: The effect of the introduction of additional thiophene moieties. Sol. Energy Mater. Sol. Cells 2016, 157, 286–294. [Google Scholar] [CrossRef]
- Aygül, U.; Peisert, H.; Batchelor, D.; Dettinger, U.; Ivanovic, M.; Tournebize, A.; Mangold, S.; Förster, M.; Dumsch, I.; Kowalski, S.; et al. Molecular orientation in polymer/fullerene blend films and the influence of annealing. Sol. Energy Mater. Sol. Cells 2014, 128, 119–125. [Google Scholar] [CrossRef]
- Borges, B.; Veiga, A.G.; Tzounis, L.; Laskarakis, A.; Logothetidis, S.; Rocco, M.L.M. Molecular Orientation and Ultrafast Charge Transfer Dynamics Studies on the P3HT:PCBM Blend. J. Phys. Chem. C 2016, 120, 25078–25082. [Google Scholar] [CrossRef]
- Hasegawa, T.; Shioya, N. MAIRS: Innovation of Molecular Orientation Analysis in a Thin Film. Bull. Chem. Soc. Jpn. 2020, 93, 1127–1138. [Google Scholar] [CrossRef]
- Shioya, N.; Shimoaka, T.; Murdey, R.; Hasegawa, T. Accurate Molecular Orientation Analysis Using Infrared p-Polarized Multiple-Angle Incidence Resolution Spectrometry (pMAIRS) Considering the Refractive Index of the Thin Film Sample. Appl. Spectrosc. 2017, 71, 1242–1248. [Google Scholar] [CrossRef]
- Anton, A.M.; Steyrleuthner, R.; Kossack, W.; Neher, D.; Kremer, F. Infrared Transition Moment Orientational Analysis on the Structural Organization of the Distinct Molecular Subunits in Thin Layers of a High Mobility n-Type Copolymer. J. Am. Chem. Soc. 2015, 137, 6034–6043. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
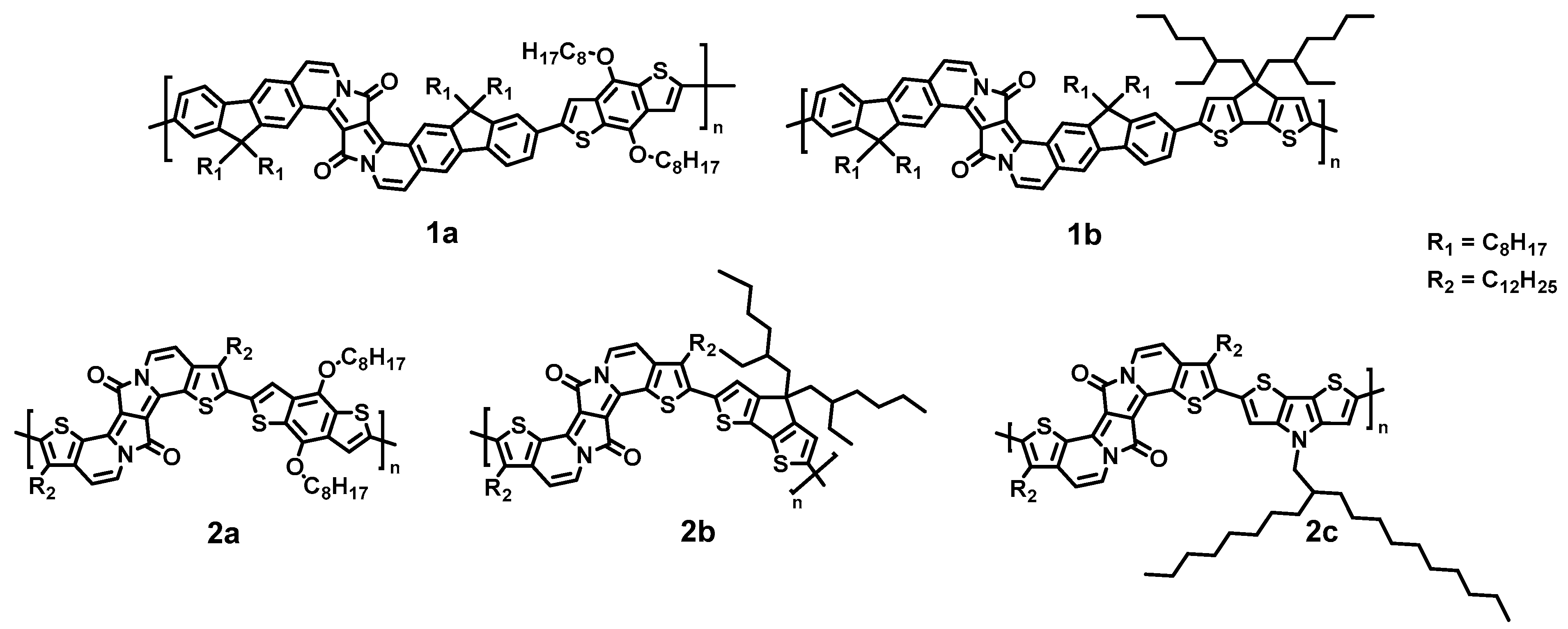
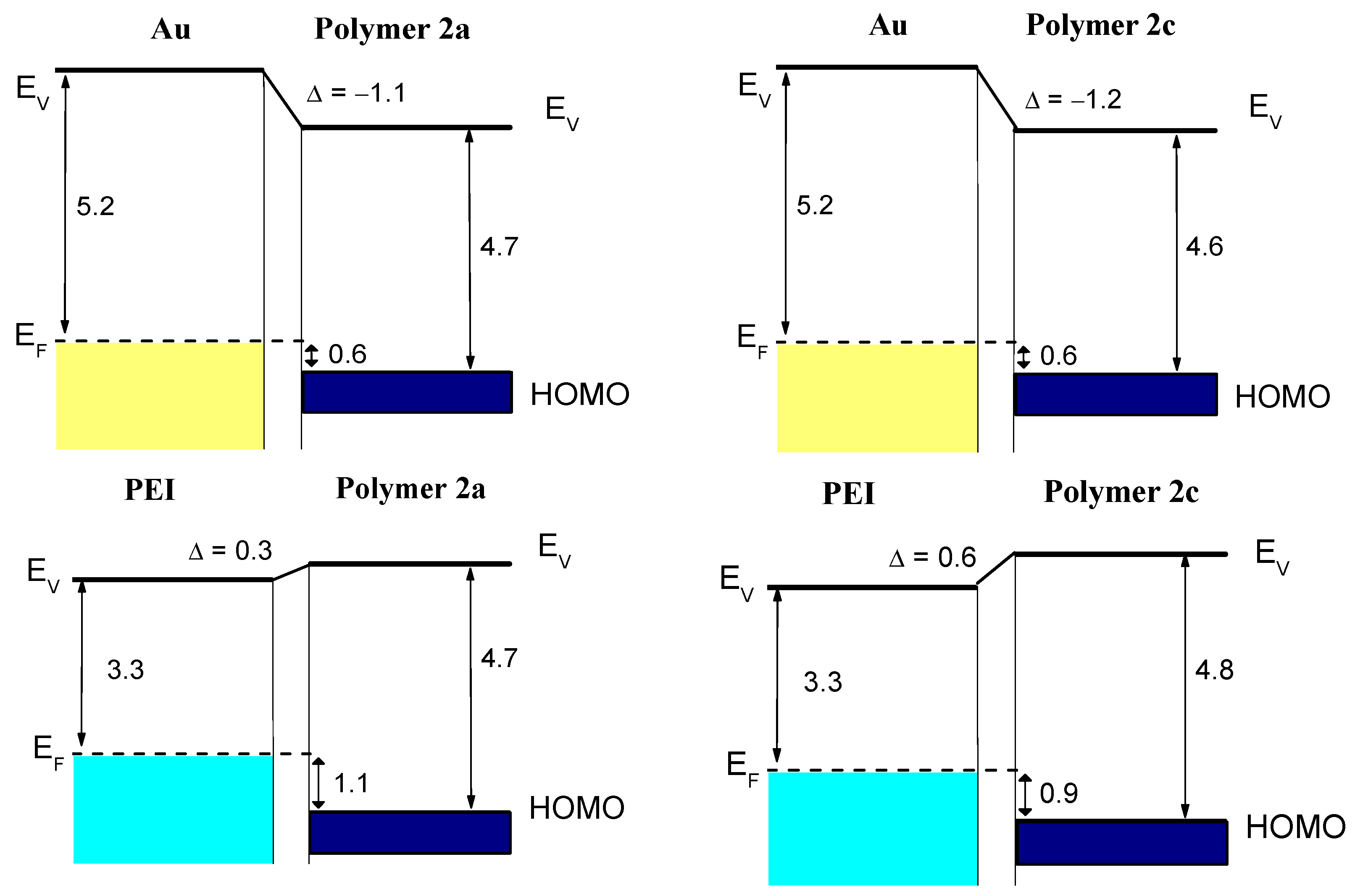
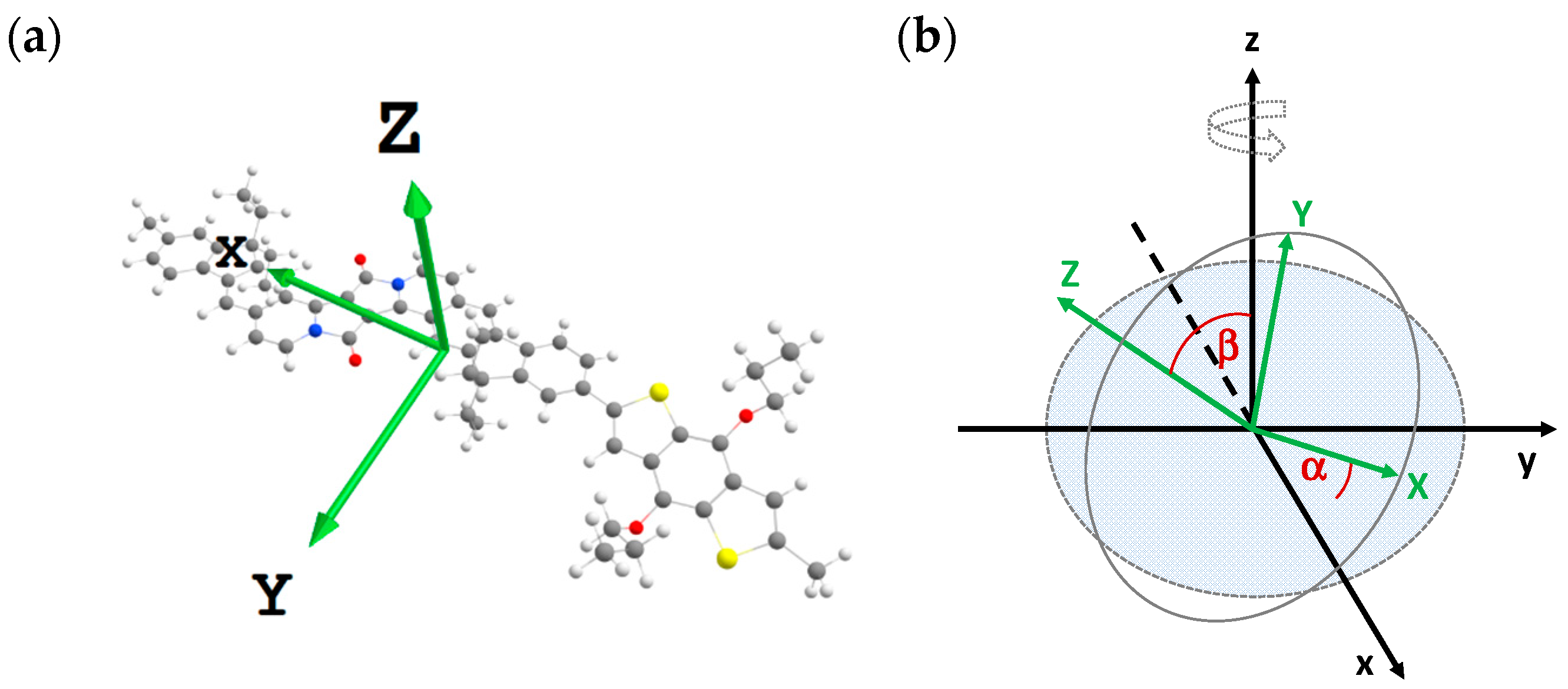
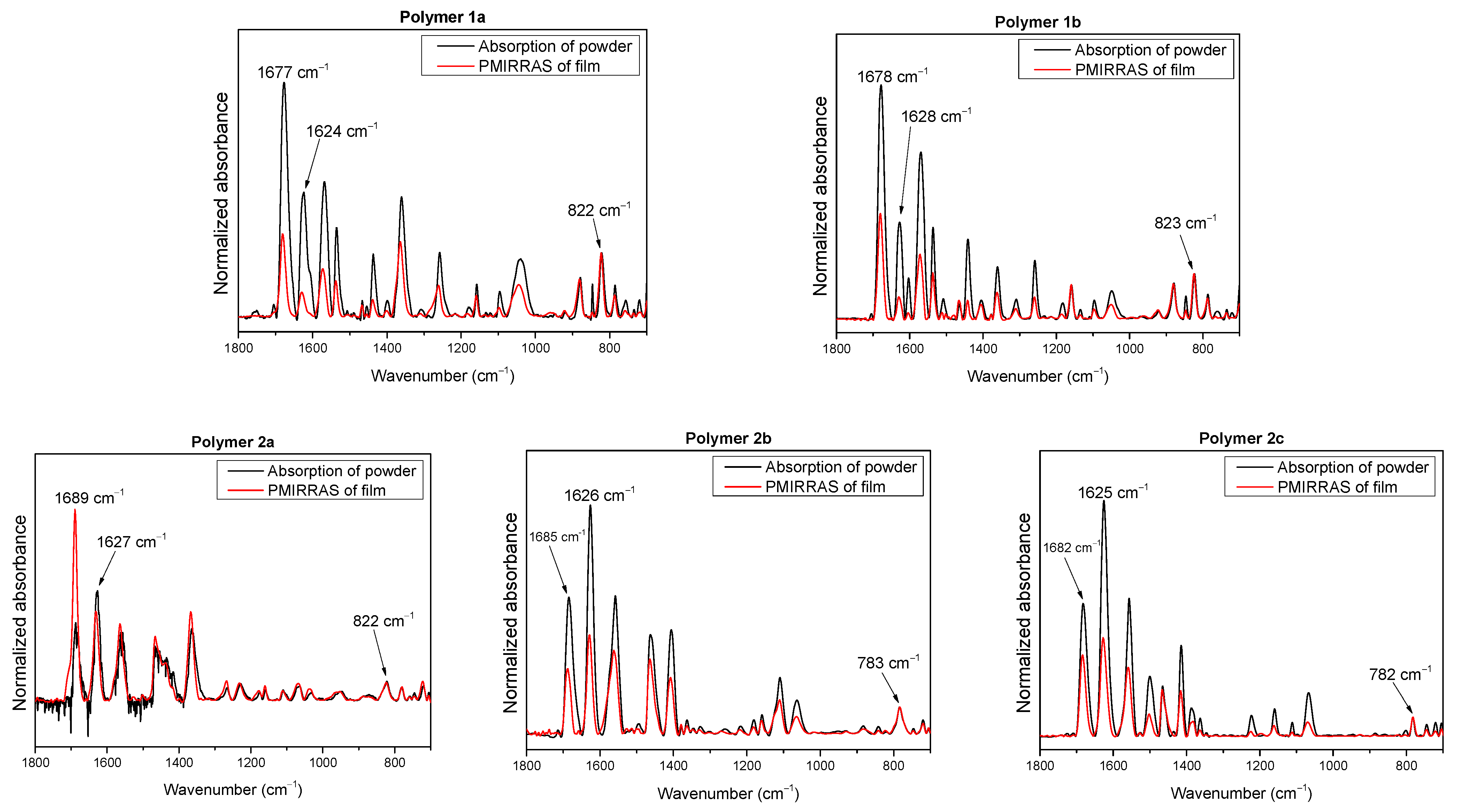

| 1a | 1b | 2a | 2b | 2c | Assignment |
|---|---|---|---|---|---|
| 785 | 785 | 781 | 783 | 782 | CH oop |
| 822 | 823 | 822 | - | - | CH oop |
| 879 | 879 | - | - | - | CH oop |
| - | 1047 | 1064 | 1064 | 1067 | C=C str. |
| 1039 | - | 1041 | - | - | C-O-C str. |
| 1158 | 1096 | 1175 | 1109 | 1110 | CH2 rocking |
| - | 1159 | - | 1160 | 1160 | CH oop |
| 1257 | 1259 | - | - | - | CH str. |
| - | - | 1267 | - | - | CH2 twisting |
| - | 1180 | - | 1405 | 1502 | CS, CC as. str. |
| 1360 | - | 1367 | 1364 | - | CH2 wagging |
| - | 1361 | - | - | 1412 | CN, CC as. str. |
| 1437 | 1441 | - | - | - | CC as. str. |
| - | - | 1465 | 1458 | 1464 | CH2 bending |
| 1535 | 1536 | - | - | - | CC as. str. |
| 1567 | 1569 | 1564 | 1557 | 1556 | CN, CC sym. str. |
| 1624 | 1628 | 1627 | 1626 | 1625 | CN, CC sym. str. |
| 1677 | 1678 | 1689 | 1685 | 1682 | C=O str. |
| Polymer | 1a | 1b | 2a | 2b | 2c |
|---|---|---|---|---|---|
| α | 21° | 21° | 26° | 28° | 27° |
| β | 29° | 31° | 50° | 30° | 33° |
| Polymer | Mn [kg/mol] | Mw [kg/mol] | Mw/Mn | DP |
|---|---|---|---|---|
| 1a | 23.4 | 111.0 | 4.74 | 17 |
| 1b | 16.2 | 21.7 | 1.34 | 12 |
| 2a | 6.6 | 13.5 | 2.05 | 6 |
| 2b | 12.0 | 20.4 | 1.71 | 11 |
| 2c | 7.4 | 10.3 | 1.38 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bölke, S.; Früh, A.; Trilling, F.; Forster, M.; Scherf, U.; Chassé, T.; Peisert, H. Influence of Backbone Ladderization and Side Chain Variation on the Orientation of Diketopyrrolopyrrole-Based Donor-Acceptor Copolymers. Molecules 2023, 28, 6435. https://doi.org/10.3390/molecules28186435
Bölke S, Früh A, Trilling F, Forster M, Scherf U, Chassé T, Peisert H. Influence of Backbone Ladderization and Side Chain Variation on the Orientation of Diketopyrrolopyrrole-Based Donor-Acceptor Copolymers. Molecules. 2023; 28(18):6435. https://doi.org/10.3390/molecules28186435
Chicago/Turabian StyleBölke, Sven, Andreas Früh, Florian Trilling, Michael Forster, Ullrich Scherf, Thomas Chassé, and Heiko Peisert. 2023. "Influence of Backbone Ladderization and Side Chain Variation on the Orientation of Diketopyrrolopyrrole-Based Donor-Acceptor Copolymers" Molecules 28, no. 18: 6435. https://doi.org/10.3390/molecules28186435









