Therapeutic Antibodies in Medicine
Abstract
:1. Introduction
1.1. Antibody Structure and Its Types

1.2. Types of Therapy with Antibodies
1.3. Evolution of Antibody Therapeutics
1.4. Nomenclature of Antibodies
1.5. Databases for Therapeutic Antibodies
2. Antibodies in Different Branches of Medicine
2.1. Antibodies in Hematological Diseases
2.2. Antibodies for Autoimmune Diseases
2.3. Antibodies Specific to Cancer
2.4. Antibodies for Pulmonary Diseases
2.5. Antibodies for Cardiovascular Diseases
2.6. Antibodies for Renal Diseases
2.7. Antibodies for Gastrointestinal Pathologies
2.8. Antibodies for Infectious Diseases
2.9. Antibodies for Endocrine Disorders
2.10. Antibodies for Neurological Disorders
2.11. Antibodies for Ophthalmological Disorders
2.12. Antibodies for Musculoskeletal Disorders
3. Critical Analysis and Adverse Effects
4. Future Directions of Antibody Therapeutics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hudson, P.J.; Souriau, C. Recombinant Antibodies for Cancer Diagnosis and Therapy. Expert Opin. Biol. Ther. 2001, 1, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Köhler, G.; Milstein, C. Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.A.; Brogden, R.N. Muromonab CD3. A Review of Its Pharmacology and Therapeutic Potential. Drugs 1989, 37, 871–899. [Google Scholar] [CrossRef] [PubMed]
- Leavy, O. Therapeutic Antibodies: Past, Present and Future. Nat. Rev. Immunol. 2010, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.; Herrmann, R. Overview of Monoclonal Antibodies in Cancer Therapy: Present and Promise. Crit. Rev. Oncol. Hematol. 2005, 54, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Zemlin, M.; Klinger, M.; Link, J.; Zemlin, C.; Bauer, K.; Engler, J.A.; Schroeder, H.W.; Kirkham, P.M. Expressed Murine and Human CDR-H3 Intervals of Equal Length Exhibit Distinct Repertoires that Differ in Their Amino Acid Composition and Predicted Range of Structures. J. Mol. Biol. 2003, 334, 733–749. [Google Scholar] [CrossRef]
- Hudson, P.J.; Souriau, C. Engineered Antibodies. Nat. Med. 2003, 9, 129–134. [Google Scholar] [CrossRef]
- Carter, P.; Presta, L.; Gorman, C.M.; Ridgway, J.B.; Henner, D.; Wong, W.L.; Rowland, A.M.; Kotts, C.; Carver, M.E.; Shepard, H.M. Humanization of an Anti-P185HER2 Antibody for Human Cancer Therapy. Proc. Natl. Acad. Sci. USA 1992, 89, 4285–4289. [Google Scholar] [CrossRef]
- Presta, L.G.; Lahr, S.J.; Shields, R.L.; Porter, J.P.; Gorman, C.M.; Fendly, B.M.; Jardieu, P.M. Humanization of an Antibody Directed against IgE. J. Immunol. 1993, 151, 2623–2632. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally Occurring Antibodies Devoid of Light Chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Asaadi, Y.; Jouneghani, F.F.; Janani, S.; Rahbarizadeh, F. A Comprehensive Comparison between Camelid Nanobodies and Single Chain Variable Fragments. Biomark. Res. 2021, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Streltsov, V.A.; Carmichael, J.A.; Nuttall, S.D. Structure of a Shark IgNAR Antibody Variable Domain and Modeling of an Early-Developmental Isotype. Protein Sci. Publ. Protein Soc. 2005, 14, 2901–2909. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sapienza, G.; Rossotti, M.A.; Tabares-da Rosa, S. Single-Domain Antibodies as Versatile Affinity Reagents for Analytical and Diagnostic Applications. Front. Immunol. 2017, 8, 977. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.B.; Ghahroudi, M.A.; Wyns, L.; Muyldermans, S. Comparison of Llama VH Sequences from Conventional and Heavy Chain Antibodies. Mol. Immunol. 1997, 34, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S.; Smider, V.V. Distinct Antibody Species: Structural Differences Creating Therapeutic Opportunities. Curr. Opin. Immunol. 2016, 40, 7–13. [Google Scholar] [CrossRef]
- Mendoza, M.N.; Jian, M.; King, M.T.; Brooks, C.L. Role of a Noncanonical Disulfide Bond in the Stability, Affinity, and Flexibility of a VHH Specific for the Listeria Virulence Factor InlB. Protein Sci. Publ. Protein Soc. 2020, 29, 1004–1017. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, G.; Lu, H.; Li, H.; Tang, M.; Tong, A. Development of Therapeutic Antibodies for the Treatment of Diseases. Mol. Biomed. 2022, 3, 35. [Google Scholar] [CrossRef]
- Carter, P. Improving the Efficacy of Antibody-Based Cancer Therapies. Nat. Rev. Cancer 2001, 1, 118–129. [Google Scholar] [CrossRef]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.-J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of Therapeutic Antibodies for the Treatment of Diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Smith, S.; Sweetenham, J.W. Iodine 131 Tositumomab in the Treatment of Non-Hodgkin’s Lymphoma. Future Oncol. 2007, 3, 255–262. [Google Scholar] [CrossRef]
- Francis, R.J.; Sharma, S.K.; Springer, C.; Green, A.J.; Hope-Stone, L.D.; Sena, L.; Martin, J.; Adamson, K.L.; Robbins, A.; Gumbrell, L.; et al. A Phase I Trial of Antibody Directed Enzyme Prodrug Therapy (ADEPT) in Patients with Advanced Colorectal Carcinoma or Other CEA Producing Tumours. Br. J. Cancer 2002, 87, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Krauss, W.C.; Park, J.W.; Kirpotin, D.B.; Hong, K.; Benz, C.C. Emerging Antibody-Based HER2 (ErbB-2/Neu) Therapeutics. Breast Dis. 2000, 11, 113–124. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P.W.H.I. Bispecific Antibodies: A Mechanistic Review of the Pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T Cell Immunotherapy for Human Cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Kochenderfer, J.N. Chimeric Antigen Receptor T-Cell Therapies for Lymphoma. Nat. Rev. Clin. Oncol. 2018, 15, 31–46. [Google Scholar] [CrossRef]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Meeker, T.C.; Lowder, J.; Maloney, D.G.; Miller, R.A.; Thielemans, K.; Warnke, R.; Levy, R. A Clinical Trial of Anti-Idiotype Therapy for B Cell Malignancy. Blood 1985, 65, 1349–1363. [Google Scholar] [CrossRef]
- Richards, J.M.; Vogelzang, N.J.; Bluestone, J.A. Neurotoxicity after Treatment with Muromonab-CD3. N. Engl. J. Med. 1990, 323, 487–488. [Google Scholar] [CrossRef]
- Faulds, D.; Sorkin, E.M. Abciximab (C7E3 Fab). A Review of Its Pharmacology and Therapeutic Potential in Ischaemic Heart Disease. Drugs 1994, 48, 583–598. [Google Scholar] [CrossRef]
- Jones, P.T.; Dear, P.H.; Foote, J.; Neuberger, M.S.; Winter, G. Replacing the Complementarity-Determining Regions in a Human Antibody with Those from a Mouse. Nature 1986, 321, 522–525. [Google Scholar] [CrossRef]
- Tsurushita, N.; Hinton, P.R.; Kumar, S. Design of Humanized Antibodies: From Anti-Tac to Zenapax. Methods 2005, 36, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Kirkman, R.; Light, S.; Bumgardner, G.; Pescovitz, M.; Halloran, P.; Neylan, J.; Wilkinson, A.; Ekberg, H.; Gaston, R.; et al. Interleukin-2-Receptor Blockade with Daclizumab to Prevent Acute Rejection in Renal Transplantation. Daclizumab Triple Therapy Study Group. N. Engl. J. Med. 1998, 338, 161–165. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, J.; Griffiths, A.D.; Winter, G.; Chiswell, D.J. Phage Antibodies: Filamentous Phage Displaying Antibody Variable Domains. Nature 1990, 348, 552–554. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Dick, A.D.; Brézin, A.P.; Nguyen, Q.D.; Thorne, J.E.; Kestelyn, P.; Barisani-Asenbauer, T.; Franco, P.; Heiligenhaus, A.; Scales, D.; et al. Adalimumab in Patients with Active Noninfectious Uveitis. N. Engl. J. Med. 2016, 375, 932–943. [Google Scholar] [CrossRef]
- Kimball, A.B.; Okun, M.M.; Williams, D.A.; Gottlieb, A.B.; Papp, K.A.; Zouboulis, C.C.; Armstrong, A.W.; Kerdel, F.; Gold, M.H.; Forman, S.B.; et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N. Engl. J. Med. 2016, 375, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Keystone, E.C.; Kavanaugh, A.F.; Sharp, J.T.; Tannenbaum, H.; Hua, Y.; Teoh, L.S.; Fischkoff, S.A.; Chartash, E.K. Radiographic, Clinical, and Functional Outcomes of Treatment with Adalimumab (a Human Anti-Tumor Necrosis Factor Monoclonal Antibody) in Patients with Active Rheumatoid Arthritis Receiving Concomitant Methotrexate Therapy: A Randomized, Placebo-Controlled, 52-Week Trial. Arthritis Rheum. 2004, 50, 1400–1411. [Google Scholar] [CrossRef]
- Kempeni, J. Preliminary Results of Early Clinical Trials with the Fully Human Anti-TNFalpha Monoclonal Antibody D2E7. Ann. Rheum. Dis. 1999, 58 (Suppl. S1), I70–I72. [Google Scholar] [CrossRef]
- Menter, A.; Tyring, S.K.; Gordon, K.; Kimball, A.B.; Leonardi, C.L.; Langley, R.G.; Strober, B.E.; Kaul, M.; Gu, Y.; Okun, M.; et al. Adalimumab Therapy for Moderate to Severe Psoriasis: A Randomized, Controlled Phase III Trial. J. Am. Acad. Dermatol. 2008, 58, 106–115. [Google Scholar] [CrossRef]
- Lonberg, N. Human Antibodies from Transgenic Animals. Nat. Biotechnol. 2005, 23, 1117–1125. [Google Scholar] [CrossRef]
- Moroni, M.; Veronese, S.; Benvenuti, S.; Marrapese, G.; Sartore-Bianchi, A.; Di Nicolantonio, F.; Gambacorta, M.; Siena, S.; Bardelli, A. Gene Copy Number for Epidermal Growth Factor Receptor (EGFR) and Clinical Response to AntiEGFR Treatment in Colorectal Cancer: A Cohort Study. Lancet Oncol. 2005, 6, 279–286. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Łuksza, M.; Riaz, N.; Makarov, V.; Balachandran, V.P.; Hellmann, M.D.; Solovyov, A.; Rizvi, N.A.; Merghoub, T.; Levine, A.J.; Chan, T.A.; et al. A Neoantigen Fitness Model Predicts Tumour Response to Checkpoint Blockade Immunotherapy. Nature 2017, 551, 517–520. [Google Scholar] [CrossRef] [PubMed]
- INN Bio Review 2019. Available online: https://www.who.int/publications-detail-redirect/who-emp-rht-tsn-2019-1 (accessed on 30 June 2023).
- Revised Monoclonal Antibody (MAb) Nomenclature Scheme. Available online: https://www.who.int/publications/m/item/inn-17-416 (accessed on 30 June 2023).
- New INN Monoclonal Antibody (MAb) Nomenclature Scheme. Available online: https://www.who.int/publications-detail-redirect/inn-21-531 (accessed on 30 June 2023).
- Center for Drug Evaluation and Research. Biosimilar Product Information. FDA. 2023. Available online: https://www.fda.gov/drugs/biosimilars/biosimilar-product-information (accessed on 30 June 2023).
- Antibody Therapeutics Approved or in Regulatory Review in the EU or US. Available online: https://www.antibodysociety.org/resources/approved-antibodies/ (accessed on 30 June 2023).
- Raybould, M. Helpful Resources for People Studying Therapeutic Antibodies; Oxford Protein Informatics Group: Oxford, UK, 2018. [Google Scholar]
- SAbDab: The Structural Antibody Database. Available online: https://opig.stats.ox.ac.uk/webapps/sabdab-sabpred/therasabdab/about/ (accessed on 30 June 2023).
- Raybould, M.I.J.; Marks, C.; Lewis, A.P.; Shi, J.; Bujotzek, A.; Taddese, B.; Deane, C.M. Thera-SAbDab: The Therapeutic Structural Antibody Database. Nucleic Acids Res. 2020, 48, D383–D388. [Google Scholar] [CrossRef] [PubMed]
- TABS Therapeutic Antibody Database. Available online: https://tabs.craic.com/users/sign_in (accessed on 30 June 2023).
- Yang, L.; Liu, W.; Yu, X.; Wu, M.; Reichert, J.M.; Ho, M. COVID-19 Antibody Therapeutics Tracker: A Global Online Database of Antibody Therapeutics for the Prevention and Treatment of COVID-19. Antib. Ther. 2020, 3, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed on 30 June 2023).
- Ghosh, K.; Ghosh, K. Monoclonal Antibodies Used for the Management of Hemataological Disorders. Expert Rev. Hematol. 2022, 15, 443–455. [Google Scholar] [CrossRef]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Mateos, C.; Alcaraz-Serna, A.; Somovilla-Crespo, B.; Muñoz-Calleja, C. Monoclonal Antibody Therapies for Hematological Malignancies: Not Just Lineage-Specific Targets. Front. Immunol. 2017, 8, 1936. [Google Scholar] [CrossRef]
- Rougé, L.; Chiang, N.; Steffek, M.; Kugel, C.; Croll, T.I.; Tam, C.; Estevez, A.; Arthur, C.P.; Koth, C.M.; Ciferri, C.; et al. Structure of CD20 in Complex with the Therapeutic Monoclonal Antibody Rituximab. Science 2020, 367, 1224–1230. [Google Scholar] [CrossRef]
- Oldenburg, J.; Mahlangu, J.N.; Kim, B.; Schmitt, C.; Callaghan, M.U.; Young, G.; Santagostino, E.; Kruse-Jarres, R.; Negrier, C.; Kessler, C.; et al. Emicizumab Prophylaxis in Hemophilia A with Inhibitors. N. Engl. J. Med. 2017, 377, 809–818. [Google Scholar] [CrossRef]
- Thornburg, C.D.; Duncan, N.A. Treatment Adherence in Hemophilia. Patient Prefer. Adherence 2017, 11, 1677–1686. [Google Scholar] [CrossRef]
- Callaghan, M.U.; Negrier, C.; Paz-Priel, I.; Chang, T.; Chebon, S.; Lehle, M.; Mahlangu, J.; Young, G.; Kruse-Jarres, R.; Mancuso, M.E.; et al. Long-Term Outcomes with Emicizumab Prophylaxis for Hemophilia A with or without FVIII Inhibitors from the HAVEN 1-4 Studies. Blood 2021, 137, 2231–2242. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Crizanlizumab: First Approval. Drugs 2020, 80, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.V. Sickle Cell Disease: Advances in Treatment. Ochsner J. 2018, 18, 377–389. [Google Scholar] [CrossRef]
- Gutsaeva, D.R.; Parkerson, J.B.; Yerigenahally, S.D.; Kurz, J.C.; Schaub, R.G.; Ikuta, T.; Head, C.A. Inhibition of Cell Adhesion by Anti–P-Selectin Aptamer: A New Potential Therapeutic Agent for Sickle Cell Disease. Blood 2011, 117, 727–735. [Google Scholar] [CrossRef]
- Ataga, K.I.; Kutlar, A.; Kanter, J.; Liles, D.; Cancado, R.; Friedrisch, J.; Guthrie, T.H.; Knight-Madden, J.; Alvarez, O.A.; Gordeuk, V.R.; et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, W.; Długosz Danecka, M.; Buske, C. Rituximab Biosimilars for Lymphoma in Europe. Expert Opin. Biol. Ther. 2019, 19, 1045–1056. [Google Scholar] [CrossRef]
- Nava-Parada, P.; Shelbaya, A.; Nabhan, C. Rituximab Biosimilars in Hematologic Malignancies: The Need for a Real-World Approach. Future Oncol. 2020, 16, 2017–2027. [Google Scholar] [CrossRef]
- Jung, S.M.; Kim, W.-U. Targeted Immunotherapy for Autoimmune Disease. Immune Netw. 2022, 22, e9. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Benjamin, O.; Goyal, A.; Lappin, S.L. Disease-Modifying Antirheumatic Drugs (DMARD). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mousavi, M.J.; Karami, J.; Aslani, S.; Tahmasebi, M.N.; Vaziri, A.S.; Jamshidi, A.; Farhadi, E.; Mahmoudi, M. Transformation of Fibroblast-like Synoviocytes in Rheumatoid Arthritis; from a Friend to Foe. Autoimmun. Highlights 2021, 12, 3. [Google Scholar] [CrossRef]
- Ogata, A.; Kato, Y.; Higa, S.; Yoshizaki, K. IL-6 Inhibitor for the Treatment of Rheumatoid Arthritis: A Comprehensive Review. Mod. Rheumatol. 2019, 29, 258–267. [Google Scholar] [CrossRef]
- Fleischmann, R.; van Adelsberg, J.; Lin, Y.; da Rocha Castelar-Pinheiro, G.; Brzezicki, J.; Hrycaj, P.; Graham, N.M.H.; van Hoogstraten, H.; Bauer, D.; Burmester, G.R. Sarilumab and Nonbiologic Disease-Modifying Antirheumatic Drugs in Patients With Active Rheumatoid Arthritis and Inadequate Response or Intolerance to Tumor Necrosis Factor Inhibitors. Arthritis Rheumatol. 2017, 69, 277–290. [Google Scholar] [CrossRef]
- Paccaly, A.J.; Kovalenko, P.; Parrino, J.; Boyapati, A.; Xu, C.; van Hoogstraten, H.; Ishii, T.; Davis, J.D.; DiCioccio, A.T. Pharmacokinetics and Pharmacodynamics of Subcutaneous Sarilumab and Intravenous Tocilizumab Following Single-Dose Administration in Patients with Active Rheumatoid Arthritis on Stable Methotrexate. J. Clin. Pharmacol. 2021, 61, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, S.; Bujkiewicz, S.; Wailoo, A.J.; Sutton, A.J.; Scott, D. The Effectiveness of Anti-TNF-Alpha Therapies When Used Sequentially in Rheumatoid Arthritis Patients: A Systematic Review and Meta-Analysis. Rheumatology 2010, 49, 2313–2321. [Google Scholar] [CrossRef]
- Kaufmann, J.; Feist, E.; Roske, A.-E.; Schmidt, W.A. Monotherapy with Tocilizumab or TNF-Alpha Inhibitors in Patients with Rheumatoid Arthritis: Efficacy, Treatment Satisfaction, and Persistence in Routine Clinical Practice. Clin. Rheumatol. 2013, 32, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Costedoat-Chalumeau, N.; Dunogué, B.; Morel, N.; Le Guern, V.; Guettrot-Imbert, G. Hydroxychloroquine: A Multifaceted Treatment in Lupus. Presse Med. 2014, 43, e167–e180. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Rovin, B.H.; Houssiau, F.; Malvar, A.; Teng, Y.K.O.; Contreras, G.; Amoura, Z.; Yu, X.; Mok, C.-C.; Santiago, M.B.; et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N. Engl. J. Med. 2020, 383, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Kalunian, K.C.; Furie, R.; Morand, E.F.; Bruce, I.N.; Manzi, S.; Tanaka, Y.; Winthrop, K.; Hupka, I.; Zhang, L.; Werther, S.; et al. A Randomized, Placebo-Controlled Phase III Extension Trial of the Long-Term Safety and Tolerability of Anifrolumab in Active Systemic Lupus Erythematosus. Arthritis Rheumatol. 2023, 75, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, H.; Lightstone, L. Rituximab in Systemic Lupus Erythematosus and Lupus Nephritis. Nephron Clin. Pract. 2014, 128, 250–254. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Zhang, J.; Han, B.; Wang, B.; Gao, W.; Zhang, N.; Zhang, C.; Yan, F.; Li, Z. Efficacy and Safety of Rituximab for Systemic Lupus Erythematosus Treatment: A Meta-Analysis. Afr. Health Sci. 2020, 20, 871–884. [Google Scholar] [CrossRef]
- van Vollenhoven, R.F.; Hahn, B.H.; Tsokos, G.C.; Wagner, C.L.; Lipsky, P.; Touma, Z.; Werth, V.P.; Gordon, R.M.; Zhou, B.; Hsu, B.; et al. Efficacy and Safety of Ustekinumab, an IL-12 and IL-23 Inhibitor, in Patients with Active Systemic Lupus Erythematosus: Results of a Multicentre, Double-Blind, Phase 2, Randomised, Controlled Study. Lancet 2018, 392, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Kalunian, K.; Furie, R.; Radhakrishnan, J.; Mathur, V.; Polu, K.; Connelly, S.; Rothman, J.; Ng, C.; Chinn, L.; Fung, M.; et al. Itolizumab, a Novel Anti-CD6 Therapy, in Systemic Lupus Erythematosus Patients: Interim Safety Results from the Phase 1b EQUALISE Dose-Escalation Study. Arthritis Rheumatol. 2021, 73. [Google Scholar]
- Tiwari, V.; Brent, L.H. Psoriatic Arthritis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ogdie, A.; Coates, L.C.; Gladman, D.D. Treatment Guidelines in Psoriatic Arthritis. Rheumatology 2020, 59, i37–i46. [Google Scholar] [CrossRef]
- Kolbinger, F.; Di Padova, F.; Deodhar, A.; Hawkes, J.E.; Huppertz, C.; Kuiper, T.; McInnes, I.B.; Ritchlin, C.T.; Rosmarin, D.; Schett, G.; et al. Secukinumab for the Treatment of Psoriasis, Psoriatic Arthritis, and Axial Spondyloarthritis: Physical and Pharmacological Properties Underlie the Observed Clinical Efficacy and Safety. Pharmacol. Ther. 2022, 229, 107925. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.; Furer, V.; Berman, M.; Isakov, O.; Zisman, D.; Haddad, A.; Elkayam, O. Treatment with Ixekizumab Following Secukinumab Failure in Patients with Psoriatic Arthritis: Real-Life Experience from a Resistant Population. Biol. Targets Ther. 2021, 15, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kawalec, P.; Holko, P.; Moćko, P.; Pilc, A. Comparative Effectiveness of Abatacept, Apremilast, Secukinumab and Ustekinumab Treatment of Psoriatic Arthritis: A Systematic Review and Network Meta-Analysis. Rheumatol. Int. 2018, 38, 189–201. [Google Scholar] [CrossRef]
- Bayer, V. An Overview of Monoclonal Antibodies. Semin. Oncol. Nurs. 2019, 35, 150927. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Koppolu, V.; Rekha Vasigala, V.K. Checkpoint Immunotherapy by Nivolumab for Treatment of Metastatic Melanoma. J. Cancer Res. Ther. 2018, 14, 1167–1175. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor Microenvironment as a Therapeutic Target in Cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Jin, M.-Z.; Jin, W.-L. The Updated Landscape of Tumor Microenvironment and Drug Repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef]
- Tang, F.; Zheng, P. Tumor Cells versus Host Immune Cells: Whose PD-L1 Contributes to PD-1/PD-L1 Blockade Mediated Cancer Immunotherapy? Cell Biosci. 2018, 8, 34. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and Challenges for the next Generation of Antibody—Drug Conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Y.; Wang, G.; Liu, M. Current Landscape and Future Directions of Bispecific Antibodies in Cancer Immunotherapy. Front. Immunol. 2022, 13, 1035276. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Enfortumab Vedotin Approved for Recurrent Bladder Cancer—NCI. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2020/enfortumab-vedotin-bladder-cancer-fda-approval (accessed on 29 June 2023).
- Van Der Weyden, C.; Dickinson, M.; Whisstock, J.; Prince, H.M. Brentuximab Vedotin in T-Cell Lymphoma. Expert Rev. Hematol. 2019, 12, 5–19. [Google Scholar] [CrossRef]
- Ansell, S.M.; Radford, J.; Connors, J.M.; Długosz-Danecka, M.; Kim, W.-S.; Gallamini, A.; Ramchandren, R.; Friedberg, J.W.; Advani, R.; Hutchings, M.; et al. Overall Survival with Brentuximab Vedotin in Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2022, 387, 310–320. [Google Scholar] [CrossRef]
- Kuruvilla, J.; Ramchandren, R.; Santoro, A.; Paszkiewicz-Kozik, E.; Gasiorowski, R.; Johnson, N.A.; Fogliatto, L.M.; Goncalves, I.; de Oliveira, J.S.R.; Buccheri, V.; et al. Pembrolizumab versus Brentuximab Vedotin in Relapsed or Refractory Classical Hodgkin Lymphoma (KEYNOTE-204): An Interim Analysis of a Multicentre, Randomised, Open-Label, Phase 3 Study. Lancet Oncol. 2021, 22, 512–524. [Google Scholar] [CrossRef]
- Yamazaki, C.M.; Yamaguchi, A.; Anami, Y.; Xiong, W.; Otani, Y.; Lee, J.; Ueno, N.T.; Zhang, N.; An, Z.; Tsuchikama, K. Antibody-Drug Conjugates with Dual Payloads for Combating Breast Tumor Heterogeneity and Drug Resistance. Nat. Commun. 2021, 12, 3528. [Google Scholar] [CrossRef]
- Pedley, R.B.; Sharma, S.K.; Boxer, G.M.; Boden, R.; Stribbling, S.M.; Davies, L.; Springer, C.J.; Begent, R.H. Enhancement of Antibody-Directed Enzyme Prodrug Therapy in Colorectal Xenografts by an Antivascular Agent. Cancer Res. 1999, 59, 3998–4003. [Google Scholar] [PubMed]
- Sécher, T.; Guilleminault, L.; Reckamp, K.; Amanam, I.; Plantier, L.; Heuzé-Vourc’h, N. Therapeutic Antibodies: A New Era in the Treatment of Respiratory Diseases? Pharmacol. Ther. 2018, 189, 149–172. [Google Scholar] [CrossRef]
- Pelaia, C.; Calabrese, C.; Terracciano, R.; de Blasio, F.; Vatrella, A.; Pelaia, G. Omalizumab, the First Available Antibody for Biological Treatment of Severe Asthma: More than a Decade of Real-Life Effectiveness. Ther. Adv. Respir. Dis. 2018, 12, 1753466618810192. [Google Scholar] [CrossRef]
- Agache, I.; Rocha, C.; Beltran, J.; Song, Y.; Posso, M.; Solà, I.; Alonso-Coello, P.; Akdis, C.; Akdis, M.; Canonica, G.W.; et al. Efficacy and Safety of Treatment with Biologicals (Benralizumab, Dupilumab and Omalizumab) for Severe Allergic Asthma: A Systematic Review for the EAACI Guidelines—Recommendations on the Use of Biologicals in Severe Asthma. Allergy 2020, 75, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Ora, J.; Cavalli, F.; Rogliani, P.; Matera, M.G. An Overview of the Safety and Efficacy of Monoclonal Antibodies for the Chronic Obstructive Pulmonary Disease. Biol. Targets Ther. 2021, 15, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Lyly, A.; Laulajainen-Hongisto, A.; Gevaert, P.; Kauppi, P.; Toppila-Salmi, S. Monoclonal Antibodies and Airway Diseases. Int. J. Mol. Sci. 2020, 21, 9477. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, R.; Mejias, A.; Ramilo, O. Monoclonal Antibodies for Prevention of Respiratory Syncytial Virus Infection. Pediatr. Infect. Dis. J. 2021, 40, S35–S39. [Google Scholar] [CrossRef]
- Que, Y.-A.; Lazar, H.; Wolff, M.; François, B.; Laterre, P.-F.; Mercier, E.; Garbino, J.; Pagani, J.-L.; Revelly, J.-P.; Mus, E.; et al. Assessment of Panobacumab as Adjunctive Immunotherapy for the Treatment of Nosocomial Pseudomonas Aeruginosa Pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2014, 33, 1861–1867. [Google Scholar] [CrossRef]
- Chastre, J.; François, B.; Bourgeois, M.; Komnos, A.; Ferrer, R.; Rahav, G.; De Schryver, N.; Lepape, A.; Koksal, I.; Luyt, C.-E.; et al. Safety, Efficacy, and Pharmacokinetics of Gremubamab (MEDI3902), an Anti-Pseudomonas Aeruginosa Bispecific Human Monoclonal Antibody, in P. Aeruginosa-Colonised, Mechanically Ventilated Intensive Care Unit Patients: A Randomised Controlled Trial. Crit. Care 2022, 26, 355. [Google Scholar] [CrossRef]
- Anti-SARS-CoV-2 Monoclonal Antibodies. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/anti-sars-cov-2-monoclonal-antibodies/ (accessed on 30 June 2023).
- What’s New. Available online: https://www.covid19treatmentguidelines.nih.gov/about-the-guidelines/whats-new/ (accessed on 30 June 2023).
- Evusheld. HHS/ASPR. Available online: https://aspr.hhs.gov:443/COVID-19/Therapeutics/Products/Evusheld/Pages/default.aspx (accessed on 30 June 2023).
- Sahebkar, A.; Watts, G.F. New Therapies Targeting ApoB Metabolism for High-Risk Patients with Inherited Dyslipidaemias: What Can the Clinician Expect? Cardiovasc. Drugs Ther. 2013, 27, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Fitchett, D.H.; Hegele, R.A.; Verma, S. Statin Intolerance. Circulation 2015, 131, e389–e391. [Google Scholar] [CrossRef] [PubMed]
- Bohula, E.A.; Giugliano, R.P.; Leiter, L.A.; Verma, S.; Park, J.-G.; Sever, P.S.; Lira Pineda, A.; Honarpour, N.; Wang, H.; Murphy, S.A.; et al. Inflammatory and Cholesterol Risk in the FOURIER Trial. Circulation 2018, 138, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Manniello, M.; Pisano, M. Alirocumab (Praluent): First in the New Class of PCSK9 Inhibitors. Pharm. Ther. 2016, 41, 28–53. [Google Scholar]
- Center for Drug Evaluation and Research. FDA Approves Add-on Therapy for Patients with Genetic Form of Severely High Cholesterol. FDA. 2021. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-add-therapy-patients-genetic-form-severely-high-cholesterol (accessed on 30 June 2023).
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Guedeney, P.; Giustino, G.; Sorrentino, S.; Claessen, B.E.; Camaj, A.; Kalkman, D.N.; Vogel, B.; Sartori, S.; De Rosa, S.; Baber, U.; et al. Efficacy and Safety of Alirocumab and Evolocumab: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. Heart J. 2022, 43, e17–e25. [Google Scholar] [CrossRef]
- Guedeney, P.; Sorrentino, S.; Giustino, G.; Chapelle, C.; Laporte, S.; Claessen, B.E.; Ollier, E.; Camaj, A.; Kalkman, D.N.; Vogel, B.; et al. Indirect Comparison of the Efficacy and Safety of Alirocumab and Evolocumab: A Systematic Review and Network Meta-Analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 225–235. [Google Scholar] [CrossRef]
- Räber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Häner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.-J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients with Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022, 327, 1771–1781. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef]
- Vallurupalli, M.; MacFadyen, J.G.; Glynn, R.J.; Thuren, T.; Libby, P.; Berliner, N.; Ridker, P.M. Effects of Interleukin-1β Inhibition on Incident Anemia. Ann. Intern. Med. 2020, 172, 523–532. [Google Scholar] [CrossRef]
- Sheppard, M.; Laskou, F.; Stapleton, P.P.; Hadavi, S.; Dasgupta, B. Tocilizumab (Actemra). Hum. Vaccines Immunother. 2017, 13, 1972–1988. [Google Scholar] [CrossRef]
- Castagné, B.; Viprey, M.; Martin, J.; Schott, A.-M.; Cucherat, M.; Soubrier, M. Cardiovascular Safety of Tocilizumab: A Systematic Review and Network Meta-Analysis. PLoS ONE 2019, 14, e0220178. [Google Scholar] [CrossRef]
- Effects of Tofacitinib and Other DMARDs on Lipid Profiles in Rheumatoid Arthritis: Implications for the Rheumatologist—ClinicalKey. Available online: https://www-clinicalkey-com.gcsom.idm.oclc.org/#!/content/playContent/1-s2.0-S0049017216000949?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0049017216000949%3Fshowall%3Dtrue&referrer= (accessed on 30 June 2023).
- Toshner, M.; Church, C.; Harbaum, L.; Rhodes, C.; Moreschi, S.S.V.; Liley, J.; Jones, R.; Arora, A.; Batai, K.; Desai, A.A.; et al. Mendelian Randomisation and Experimental Medicine Approaches to Interleukin-6 as a Drug Target in Pulmonary Arterial Hypertension. Eur. Respir. J. 2022, 59, 2002463. [Google Scholar] [CrossRef]
- Santoro, D.; Pellicanò, V.; Visconti, L.; Trifirò, G.; Cernaro, V.; Buemi, M. Monoclonal Antibodies for Renal Diseases: Current Concepts and Ongoing Treatments. Expert Opin. Biol. Ther. 2015, 15, 1119–1143. [Google Scholar] [CrossRef] [PubMed]
- Ravani, P.; Bonanni, A.; Rossi, R.; Caridi, G.; Ghiggeri, G.M. Anti-CD20 Antibodies for Idiopathic Nephrotic Syndrome in Children. Clin. J. Am. Soc. Nephrol. 2016, 11, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Kattah, A.G.; Fervenza, F.C. Rituximab: Emerging Treatment Strategies of Immune-Mediated Glomerular Disease. Expert Rev. Clin. Immunol. 2012, 8, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Siligato, R.; Cernaro, V.; Nardi, C.; De Gregorio, F.; Gembillo, G.; Costantino, G.; Conti, G.; Buemi, M.; Santoro, D. Emerging Therapeutic Strategies for Minimal Change Disease and Focal and Segmental Glomerulosclerosis. Expert Opin. Investig. Drugs 2018, 27, 839–879. [Google Scholar] [CrossRef] [PubMed]
- Manrique, J.; Cravedi, P. Role of Monoclonal Antibodies in the Treatment of Immune-Mediated Glomerular Diseases. Nefrol. Publ. Soc. Esp. Nefrol. 2014, 34, 388–397. [Google Scholar] [CrossRef]
- Abbas, A.; Mirza, M.M.; Ganti, A.K.; Tendulkar, K. Renal Toxicities of Targeted Therapies. Target. Oncol. 2015, 10, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef]
- de Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current State of the Art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and Adaptive Immunity in Inflammatory Bowel Disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Van Deventer, S.J. Tumour Necrosis Factor and Crohn’s Disease. Gut 1997, 40, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Brynskov, J.; Nielsen, O.H.; Ahnfelt-Rønne, I.; Bendtzen, K. Cytokines (Immunoinflammatory Hormones) and Their Natural Regulation in Inflammatory Bowel Disease (Crohn’s Disease and Ulcerative Colitis): A Review. Dig. Dis. 2008, 12, 290–304. [Google Scholar] [CrossRef]
- Liang, S.; Dai, J.; Hou, S.; Su, L.; Zhang, D.; Guo, H.; Hu, S.; Wang, H.; Rao, Z.; Guo, Y.; et al. Structural Basis for Treating Tumor Necrosis Factor α (TNFα)-Associated Diseases with the Therapeutic Antibody Infliximab. J. Biol. Chem. 2013, 288, 13799–13807. [Google Scholar] [CrossRef]
- Knight, D.M.; Trinh, H.; Le, J.; Siegel, S.; Shealy, D.; McDonough, M.; Scallon, B.; Moore, M.A.; Vilcek, J.; Daddona, P. Construction and Initial Characterization of a Mouse-Human Chimeric Anti-TNF Antibody. Mol. Immunol. 1993, 30, 1443–1453. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Feagan, B.G.; Lichtenstein, G.R.; Mayer, L.F.; Schreiber, S.; Colombel, J.F.; Rachmilewitz, D.; Wolf, D.C.; Olson, A.; Bao, W.; et al. Maintenance Infliximab for Crohn’s Disease: The ACCENT I Randomised Trial. Lancet 2002, 359, 1541–1549. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef]
- Rutgeerts, P.; D’Haens, G.; Targan, S.; Vasiliauskas, E.; Hanauer, S.B.; Present, D.H.; Mayer, L.; Van Hogezand, R.A.; Braakman, T.; DeWoody, K.L.; et al. Efficacy and Safety of Retreatment with Anti-Tumor Necrosis Factor Antibody (Infliximab) to Maintain Remission in Crohn’s Disease. Gastroenterology 1999, 117, 761–769. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Rutgeerts, P.; Sandborn, W.J.; Sands, B.E.; Diamond, R.H.; Blank, M.; Montello, J.; Tang, L.; Cornillie, F.; Colombel, J.-F. A Pooled Analysis of Infections, Malignancy, and Mortality in Infliximab- and Immunomodulator-Treated Adult Patients with Inflammatory Bowel Disease. Am. J. Gastroenterol. 2012, 107, 1051–1063. [Google Scholar] [CrossRef]
- Fidder, H.; Schnitzler, F.; Ferrante, M.; Noman, M.; Katsanos, K.; Segaert, S.; Henckaerts, L.; Van Assche, G.; Vermeire, S.; Rutgeerts, P. Long-Term Safety of Infliximab for the Treatment of Inflammatory Bowel Disease: A Single-Centre Cohort Study. Gut 2009, 58, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, S.B.; Sandborn, W.J.; Rutgeerts, P.; Fedorak, R.N.; Lukas, M.; MacIntosh, D.; Panaccione, R.; Wolf, D.; Pollack, P. Human Anti-Tumor Necrosis Factor Monoclonal Antibody (Adalimumab) in Crohn’s Disease: The CLASSIC-I Trial. Gastroenterology 2006, 130, 323–333, quiz 591. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.-F.; Sandborn, W.J.; Rutgeerts, P.; Enns, R.; Hanauer, S.B.; Panaccione, R.; Schreiber, S.; Byczkowski, D.; Li, J.; Kent, J.D.; et al. Adalimumab for Maintenance of Clinical Response and Remission in Patients with Crohn’s Disease: The CHARM Trial. Gastroenterology 2007, 132, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Hanauer, S.B.; Rutgeerts, P.; Fedorak, R.N.; Lukas, M.; MacIntosh, D.G.; Panaccione, R.; Wolf, D.; Kent, J.D.; Bittle, B.; et al. Adalimumab for Maintenance Treatment of Crohn’s Disease: Results of the CLASSIC II Trial. Gut 2007, 56, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; van Assche, G.; Reinisch, W.; Colombel, J.; D’Haens, G.; Wolf, D.C.; Kron, M.; Tighe, M.B.; Lazar, A.; Thakkar, R.B. Adalimumab Induces and Maintains Clinical Remission in Patients with Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2012, 142, 257–265.e3. [Google Scholar] [CrossRef]
- Reinisch, W.; Sandborn, W.J.; Hommes, D.W.; D’Haens, G.; Hanauer, S.; Schreiber, S.; Panaccione, R.; Fedorak, R.N.; Tighe, M.B.; Huang, B.; et al. Adalimumab for Induction of Clinical Remission in Moderately to Severely Active Ulcerative Colitis: Results of a Randomised Controlled Trial. Gut 2011, 60, 780–787. [Google Scholar] [CrossRef]
- Afif, W.; Leighton, J.A.; Hanauer, S.B.; Loftus, E.V., Jr.; Faubion, W.A.; Pardi, D.S.; Tremaine, W.J.; Kane, S.V.; Bruining, D.H.; Cohen, R.D.; et al. Open-Label Study of Adalimumab in Patients with Ulcerative Colitis Including Those with Prior Loss of Response or Intolerance to Infliximab. Inflamm. Bowel Dis. 2009, 15, 1302–1307. [Google Scholar] [CrossRef]
- Osterman, M.T.; Haynes, K.; Delzell, E.; Zhang, J.; Bewtra, M.; Brensinger, C.; Chen, L.; Xie, F.; Curtis, J.R.; Lewis, J.D. Comparative Effectiveness of Infliximab and Adalimumab for Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2014, 12, 811–817.e3. [Google Scholar] [CrossRef]
- Lee, Y.I.; Park, Y.; Park, S.J.; Kim, T.I.; Kim, W.H.; Cheon, J.H. Comparison of Long-Term Outcomes of Infliximab versus Adalimumab Treatment in Biologic-Naïve Patients with Ulcerative Colitis. Gut Liver 2021, 15, 232–242. [Google Scholar] [CrossRef]
- Baert, F.; Kondragunta, V.; Lockton, S.; Casteele, N.V.; Hauenstein, S.; Singh, S.; Karmiris, K.; Ferrante, M.; Gils, A.; Vermeire, S. Antibodies to Adalimumab Are Associated with Future Inflammation in Crohn’s Patients Receiving Maintenance Adalimumab Therapy: A Post Hoc Analysis of the Karmiris Trial. Gut 2016, 65, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Baert, F.; Noman, M.; Vermeire, S.; Van Assche, G.; D’ Haens, G.; Carbonez, A.; Rutgeerts, P. Influence of Immunogenicity on the Long-Term Efficacy of Infliximab in Crohn’s Disease. N. Engl. J. Med. 2003, 348, 601–608. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Marín, A.C.; McNicholl, A.G.; Chaparro, M. Systematic Review with Meta-Analysis: The Efficacy of a Second Anti-TNF in Patients with Inflammatory Bowel Disease Whose Previous Anti-TNF Treatment Has Failed. Aliment. Pharmacol. Ther. 2015, 41, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Roblin, X.; Vérot, C.; Paul, S.; Duru, G.; Williet, N.; Boschetti, G.; Del Tedesco, E.; Peyrin-Biroulet, L.; Phelip, J.M.; Nancey, S.; et al. Is the Pharmacokinetic Profile of a First Anti-TNF Predictive of the Clinical Outcome and Pharmacokinetics of a Second Anti-TNF? Inflamm. Bowel Dis. 2018, 24, 2078–2085. [Google Scholar] [CrossRef]
- Ong, D.E.H.; Kamm, M.A.; Hartono, J.L.; Lust, M. Addition of Thiopurines Can Recapture Response in Patients with Crohn’s Disease Who Have Lost Response to Anti-Tumor Necrosis Factor Monotherapy. J. Gastroenterol. Hepatol. 2013, 28, 1595–1599. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Waterman, M.; Kopylov, U.; Yavzori, M.; Picard, O.; Fudim, E.; Awadie, H.; Weiss, B.; Chowers, Y. Addition of an Immunomodulator to Infliximab Therapy Eliminates Antidrug Antibodies in Serum and Restores Clinical Response of Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2013, 11, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, Azathioprine, or Combination Therapy for Crohn’s Disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef]
- Panaccione, R.; Ghosh, S.; Middleton, S.; Márquez, J.R.; Scott, B.B.; Flint, L.; van Hoogstraten, H.J.F.; Chen, A.C.; Zheng, H.; Danese, S.; et al. Combination Therapy with Infliximab and Azathioprine Is Superior to Monotherapy with Either Agent in Ulcerative Colitis. Gastroenterology 2014, 146, 392–400.e3. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Isaacs, K.L.; Schneider, Y.; Siddique, S.M.; Falck-Ytter, Y.; Singh, S.; Chachu, K.; Day, L.; Lebwohl, B.; Muniraj, T.; et al. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1450–1461. [Google Scholar] [CrossRef]
- Kirchgesner, J.; Lemaitre, M.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Risk of Serious and Opportunistic Infections Associated with Treatment of Inflammatory Bowel Diseases. Gastroenterology 2018, 155, 337–346.e10. [Google Scholar] [CrossRef]
- Lemaitre, M.; Kirchgesner, J.; Rudnichi, A.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Association between Use of Thiopurines or Tumor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients with Inflammatory Bowel Disease. JAMA 2017, 318, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; So, R.; Matsuoka, K.; Kobayashi, T.; Shinzaki, S.; Matsuura, M.; Okabayashi, S.; Kataoka, Y.; Tsujimoto, Y.; Furukawa, T.A.; et al. Certolizumab Pegol for Induction of Remission in Crohn’s Disease. Cochrane Database Syst. Rev. 2019, 8, CD012893. [Google Scholar] [CrossRef]
- Goel, N.; Stephens, S. Certolizumab Pegol. mAbs 2010, 2, 137–147. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Stoinov, S.; Honiball, P.J.; Rutgeerts, P.; Mason, D.; Bloomfield, R.; Schreiber, S. Certolizumab Pegol for the Treatment of Crohn’s Disease. N. Engl. J. Med. 2007, 357, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Khaliq-Kareemi, M.; Lawrance, I.C.; Thomsen, O.Ø.; Hanauer, S.B.; McColm, J.; Bloomfield, R.; Sandborn, W.J. PRECISE 2 Study Investigators Maintenance Therapy with Certolizumab Pegol for Crohn’s Disease. N. Engl. J. Med. 2007, 357, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Lang, L. FDA Approves Cimzia to Treat Crohn’s Disease. Gastroenterology 2008, 134, 1819. [Google Scholar] [CrossRef]
- Chapman, A.P.; Antoniw, P.; Spitali, M.; West, S.; Stephens, S.; King, D.J. Therapeutic Antibody Fragments with Prolonged in Vivo Half-Lives. Nat. Biotechnol. 1999, 17, 780–783. [Google Scholar] [CrossRef]
- Blick, S.K.A.; Curran, M.P. Certolizumab Pegol. BioDrugs 2007, 21, 195–201. [Google Scholar] [CrossRef]
- de Bourayne, M.; Meunier, S.; Bitoun, S.; Correia, E.; Mariette, X.; Nozach, H.; Maillère, B. Pegylation Reduces the Uptake of Certolizumab Pegol by Dendritic Cells and Epitope Presentation to T-Cells. Front. Immunol. 2022, 13, 808606. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.-F.; Reinisch, W.; et al. Subcutaneous Golimumab Induces Clinical Response and Remission in Patients with Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2014, 146, 85–95, quiz e14–15. [Google Scholar] [CrossRef]
- Flamant, M.; Paul, S.; Roblin, X. Golimumab for the Treatment of Ulcerative Colitis. Expert Opin. Biol. Ther. 2017, 17, 879–886. [Google Scholar] [CrossRef]
- Danese, S.; Fiorino, G.; Peyrin-Biroulet, L.; Lucenteforte, E.; Virgili, G.; Moja, L.; Bonovas, S. Biological Agents for Moderately to Severely Active Ulcerative Colitis: A Systematic Review and Network Meta-Analysis. Ann. Intern. Med. 2014, 160, 704–711. [Google Scholar] [CrossRef]
- Stidham, R.W.; Lee, T.C.H.; Higgins, P.D.R.; Deshpande, A.R.; Sussman, D.A.; Singal, A.G.; Elmunzer, B.J.; Saini, S.D.; Vijan, S.; Waljee, A.K. Systematic Review with Network Meta-Analysis: The Efficacy of Anti-TNF Agents for the Treatment of Crohn’s Disease. Aliment. Pharmacol. Ther. 2014, 39, 1349–1362. [Google Scholar] [CrossRef]
- Thomas, S.S.; Borazan, N.; Barroso, N.; Duan, L.; Taroumian, S.; Kretzmann, B.; Bardales, R.; Elashoff, D.; Vangala, S.; Furst, D.E. Comparative Immunogenicity of TNF Inhibitors: Impact on Clinical Efficacy and Tolerability in the Management of Autoimmune Diseases. A Systematic Review and Meta-Analysis. BioDrugs 2015, 29, 241–258. [Google Scholar] [CrossRef]
- Vermeire, S.; Gils, A.; Accossato, P.; Lula, S.; Marren, A. Immunogenicity of Biologics in Inflammatory Bowel Disease. Ther. Adv. Gastroenterol. 2018, 11, 1756283X17750355. [Google Scholar] [CrossRef]
- Nakamura, K.; Honda, K.; Mizutani, T.; Akiho, H.; Harada, N. Novel Strategies for the Treatment of Inflammatory Bowel Disease: Selective Inhibition of Cytokines and Adhesion Molecules. World J. Gastroenterol. 2006, 12, 4628–4635. [Google Scholar] [CrossRef]
- Guagnozzi, D.; Caprilli, R. Natalizumab in the Treatment of Crohn’s Disease. Biol. Targets Ther. 2008, 2, 275–284. [Google Scholar]
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.-F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2013, 369, 711–721. [Google Scholar] [CrossRef]
- Nelson, S.M.; Nguyen, T.M.; McDonald, J.W.; MacDonald, J.K. Natalizumab for Induction of Remission in Crohn’s Disease. Cochrane Database Syst. Rev. 2018, 2018, CD006097. [Google Scholar] [CrossRef]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Off. J. Am. Coll. Gastroenterol. ACG 2019, 114, 384. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Panés, J.; Danese, S.; Sharafali, Z.; Hassanali, A.; Jacob-Moffatt, R.; Eden, C.; Daperno, M.; Valentine, J.F.; Laharie, D.; et al. Etrolizumab as Induction and Maintenance Therapy in Patients with Moderately to Severely Active Crohn’s Disease (BERGAMOT): A Randomised, Placebo-Controlled, Double-Blind, Phase 3 Trial. Lancet Gastroenterol. Hepatol. 2023, 8, 43–55. [Google Scholar] [CrossRef]
- Laurel, P.I.; Gardenia, P.I.; Hickory, P.I.; Bergamot, P.I. Genentech Provides Update on Phase III Studies of Etrolizumab in People with Moderately to Severely Active Ulcerative Colitis; Bloomberg.com: New York, NY, USA, 2020; Available online: https://www.businesswire.com/news/home/20200809005022/en/Genentech-Provides-Update-on-Phase-III-Studies-of-Etrolizumab-in-People-With-Moderately-to-Severely-Active-Ulcerative-Colitis (accessed on 30 June 2023).
- Caron, B.; Peyrin-Biroulet, L.; Pariente, B.; Bouhnik, Y.; Seksik, P.; Bouguen, G.; Caillo, L.; Laharie, D.; Carbonnel, F.; Altwegg, R.; et al. Vedolizumab Therapy Is Ineffective for Primary Sclerosing Cholangitis in Patients with Inflammatory Bowel Disease: A GETAID Multicentre Cohort Study. J. Crohns Colitis 2019, 13, 1239–1247. [Google Scholar] [CrossRef]
- Lynch, K.D.; Chapman, R.W.; Keshav, S.; Montano-Loza, A.J.; Mason, A.L.; Kremer, A.E.; Vetter, M.; de Krijger, M.; Ponsioen, C.Y.; Trivedi, P.; et al. Effects of Vedolizumab in Patients with Primary Sclerosing Cholangitis and Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 179–187.e6. [Google Scholar] [CrossRef]
- Laborda, T.J.; Ricciuto, A.; Aumar, M.; Carman, N.; DiGuglielmo, M.; Draijer, L.G.; Furuya, K.N.; Gupta, N.; Koot, B.G.P.; Loomes, K.M.; et al. Vedolizumab Therapy in Children With Primary Sclerosing Cholangitis: Data from the Pediatric Primary Sclerosing Cholangitis Consortium. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 459. [Google Scholar] [CrossRef]
- Eksteen, B.; Grant, A.J.; Miles, A.; Curbishley, S.M.; Lalor, P.F.; Hübscher, S.G.; Briskin, M.; Salmon, M.; Adams, D.H. Hepatic Endothelial CCL25 Mediates the Recruitment of CCR9+ Gut-Homing Lymphocytes to the Liver in Primary Sclerosing Cholangitis. J. Exp. Med. 2004, 200, 1511–1517. [Google Scholar] [CrossRef]
- Grant, A.J.; Lalor, P.F.; Hübscher, S.G.; Briskin, M.; Adams, D.H. MAdCAM-1 Expressed in Chronic Inflammatory Liver Disease Supports Mucosal Lymphocyte Adhesion to Hepatic Endothelium (MAdCAM-1 in Chronic Inflammatory Liver Disease). Hepatology 2001, 33, 1065–1072. [Google Scholar] [CrossRef]
- Muir, A.J.; Levy, C.; Janssen, H.L.A.; Montano-Loza, A.J.; Shiffman, M.L.; Caldwell, S.; Luketic, V.; Ding, D.; Jia, C.; McColgan, B.J.; et al. Simtuzumab for Primary Sclerosing Cholangitis: Phase 2 Study Results with Insights on the Natural History of the Disease. Hepatology 2019, 69, 684–698. [Google Scholar] [CrossRef]
- Harrison, S.A.; Abdelmalek, M.F.; Caldwell, S.; Shiffman, M.L.; Diehl, A.M.; Ghalib, R.; Lawitz, E.J.; Rockey, D.C.; Schall, R.A.; Jia, C.; et al. Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology 2018, 155, 1140–1153. [Google Scholar] [CrossRef]
- Segal-Salto, M.; Barashi, N.; Katav, A.; Edelshtein, V.; Aharon, A.; Hashmueli, S.; George, J.; Maor, Y.; Pinzani, M.; Haberman, D.; et al. A Blocking Monoclonal Antibody to CCL24 Alleviates Liver Fibrosis and Inflammation in Experimental Models of Liver Damage. JHEP Rep. 2020, 2, 100064. [Google Scholar] [CrossRef]
- Roufosse, F. Targeting the Interleukin-5 Pathway for Treatment of Eosinophilic Conditions Other than Asthma. Front. Med. 2018, 5, 49. [Google Scholar] [CrossRef]
- Cases, Data, and Surveillance. Available online: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html (accessed on 30 June 2023).
- Richards, F.; Kodjamanova, P.; Chen, X.; Li, N.; Atanasov, P.; Bennetts, L.; Patterson, B.J.; Yektashenas, B.; Mesa-Frias, M.; Tronczynski, K.; et al. Economic Burden of COVID-19: A Systematic Review. Clin. Outcomes Res. 2022, 14, 293–307. [Google Scholar] [CrossRef]
- Bloom, D.E.; Kuhn, M.; Prettner, K. Modern Infectious Diseases: Macroeconomic Impacts and Policy Responses. J. Econ. Lit. 2022, 60, 85–131. [Google Scholar] [CrossRef]
- Young, M.; Smitherman, L. Socioeconomic Impact of RSV Hospitalization. Infect. Dis. Ther. 2021, 10, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Crank, M.C.; Ruckwardt, T.J.; Chen, M.; Morabito, K.M.; Phung, E.; Costner, P.J.; Holman, L.A.; Hickman, S.P.; Berkowitz, N.M.; Gordon, I.J.; et al. A Proof of Concept for Structure-Based Vaccine Design Targeting RSV in Humans. Science 2019, 365, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Boyoglu-Barnum, S.; Chirkova, T.; Anderson, L.J. Biology of Infection and Disease Pathogenesis to Guide RSV Vaccine Development. Front. Immunol. 2019, 10, 1675. [Google Scholar] [CrossRef]
- Garegnani, L.; Styrmisdóttir, L.; Rodriguez, P.R.; Liquitay, C.M.E.; Esteban, I.; Franco, J.V. Palivizumab for Preventing Severe Respiratory Syncytial Virus (RSV) Infection in Children. Cochrane Database Syst. Rev. 2021, 11, CD013757. [Google Scholar] [CrossRef]
- Scott, L.J.; Lamb, H.M. Palivizumab. Drugs 1999, 58, 305–311. [Google Scholar] [CrossRef]
- Mac, S.; Sumner, A.; Duchesne-Belanger, S.; Stirling, R.; Tunis, M.; Sander, B. Cost-Effectiveness of Palivizumab for Respiratory Syncytial Virus: A Systematic Review. Pediatrics 2019, 143, e20184064. [Google Scholar] [CrossRef]
- Lee, A. Ansuvimab: First Approval. Drugs 2021, 81, 595–598. [Google Scholar] [CrossRef]
- Markham, A. REGN-EB3: First Approval. Drugs 2021, 81, 175–178. [Google Scholar] [CrossRef]
- Rayaprolu, V.; Fulton, B.; Rafique, A.; Arturo, E.; Williams, D.; Hariharan, C.; Callaway, H.; Parvate, A.; Schendel, S.L.; Parekh, D.; et al. Structure of the Inmazeb Cocktail and Resistance to Escape against Ebola Virus. bioRxiv 2022. [Google Scholar] [CrossRef]
- Pantaleo, G.; Correia, B.; Fenwick, C.; Joo, V.S.; Perez, L. Antibodies to Combat Viral Infections: Development Strategies and Progress. Nat. Rev. Drug Discov. 2022, 21, 676–696. [Google Scholar] [CrossRef] [PubMed]
- Andreano, E.; Seubert, A.; Rappuoli, R. Human Monoclonal Antibodies for Discovery, Therapy, and Vaccine Acceleration. Curr. Opin. Immunol. 2019, 59, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Tisch, R.; McDevitt, H. Insulin-Dependent Diabetes Mellitus. Cell 1996, 85, 291–297. [Google Scholar] [CrossRef]
- Van Belle, T.L.; Coppieters, K.T.; Von Herrath, M.G. Type 1 Diabetes: Etiology, Immunology, and Therapeutic Strategies. Physiol. Rev. 2011, 91, 79–118. [Google Scholar] [CrossRef]
- Eisenbarth, G.S. Type 1 Diabetes: Molecular, Cellular and Clinical Immunology. Adv. Exp. Med. Biol. 2004, 552, 306–310. [Google Scholar]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of Diabetes and Diabetes-Related Complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- Evans-Molina, C.; Oram, R.A. Teplizumab Approval for Type 1 Diabetes in the USA. Lancet Diabetes Endocrinol. 2023, 11, 76–77. [Google Scholar] [CrossRef]
- Long, S.A.; Thorpe, J.; DeBerg, H.A.; Gersuk, V.; Eddy, J.A.; Harris, K.M.; Ehlers, M.; Herold, K.C.; Nepom, G.T.; Linsley, P.S. Partial Exhaustion of CD8 T Cells and Clinical Response to Teplizumab in New-Onset Type 1 Diabetes. Sci. Immunol. 2016, 1, eaai7793. [Google Scholar] [CrossRef]
- Tooley, J.E.; Vudattu, N.; Choi, J.; Cotsapas, C.; Devine, L.; Raddassi, K.; Ehlers, M.R.; McNamara, J.G.; Harris, K.M.; Kanaparthi, S.; et al. Changes in T-Cell Subsets Identify Responders to FcR-Nonbinding Anti-CD3 MAb (Teplizumab) in Patients with Type 1 Diabetes. Eur. J. Immunol. 2016, 46, 230–241. [Google Scholar] [CrossRef]
- Kuhn, C.; You, S.; Valette, F.; Hale, G.; van Endert, P.; Bach, J.-F.; Waldmann, H.; Chatenoud, L. Human CD3 Transgenic Mice: Preclinical Testing of Antibodies Promoting Immune Tolerance. Sci. Transl. Med. 2011, 3, 68ra10. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Leforban, B.; Garcia, C.; Bach, J.-F.; Bluestone, J.A.; Chatenoud, L. Adaptive TGF-β-Dependent Regulatory T Cells Control Autoimmune Diabetes and Are a Privileged Target of Anti-CD3 Antibody Treatment. Proc. Natl. Acad. Sci. USA 2007, 104, 6335–6340. [Google Scholar] [CrossRef]
- Waldron-Lynch, F.; Henegariu, O.; Deng, S.; Preston-Hurlburt, P.; Tooley, J.; Flavell, R.; Herold, K.C. Teplizumab Induces Human Gut-Tropic Regulatory Cells in Humanized Mice and Patients. Sci. Transl. Med. 2012, 4, 118ra12. [Google Scholar] [CrossRef] [PubMed]
- Sims, E.K.; Bundy, B.N.; Stier, K.; Serti, E.; Lim, N.; Long, S.A.; Geyer, S.M.; Moran, A.; Greenbaum, C.J.; Evans-Molina, C.; et al. Teplizumab Improves and Stabilizes Beta Cell Function in Antibody-Positive High-Risk Individuals. Sci. Transl. Med. 2021, 13, eabc8980. [Google Scholar] [CrossRef]
- Perdigoto, A.L.; Preston-Hurlburt, P.; Clark, P.; Long, S.A.; Linsley, P.S.; Harris, K.M.; Gitelman, S.E.; Greenbaum, C.J.; Gottlieb, P.A.; Hagopian, W.; et al. Treatment of Type 1 Diabetes with Teplizumab: Clinical and Immunological Follow-up after 7 Years from Diagnosis. Diabetologia 2019, 62, 655–664. [Google Scholar] [CrossRef]
- Nourelden, A.Z.; Elshanbary, A.A.; El-Sherif, L.; Benmelouka, A.Y.; Rohim, H.I.; Helmy, S.K.; Sayed, M.K.; Ismail, A.; Ali, A.S.; Ragab, K.M.; et al. Safety and Efficacy of Teplizumab for Treatment of Type One Diabetes Mellitus: A Systematic Review and Meta-Analysis. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets Immune Endocr. Metab. Disord.) 2021, 21, 1895–1904. [Google Scholar] [CrossRef]
- Herold, K.C.; Gitelman, S.E.; Ehlers, M.R.; Gottlieb, P.A.; Greenbaum, C.J.; Hagopian, W.; Boyle, K.D.; Keyes-Elstein, L.; Aggarwal, S.; Phippard, D.; et al. Teplizumab (Anti-CD3 MAb) Treatment Preserves C-Peptide Responses in Patients with New-Onset Type 1 Diabetes in a Randomized Controlled Trial: Metabolic and Immunologic Features at Baseline Identify a Subgroup of Responders. Diabetes 2013, 62, 3766–3774. [Google Scholar] [CrossRef]
- Sherry, N.; Hagopian, W.; Ludvigsson, J.; Jain, S.M.; Wahlen, J.; Ferry, R.J.; Bode, B.; Aronoff, S.; Holland, C.; Carlin, D.; et al. Teplizumab for Treatment of Type 1 Diabetes (Protégé Study): 1-Year Results from a Randomised, Placebo-Controlled Trial. Lancet 2011, 378, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Herold, K.C.; Bundy, B.N.; Long, S.A.; Bluestone, J.A.; DiMeglio, L.A.; Dufort, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Krischer, J.P.; Linsley, P.S.; et al. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N. Engl. J. Med. 2019, 381, 603–613. [Google Scholar] [CrossRef]
- Vlasakakis, G.; Napolitano, A.; Barnard, R.; Brown, K.; Bullman, J.; Inman, D.; Keymeulen, B.; Lanham, D.; Leirens, Q.; MacDonald, A.; et al. Target Engagement and Cellular Fate of Otelixizumab: A Repeat Dose Escalation Study of an Anti-CD3ε MAb in New-Onset Type 1 Diabetes Mellitus Patients. Br. J. Clin. Pharmacol. 2019, 85, 704–714. [Google Scholar] [CrossRef]
- Keymeulen, B.; van Maurik, A.; Inman, D.; Oliveira, J.; McLaughlin, R.; Gittelman, R.M.; Roep, B.O.; Gillard, P.; Hilbrands, R.; Gorus, F.; et al. A Randomised, Single-Blind, Placebo-Controlled, Dose-Finding Safety and Tolerability Study of the Anti-CD3 Monoclonal Antibody Otelixizumab in New-Onset Type 1 Diabetes. Diabetologia 2021, 64, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Keymeulen, B.; Vandemeulebroucke, E.; Ziegler, A.G.; Mathieu, C.; Kaufman, L.; Hale, G.; Gorus, F.; Goldman, M.; Walter, M.; Candon, S.; et al. Insulin Needs after CD3-Antibody Therapy in New-Onset Type 1 Diabetes. N. Engl. J. Med. 2005, 352, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Gitelman, S.E.; Gottlieb, P.A.; Rigby, M.R.; Felner, E.I.; Willi, S.M.; Fisher, L.K.; Moran, A.; Gottschalk, M.; Moore, W.V.; Pinckney, A.; et al. Antithymocyte Globulin Treatment for Patients with Recent-Onset Type 1 Diabetes: 12-Month Results of a Randomised, Placebo-Controlled, Phase 2 Trial. Lancet Diabetes Endocrinol. 2013, 1, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Gitelman, S.E.; Gottlieb, P.A.; Felner, E.I.; Willi, S.M.; Fisher, L.K.; Moran, A.; Gottschalk, M.; Moore, W.V.; Pinckney, A.; Keyes-Elstein, L.; et al. Antithymocyte Globulin Therapy for Patients with Recent-Onset Type 1 Diabetes: 2 Year Results of a Randomised Trial. Diabetologia 2016, 59, 1153–1161. [Google Scholar] [CrossRef]
- Haller, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Michels, A.W.; Rosenthal, S.M.; Shuster, J.J.; Zou, B.; Brusko, T.M.; Hulme, M.A.; Wasserfall, C.H.; et al. Anti-Thymocyte Globulin/G-CSF Treatment Preserves β Cell Function in Patients with Established Type 1 Diabetes. J. Clin. Investig. 2015, 125, 448–455. [Google Scholar] [CrossRef]
- Haller, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Michels, A.W.; Perry, D.J.; Schultz, A.R.; Hulme, M.A.; Shuster, J.J.; Zou, B.; Wasserfall, C.H.; et al. Antithymocyte Globulin Plus G-CSF Combination Therapy Leads to Sustained Immunomodulatory and Metabolic Effects in a Subset of Responders With Established Type 1 Diabetes. Diabetes 2016, 65, 3765–3775. [Google Scholar] [CrossRef]
- Haller, M.J.; Atkinson, M.A.; Wasserfall, C.H.; Brusko, T.M.; Mathews, C.E.; Hulme, M.; Cintron, M.; Shuster, J.; McGrail, K.; Posgai, A.; et al. Mobilization without Immune Depletion Fails to Restore Immunological Tolerance or Preserve Beta Cell Function in Recent Onset Type 1 Diabetes. Clin. Exp. Immunol. 2016, 183, 350–357. [Google Scholar] [CrossRef]
- Haller, M.J.; Schatz, D.A.; Skyler, J.S.; Krischer, J.P.; Bundy, B.N.; Miller, J.L.; Atkinson, M.A.; Becker, D.J.; Baidal, D.; DiMeglio, L.A.; et al. Low-Dose Anti-Thymocyte Globulin (ATG) Preserves β-Cell Function and Improves HbA1c in New-Onset Type 1 Diabetes. Diabetes Care 2018, 41, 1917–1925. [Google Scholar] [CrossRef]
- Haller, M.J.; Long, S.A.; Blanchfield, J.L.; Schatz, D.A.; Skyler, J.S.; Krischer, J.P.; Bundy, B.N.; Geyer, S.M.; Warnock, M.V.; Miller, J.L.; et al. Low-Dose Anti-Thymocyte Globulin Preserves C-Peptide, Reduces HbA1c, and Increases Regulatory to Conventional T-Cell Ratios in New-Onset Type 1 Diabetes: Two-Year Clinical Trial Data. Diabetes 2019, 68, 1267–1276. [Google Scholar] [CrossRef]
- Jacobsen, L.M.; Bundy, B.N.; Greco, M.N.; Schatz, D.A.; Atkinson, M.A.; Brusko, T.M.; Mathews, C.E.; Herold, K.C.; Gitelman, S.E.; Krischer, J.P.; et al. Comparing Beta Cell Preservation across Clinical Trials in Recent-Onset Type 1 Diabetes. Diabetes Technol. Ther. 2020, 22, 948–953. [Google Scholar] [CrossRef]
- Pescovitz, M.D.; Greenbaum, C.J.; Krause-Steinrauf, H.; Becker, D.J.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Marks, J.B.; McGee, P.F.; Moran, A.M.; et al. Rituximab, B-Lymphocyte Depletion, and Preservation of Beta-Cell Function. N. Engl. J. Med. 2009, 361, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Pescovitz, M.D.; Greenbaum, C.J.; Bundy, B.; Becker, D.J.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Marks, J.B.; Moran, A.; Raskin, P.; et al. B-Lymphocyte Depletion With Rituximab and β-Cell Function: Two-Year Results. Diabetes Care 2014, 37, 453–459. [Google Scholar] [CrossRef]
- Garg, N.; Smith, T.W. An Update on Immunopathogenesis, Diagnosis, and Treatment of Multiple Sclerosis. Brain Behav. 2015, 5, e00362. [Google Scholar] [CrossRef] [PubMed]
- Selewski, D.T.; Shah, G.V.; Segal, B.M.; Rajdev, P.A.; Mukherji, S.K. Natalizumab (Tysabri). AJNR Am. J. Neuroradiol. 2010, 31, 1588–1590. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Bartholomé, E. Treatment Effect of Natalizumab on Relapse Outcomes in Multiple Sclerosis Patients despite Ongoing MRI Activity. J. Neurol. Neurosurg. Psychiatry 2012, 83, 55–60. [Google Scholar] [CrossRef]
- Clerico, M.; Artusi, C.A.; Liberto, A.D.; Rolla, S.; Bardina, V.; Barbero, P.; Mercanti, S.F.D.; Durelli, L. Natalizumab in Multiple Sclerosis: Long-Term Management. Int. J. Mol. Sci. 2017, 18, 940. [Google Scholar] [CrossRef]
- Ho, P.-R.; Koendgen, H.; Campbell, N.; Haddock, B.; Richman, S.; Chang, I. Risk of Natalizumab-Associated Progressive Multifocal Leukoencephalopathy in Patients with Multiple Sclerosis: A Retrospective Analysis of Data from Four Clinical Studies. Lancet Neurol. 2017, 16, 925–933. [Google Scholar] [CrossRef]
- Morrow, S.A.; Clift, F.; Devonshire, V.; Lapointe, E.; Schneider, R.; Stefanelli, M.; Vosoughi, R. Use of Natalizumab in Persons with Multiple Sclerosis: 2022 Update. Mult. Scler. Relat. Disord. 2022, 65, 103995. [Google Scholar] [CrossRef]
- Rommer, P.S.; Dudesek, A.; Stüve, O.; Zettl, U. Monoclonal Antibodies in Treatment of Multiple Sclerosis. Clin. Exp. Immunol. 2014, 175, 373–384. [Google Scholar] [CrossRef]
- Rostagno, A.A. Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 107. [Google Scholar] [CrossRef]
- Lacorte, E.; Ancidoni, A.; Zaccaria, V.; Remoli, G.; Tariciotti, L.; Bellomo, G.; Sciancalepore, F.; Corbo, M.; Lombardo, F.L.; Bacigalupo, I.; et al. Safety and Efficacy of Monoclonal Antibodies for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Published and Unpublished Clinical Trials. J. Alzheimers Dis. 2022, 87, 101–129. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Silvestre, S. Alzheimer’s Disease: Recent Treatment Strategies. Eur. J. Pharmacol. 2020, 887, 173554. [Google Scholar] [CrossRef] [PubMed]
- Kastanenka, K.V.; Bussiere, T.; Shakerdge, N.; Qian, F.; Weinreb, P.H.; Rhodes, K.; Bacskai, B.J. Immunotherapy with Aducanumab Restores Calcium Homeostasis in Tg2576 Mice. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 12549–12558. [Google Scholar] [CrossRef] [PubMed]
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The Antibody Aducanumab Reduces Aβ Plaques in Alzheimer’s Disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of Anti-Amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef]
- Panza, F.; Frisardi, V.; Imbimbo, B.P.; Seripa, D.; Paris, F.; Santamato, A.; D’Onofrio, G.; Logroscino, G.; Pilotto, A.; Solfrizzi, V. Anti-β-Amyloid Immunotherapy for Alzheimer’s Disease: Focus on Bapineuzumab. Curr. Alzheimer Res. 2011, 8, 808–817. [Google Scholar] [CrossRef]
- Salloway, S.; Sperling, R.; Fox, N.C.; Blennow, K.; Klunk, W.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; Ferris, S.; et al. Two Phase 3 Trials of Bapineuzumab in Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2014, 370, 322–333. [Google Scholar] [CrossRef]
- La Porte, S.L.; Bollini, S.S.; Lanz, T.A.; Abdiche, Y.N.; Rusnak, A.S.; Ho, W.-H.; Kobayashi, D.; Harrabi, O.; Pappas, D.; Mina, E.W.; et al. Structural Basis of C-Terminal β-Amyloid Peptide Binding by the Antibody Ponezumab for the Treatment of Alzheimer’s Disease. J. Mol. Biol. 2012, 421, 525–536. [Google Scholar] [CrossRef]
- Landen, J.W.; Andreasen, N.; Cronenberger, C.L.; Schwartz, P.F.; Börjesson-Hanson, A.; Östlund, H.; Sattler, C.A.; Binneman, B.; Bednar, M.M. Ponezumab in Mild-to-Moderate Alzheimer’s Disease: Randomized Phase II PET-PIB Study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 393–401. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Südhof, T.C. Cell Biology and Pathophysiology of α-Synuclein. Cold Spring Harb. Perspect. Med. 2018, 8, a024091. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Taylor, K.I.; Anzures-Cabrera, J.; Marchesi, M.; Simuni, T.; Marek, K.; Postuma, R.B.; Pavese, N.; Stocchi, F.; Azulay, J.-P.; et al. Trial of Prasinezumab in Early-Stage Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.L. Migraine Overview and Summary of Current and Emerging Treatment Options. Am. J. Manag. Care 2019, 25, S23–S34. [Google Scholar] [PubMed]
- Nappi, R.E.; Tiranini, L.; Sacco, S.; De Matteis, E.; De Icco, R.; Tassorelli, C. Role of Estrogens in Menstrual Migraine. Cells 2022, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Reuter, U.; Hallström, Y.; Broessner, G.; Bonner, J.H.; Zhang, F.; Sapra, S.; Picard, H.; Mikol, D.D.; Lenz, R.A. A Controlled Trial of Erenumab for Episodic Migraine. N. Engl. J. Med. 2017, 377, 2123–2132. [Google Scholar] [CrossRef]
- Detke, H.C.; Goadsby, P.J.; Wang, S.; Friedman, D.I.; Selzler, K.J.; Aurora, S.K. Galcanezumab in Chronic Migraine: The Randomized, Double-Blind, Placebo-Controlled REGAIN Study. Neurology 2018, 91, e2211. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Dodick, D.W.; Bigal, M.E.; Yeung, P.P.; Goadsby, P.J.; Blankenbiller, T.; Grozinski-Wolff, M.; Yang, R.; Ma, Y.; Aycardi, E. Fremanezumab for the Preventive Treatment of Chronic Migraine. N. Engl. J. Med. 2017, 377, 2113–2122. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Silberstein, S.D.; Yeung, P.P.; Cohen, J.M.; Ning, X.; Yang, R.; Dodick, D.W. Long-Term Safety, Tolerability, and Efficacy of Fremanezumab in Migraine: A Randomized Study. Neurology 2020, 95, e2487–e2499. [Google Scholar] [CrossRef]
- Gao, B.; Sun, N.; Yang, Y.; Sun, Y.; Chen, M.; Chen, Z.; Wang, Z. Safety and Efficacy of Fremanezumab for the Prevention of Migraine: A Meta-Analysis from Randomized Controlled Trials. Front. Neurol. 2020, 11, 435. [Google Scholar] [CrossRef]
- Lee, A.; Shirley, M. Ranibizumab: A Review in Retinopathy of Prematurity. Paediatr. Drugs 2021, 23, 111–117. [Google Scholar] [CrossRef]
- Kaushal, M.; Razak, A.; Patel, W.; Pullattayil, A.K.; Kaushal, A. Neurodevelopmental Outcomes Following Bevacizumab Treatment for Retinopathy of Prematurity: A Systematic Review and Meta-Analysis. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2021, 41, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Naxitamab: First Approval. Drugs 2021, 81, 291–296. [Google Scholar] [CrossRef]
- Hanley, D.A.; Adachi, J.D.; Bell, A.; Brown, V. Denosumab: Mechanism of Action and Clinical Outcomes. Int. J. Clin. Pract. 2012, 66, 1139–1146. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dutta, S.; Khasbage, S.; Kumar, T.; Sachin, J.; Sharma, J.; Varthya, S.B. A Systematic Review and Meta-Analysis of Efficacy and Safety of Romosozumab in Postmenopausal Osteoporosis. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2022, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kobayakawa, T.; Miyazaki, A.; Saito, M.; Suzuki, T.; Takahashi, J.; Nakamura, Y. Denosumab versus Romosozumab for Postmenopausal Osteoporosis Treatment. Sci. Rep. 2021, 11, 11801. [Google Scholar] [CrossRef]
- Imel, E.A. Burosumab for Pediatric X-Linked Hypophosphatemia. Curr. Osteoporos. Rep. 2021, 19, 271–277. [Google Scholar] [CrossRef]
- Schindeler, A.; Biggin, A.; Munns, C.F. Clinical Evidence for the Benefits of Burosumab Therapy for X-Linked Hypophosphatemia (XLH) and Other Conditions in Adults and Children. Front. Endocrinol. 2020, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L.; Jan de Beur, S.; Briot, K.; Carpenter, T.; Cheong, H.I.; Cohen-Solal, M.; Crowley, R.K.; Eastell, R.; Imanishi, Y.; Imel, E.A.; et al. Efficacy of Burosumab in Adults with X-Linked Hypophosphatemia (XLH): A Post Hoc Subgroup Analysis of a Randomized Double-Blind Placebo-Controlled Phase 3 Study. Calcif. Tissue Int. 2022, 111, 409–418. [Google Scholar] [CrossRef]
- Tannemaat, M.R.; Verschuuren, J.J.G.M. Emerging Therapies for Autoimmune Myasthenia Gravis: Towards Treatment without Corticosteroids. Neuromuscul. Disord. 2020, 30, 111–119. [Google Scholar] [CrossRef]
- Shapiro, A.D. Concizumab: A Novel Anti-TFPI Therapeutic for Hemophilia. Blood Adv. 2021, 5, 279. [Google Scholar] [CrossRef] [PubMed]
- Röth, A.; Barcellini, W.; D’Sa, S.; Miyakawa, Y.; Broome, C.M.; Michel, M.; Kuter, D.J.; Jilma, B.; Tvedt, T.H.A.; Fruebis, J.; et al. Sutimlimab in Cold Agglutinin Disease. N. Engl. J. Med. 2021, 384, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Cataland, S.R.; Peyvandi, F.; Coppo, P.; Knöbl, P.; Kremer Hovinga, J.A.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Callewaert, F.; et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2019, 380, 335–346. [Google Scholar] [CrossRef]
- Stern, R.M.; Connell, N.T. Ravulizumab: A Novel C5 Inhibitor for the Treatment of Paroxysmal Nocturnal Hemoglobinuria. Ther. Adv. Hematol. 2019, 10, 2040620719874728. [Google Scholar] [CrossRef] [PubMed]
- Nooka, A.K.; Kaufman, J.L.; Hofmeister, C.C.; Joseph, N.S.; Heffner, T.L.; Gupta, V.A.; Sullivan, H.C.; Neish, A.S.; Dhodapkar, M.V.; Lonial, S. Daratumumab in Multiple Myeloma. Cancer 2019, 125, 2364–2382. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Richardson, P.G.; Bahlis, N.J.; Grosicki, S.; Cavo, M.; Beksaç, M.; Legieć, W.; Liberati, A.M.; Goldschmidt, H.; Belch, A.; et al. Addition of Elotuzumab to Lenalidomide and Dexamethasone for Patients with Newly Diagnosed, Transplantation Ineligible Multiple Myeloma (ELOQUENT-1): An Open-Label, Multicentre, Randomised, Phase 3 Trial. Lancet Haematol. 2022, 9, e403–e414. [Google Scholar] [CrossRef] [PubMed]
- Pollack, C.V.; Reilly, P.A.; van Ryn, J.; Eikelboom, J.W.; Glund, S.; Bernstein, R.A.; Dubiel, R.; Huisman, M.V.; Hylek, E.M.; Kam, C.-W.; et al. Idarucizumab for Dabigatran Reversal—Full Cohort Analysis. N. Engl. J. Med. 2017, 377, 431–441. [Google Scholar] [CrossRef]
- Brodsky, R.A.; Young, N.S.; Antonioli, E.; Risitano, A.M.; Schrezenmeier, H.; Schubert, J.; Gaya, A.; Coyle, L.; de Castro, C.; Fu, C.-L.; et al. Multicenter Phase 3 Study of the Complement Inhibitor Eculizumab for the Treatment of Patients with Paroxysmal Nocturnal Hemoglobinuria. Blood 2008, 111, 1840–1847. [Google Scholar] [CrossRef]
- Griffin, M.M.; Morley, N. Rituximab in the Treatment of Non-Hodgkin’s Lymphoma—A Critical Evaluation of Randomized Controlled Trials. Expert Opin. Biol. Ther. 2013, 13, 803–811. [Google Scholar] [CrossRef]
- Grosicki, S.; Bednarczyk, M.; Kociszewska, K. Elranatamab: A New Promising BispAb in Multiple Myeloma Treatment. Expert Rev. Anticancer Ther. 2023, 23, 775–782. [Google Scholar] [CrossRef]
- Chari, A.; Minnema, M.C.; Berdeja, J.G.; Oriol, A.; van de Donk, N.W.C.J.; Rodríguez-Otero, P.; Askari, E.; Mateos, M.-V.; Costa, L.J.; Caers, J.; et al. Talquetamab, a T-Cell-Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. N. Engl. J. Med. 2022, 387, 2232–2244. [Google Scholar] [CrossRef] [PubMed]
- Thieblemont, C.; Phillips, T.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Do, Y.R.; Feldman, T.; Gasiorowski, R.; Jurczak, W.; et al. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 2238–2247. [Google Scholar] [CrossRef]
- Dickinson, M.J.; Carlo-Stella, C.; Morschhauser, F.; Bachy, E.; Corradini, P.; Iacoboni, G.; Khan, C.; Wróbel, T.; Offner, F.; Trněný, M.; et al. Glofitamab for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 387, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Coles, A.J.; Twyman, C.L.; Arnold, D.L.; Cohen, J.A.; Confavreux, C.; Fox, E.J.; Hartung, H.-P.; Havrdova, E.; Selmaj, K.W.; Weiner, H.L.; et al. Alemtuzumab for Patients with Relapsing Multiple Sclerosis after Disease-Modifying Therapy: A Randomised Controlled Phase 3 Trial. Lancet 2012, 380, 1829–1839. [Google Scholar] [CrossRef]
- Fox, E.; Lovett-Racke, A.E.; Gormley, M.; Liu, Y.; Petracca, M.; Cocozza, S.; Shubin, R.; Wray, S.; Weiss, M.S.; Bosco, J.A.; et al. A Phase 2 Multicenter Study of Ublituximab, a Novel Glycoengineered Anti-CD20 Monoclonal Antibody, in Patients with Relapsing Forms of Multiple Sclerosis. Mult. Scler. Houndmills Basingstoke Engl. 2021, 27, 420–429. [Google Scholar] [CrossRef]
- Moreau, P.; Garfall, A.L.; van de Donk, N.W.C.J.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Budde, L.E.; Sehn, L.H.; Matasar, M.; Schuster, S.J.; Assouline, S.; Giri, P.; Kuruvilla, J.; Canales, M.; Dietrich, S.; Fay, K.; et al. Safety and Efficacy of Mosunetuzumab, a Bispecific Antibody, in Patients with Relapsed or Refractory Follicular Lymphoma: A Single-Arm, Multicentre, Phase 2 Study. Lancet Oncol. 2022, 23, 1055–1065. [Google Scholar] [CrossRef]
- Kim, Y.H.; Bagot, M.; Pinter-Brown, L.; Rook, A.H.; Porcu, P.; Horwitz, S.M.; Whittaker, S.; Tokura, Y.; Vermeer, M.; Zinzani, P.L.; et al. Mogamulizumab versus Vorinostat in Previously Treated Cutaneous T-Cell Lymphoma (MAVORIC): An International, Open-Label, Randomised, Controlled Phase 3 Trial. Lancet Oncol. 2018, 19, 1192–1204. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, J.; Zhang, Y.; Luo, J.; Shi, S. Ocrelizumab for Multiple Sclerosis. Cochrane Database Syst. Rev. 2022, 5, CD013247. [Google Scholar] [CrossRef]
- Reagan, J.L.; Castillo, J.J. Ofatumumab as Front-Line Therapy in Untreated Chronic Lymphocytic Leukemia. Future Oncol. 2014, 10, 1147–1155. [Google Scholar] [CrossRef]
- Caimi, P.F.; Ai, W.; Alderuccio, J.P.; Ardeshna, K.M.; Hamadani, M.; Hess, B.; Kahl, B.S.; Radford, J.; Solh, M.; Stathis, A.; et al. Loncastuximab Tesirine in Relapsed or Refractory Diffuse Large B-Cell Lymphoma (LOTIS-2): A Multicentre, Open-Label, Single-Arm, Phase 2 Trial. Lancet Oncol. 2021, 22, 790–800. [Google Scholar] [CrossRef]
- Offidani, M.; Corvatta, L.; Morè, S.; Olivieri, A. Belantamab Mafodotin for the Treatment of Multiple Myeloma: An Overview of the Clinical Efficacy and Safety. Drug Des. Devel. Ther. 2021, 15, 2401–2415. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Duell, J.; González Barca, E.; Tournilhac, O.; Jurczak, W.; Liberati, A.M.; Nagy, Z.; Obr, A.; Gaidano, G.; André, M.; et al. Tafasitamab plus Lenalidomide in Relapsed or Refractory Diffuse Large B-Cell Lymphoma (L-MIND): A Multicentre, Prospective, Single-Arm, Phase 2 Study. Lancet Oncol. 2020, 21, 978–988. [Google Scholar] [CrossRef]
- Moreau, P.; Dimopoulos, M.-A.; Mikhael, J.; Yong, K.; Capra, M.; Facon, T.; Hajek, R.; Špička, I.; Baker, R.; Kim, K.; et al. Isatuximab, Carfilzomib, and Dexamethasone in Relapsed Multiple Myeloma (IKEMA): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet 2021, 397, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.; Dinner, S.N. Moxetumomab Pasudotox for Hairy Cell Leukemia: Preclinical Development to FDA Approval. Blood Adv. 2019, 3, 2905–2910. [Google Scholar] [CrossRef]
- Al-Salama, Z.T. Inotuzumab Ozogamicin: A Review in Relapsed/Refractory B-Cell Acute Lymphoblastic Leukaemia. Target. Oncol. 2018, 13, 525–532. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.-M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef]
- Reda, G.; Orofino, N.; Cassin, R.; Sciumè, M.; Fattizzo, B.; Cortelezzi, A. Treating Chronic Lymphocytic Leukemia with Obinutuzumab: Safety and Efficacy Considerations. Expert Opin. Drug Saf. 2016, 15, 865–873. [Google Scholar] [CrossRef]
- Straus, D.J.; Długosz-Danecka, M.; Connors, J.M.; Alekseev, S.; Illés, Á.; Picardi, M.; Lech-Maranda, E.; Feldman, T.; Smolewski, P.; Savage, K.J.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Classical Hodgkin Lymphoma (ECHELON-1): 5-Year Update of an International, Open-Label, Randomised, Phase 3 Trial. Lancet Haematol. 2021, 8, e410–e421. [Google Scholar] [CrossRef]
- Cheung, M.C.; MacEachern, J.A.; Haynes, A.E.; Meyer, R.M.; Imrie, K. 131I–Tositumomab in Lymphoma. Curr. Oncol. 2009, 16, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Mondello, P.; Cuzzocrea, S.; Navarra, M.; Mian, M. 90 Y-Ibritumomab Tiuxetan: A Nearly Forgotten Opportunity. Oncotarget 2015, 7, 7597–7609. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.; Wang, E.S. Gemtuzumab Ozogamicin for the Treatment of Acute Myeloid Leukemia. Expert Rev. Clin. Pharmacol. 2018, 11, 549–559. [Google Scholar] [CrossRef]
- Baum, P.; Visvanathan, S.; Garcet, S.; Roy, J.; Schmid, R.; Bossert, S.; Lang, B.; Bachelez, H.; Bissonnette, R.; Thoma, C.; et al. Pustular Psoriasis: Molecular Pathways and Effects of Spesolimab in Generalized Pustular Psoriasis. J. Allergy Clin. Immunol. 2022, 149, 1402–1412. [Google Scholar] [CrossRef]
- Morand, E.F.; Furie, R.; Tanaka, Y.; Bruce, I.N.; Askanase, A.D.; Richez, C.; Bae, S.-C.; Brohawn, P.Z.; Pineda, L.; Berglind, A.; et al. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N. Engl. J. Med. 2020, 382, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Warren, R.B.; Lebwohl, M.; Gooderham, M.; Strober, B.; Langley, R.G.; Paul, C.; De Cuyper, D.; Vanvoorden, V.; Madden, C.; et al. Bimekizumab versus Secukinumab in Plaque Psoriasis. N. Engl. J. Med. 2021, 385, 142–152. [Google Scholar] [CrossRef]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.-P.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for Moderate-to-Severe Atopic Dermatitis: Results from Two 52-Week, Randomized, Double-Blind, Multicentre, Placebo-Controlled Phase III Trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef]
- Papp, K.A.; Blauvelt, A.; Bukhalo, M.; Gooderham, M.; Krueger, J.G.; Lacour, J.-P.; Menter, A.; Philipp, S.; Sofen, H.; Tyring, S.; et al. Risankizumab versus Ustekinumab for Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2017, 376, 1551–1560. [Google Scholar] [CrossRef]
- Locatelli, F.; Jordan, M.B.; Allen, C.; Cesaro, S.; Rizzari, C.; Rao, A.; Degar, B.; Garrington, T.P.; Sevilla, J.; Putti, M.-C.; et al. Emapalumab in Children with Primary Hemophagocytic Lymphohistiocytosis. N. Engl. J. Med. 2020, 382, 1811–1822. [Google Scholar] [CrossRef]
- Banerji, A.; Riedl, M.A.; Bernstein, J.A.; Cicardi, M.; Longhurst, H.J.; Zuraw, B.L.; Busse, P.J.; Anderson, J.; Magerl, M.; Martinez-Saguer, I.; et al. Effect of Lanadelumab Compared With Placebo on Prevention of Hereditary Angioedema Attacks: A Randomized Clinical Trial. JAMA 2018, 320, 2108–2121. [Google Scholar] [CrossRef]
- Reich, K.; Papp, K.A.; Blauvelt, A.; Tyring, S.K.; Sinclair, R.; Thaçi, D.; Nograles, K.; Mehta, A.; Cichanowitz, N.; Li, Q.; et al. Tildrakizumab versus Placebo or Etanercept for Chronic Plaque Psoriasis (ReSURFACE 1 and ReSURFACE 2): Results from Two Randomised Controlled, Phase 3 Trials. Lancet 2017, 390, 276–288. [Google Scholar] [CrossRef]
- Beccari, M.V.; Mogle, B.T.; Sidman, E.F.; Mastro, K.A.; Asiago-Reddy, E.; Kufel, W.D. Ibalizumab, a Novel Monoclonal Antibody for the Management of Multidrug-Resistant HIV-1 Infection. Antimicrob. Agents Chemother. 2019, 63, e00110-19. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Costanzo, A.; Fargnoli, M.C.; Malagoli, P.; Piaserico, S.; Amerio, P.; Argenziano, G.; Balato, N.; Bardazzi, F.; Bianchi, L.; et al. Guselkumab: An Anti-IL-23 Antibody for the Treatment of Moderate-to-Severe Plaque Psoriasis. Eur. J. Dermatol. 2021, 31, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N.; Deeks, E.D. Sarilumab: A Review in Moderate to Severe Rheumatoid Arthritis. Drugs 2018, 78, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Thaçi, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab Treatment in Adults with Moderate-to-Severe Atopic Dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Girolomoni, G. Brodalumab in the Treatment of Chronic Plaque Psoriasis. Expert Opin. Biol. Ther. 2020, 20, 1175–1186. [Google Scholar] [CrossRef]
- Gordon, K.B.; Blauvelt, A.; Papp, K.A.; Langley, R.G.; Luger, T.; Ohtsuki, M.; Reich, K.; Amato, D.; Ball, S.G.; Braun, D.K.; et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2016, 375, 345–356. [Google Scholar] [CrossRef]
- Blair, H.A. Secukinumab: A Review in Moderate to Severe Pediatric Plaque Psoriasis. Paediatr. Drugs 2021, 23, 601–608. [Google Scholar] [CrossRef]
- Barquero, N. Siltuximab: A New Option for the Management of Castleman’s Disease. Drugs Today Barc. Spain 1998 2015, 51, 21–28. [Google Scholar] [CrossRef]
- Singh, J.A.; Shah, N.P.; Mudano, A.S. Belimumab for Systemic Lupus Erythematosus. Cochrane Database Syst. Rev. 2021, 2, CD010668. [Google Scholar] [CrossRef]
- Gabay, C.; Emery, P.; van Vollenhoven, R.; Dikranian, A.; Alten, R.; Pavelka, K.; Klearman, M.; Musselman, D.; Agarwal, S.; Green, J.; et al. Tocilizumab Monotherapy versus Adalimumab Monotherapy for Treatment of Rheumatoid Arthritis (ADACTA): A Randomised, Double-Blind, Controlled Phase 4 Trial. Lancet 2013, 381, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Dhimolea, E. Canakinumab. mAbs 2010, 2, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Epstein, D.; Bojke, L.; Craig, D.; Light, K.; Bruce, I.; Sculpher, M.; Woolacott, N. Golimumab for the Treatment of Psoriatic Arthritis. Health Technol. Assess. Winch. Engl. 2011, 15 (Suppl. 1), 87–95. [Google Scholar] [CrossRef]
- Pelechas, E.; Voulgari, P.V.; Drosos, A.A. Golimumab for Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 387. [Google Scholar] [CrossRef]
- Yiu, Z.Z.; Warren, R.B. Ustekinumab for the Treatment of Psoriasis: An Evidence Update. Semin. Cutan. Med. Surg. 2018, 37, 143–147. [Google Scholar] [CrossRef]
- Gupta, A.K.; Cherman, A.M. Efalizumab in the Treatment of Psoriasis. J. Cutan. Med. Surg. 2006, 10, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Voulgari, P.V.; Drosos, A.A. Adalimumab for Rheumatoid Arthritis. Expert Opin. Biol. Ther. 2006, 6, 1349–1360. [Google Scholar] [CrossRef]
- Onrust, S.V.; Wiseman, L.R. Basiliximab. Drugs 1999, 57, 207–213; discussion 214. [Google Scholar] [CrossRef]
- Wiseman, L.R.; Faulds, D. Daclizumab: A Review of Its Use in the Prevention of Acute Rejection in Renal Transplant Recipients. Drugs 1999, 58, 1029–1042. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Guttman-Yassky, E.; Thaçi, D.; Irvine, A.D.; Stein Gold, L.; Blauvelt, A.; Simpson, E.L.; Chu, C.-Y.; Liu, Z.; Gontijo Lima, R.; et al. Two Phase 3 Trials of Lebrikizumab for Moderate-to-Severe Atopic Dermatitis. N. Engl. J. Med. 2023, 388, 1080–1091. [Google Scholar] [CrossRef]
- Hanna, K.S. Enfortumab Vedotin to Treat Urothelial Carcinoma. Drugs Today Barc. Spain 1998 2020, 56, 329–335. [Google Scholar] [CrossRef]
- Frampton, J.E. Catumaxomab: In Malignant Ascites. Drugs 2012, 72, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Culver, M.E.; Gatesman, M.L.; Mancl, E.E.; Lowe, D.K. Ipilimumab: A Novel Treatment for Metastatic Melanoma. Ann. Pharmacother. 2011, 45, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Damato, B.E.; Dukes, J.; Goodall, H.; Carvajal, R.D. Tebentafusp: T Cell Redirection for the Treatment of Metastatic Uveal Melanoma. Cancers 2019, 11, 971. [Google Scholar] [CrossRef]
- Paik, J. Nivolumab Plus Relatlimab: First Approval. Drugs 2022, 82, 925–931. [Google Scholar] [CrossRef]
- Keam, S.J. Tremelimumab: First Approval. Drugs 2023, 83, 93–102. [Google Scholar] [CrossRef]
- Kang, C. Retifanlimab: First Approval. Drugs 2023, 83, 731–737. [Google Scholar] [CrossRef]
- Zheng, Y.; Mislang, A.R.A.; Coward, J.; Cosman, R.; Cooper, A.; Underhill, C.; Zhu, J.; Xiong, J.; Jiang, O.; Wang, H.; et al. Penpulimab, an Anti-PD1 IgG1 Antibody in the Treatment of Advanced or Metastatic Upper Gastrointestinal Cancers. Cancer Immunol. Immunother. 2022, 71, 2371–2379. [Google Scholar] [CrossRef]
- Mai, H.-Q.; Chen, Q.-Y.; Chen, D.; Hu, C.; Yang, K.; Wen, J.; Li, J.; Shi, Y.-R.; Jin, F.; Xu, R.; et al. Toripalimab or Placebo plus Chemotherapy as First-Line Treatment in Advanced Nasopharyngeal Carcinoma: A Multicenter Randomized Phase 3 Trial. Nat. Med. 2021, 27, 1536–1543. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Cui, C.; Yao, J.; Zhang, Y.; Li, M.; Feng, J.; Yang, S.; Fan, Y.; Shi, J.; Zhang, X.; et al. Toripalimab plus Chemotherapy in Treatment-Naïve, Advanced Esophageal Squamous Cell Carcinoma (JUPITER-06): A Multi-Center Phase 3 Trial. Cancer Cell 2022, 40, 277–288.e3. [Google Scholar] [CrossRef]
- Shen, L.; Kato, K.; Kim, S.-B.; Ajani, J.A.; Zhao, K.; He, Z.; Yu, X.; Shu, Y.; Luo, Q.; Wang, J.; et al. Tislelizumab Versus Chemotherapy as Second-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma (RATIONALE-302): A Randomized Phase III Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 3065–3076. [Google Scholar] [CrossRef] [PubMed]
- Clingan, P.R.; Brungs, D.; Arnold, S.; Coward, J.; Fourie, S.J.; Harris, D.L.; Kurochkin, A.; Ladwa, R.; Malan, N.; Mant, A.M.; et al. Efficacy and Safety of Cosibelimab, an Anti–PD-L1 Antibody, in Patients with Metastatic Cutaneous Squamous Cell Carcinoma. J. Clin. Oncol. 2022, 40, 9537. [Google Scholar] [CrossRef]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab Duocarmazine in Locally Advanced and Metastatic Solid Tumours and HER2-Expressing Breast Cancer: A Phase 1 Dose-Escalation and Dose-Expansion Study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef]
- Heo, Y.-A. Mirvetuximab Soravtansine: First Approval. Drugs 2023, 83, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Lorusso, D.; Gennigens, C.; González-Martín, A.; Randall, L.; Cibula, D.; Lund, B.; Woelber, L.; Pignata, S.; Forget, F.; et al. Efficacy and Safety of Tisotumab Vedotin in Previously Treated Recurrent or Metastatic Cervical Cancer (InnovaTV 204/GOG-3023/ENGOT-Cx6): A Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol. 2021, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.-Y.; Kim, S.-W.; Lee, C.K.; et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results from the CHRYSALIS Phase I Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 3391–3402. [Google Scholar] [CrossRef]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; dePont Christensen, R.; Novák, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef]
- Markham, A. Margetuximab: First Approval. Drugs 2021, 81, 599–604. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Nakada, T.; Sugihara, K.; Jikoh, T.; Abe, Y.; Agatsuma, T. The Latest Research and Development into the Antibody-Drug Conjugate, [Fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem. Pharm. Bull. 2019, 67, 173–185. [Google Scholar] [CrossRef]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in Locally Advanced Cutaneous Squamous Cell Carcinoma: Results from an Open-Label, Phase 2, Single-Arm Trial. Lancet Oncol. 2020, 21, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Lavaud, P.; Hamilou, Z.; Loriot, Y.; Massard, C. Durvalumab in Urothelial Cancers. Expert Rev. Anticancer Ther. 2018, 18, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in Patients with Chemotherapy-Refractory Metastatic Merkel Cell Carcinoma: A Multicentre, Single-Group, Open-Label, Phase 2 Trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Hamilou, Z.; Lavaud, P.; Loriot, Y. Atezolizumab in Urothelial Bladder Carcinoma. Future Oncol. 2018, 14, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Pender, A.; Jones, R.L. Olaratumab for the Treatment of Soft-Tissue Sarcoma. Future Oncol. 2017, 13, 2151–2157. [Google Scholar] [CrossRef]
- Hoy, S.M. Dinutuximab: A Review in High-Risk Neuroblastoma. Target. Oncol. 2016, 11, 247–253. [Google Scholar] [CrossRef]
- Luke, J.J.; Rutkowski, P.; Queirolo, P.; Del Vecchio, M.; Mackiewicz, J.; Chiarion-Sileni, V.; de la Cruz Merino, L.; Khattak, M.A.; Schadendorf, D.; Long, G.V.; et al. Pembrolizumab versus Placebo as Adjuvant Therapy in Completely Resected Stage IIB or IIC Melanoma (KEYNOTE-716): A Randomised, Double-Blind, Phase 3 Trial. Lancet 2022, 399, 1718–1729. [Google Scholar] [CrossRef]
- Shitara, K.; Ohtsu, A. Ramucirumab for Gastric Cancer. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 133–139. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Iwata, H.; Taira, N.; Masuda, N.; Takahashi, M.; Yoshinami, T.; Ueno, T.; Toyama, T.; Yamanaka, T.; Takano, T.; et al. Pertuzumab Retreatment for HER2-Positive Advanced Breast Cancer: A Randomized, Open-Label Phase III Study (PRECIOUS). Cancer Sci. 2022, 113, 3169–3179. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Pietrantonio, F.; Lonardi, S.; Mussolin, B.; Rua, F.; Crisafulli, G.; Bartolini, A.; Fenocchio, E.; Amatu, A.; Manca, P.; et al. Circulating Tumor DNA to Guide Rechallenge with Panitumumab in Metastatic Colorectal Cancer: The Phase 2 CHRONOS Trial. Nat. Med. 2022, 28, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Welch, S.; Spithoff, K.; Rumble, R.B.; Maroun, J. Gastrointestinal Cancer Disease Site Group Bevacizumab Combined with Chemotherapy for Patients with Advanced Colorectal Cancer: A Systematic Review. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mo, J.; Dai, J.; Ye, C.; Cen, W.; Zheng, X.; Jiang, L.; Ye, L. Cetuximab Promotes RSL3-Induced Ferroptosis by Suppressing the Nrf2/HO-1 Signalling Pathway in KRAS Mutant Colorectal Cancer. Cell Death Dis. 2021, 12, 1079. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, B.; Yang, J. Evinacumab for the Treatment of Homozygous Familial Hypercholesterolemia. Expert Rev. Clin. Pharmacol. 2022, 15, 139–145. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- De Luca, G.; Suryapranata, H.; Grimaldi, R.; Chiariello, M. Coronary Stenting and Abciximab in Primary Angioplasty for ST-Segment-Elevation Myocardial Infarction. QJM Mon. J. Assoc. Physicians 2005, 98, 633–641. [Google Scholar] [CrossRef]
- Douglas, R.S.; Kahaly, G.J.; Patel, A.; Sile, S.; Thompson, E.H.Z.; Perdok, R.; Fleming, J.C.; Fowler, B.T.; Marcocci, C.; Marinò, M.; et al. Teprotumumab for the Treatment of Active Thyroid Eye Disease. N. Engl. J. Med. 2020, 382, 341–352. [Google Scholar] [CrossRef]
- Ozen, A.; Chongsrisawat, V.; Sefer, A.P.; Kolukisa, B.; Jalbert, J.J.; Miller, J.L.; Meagher, K.A.; Brackin, T.; Feldman, H.B.; Adiv, O.E.; et al. A Phase 2/3 Study Evaluating the Efficacy and Safety of Pozelimab in Patients with CD55 Deficiency with Hyperactivation of Complement, Angiopathic Thrombosis, and Protein-Losing Enteropathy (CHAPLE Disease). Lancet 2023. [Google Scholar] [CrossRef]
- D’Haens, G.; Dubinsky, M.; Kobayashi, T.; Irving, P.M.; Howaldt, S.; Pokrotnieks, J.; Krueger, K.; Laskowski, J.; Li, X.; Lissoos, T.; et al. Mirikizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2023, 388, 2444–2455. [Google Scholar] [CrossRef]
- Ha, C.; Kornbluth, A. Vedolizumab as a Treatment for Crohn’s Disease and Ulcerative Colitis. Gastroenterol. Hepatol. 2014, 10, 793–800. [Google Scholar]
- Hemperly, A.; Vande Casteele, N. Clinical Pharmacokinetics and Pharmacodynamics of Infliximab in the Treatment of Inflammatory Bowel Disease. Clin. Pharmacokinet. 2018, 57, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ren, Y.; Chen, L.; Sun, T.; Zhang, W.; Sun, B.; Zhu, L.; Xiong, F.; Zheng, C. Transarterial Chemoembolization Combined with Camrelizumab for Recurrent Hepatocellular Carcinoma. BMC Cancer 2022, 22, 270. [Google Scholar] [CrossRef] [PubMed]
- Haller, D.G. Update of Clinical Trials with Edrecolomab: A Monoclonal Antibody Therapy for Colorectal Cancer. Semin. Oncol. 2001, 28, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium Difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef]
- Kummerfeldt, C.E. Raxibacumab: Potential Role in the Treatment of Inhalational Anthrax. Infect. Drug Resist. 2014, 7, 101–109. [Google Scholar] [CrossRef]
- Romano, M.J.; Kearns, G.L.; Kaplan, S.L.; Jacobs, R.F.; Killian, A.; Bradley, J.S.; Moss, M.M.; Van Dyke, R.; Rodriguez, W.; Straube, R.C. Single-Dose Pharmacokinetics and Safety of HA-1A, a Human IgM Anti-Lipid-A Monoclonal Antibody, in Pediatric Patients with Sepsis Syndrome. J. Pediatr. 1993, 122, 974–981. [Google Scholar] [CrossRef]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Kramer, K.; Pandit-Taskar, N.; Kushner, B.H.; Zanzonico, P.; Humm, J.L.; Tomlinson, U.; Donzelli, M.; Wolden, S.L.; Haque, S.; Dunkel, I.; et al. Phase 1 Study of Intraventricular 131I-Omburtamab Targeting B7H3 (CD276)-Expressing CNS Malignancies. J. Hematol. Oncol. 2022, 15, 165. [Google Scholar] [CrossRef]
- Nicolò, M.; Ferro Desideri, L.; Vagge, A.; Traverso, C.E. Faricimab: An Investigational Agent Targeting the Tie-2/Angiopoietin Pathway and VEGF-A for the Treatment of Retinal Diseases. Expert Opin. Investig. Drugs 2021, 30, 193–200. [Google Scholar] [CrossRef]
- Yamamura, T.; Kleiter, I.; Fujihara, K.; Palace, J.; Greenberg, B.; Zakrzewska-Pniewska, B.; Patti, F.; Tsai, C.-P.; Saiz, A.; Yamazaki, H.; et al. Trial of Satralizumab in Neuromyelitis Optica Spectrum Disorder. N. Engl. J. Med. 2019, 381, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Cree, B.A.C.; Bennett, J.L.; Kim, H.J.; Weinshenker, B.G.; Pittock, S.J.; Wingerchuk, D.M.; Fujihara, K.; Paul, F.; Cutter, G.R.; Marignier, R.; et al. Inebilizumab for the Treatment of Neuromyelitis Optica Spectrum Disorder (N-MOmentum): A Double-Blind, Randomised Placebo-Controlled Phase 2/3 Trial. Lancet 2019, 394, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Saper, J.; Cady, R.; Schaeffler, B.A.; Biondi, D.M.; Hirman, J.; Pederson, S.; Allan, B.; Smith, J. Eptinezumab in Episodic Migraine: A Randomized, Double-Blind, Placebo-Controlled Study (PROMISE-1). Cephalalgia Int. J. Headache 2020, 40, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G.; et al. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, L.M.; Friedman, D.I. Galcanezumab for the Prevention of Migraine. Pain Manag. 2021, 11, 101–112. [Google Scholar] [CrossRef]
- Reuter, U.; Ehrlich, M.; Gendolla, A.; Heinze, A.; Klatt, J.; Wen, S.; Hours-Zesiger, P.; Nickisch, J.; Sieder, C.; Hentschke, C.; et al. Erenumab versus Topiramate for the Prevention of Migraine—A Randomised, Double-Blind, Active-Controlled Phase 4 Trial. Cephalalgia Int. J. Headache 2022, 42, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Dhoot, D.S.; Kaiser, P.K. Ranibizumab for Age-Related Macular Degeneration. Expert Opin. Biol. Ther. 2012, 12, 371–381. [Google Scholar] [CrossRef]
- Khoy, K.; Mariotte, D.; Defer, G.; Petit, G.; Toutirais, O.; Le Mauff, B. Natalizumab in Multiple Sclerosis Treatment: From Biological Effects to Immune Monitoring. Front. Immunol. 2020, 11, 549842. [Google Scholar] [CrossRef]
- Cheng, Y.; Han, L.; Wu, L.; Chen, J.; Sun, H.; Wen, G.; Ji, Y.; Dvorkin, M.; Shi, J.; Pan, Z.; et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients With Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 2022, 328, 1223–1232. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Z.; Sun, Y.; Cao, L.; Ma, Z.; Wu, R.; Yu, Y.; Yao, W.; Chang, J.; Chen, J.; et al. Sugemalimab versus Placebo, in Combination with Platinum-Based Chemotherapy, as First-Line Treatment of Metastatic Non-Small-Cell Lung Cancer (GEMSTONE-302): Interim and Final Analyses of a Double-Blind, Randomised, Phase 3 Clinical Trial. Lancet Oncol. 2022, 23, 220–233. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, W.; Tan, F.; Li, N.; Xue, Q.; Gao, S.; Gao, Y.; He, J. Sintilimab for the Treatment of Non-Small Cell Lung Cancer. Biomark. Res. 2022, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef] [PubMed]
- ACTIV-3–Therapeutics for Inpatients with COVID-19 (TICO) Study Group. Tixagevimab-Cilgavimab for Treatment of Patients Hospitalised with COVID-19: A Randomised, Double-Blind, Phase 3 Trial. Lancet Respir. Med. 2022, 10, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef]
- Syed, Y.Y. Regdanvimab: First Approval. Drugs 2021, 81, 2133–2137. [Google Scholar] [CrossRef]
- Deeks, E.D. Casirivimab/Imdevimab: First Approval. Drugs 2021, 81, 2047–2055. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, Å.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Engl. J. Med. 2021, 384, 1800–1809. [Google Scholar] [CrossRef]
- Kavanagh, J.E.; Hearn, A.P.; Dhariwal, J.; d’Ancona, G.; Douiri, A.; Roxas, C.; Fernandes, M.; Green, L.; Thomson, L.; Nanzer, A.M.; et al. Real-World Effectiveness of Benralizumab in Severe Eosinophilic Asthma. Chest 2021, 159, 496–506. [Google Scholar] [CrossRef]
- Deeks, E.D.; Brusselle, G. Reslizumab in Eosinophilic Asthma: A Review. Drugs 2017, 77, 777–784. [Google Scholar] [CrossRef]
- Greig, S.L. Obiltoxaximab: First Global Approval. Drugs 2016, 76, 823–830. [Google Scholar] [CrossRef]
- Díaz-Serrano, A.; Sánchez-Torre, A.; Paz-Ares, L. Necitumumab for the Treatment of Advanced Non-Small-Cell Lung Cancer. Future Oncol. 2019, 15, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Serna-Blasco, R.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM Phase II Trial). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 2924–2933. [Google Scholar] [CrossRef]
- Dell’Aquila, E.; Armento, G.; Iuliani, M.; Simonetti, S.; D’Onofrio, L.; Zeppola, T.; Madaudo, C.; Russano, M.; Citarella, F.; Ribelli, G.; et al. Denosumab for Cancer-Related Bone Loss. Expert Opin. Biol. Ther. 2020, 20, 1261–1274. [Google Scholar] [CrossRef]
- Bril, V.; Drużdż, A.; Grosskreutz, J.; Habib, A.A.; Mantegazza, R.; Sacconi, S.; Utsugisawa, K.; Vissing, J.; Vu, T.; Boehnlein, M.; et al. Safety and Efficacy of Rozanolixizumab in Patients with Generalised Myasthenia Gravis (MycarinG): A Randomised, Double-Blind, Placebo-Controlled, Adaptive Phase 3 Study. Lancet Neurol. 2023, 22, 383–394. [Google Scholar] [CrossRef]
- Gklinos, P.; Papadopoulou, M.; Stanulovic, V.; Mitsikostas, D.D.; Papadopoulos, D. Monoclonal Antibodies as Neurological Therapeutics. Pharmaceuticals 2021, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J. Complement Activation-Related Pseudoallergy: A New Class of Drug-Induced Acute Immune Toxicity. Toxicology 2005, 216, 106–121. [Google Scholar] [CrossRef]
- Szebeni, J. Complement Activation-Related Pseudoallergy: A Stress Reaction in Blood Triggered by Nanomedicines and Biologicals. Mol. Immunol. 2014, 61, 163–173. [Google Scholar] [CrossRef]
- Bugelski, P.J.; Achuthanandam, R.; Capocasale, R.J.; Treacy, G.; Bouman-Thio, E. Monoclonal Antibody-Induced Cytokine-Release Syndrome. Expert Rev. Clin. Immunol. 2009, 5, 499–521. [Google Scholar] [CrossRef]
- Butzkueven, H.; Kappos, L.; Wiendl, H.; Trojano, M.; Spelman, T.; Chang, I.; Kasliwal, R.; Jaitly, S.; Campbell, N.; Ho, P.-R.; et al. Long-Term Safety and Effectiveness of Natalizumab Treatment in Clinical Practice: 10 Years of Real-World Data from the Tysabri Observational Program (TOP). J. Neurol. Neurosurg. Psychiatry 2020, 91, 660–668. [Google Scholar] [CrossRef]
- Dodick, D.W.; Ashina, M.; Brandes, J.L.; Kudrow, D.; Lanteri-Minet, M.; Osipova, V.; Palmer, K.; Picard, H.; Mikol, D.D.; Lenz, R.A. ARISE: A Phase 3 Randomized Trial of Erenumab for Episodic Migraine. Cephalalgia Int. J. Headache 2018, 38, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Tepper, S.; Ashina, M.; Reuter, U.; Brandes, J.L.; Doležil, D.; Silberstein, S.; Winner, P.; Leonardi, D.; Mikol, D.; Lenz, R. Safety and Efficacy of Erenumab for Preventive Treatment of Chronic Migraine: A Randomised, Double-Blind, Placebo-Controlled Phase 2 Trial. Lancet Neurol. 2017, 16, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Dodick, D.; Goadsby, P.J.; Reuter, U.; Silberstein, S.; Zhang, F.; Gage, J.R.; Cheng, S.; Mikol, D.D.; Lenz, R.A. Erenumab (AMG 334) in Episodic Migraine: Interim Analysis of an Ongoing Open-Label Study. Neurology 2017, 89, 1237–1243. [Google Scholar] [CrossRef]
- Valenzuela, R.M.; Pula, J.H.; Garwacki, D.; Cotter, J.; Kattah, J.C. Cryptococcal Meningitis in a Multiple Sclerosis Patient Taking Natalizumab. J. Neurol. Sci. 2014, 340, 109–111. [Google Scholar] [CrossRef]
- EMA Ocrevus. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ocrevus (accessed on 4 August 2023).
- Lancet, T. End of the Road for Daclizumab in Multiple Sclerosis. Lancet 2018, 391, 1000. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khalili, S.; Baradaran, B.; Bidar, N.; Shahbazi, M.-A.; Mosafer, J.; Hashemzaei, M.; Mokhtarzadeh, A.; Hamblin, M.R. Bispecific Monoclonal Antibodies for Targeted Immunotherapy of Solid Tumors: Recent Advances and Clinical Trials. Int. J. Biol. Macromol. 2021, 167, 1030–1047. [Google Scholar] [CrossRef]
- Jin, S.; Sun, Y.; Liang, X.; Gu, X.; Ning, J.; Xu, Y.; Chen, S.; Pan, L. Emerging New Therapeutic Antibody Derivatives for Cancer Treatment. Signal Transduct. Target. Ther. 2022, 7, 39. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The Blood-Brain Barrier: Physiology and Strategies for Drug Delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Jovčevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2020, 34, 11–26. [Google Scholar] [CrossRef]
- Cavaco, M.; Frutos, S.; Oliete, P.; Valle, J.; Andreu, D.; Castanho, M.A.R.B.; Vila-Perelló, M.; Neves, V. Conjugation of a Blood Brain Barrier Peptide Shuttle to an Fc Domain for Brain Delivery of Therapeutic Biomolecules. ACS Med. Chem. Lett. 2021, 12, 1663–1668. [Google Scholar] [CrossRef]
- Di, J.; Xie, F.; Xu, Y. When Liposomes Met Antibodies: Drug Delivery and Beyond. Adv. Drug Deliv. Rev. 2020, 154–155, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Parray, H.A.; Shukla, S.; Perween, R.; Khatri, R.; Shrivastava, T.; Singh, V.; Murugavelu, P.; Ahmed, S.; Samal, S.; Sharma, C.; et al. Inhalation Monoclonal Antibody Therapy: A New Way to Treat and Manage Respiratory Infections. Appl. Microbiol. Biotechnol. 2021, 105, 6315–6332. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; McFee, M.; Fang, Q.; Abdin, O.; Kim, P.M. Computational and Artificial Intelligence-Based Methods for Antibody Development. Trends Pharmacol. Sci. 2023, 44, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Prihoda, D.; Maamary, J.; Waight, A.; Juan, V.; Fayadat-Dilman, L.; Svozil, D.; Bitton, D.A. BioPhi: A Platform for Antibody Design, Humanization, and Humanness Evaluation Based on Natural Antibody Repertoires and Deep Learning. mAbs 2022, 14, 2020203. [Google Scholar] [CrossRef]
- Marks, C.; Hummer, A.M.; Chin, M.; Deane, C.M. Humanization of Antibodies Using a Machine Learning Approach on Large-Scale Repertoire Data. Bioinformatics 2021, 37, 4041–4047. [Google Scholar] [CrossRef]
- Choi, Y. Artificial Intelligence for Antibody Reading Comprehension: AntiBERTa. Patterns 2022, 3, 100535. [Google Scholar] [CrossRef]
- Shanehsazzadeh, A.; Bachas, S.; McPartlon, M.; Kasun, G.; Sutton, J.M.; Steiger, A.K.; Shuai, R.; Kohnert, C.; Rakocevic, G.; Gutierrez, J.M.; et al. Unlocking de Novo Antibody Design with Generative Artificial Intelligence. bioRxiv 2023. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.; Zhu, X.; He, Y.; Wu, Y.; Ying, T.; Xie, Z. Artificial Intelligence in Cancer Immunotherapy: Applications in Neoantigen Recognition, Antibody Design and Immunotherapy Response Prediction. Semin. Cancer Biol. 2023, 91, 50–69. [Google Scholar] [CrossRef]
- Bachas, S.; Rakocevic, G.; Spencer, D.; Sastry, A.V.; Haile, R.; Sutton, J.M.; Kasun, G.; Stachyra, A.; Gutierrez, J.M.; Yassine, E.; et al. Antibody Optimization Enabled by Artificial Intelligence Predictions of Binding Affinity and Naturalness. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hie, B.L.; Shanker, V.R.; Xu, D.; Bruun, T.U.J.; Weidenbacher, P.A.; Tang, S.; Wu, W.; Pak, J.E.; Kim, P.S. Efficient Evolution of Human Antibodies from General Protein Language Models. Nat. Biotechnol. 2023, 1–9. [Google Scholar] [CrossRef]
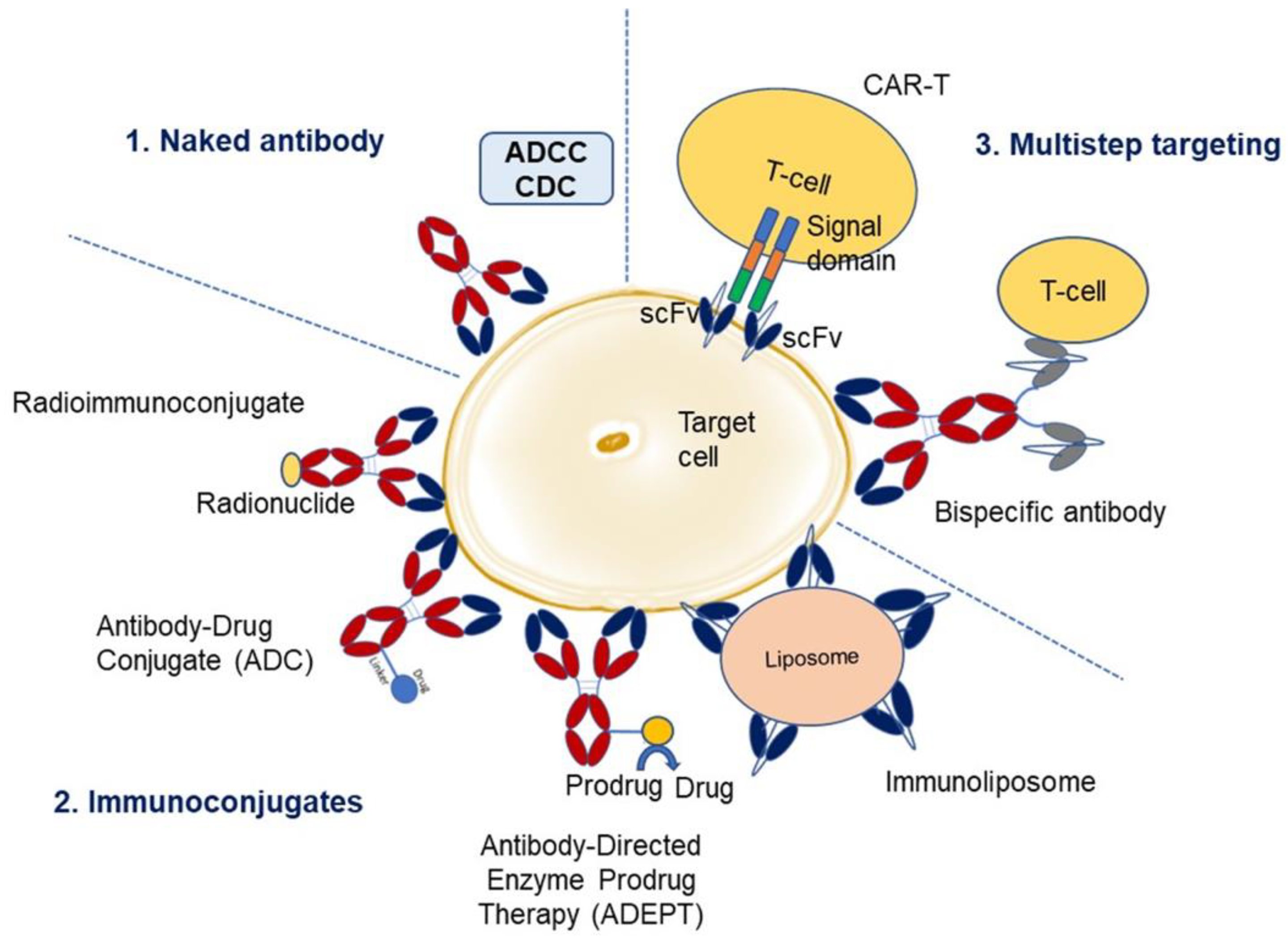
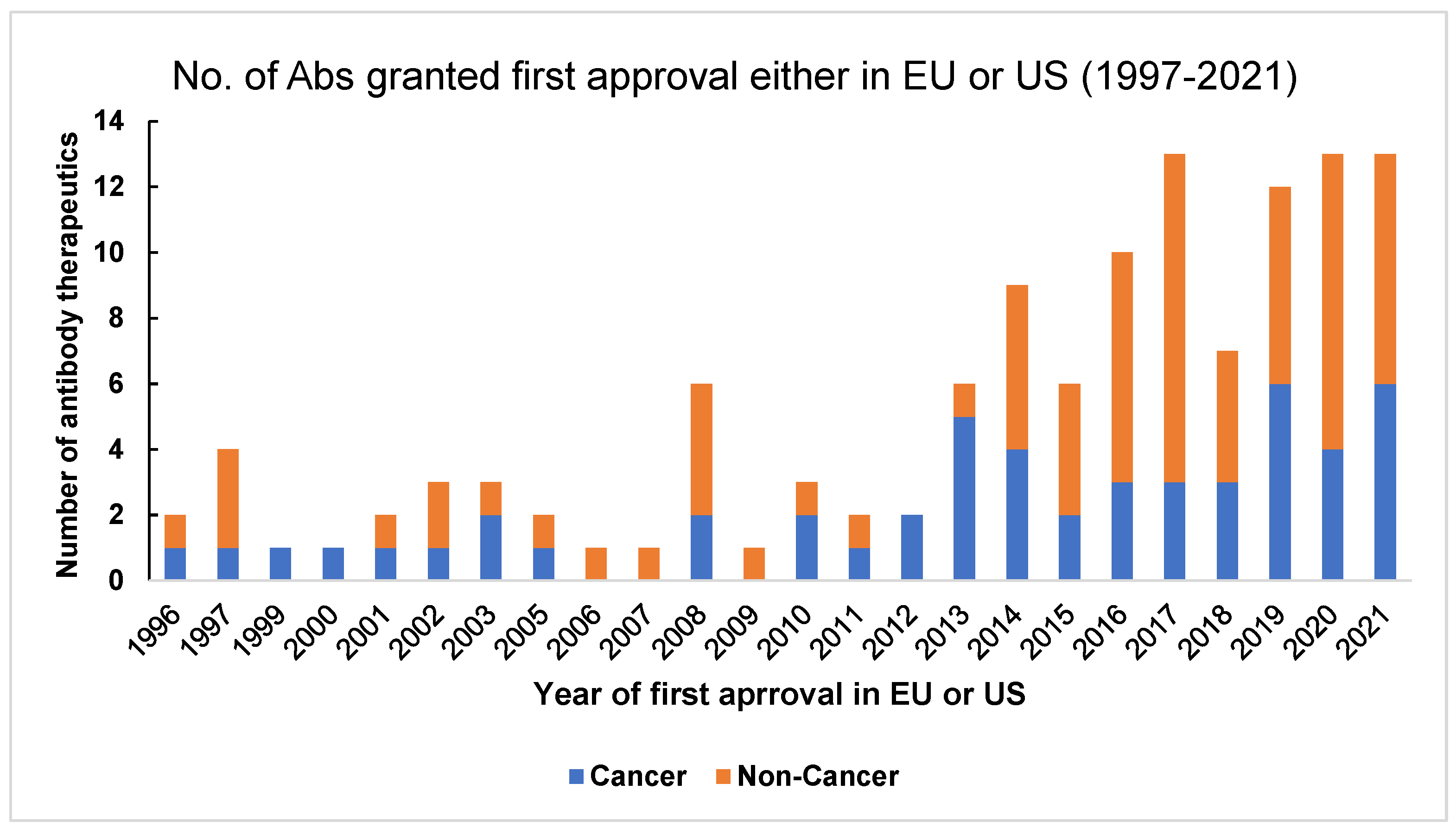
| Therapeutic Area | INN | Brand Name | Target | Format | Use | Ref | US Approval Year | EU Approval Year |
|---|---|---|---|---|---|---|---|---|
| Hematologic | Concizumab | Alhemo™ | Tissue factor pathway inhibitor | Humanized IgG4 | Hemophilia | [280] | Review | Review |
| Hematologic | Sutimlimab | Enjaymo | C1s | Humanized IgG4 | Cold agglutinin disease | [281] | 2022 | 2022 |
| Hematologic | Emicizumab | Hemlibra | Factor IXa, X | Humanized IgG4, bispecific | Hemophilia A | [58] | 2017 | 2018 |
| Hematologic | Crizanlizumab | Adakveo | P-selectin | Humanized IgG2 | Sickle cell disease | [64] | 2019 | 2020 |
| Hematologic | Caplacizumab | Cablivi | von Willebrand factor | Humanized Nanobody | Acquired thrombotic thrombo- cytopenic purpura | [282] | 2019 | 2018 |
| Hematologic | Ravulizumab | Ultomiris | C5 | Humanized IgG2/4 | Paroxysmal nocturnal hemoglobinuria | [283] | 2018 | 2019 |
| Hematologic | Daratumumab | Darzalex | CD38 | Human IgG1 | Multiple myeloma | [284] | 2015 | 2016 |
| Hematologic | Elotuzumab | Empliciti | SLAMF7 | Humanized IgG1 | Multiple myeloma | [285] | 2015 | 2016 |
| Hematologic | Idarucizumab | Praxbind | Dabigatran | Humanized Fab | Reversal of dabigatran-induced anticoagulation | [286] | 2015 | 2015 |
| Hematologic | Eculizumab | Soliris | C5 | Humanized IgG2/4 | Paroxysmal nocturnal hemoglobinuria | [287] | 2007 | 2007 |
| Hematologic | Rituximab | MabThera, Rituxan | CD20 | Chimeric IgG1 | Non-Hodgkin lymphoma | [288] | 1997 | 1998 |
| Hematologic | Elranatamab | (Pending) | BCMA, CD3 | Humanized IgG2 | Multiple myeloma | [289] | Review | Review |
| Hematologic | Talquetamab | (Pending) | G protein-coupled receptor 5D, CD3 | Humanized IgG4 bispecific | Multiple myeloma | [290] | Review | Review |
| Hematologic | Epcoritamab | EPKINLY™ | CD20, CD3 | Bispecific humanized IgG1 | Diffuse large B-cell lymphoma | [291] | 2023 | Review |
| Hematologic | Glofitamab | (Pending) | CD20, CD3e | Bispecific 2 + 1 IgG1 CrossMab | Diffuse large B-cell lymphoma | [292] | 2023 | Pos Opinion |
| Hematologic | Alemtuzumab | MabCampath, Campath-1H; Lemtrada | CD52 | Humanized IgG1 | Multiple sclerosis | [293] | 2014 | 2013 |
| Hematologic | Ublituximab | BRIUMVI | CD20 | Chimeric IgG1 | Multiple sclerosis | [294] | 2022 | 2023 |
| Hematologic | Teclistamab | TECVAYLI | BCMA, CD3 | Humanized bispecific IgG4 | Multiple myeloma | [295] | 2022 | 2022 |
| Hematologic | Mosunetuzumab | Lunsumio | CD20, CD3 | Humanized bispecific IgG1 | Follicular lymphoma | [296] | 2022 | 2022 |
| Hematologic | Mogamulizumab | Poteligeo | CCR4 | Humanized IgG1 | Cutaneous T-cell lymphoma | [297] | 2018 | 2018 |
| Hematologic | Ocrelizumab | OCREVUS | CD20 | Humanized IgG1 | Multiple sclerosis | [298] | 2017 | 2018 |
| Hematologic | Ofatumumab | Arzerra | CD20 | Human IgG1 | Chronic lymphocytic leukemia | [299] | 2009 | 2010# |
| Hematologic | Loncastuximab tesirine | Zynlonta | CD19 | Humanized IgG1 ADC | Diffuse large B-cell lymphoma | [300] | 2021 | 2022 |
| Hematologic | Belantamab mafodotin | BLENREP | BCMA | Humanized IgG1 ADC | Multiple myeloma | [301] | 2020 | 2020 |
| Hematologic | Tafasitamab | Monjuvi, Minjuvi | CD19 | Humanized IgG1 | Diffuse large B-cell lymphoma | [302] | 2020 | 2021 |
| Hematologic | Isatuximab | Sarclisa | CD38 | Chimeric IgG1 | Multiple myeloma | [303] | 2020 | 2020 |
| Hematologic | Polatuzumab vedotin | Polivy | CD79b | Humanized IgG1 ADC | Diffuse large B-cell lymphoma | [304] | 2019 | 2020 |
| Hematologic | Moxetumomab pasudotox | Lumoxiti | CD22 | Murine IgG1 dsFv immunotoxin | Hairy cell leukemia | [305] | 2018 | 2021 |
| Hematologic | Inotuzumab ozogamicin | BESPONSA | CD22 | Humanized IgG4, ADC | Hematological malignancy | [306] | 2017 | 2017 |
| Hematologic | Blinatumomab | Blincyto | CD19, CD3 | Murine bispecific tandem scFv | Acute lymphoblastic leukemia | [307] | 2014 | 2015 |
| Hematologic | Obinutuzumab | Gazyva | CD20 | Humanized IgG1; Glycoengineered | Chronic lymphocytic leukemia | [308] | 2013 | 2014 |
| Hematologic | Brentuximab vedotin | Adcetris | CD30 | Chimeric IgG1, ADC | Hodgkin lymphoma, systemic anaplastic large-cell lymphoma | [309] | 2011 | 2012 |
| Hematologic | Tositumomab-I131 | Bexxar | CD20 | Murine IgG2a | Non-Hodgkin lymphoma | [310] | 2003 # | NA |
| Hematologic | Ibritumomab tiuxetan | Zevalin | CD20 | Murine IgG1 | Non-Hodgkin lymphoma | [311] | 2002 | 2004 |
| Hematologic | Gemtuzumab ozogamicin | Mylotarg | CD33 | Humanized IgG4, ADC | Acute myeloid leukemia | [312] | 2017; 2000 # | 2018 |
| Immunologic | Spesolimab | SPEVIGO® | IL-36 receptor | Humanized IgG1 | Generalized pustular psoriasis | [313] | 2022 | 2022 |
| Immunologic | Anifrolumab | Saphnelo | IFNAR1 | Human IgG1 | Systemic lupus erythematosus | [314] | 2021 | 2022 |
| Immunologic | Bimekizumab | Bimzelx | IL-17A,F | Humanized IgG1 | Psoriasis | [315] | 2nd cycle review | 2021 |
| Immunologic | Tralokinumab | Adtralza | IL-13 | Human IgG4 | Atopic dermatitis | [316] | 2021 | 2021 |
| Immunologic | Risankizumab | Skyrizi | IL-23p19 | Humanized IgG1 | Plaque psoriasis | [317] | 2019 | 2019 |
| Immunologic | Emapalumab | Gamifant | IFN-ɣ (gamma) | Human IgG1 | Primary hemophagocytic lymphohistiocytosis | [318] | 2018 | NA |
| Immunologic | Lanadelumab | Takhzyro | Plasma kallikrein | Human IgG1 | Hereditary angioedema attacks | [319] | 2018 | 2018 |
| Immunologic | Tildrakizumab | Ilumya | IL-23p19 | Humanized IgG1 | Plaque psoriasis | [320] | 2018 | 2018 |
| Immunologic | Ibalizumab | Trogarzo | CD4 | Humanized IgG4 | HIV infection | [321] | 2018 | 2019 |
| Immunologic | Guselkumab | TREMFYA | IL-23 P19 | Human IgG1 | Plaque psoriasis | [322] | 2017 | 2017 |
| Immunologic | Sarilumab | Kevzara | IL-6R | Human IgG1 | Rheumatoid arthritis | [323] | 2017 | 2017 |
| Immunologic | Dupilumab | Dupixent | IL-4Rα | Human IgG4 | Atopic dermatitis | [324] | 2017 | 2017 |
| Immunologic | Brodalumab | Siliq, LUMICEF | IL-17R | Human IgG2 | Plaque psoriasis | [325] | 2017 | 2017 |
| Immunologic | Ixekizumab | Taltz | IL-17a | Humanized IgG4 | Psoriasis | [326] | 2016 | 2016 |
| Immunologic | Secukinumab | Cosentyx | IL-17a | Human IgG1 | Psoriasis | [327] | 2015 | 2015 |
| Immunologic | Siltuximab | Sylvant | IL-6 | Chimeric IgG1 | Castleman disease | [328] | 2014 | 2014 |
| Immunologic | Belimumab | Benlysta | BLyS | Human IgG1 | Systemic lupus erythematosus | [329] | 2011 | 2011 |
| Immunologic | Tocilizumab | RoActemra, Actemra | IL-6R | Humanized IgG1 | Rheumatoid arthritis | [330] | 2010 | 2009 |
| Immunologic | Canakinumab | Ilaris | IL-1β | Human IgG1 | Muckle-Wells syndrome | [331] | 2009 | 2009 |
| Immunologic | Golimumab | Simponi | TNF | Human IgG1 | Rheumatoid and psoriatic arthritis, ankylosing spondylitis | [332], [333] | 2009 | 2009 |
| Immunologic | Ustekinumab | Stelara | IL-12/23 | Human IgG1 | Psoriasis | [334] | 2009 | 2009 |
| Immunologic | Efalizumab | Raptiva | CD11a | Humanized IgG1 | Psoriasis | [335] | 2003 # | 2004 # |
| Immunologic | Adalimumab | Humira | TNF | Human IgG1 | Rheumatoid arthritis | [336] | 2002 | 2003 |
| Immunologic | Muromonab-CD3 | Orthoclone Okt3 | CD3 | Murine IgG2a | Reversal of kidney transplant rejection | [3] | 1986 # | 1986 * |
| Immunologic | Basiliximab | Simulect | IL-2R | Chimeric IgG1 | Prevention of kidney transplant rejection | [337] | 1998 | 1998 |
| Immunologic | Daclizumab | Zenapax; Zinbryta | IL-2R | Humanized IgG1 | Prevention of kidney transplant rejection; multiple sclerosis | [338] | 2016; 1997 # | 2016; 1999 # |
| Immunologic | Lebrikizumab | (Pending) | IL-13 | humanized IgG4 | Atopic dermatitis | [339] | NA | Review |
| Oncologic | Enfortumab vedotin | Padcev | Nectin-4 | Human IgG1 ADC | Urothelial cancer | [340] | 2019 | 2022 |
| Oncologic | Catumaxomab | Removab | EPCAM/CD3 | Rat/mouse bispecific mAb | Malignant ascites | [341] | NA | 2009 # |
| Oncologic | Ipilimumab | Yervoy | CTLA-4 | Human IgG1 | Metastatic melanoma | [342] | 2011 | 2011 |
| Oncologic | Tebentafusp | KIMMTRAK | gp100, CD3 | Bispecific immunoconjugate (TCR-scFv) | Metastatic uveal melanoma | [343] | 2022 | 2022 |
| Oncologic | Relatlimab | Opdualag (relatlimab + nivolumab combo) | LAG-3 | Human IgG4 | Melanoma | [344] | 2022 | 2022 |
| Oncologic | Tremelimumab | Imjudo | CTLA-4 | Human IgG2A | Antineoplastic; liver cancer | [345] | 2022 | 2023 |
| Oncologic | Retifanlimab | Zynyz | PD-1 | Humanized IgG4 | Merkel-cell carcinoma | [346] | 2023 | In review |
| Oncologic | Penpulimab | (Pending) | PD-1 | Humanized IgG1 | Metastatic nasopharyngeal carcinoma | [347] | Review status unknown | NA |
| Oncologic | Toripalimab | Tuoyi | PD-1 | Humanized IgG4 | Nasopharyneal carcinoma, esophageal squamous-cell carcinoma | [348], [349] | Decision delayed | Review |
| Oncologic | Tislelizumab | (Pending) | PD-1 | Humanized IgG4 | Esophageal squamous-cell carcinoma | [350] | 2nd cycle review | Review |
| Oncologic | Cosibelimab | (Pending) | PD-L1 | Human IgG1 | Squamous-cell carcinoma | [351] | Review | NA |
| Oncologic | Naxitamab | DANYELZA | GD2 | Humanized IgG1 | High-risk neuroblastoma and refractory osteomedullary disease | [271] | 2020 | NA |
| Oncologic | Trastuzumab duocarmazine | (Pending) | HER2 | Humanized IgG1 ADC | Breast cancer | [352] | 2nd cycle review | Review |
| Oncologic | Mirvetuximab soravtansine | ELAHERE | Folate receptor α | Humanized IgG1 ADC | Ovarian cancer | [353] | 2022 | NA |
| Oncologic | Tisotumab vedotin | TIVDAK | Tissue factor | Human IgG1 ADC | Cervical cancer | [354] | 2021 | NA |
| Oncologic | Amivantamab | Rybrevant | EGFR, cMET | Human bispecific IgG1 | NSCLC w/EGFR exon 20 insertion mutations | [355] | 2021 | 2021 |
| Oncologic | Dostarlimab | Jemperli | PD-1 | Humanized IgG4 | Endometrial cancer | [356] | 2021 | 2021 |
| Oncologic | Margetuximab | MARGENZA | HER2 | Chimeric IgG1 | HER2+ breast cancer | [357] | 2020 | NA |
| Oncologic | Sacituzumab govitecan | Trodelvy | TROP-2 | Humanized IgG1 ADC | Triple-neg. breast cancer | [358] | 2020 | 2021 |
| Oncologic | [fam]-trastuzumab deruxtecan | Enhertu | HER2 | Humanized IgG1 ADC | HER2+ breast cancer | [359] | 2029 | 2021 |
| Oncologic | Cemiplimab | Libtayo | PD-1 | Human IgG4 | Cutaneous squamous-cell carcinoma | [360] | 2018 | 2019 |
| Oncologic | Durvalumab | IMFINZI | PD-L1 | Human IgG1 | Bladder cancer | [361] | 2017 | 2018 |
| Oncologic | Avelumab | Bavencio | PD-L1 | Human IgG1 | Merkel-cell carcinoma | [362] | 2017 | 2017 |
| Oncologic | Atezolizumab | Tecentriq | PD-L1 | Humanized IgG1 | Bladder cancer | [363] | 2016 | 2017 |
| Oncologic | Olaratumab | Lartruvo | PDGRFα | Human IgG1 | Soft tissue sarcoma | [364] | 2016 # | 2016 # |
| Oncologic | Dinutuximab | Qarziba; Unituxin | GD2 | Chimeric IgG1 | Neuroblastoma | [365] | 2015 | 2017; 2015 # |
| Oncologic | Pembrolizumab | Keytruda | PD1 | Humanized IgG4 | Melanoma | [366] | 2014 | 2015 |
| Oncologic | Ramucirumab | Cyramza | VEGFR2 | Human IgG1 | Gastric cancer | [367] | 2014 | 2014 |
| Oncologic | Ado-trastuzumab emtansine | Kadcyla | HER2 | Humanized IgG1, ADC | Breast cancer | [368] | 2013 | 2013 |
| Oncologic | Pertuzumab | Perjeta | HER2 | Humanized IgG1 | Breast Cancer | [369] | 2012 | 2013 |
| Oncologic | Panitumumab | Vectibix | EGFR | Human IgG2 | Colorectal cancer | [370] | 2006 | 2007 |
| Oncologic | Bevacizumab | Avastin | VEGF | Humanized IgG1 | Colorectal cancer | [371] | 2004 | 2005 |
| Oncologic | Cetuximab | Erbitux | EGFR | Chimeric IgG1 | Colorectal cancer | [372] | 2004 | 2004 |
| Oncologic | Trastuzumab | Herceptin | HER2 | Humanized IgG1 | Breast cancer | [97] | 1998 | 2000 |
| Cardiovascular | Evinacumab | Evkeeza | Angiopoietin-like 3 | Human IgG4 | Homozygous familial hypercholesterolemia | [373] | 2021 | 2021 |
| Cardiovascular | Alirocumab | Praluent | PCSK9 | Human IgG1 | High cholesterol | [124] | 2015 | 2015 |
| Cardiovascular | Evolocumab | Repatha | PCSK9 | Human IgG2 | High cholesterol | [374] | 2015 | 2015 |
| Cardiovascular | Abciximab | Reopro | GPIIb/IIIa | Chimeric IgG1 Fab | Prevention of blood clots in angioplasty | [375] | 1994 | 1995 * |
| Endocrine | Teprotumumab | Tepezza | IGF-1R | Human IgG1 | Thyroid eye disease | [376] | 2020 | NA |
| Gastrointestinal | Pozelimab | (Pending) | Complement 5 | Human IgG4 | CHAPLE disease | [377] | Review | NA |
| Gastrointestinal | Mirikizumab | (Pending) | IL-23p19 | Humanized IgG4 | Ulcerative Colitis | [378] | Review | Review |
| Gastrointestinal | Vedolizumab | Entyvio | A4β7 integrin | Humanized IgG1 | Ulcerative Colitis, Crohn’s Disease | [379] | 2014 | 2014 |
| Gastrointestinal | Certolizumab pegol | Cimzia | NF | Humanized Fab, pegylated | Crohn’s Disease | [170] | 2008 | 2009 |
| Gastrointestinal | Infliximab | Remicade | TNF | Chimeric IgG1 | Crohn’s Disease | [380] | 1998 | 1999 |
| Gastrointestinal | Camrelizumab | (Pending) | PD-1 | Human IgG4 | Hepatocellular carcinoma | [381] | Review | NA |
| Gastrointestinal | Edrecolomab | Panorex | EpCAM | Murine IgG2a | Colorectal cancer | [382] | NA | 1995 *# |
| Gastrointestinal | Bezlotoxumab | Zinplava | Clostridium difficile enterotoxin B | Human IgG1 | Prevention of Clostridium difficile infection recurrence | [383] | 2016 | 2017 |
| Infectious disease | Ansuvimab | Ebanga | Ebola virus | Human IgG1 | Ebola infection | [208] | 2020 | NA |
| Infectious disease | Atoltivimab, Maftivimab, and Odesivimab-ebgn | Inmazeb | Ebola virus | mixture of 3 human IgG1 | Ebola virus infection | [209] | 2020 | NA |
| Infectious disease | Raxibacumab | (Pending) | B. anthrasis PA | Human IgG1 | Anthrax infection | [384] | 2012 | NA |
| Infectious disease | Nebacumab | Centoxin | Endotoxin | Human IgM | Gram-negative sepsis | [385] | NA | 1991 *# |
| Neurologic | Donanemab | (Pending) | Amyloid β | Humanized IgG1 | Alzheimer’s disease | [386] | 2nd cycle review | NA |
| Neurologic | Lecanemab | Leqembi | Amyloid β protofibrils | Humanized IgG1 | Alzheimer’s disease | [387] | 2023 | Review |
| Neurologic | Omburtamab | (Pending) | B7-H3 | Murine IgG1 | CNS/leptomeningeal metastasis from neuroblastoma | [388] | 2nd cycle review | Neg. opinion |
| Neurologic | Faricimab | Vabysmo | VEGF-A, Ang-2 | Human/humanized IgG1 κ (kappa)/lambda, with domain crossover | wAMD, DME | [389] | 2022 | 2022 |
| Neurologic | Aducanumab | Aduhelm | Amyloid β | Human IgG1 | Alzheimer’s disease | [254] | 2021 | MAA withdrawn |
| Neurologic | Satralizumab | Enspryng | IL-6R | Humanized IgG2 | Neuromyelitis optica and neuromyelitis optica spectrum disorders | [390] | 2020 | 2021 |
| Neurologic | Inebilizumab | Uplizna | CD19 | Humanized IgG1 | Neuromyelitis optica and neuromyelitis optica spectrum disorders | [391] | 2020 | 2022 |
| Neurologic | Eptinezumab | Vyepti | CGRP | Humanized IgG1 | Migraine prevention | [392] | 2020 | 2022 |
| Neurologic | Brolucizumab | BEOVU | VEGF-A | Humanized scFv | Macular degeneration | [393] | 2019 | 2020 |
| Neurologic | Fremanezumab | Ajovy | CGRP | Human IgG2 | Migraine prevention | [266] | 2018 | 2019 |
| Neurologic | Galcanezumab | Emgality | CGRP | Human IgG4 | Migraine prevention | [394] | 2018 | 2018 |
| Neurologic | Erenumab | Aimovig | CGRP receptor | Human IgG2 | Migraine prevention | [395] | 2018 | 2018 |
| Neurologic | Ranibizumab | Lucentis | VEGF | Humanized IgG1 Fab | Macular degeneration | [396] | 2006 | 2007 |
| Neurologic | Natalizumab | Tysabri | a4 integrin | Humanized IgG4 | Multiple sclerosis | [397] | 2004 | 2006 |
| Pulmonary | Serplulimab | (Pending) | PD-1 | Human IgG4 | Small-cell lung cancer | [398] | NA | Review |
| Pulmonary | Sugemalimab | (Pending) | PD-1 | Human IgG4 | Non-small-cell cancer | [399] | NA | Review |
| Pulmonary | Sintilimab | (Pending) | PD-1 | Human IgG4 | Non-small-cell lung cancer | [400] | 2nd Cycle Review | NA |
| Pulmonary | Nirsevimab | Beyfortus | RSV | Human IgG1 | RSV Infection | [401] | Review | 2022 |
| Pulmonary | Tixagevimab, cilgavimab | Evusheld | SARS-CoV-2 | Human IgG1 | COVID-19 | [402] | EUA | 2022 |
| Pulmonary | Sotrovimab | Xevudy | SARS-CoV-2 | Human IgG1 | COVID-19 | [403] | NA | 2021 |
| Pulmonary | Regdanvimab | Regkirona | SARS-CoV-2 | Human IgG1 | COVID-19 | [404] | NA | 2021 |
| Pulmonary | Casirivimab + imdevimab | REGEN-COV, Ronapreve | SARS-CoV-2 | Human IgG1 | COVID-19 | [405] | Review | 2021 |
| Pulmonary | Tezepelumab | Tezspire | Thymic stromal lymphopoeitin | Human IgG2 | Severe Asthma | [406] | 2021 | 2022 |
| Pulmonary | Benralizumab | Fasenra | IL-5R α | Humanized IgG1 | Asthma | [407] | 2017 | 2018 |
| Pulmonary | Reslizumab | Cingaero, Cingair | IL-5 | Humanized IgG4 | Asthma | [408] | 2016 | 2016 |
| Pulmonary | Obiltoxaximab | Anthim | Protective antigen of B.anthracis exotoxin | Chimeric IgG1 | Preventionof inhalation anthrax | [409] | 2016 | 2020 |
| Pulmonary | Necitumumab | Portazza | EGFR | Human IgG1 | Non-small-cell lung cancer | [410] | 2015 | 2015 |
| Pulmonary | Mepolizumab | Nucala | IL-5 | Humanized IgG1 | Severe eosinophilic asthma | [411] | 2015 | 2015 |
| Pulmonary | Nivolumab | Opdivo | PD1 | Human IgG4 | Non-small-cell lung cancer, melanoma | [90,412] | 2014 | 2015 |
| Pulmonary | Omalizumab | Xolair | IgE | Humanized IgG1 | Asthma | [106] | 2003 | 2005 |
| Pulmonary | Palivizumab | Synagis | RSV | Humanized IgG1 | Prevention of RSV infection | [205] | 1998 | 1999 |
| Muscoloskeletal | Romosozumab | Evenity | Sclerostin | Humanized IgG2 | Osteoporosis in postmenopausal women at risk of fracture | [273] | 2019 | 2019 |
| Musculoskeletal | Burosumab | Crysvita | FGF23 | Human IgG1 | X-linked hypophosphatemia | [276] | 2018 | 2018 |
| Musculoskeletal | Denosumab | Prolia | RANK-L | Human IgG2 | Bone Loss | [413] | 2010 | 2010 |
| Musculoskeletal | Rozanolixizumab | RYSTIGGO® | FcRn | Humanized IgG4 | Generalized myasthenia gravis | [414] | 2023 | Review |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, P.; Joshi, R.V.; Pritchard, R.; Xu, K.; Eicher, M.A. Therapeutic Antibodies in Medicine. Molecules 2023, 28, 6438. https://doi.org/10.3390/molecules28186438
Sharma P, Joshi RV, Pritchard R, Xu K, Eicher MA. Therapeutic Antibodies in Medicine. Molecules. 2023; 28(18):6438. https://doi.org/10.3390/molecules28186438
Chicago/Turabian StyleSharma, Prerna, Rahul V. Joshi, Robert Pritchard, Kevin Xu, and Maya A. Eicher. 2023. "Therapeutic Antibodies in Medicine" Molecules 28, no. 18: 6438. https://doi.org/10.3390/molecules28186438





