Exploring Bioactivities and Peptide Content of Body Mucus from the Lusitanian Toadfish Halobatrachus didactylus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Peptide Profile
2.2. Total Protein and Antioxidant Activity
2.3. Antihypertensive Activity
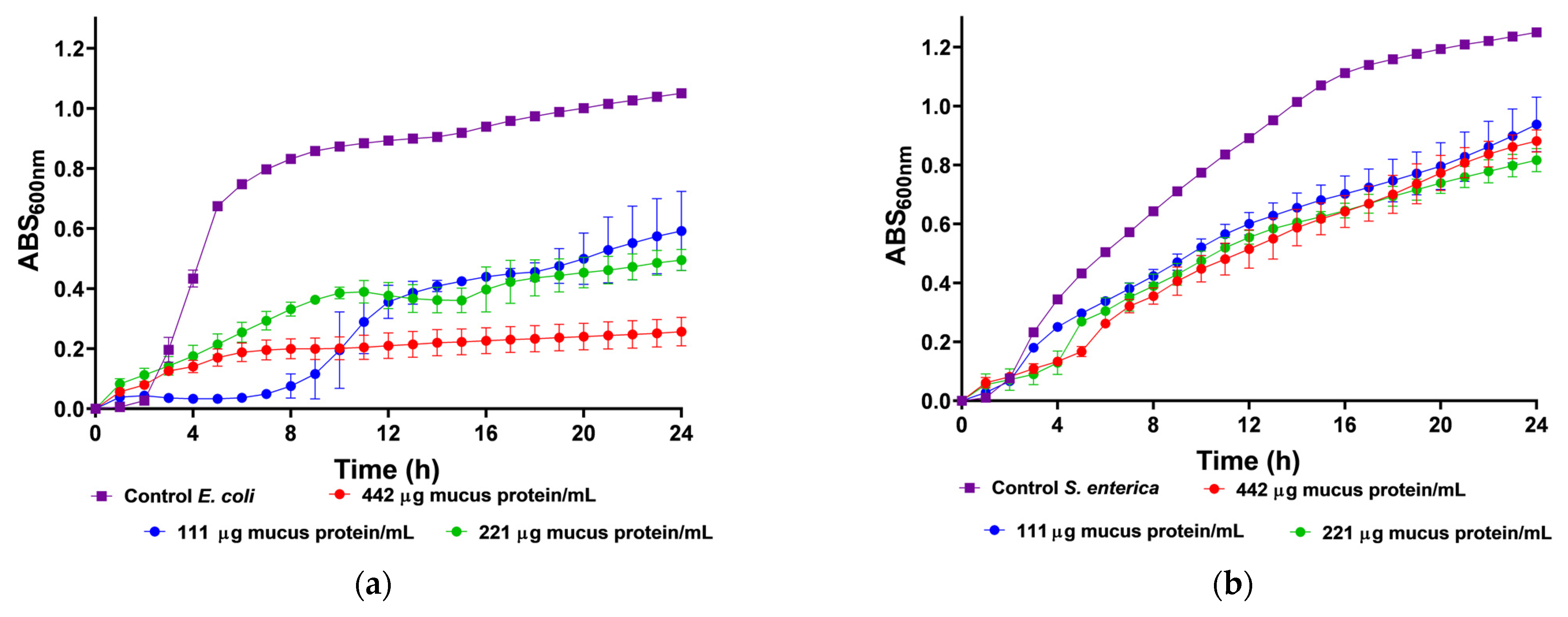
2.4. Antimicrobial Activity
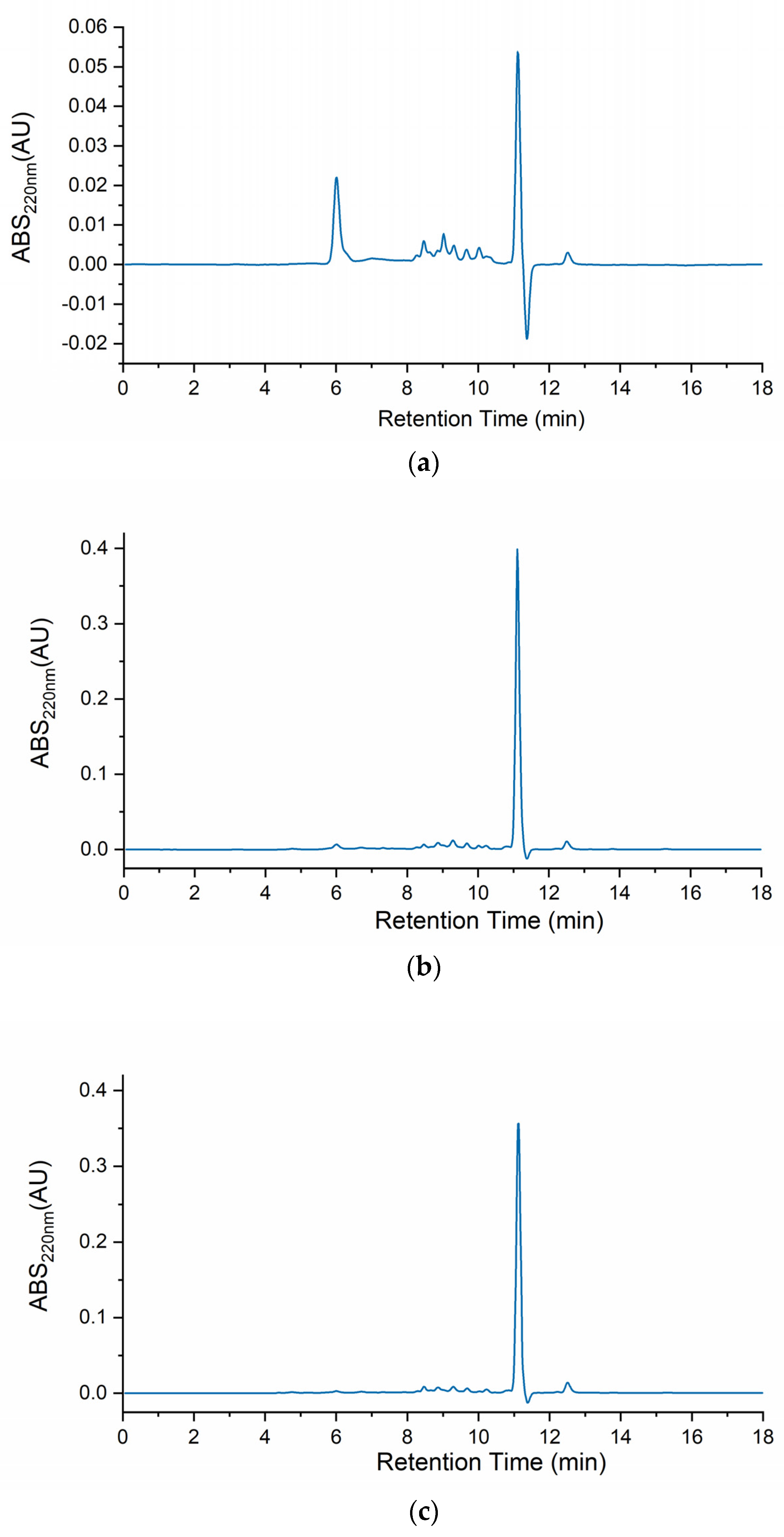
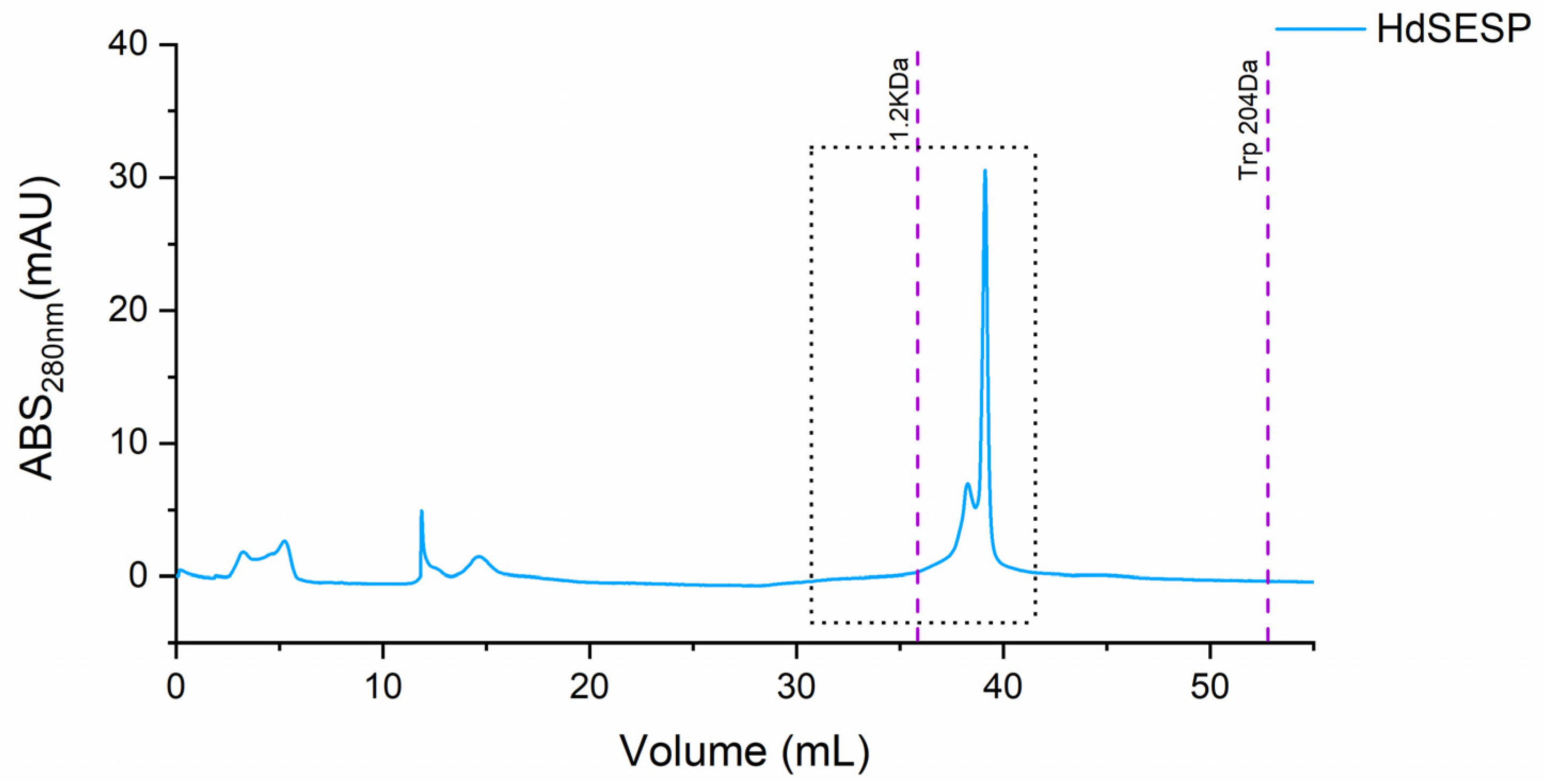
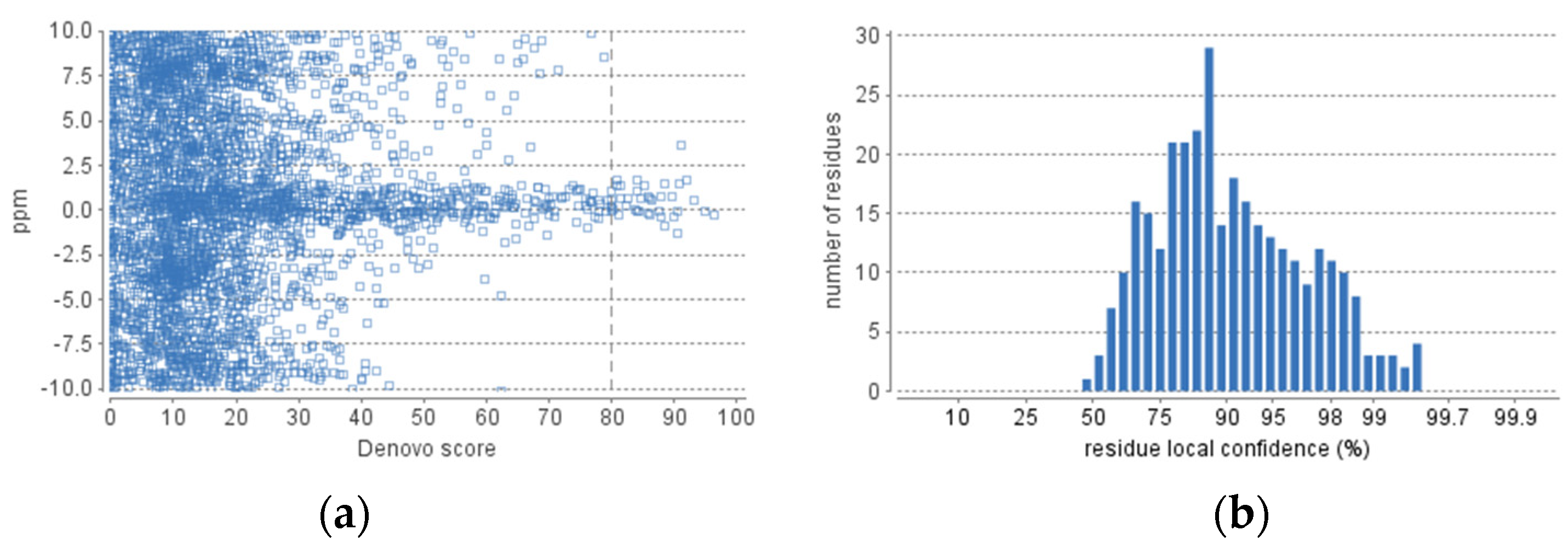
2.5. Fractionation by Size Exclusion Chromatography and Peptidomics of the Peptide Fraction
3. Materials and Methods
3.1. Materials
3.2. Mucus Collection
3.3. Peptide Size Profile
3.4. Soluble Protein Content
3.5. Antioxidant Activity
3.5.1. ABTS Assay
3.5.2. ORAC Assay
3.6. Antihypertensive Activity
3.7. Antimicrobial Activity
3.8. Preparative Size Exclusion Chromatography
3.9. Peptidomic Analysis
3.10. Statistical Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic Resistance in Microbes: History, Mechanisms, Therapeutic Strategies and Future Prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Next-Generation Personalized Medicine: Implementation of Variability Patterns for Overcoming Drug Resistance in Chronic Diseases. J. Pers. Med. 2022, 12, 1303. [Google Scholar] [CrossRef] [PubMed]
- Durand, G.A.; Raoult, D.; Dubourg, G. Antibiotic Discovery: History, Methods and Perspectives. Int. J. Antimicrob. Agents 2019, 53, 371–382. [Google Scholar] [CrossRef]
- Du, Z.; Comer, J.; Li, Y. Bioinformatics Approaches to Discovering Food-Derived Bioactive Peptides: Reviews and Perspectives. TrAC Trends Anal. Chem. 2023, 162, 117051. [Google Scholar] [CrossRef]
- Grosberg, R.K.; Vermeij, G.J.; Wainwright, P.C. Biodiversity in Water and on Land. Curr. Biol. 2012, 22, R900–R903. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural Products: A Continuing Source of Novel Drug Leads. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Kim, S.K.; Wijesekara, I. Development and Biological Activities of Marine-Derived Bioactive Peptides: A Review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Smith, W.L.; Wheeler, W.C. Venom Evolution Widespread in Fishes: A Phylogenetic Road Map for the Bioprospecting of Piscine Venoms. J. Hered. 2006, 97, 206–217. [Google Scholar] [CrossRef]
- Rajanbabu, V.; Chen, J.Y. Applications of Antimicrobial Peptides from Fish and Perspectives for the Future. Peptides 2011, 32, 415–420. [Google Scholar] [CrossRef]
- Sridhar, A.; Manikandan, D.B.; Marimuthu, S.K.; Murugesan, M.; Ramasamy, T. Methanol Skin Mucus Extract of Mrigal (Cirrhinus mrigala) Fish Peptide Targeting Viral Particles of Infectious Pancreatic Necrosis Virus (IPNV) and Infectious Salmon Anemia Virus (ISAV): An in Silico Approach. Int. J. Pept. Res. Ther. 2021, 27, 1429–1440. [Google Scholar] [CrossRef]
- Ziegman, R.; Alewood, P. Bioactive Components in Fish Venoms. Toxins 2015, 7, 1497–1531. [Google Scholar] [CrossRef] [PubMed]
- Qadiri, S.S.N.; Makesh, M.; Rajendran, K.V.; Rathore, G.; Purushothaman, C.S. Specific Immune Response in Mucosal and Systemic Compartments of Cirrhinus mrigala Vaccinated against Edwardsiella tarda: In Vivo Kinetics Using Different Antigen Delivery Routes. J. World Aquac. Soc. 2019, 50, 856–865. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Jahazi, M.A.; Nikdehghan, N.; Van Doan, H.; Volpe, M.G.; Paolucci, M. Effects of Dietary Polyphenols from Agricultural By-Products on Mucosal and Humoral Immune and Antioxidant Responses of Convict Cichlid (Amatitlania nigrofasciata). Aquaculture 2020, 517, 734790. [Google Scholar] [CrossRef]
- Heydari, M.; Firouzbakhsh, F.; Paknejad, H. Effects of Mentha longifolia Extract on Some Blood and Immune Parameters, and Disease Resistance against Yersiniosis in Rainbow Trout. Aquaculture 2020, 515, 734586. [Google Scholar] [CrossRef]
- Mohammadi, G.; Adorian, T.J.; Rafiee, G. Beneficial Effects of Bacillus subtilis on Water Quality, Growth, Immune Responses, Endotoxemia and Protection against Lipopolysaccharide-Induced Damages in Oreochromis niloticus under Biofloc Technology System. Aquac. Nutr. 2020, 26, 1476–1492. [Google Scholar] [CrossRef]
- Oliveira, M.; Tvarijonaviciute, A.; Trindade, T.; Soares, A.; Tort, L.; Teles, M. Can Non-Invasive Methods Be Used to Assess Effects of Nanoparticles in Fish? Ecol. Indic. 2018, 95, 1118–1127. [Google Scholar] [CrossRef]
- Valero, Y.; Cortés, J.; Mercado, L. NK-Lysin from Skin-Secreted Mucus of Atlantic Salmon and Its Potential Role in Bacteriostatic Activity. Fish Shellfish Immunol. 2019, 87, 410–413. [Google Scholar] [CrossRef]
- Li, T.; Liu, Q.; Wang, D.; Li, J. Characterization and Antimicrobial Mechanism of CF-14, a New Antimicrobial Peptide from the Epidermal Mucus of Catfish. Fish Shellfish Immunol. 2019, 92, 881–888. [Google Scholar] [CrossRef]
- Go, H.-J.; Kim, C.-H.; Park, J.B.; Kim, T.Y.; Lee, T.K.; Oh, H.Y.; Park, N.G. Biochemical and Molecular Identification of a Novel Hepcidin Type 2-like Antimicrobial Peptide in the Skin Mucus of the Pufferfish Takifugu pardalis. Fish Shellfish Immunol. 2019, 93, 683–693. [Google Scholar] [CrossRef]
- Lopes-Ferreira, M.; Barbaro, K.C.; Cardoso, D.F.; Moura-Da-Silva, A.M.; Mota, I. Thalassophryne nattereri Fish Venom: Biological and Biochemical Characterixation and Serum Neutralization of Its Toxic Activities. Toxicon 1998, 36, 405–410. [Google Scholar] [CrossRef]
- Magalhães, G.S.; Lopes-Ferreira, M.; Junqueira-De-Azevedo, I.L.M.; Spencer, P.J.; Araújo, M.S.; Portaro, F.C.V.; Ma, L.; Valente, R.H.; Juliano, L.; Fox, J.W.; et al. Natterins, a New Class of Proteins with Kininogenase Activity Characterized from Thalassophryne nattereri Fish Venom. Biochimie 2005, 87, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S.R.; Leong, I.; Nayar, M.S.B. Ichthyotoxins from the Oyster Toadfish, Opsanus tau (Linnaeus). Toxicon 1982, 20, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.F.; Santos, M.J.; Costa, J.L.; Costa, M.J.; Cabral, H.N. Metazoan Parasites as Biological Indicators of Population Structure of Halobatrachus didactylus on the Portuguese Coast. J. Appl. Ichthyol. 2005, 21, 220–224. [Google Scholar] [CrossRef]
- Cotter, J.C.; Pereira, T.J.; Costa, M.J.; Costa, J.L. Distribution, Abundance, Population Structure and Activity of Halobatrachus didactylus in the Tagus Estuary (Portugal) and Adjacent Coastal Waters. J. Mar. Biol. Assoc. UK 2013, 93, 405–412. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Tagami, M.; Kuwahara, J. Evaluation of Antioxidant Activity and Amino Acids in the Mucus of Mackerel for Cosmetic Applications. J. Oleo Sci. 2020, 69, 1133–1138. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Plant Protein-Derived Antioxidant Peptides: Isolation, Identification, Mechanism of Action and Application in Food Systems: A Review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Brassesco, M.E.; Pintado, M. Collagen-Based Bioactive Bromelain Hydrolysate from Salt-Cured Cod Skin. Appl. Sci. 2021, 11, 8538. [Google Scholar] [CrossRef]
- Kim, S.K.; Ngo, D.H.; Vo, T.S. Marine Fish-Derived Bioactive Peptides as Potential Antihypertensive Agents, 1st ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 65, ISBN 9780124160033. [Google Scholar]
- Abdelhedi, O.; Nasri, M. Basic and Recent Advances in Marine Antihypertensive Peptides: Production, Structure-Activity Relationship and Bioavailability. Trends Food Sci. Technol. 2019, 88, 543–557. [Google Scholar] [CrossRef]
- Yathisha, U.G.; Bhat, I.; Karunasagar, I.; Mamatha, B.S. Antihypertensive Activity of Fish Protein Hydrolysates and Its Peptides. Crit. Rev. Food Sci. Nutr. 2019, 59, 2363–2374. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Otte, J.; De Gobba, C.; Osman, A.; Hamad, E. Angiotensin I-Converting Enzyme Inhibitory Activity and Antioxidant Capacity of Bioactive Peptides Derived from Enzymatic Hydrolysis of Buffalo Milk Proteins. Int. Dairy J. 2017, 66, 91–98. [Google Scholar] [CrossRef]
- Abachi, S.; Bazinet, L.; Beaulieu, L. Antihypertensive and Angiotensin-i-Converting Enzyme (ACE)-Inhibitory Peptides from Fish as Potential Cardioprotective Compounds. Mar. Drugs 2019, 17, 613. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Rajeshwaran, T.; Priya, P.; Kailasam, M.; Biswas, G.; Ghoshal, T.K.; Vijayan, K.K.; Arasu, A.R.T. Comparative Immunological and Biochemical Properties of the Epidermal Mucus from Three Brackishwater Fishes. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 95–103. [Google Scholar] [CrossRef]
- Guluarte, C.; Reyes-Becerril, M.; Gonzalez-Silvera, D.; Cuesta, A.; Angulo, C.; Esteban, M.Á. Probiotic Properties and Fatty Acid Composition of the Yeast Kluyveromyces lactis M3. In Vivo Immunomodulatory Activities in Gilthead Seabream (Sparus Aurata). Fish Shellfish Immunol. 2019, 94, 389–397. [Google Scholar] [CrossRef]
- Soltanian, S.; Gholamhosseini, A. The Effects of Starvation on Some Epidermal Mucus Immune Parameters in Rainbow Trout, Oncorhynchus Mykiss. Int. J. Aquat. Biol. 2019, 7, 291–300. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Osman, A.; Al-Mohammadi, A.-R.; Enan, G.; Kamal, N.; Sitohy, M. Biochemical, Biological Characteristics and Antibacterial Activity of Glycoprotein Extracted from the Epidermal Mucus of African Catfish (Clarias gariepinus). Int. J. Biol. Macromol. 2019, 138, 773–780. [Google Scholar] [CrossRef]
- Patel, M.; Ashraf, M.S.; Siddiqui, A.J.; Ashraf, S.A.; Sachidanandan, M.; Snoussi, M.; Adnan, M.; Hadi, S. Profiling and Role of Bioactive Molecules from Puntius sophore (Freshwater/Brackish Fish) Skin Mucus with Its Potent Antibacterial, Antiadhesion, and Antibiofilm Activities. Biomolecules 2020, 10, 920. [Google Scholar] [CrossRef]
- Fuochi, V.; Li Volti, G.; Camiolo, G.; Tiralongo, F.; Giallongo, C.; Distefano, A.; Petronio, G.P.; Barbagallo, I.; Viola, M.; Furneri, P.M.; et al. Antimicrobial and Anti-Proliferative Effects of Skin Mucus Derived from Dasyatis pastinaca (Linnaeus, 1758). Mar. Drugs 2017, 15, 342. [Google Scholar] [CrossRef]
- Cantisani, M.; Finamore, E.; Mignogna, E.; Falanga, A.; Nicoletti, G.F.; Pedone, C.; Morelli, G.; Leone, M.; Galdiero, M.; Galdiero, S. Structural Insights into and Activity Analysis of the Antimicrobial Peptide Myxinidin. Antimicrob. Agents Chemother. 2014, 58, 5280–5290. [Google Scholar] [CrossRef]
- Jiang, Z.; Vasil, A.I.; Gera, L.; Vasil, M.L.; Hodges, R.S. Rational Design of α-Helical Antimicrobial Peptides to Target Gram-Negative Pathogens, Acinetobacter Baumannii and Pseudomonas Aeruginosa: Utilization of Charge, “Specificity Determinants,” Total Hydrophobicity, Hydrophobe Type and Location as Design Parameters to Improve the Therapeutic Ratio. Chem. Biol. Drug Des. 2011, 77, 225–240. [Google Scholar] [CrossRef]
- Najm, A.A.K.; Azfaralariff, A.; Dyari, H.R.E.; Othman, B.A.; Shahid, M.; Khalili, N.; Law, D.; Syed Alwi, S.S.; Fazry, S. Anti-Breast Cancer Synthetic Peptides Derived from the Anabas Testudineus Skin Mucus Fractions. Sci. Rep. 2021, 11, 23182. [Google Scholar] [CrossRef] [PubMed]
- Coscueta, E.R.; Batista, P.; Gomes, J.E.G.; da Silva, R.; Pintado, M.M. Screening of Novel Bioactive Peptides from Goat Casein: In Silico to In Vitro Validation. Int. J. Mol. Sci. 2022, 23, 2439. [Google Scholar] [CrossRef]
- Ma, C.; Liu, D.; Hao, H.; Wu, X. Identification of the DPP-IV Inhibitory Peptides from Donkey Blood and Regulatory Effect on the Gut Microbiota of Type 2 Diabetic Mice. Foods 2022, 11, 2148. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Fitzgerald, R.J. An in Silico Model to Predict the Potential of Dietary Proteins as Sources of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides. Food Chem. 2014, 165, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.C.; Chauhan, S.S. Dipeptidyl Peptidase III: A Multifaceted Oligopeptide N-End Cutter. FEBS J. 2011, 278, 3256–3276. [Google Scholar] [CrossRef]
- Abramić, M.; Agić, D. Survey of Dipeptidyl Peptidase III Inhibitors: From Small Molecules of Microbial or Synthetic Origin to Aprotinin. Molecules 2022, 27, 3006. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A Review of Alpha-Glucosidase Inhibitors from Plants as Potential Candidates for the Treatment of Type-2 Diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef]
- Massolini, B.D.; Contieri, S.S.G.; Lazarini, G.S.; Bellacosa, P.A.; Dobre, M.; Petroianu, G.; Brateanu, A.; Campos, L.A.; Baltatu, O.C. Therapeutic Renin Inhibition in Diabetic Nephropathy—A Review of the Physiological Evidence. Front. Physiol. 2020, 11, 190. [Google Scholar] [CrossRef]
- Gonçalves, B.; Falco, V.; Moutinho-Pereira, J.; Bacelar, E.; Peixoto, F.; Correia, C. Effects of Elevated CO2 on Grapevine (Vitis vinifera L.): Volatile Composition, Phenolic Content, and in Vitro Antioxidant Activity of Red Wine. J. Agric. Food Chem. 2009, 57, 265–273. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Campos, D.A.; Osório, H.; Nerli, B.B.; Pintado, M. Enzymatic Soy Protein Hydrolysis: A Tool for Biofunctional Food Ingredient Production. Food Chem. X 2019, 1, 100006. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial Activity of Pomegranate Peel Extracts Performed by High Pressure and Enzymatic Assisted Extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Osório, H.; Silva, C.; Ferreira, M.; Gullo, I.; Máximo, V.; Barros, R.; Mendonça, F.; Oliveira, C.; Carneiro, F. Proteomics Analysis of Gastric Cancer Patients with Diabetes Mellitus. J. Clin. Med. 2021, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- CAMP: Collection of Sequences and Structures of Antimicrobial Peptides. Available online: http://www.camp3.bicnirrh.res.in/ (accessed on 25 July 2022).
- PeptideRanker. Available online: http://distilldeep.ucd.ie/PeptideRanker/ (accessed on 25 July 2022).
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the Improved Discovery and Design of Functional Peptides: Common Features of Diverse Classes Permit Generalized Prediction of Bioactivity. PLoS ONE 2012, 7, e45012. [Google Scholar] [CrossRef]
- No BIOPEP-UWM—Katedra Biochemii Zywno’sci. Available online: https://biochemia.uwm.edu.pl/biopep-uwm/ (accessed on 25 July 2022).
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Dziuba, J.; Iwaniak, A.; Dziuba, M.; Darewicz, M. BIOPEP Database and Other Programs for Processing Bioactive Peptide Sequences. J. AOAC Int. 2008, 91, 965–980. [Google Scholar] [CrossRef]

| Sample | Protein (µg BSA/mL) | ORAC (µmol TE/g Mucus Protein) | ABTS (µmol TE/g Mucus Protein) |
|---|---|---|---|
| HdTAG | 13,260 ± 342 a | 2371 ± 97 a | 112 ± 3 a |
| HdSES1 | 344 ± 4 b | 164 ± 28 b | 154 ± 6 b |
| HdSES2 | 447 ± 98 b | 188 ± 6 b | 129 ± 4 a |
| Mass (Da) | Area | Seq. 1 | Class 2,3 | |||
|---|---|---|---|---|---|---|
| SVM | RF | ANN | DA | |||
| 1589.8 | 2.89 × 108 | EDNSELGQETPTLR | AMP | NAMP | NAMP | NAMP |
| 1101.6 | 1.26 × 108 | VYPFPGPLPN | NAMP | NAMP | AMP | NAMP |
| 839.5 | 9.33 × 107 | PFPGPLPN | NAMP | NAMP | AMP | NAMP |
| 839.5 | 9.33 × 107 | PFGPPLPN | NAMP | NAMP | AMP | NAMP |
| 889.5 | 7.54 × 107 | EVGDEVLK | NAMP | NAMP | NAMP | NAMP |
| 887.5 | 5.67 × 107 | ELGDEVPK | NAMP | NAMP | AMP | NAMP |
| 960.5 | 4.81 × 107 | AEVGDEVLK | NAMP | NAMP | NAMP | NAMP |
| 760.4 | 2.89 × 107 | VGDEVKL | NAMP | NAMP | NAMP | NAMP |
| 760.4 | 2.89 × 107 | VGDEVLK | NAMP | NAMP | NAMP | NAMP |
| 1015.6 | 2.40 × 107 | QETPTLRVA | AMP | NAMP | NAMP | NAMP |
| 1101.6 | 2.33 × 107 | DDVGDKELLP | NAMP | NAMP | NAMP | NAMP |
| 785.5 | 2.28 × 107 | EVVLVEP | AMP | NAMP | NAMP | NAMP |
| 1086.6 | 2.12 × 107 | QAELGDEVPK | NAMP | NAMP | NAMP | NAMP |
| 895.5 | 1.59 × 107 | DPPNPKNL | AMP | AMP | AMP | AMP |
| 895.5 | 1.59 × 107 | DPPNPKLN | AMP | AMP | AMP | AMP |
| 1474.7 | 1.44 × 107 | APAGATDAKDEDLVT | NAMP | NAMP | NAMP | NAMP |
| 887.4 | 6.53 × 106 | TDPPELNT | AMP | NAMP | NAMP | NAMP |
| 950.5 | 4.19 × 106 | VAPPNPQNL | NAMP | NAMP | NAMP | NAMP |
| 1026.6 | 4.13 × 106 | PPFLQVVPE | NAMP | NAMP | NAMP | NAMP |
| 993.5 | 3.87 × 106 | PLVNHEGAGV | NAMP | NAMP | NAMP | NAMP |
| 985.4 | 3.41 × 106 | DGGPPSPDNE | NAMP | AMP | NAMP | NAMP |
| 791.4 | 3.04 × 106 | PFYPGPL | NAMP | NAMP | AMP | NAMP |
| 770.4 | 2.05 × 106 | PAPPPPPP | AMP | AMP | NAMP | NAMP |
| 845.5 | 6.27 × 105 | QETPTLR | AMP | NAMP | NAMP | NAMP |
| 844.5 | 3.56 × 105 | NPATVLKT | NAMP | NAMP | AMP | NAMP |
| 832.5 | 1.02 × 105 | LTKVLEE | NAMP | NAMP | NAMP | NAMP |
| Seq. 1 | PeptideRanker 2 | BIOPEP (A) 3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACE inhibitor | Alpha-glucosidase Inhibitor | Antiamnestic | Antioxidative | Antithrombotic | Chemotactic | Dipeptidyl Peptidase III Inhibitor | Dipeptidyl Peptidase IV Inhibitor | Immunomodulating | Inhibitor | Neuropeptide | Opioid Agonist | Opioid | Regulating | Renin Inhibitor | Stimulating | ||
| DPPNPKNL | 0.73075 | 0.125 | 0.125 | 0.750 | |||||||||||||
| DPPNPKLN | 0.68444 | 0.375 | 0.125 | 0.750 | |||||||||||||
| PAPPPPPP | 0.96263 | 1.375 | 0.625 | 1.250 | |||||||||||||
| EDNSELGQETPTLR | 0.04606 | 0.357 | 0.071 | 0.071 | 0.429 | 0.071 | 0.071 | 0.071 | |||||||||
| VYPFPGPLPN | 0.96638 | 1.200 | 0.100 | 0.300 | 0.100 | 0.300 | 0.100 | 0.200 | 0.900 | 0.200 | 0.100 | 0.100 | 0.200 | 0.300 | |||
| PFPGPLPN | 0.91394 | 1.000 | 0.375 | 0.375 | 0.125 | 0.125 | 0.875 | 0.125 | 0.375 | ||||||||
| PFGPPLPN | 0.91526 | 1.000 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.875 | 0.125 | 0.125 | |||||||
| ELGDEVPK | 0.19243 | 0.625 | 0.125 | 0.375 | |||||||||||||
| QETPTLRVA | 0.19192 | 0.333 | 0.111 | 0.222 | 0.667 | 0.111 | |||||||||||
| EVVLVEP | 0.18655 | 0.571 | 0.143 | 0.857 | 0.286 | ||||||||||||
| TDPPELNT | 0.09808 | 0.250 | 0.250 | 0.250 | 0.250 | 0.625 | |||||||||||
| DGGPPSPDNE | 0.37634 | 0.500 | 0.100 | 0.100 | 0.100 | 0.100 | 0.700 | 0.100 | 0.100 | ||||||||
| PFYPGPL | 0.11515 | 1.000 | 0.286 | 0.429 | 0.429 | 0.143 | 0.143 | 0.714 | 0.143 | 0.429 | |||||||
| QETPTLR | 0.78508 | 0.429 | 0.143 | 0.143 | 0.714 | 0.143 | |||||||||||
| NPATVLKT | 0.14493 | 0.125 | 0.750 | 0.125 | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez Cunha, M.; Coscueta, E.R.; Brassesco, M.E.; Marques, R.; Neto, J.; Almada, F.; Gonçalves, D.; Pintado, M. Exploring Bioactivities and Peptide Content of Body Mucus from the Lusitanian Toadfish Halobatrachus didactylus. Molecules 2023, 28, 6458. https://doi.org/10.3390/molecules28186458
Fernandez Cunha M, Coscueta ER, Brassesco ME, Marques R, Neto J, Almada F, Gonçalves D, Pintado M. Exploring Bioactivities and Peptide Content of Body Mucus from the Lusitanian Toadfish Halobatrachus didactylus. Molecules. 2023; 28(18):6458. https://doi.org/10.3390/molecules28186458
Chicago/Turabian StyleFernandez Cunha, Marta, Ezequiel R. Coscueta, María Emilia Brassesco, Rita Marques, José Neto, Frederico Almada, David Gonçalves, and Manuela Pintado. 2023. "Exploring Bioactivities and Peptide Content of Body Mucus from the Lusitanian Toadfish Halobatrachus didactylus" Molecules 28, no. 18: 6458. https://doi.org/10.3390/molecules28186458
APA StyleFernandez Cunha, M., Coscueta, E. R., Brassesco, M. E., Marques, R., Neto, J., Almada, F., Gonçalves, D., & Pintado, M. (2023). Exploring Bioactivities and Peptide Content of Body Mucus from the Lusitanian Toadfish Halobatrachus didactylus. Molecules, 28(18), 6458. https://doi.org/10.3390/molecules28186458












