Optical Imaging Opportunities to Inspect the Nature of Cytosolic Iron Pools
Abstract
:1. Introduction
2. Chemical Components of LIP
3. The Nature of the Labile Iron Pool in Different Intracellular Organelles
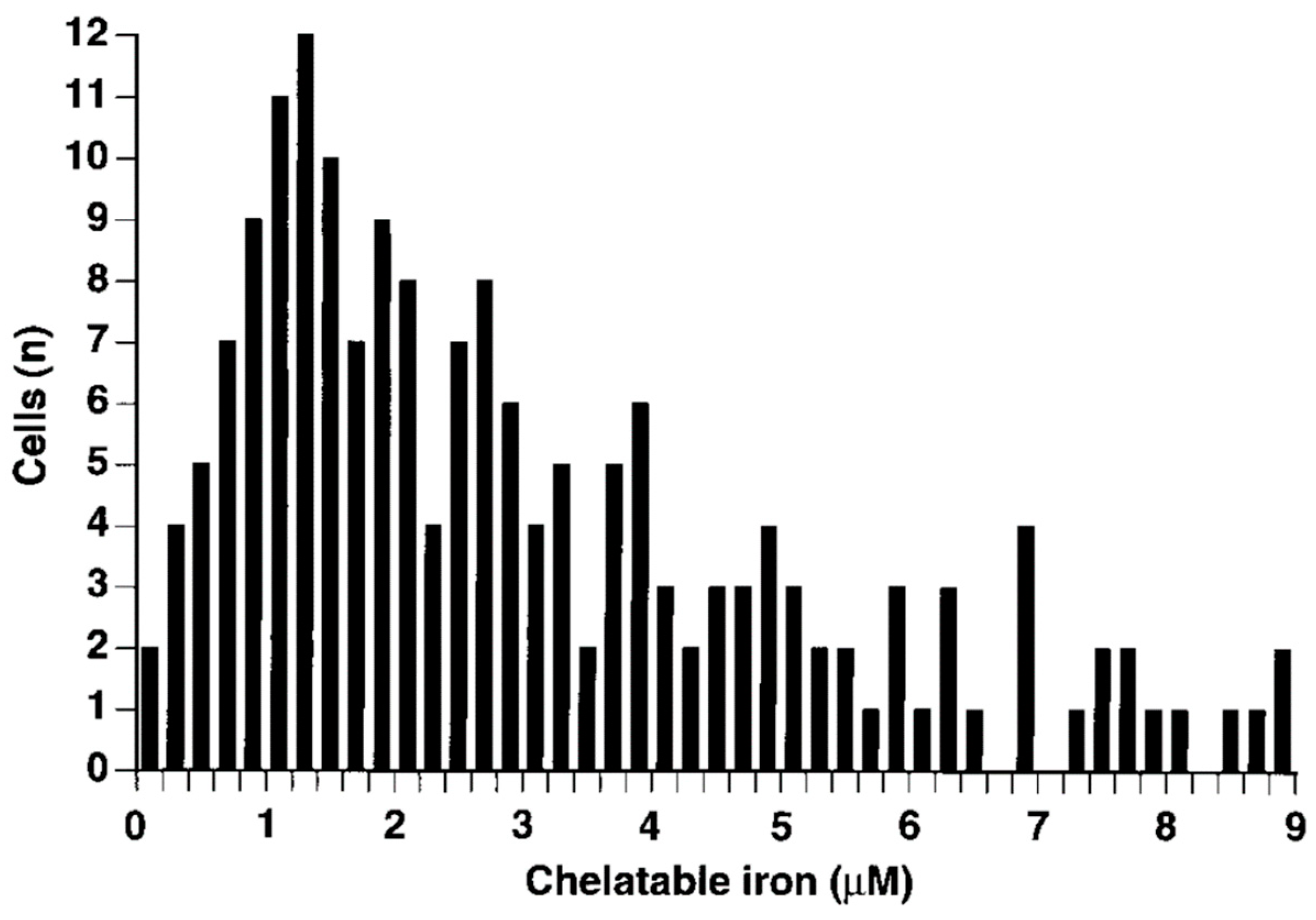
4. Isolation and Characterization of Labile Iron Species
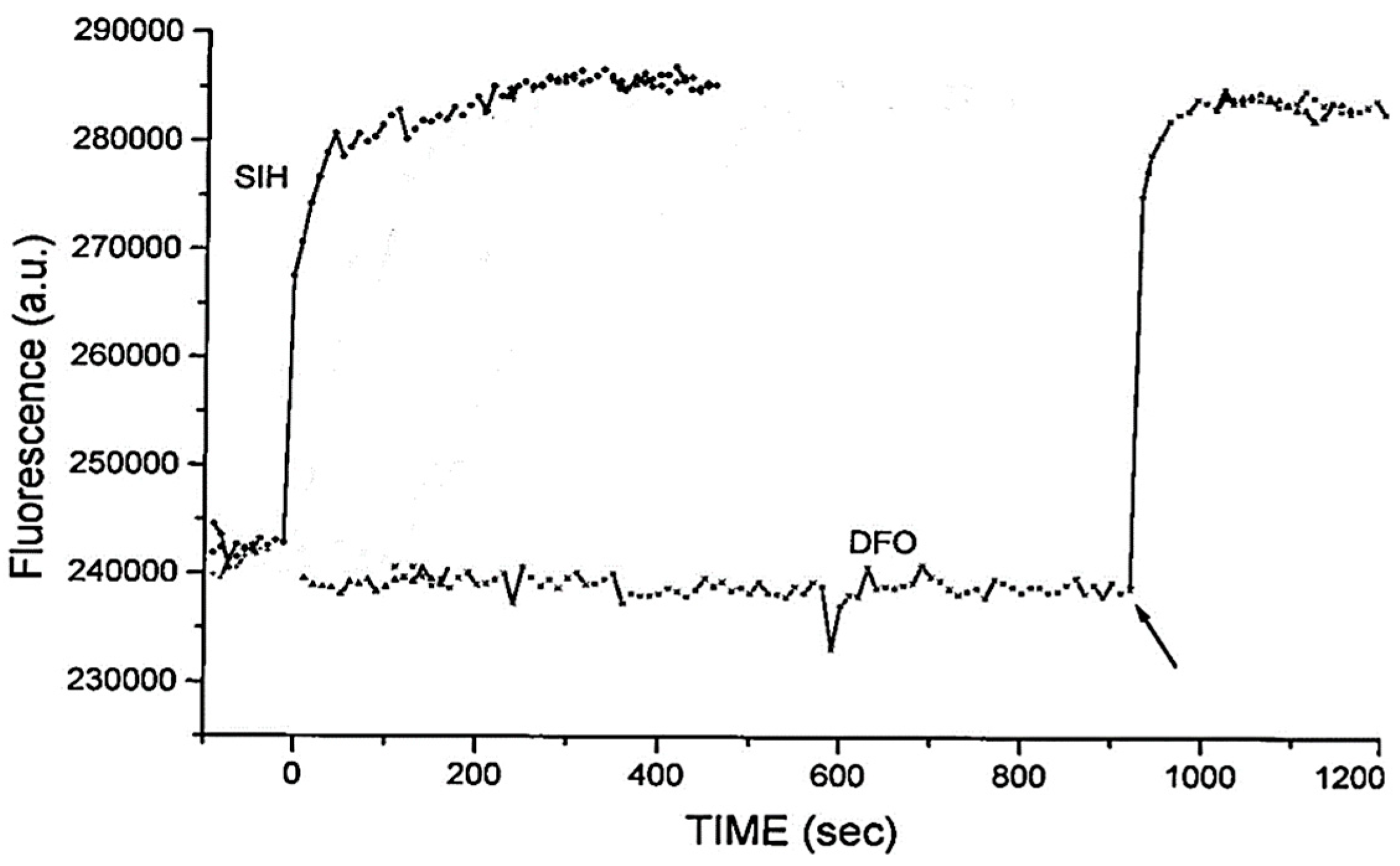
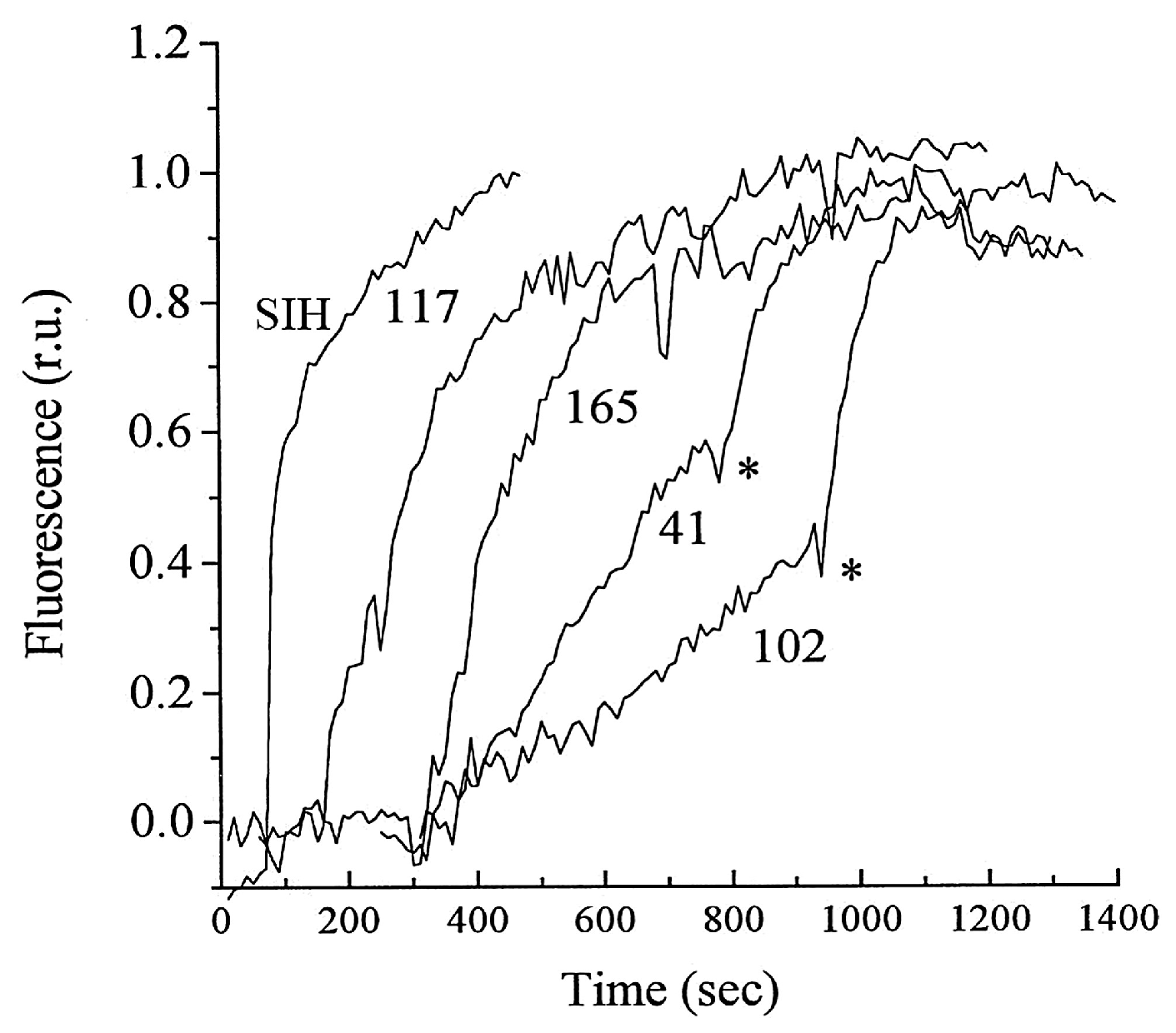
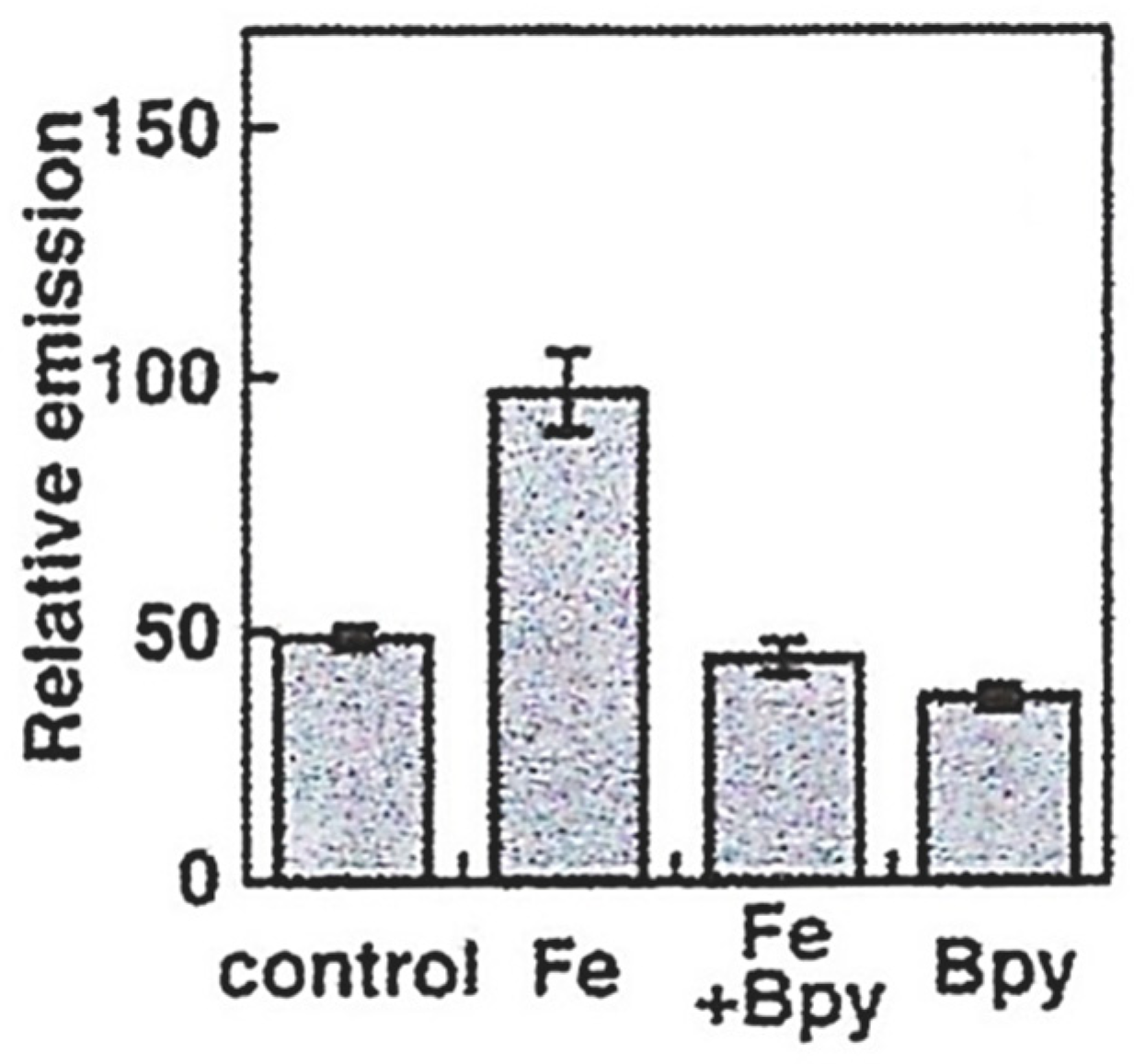
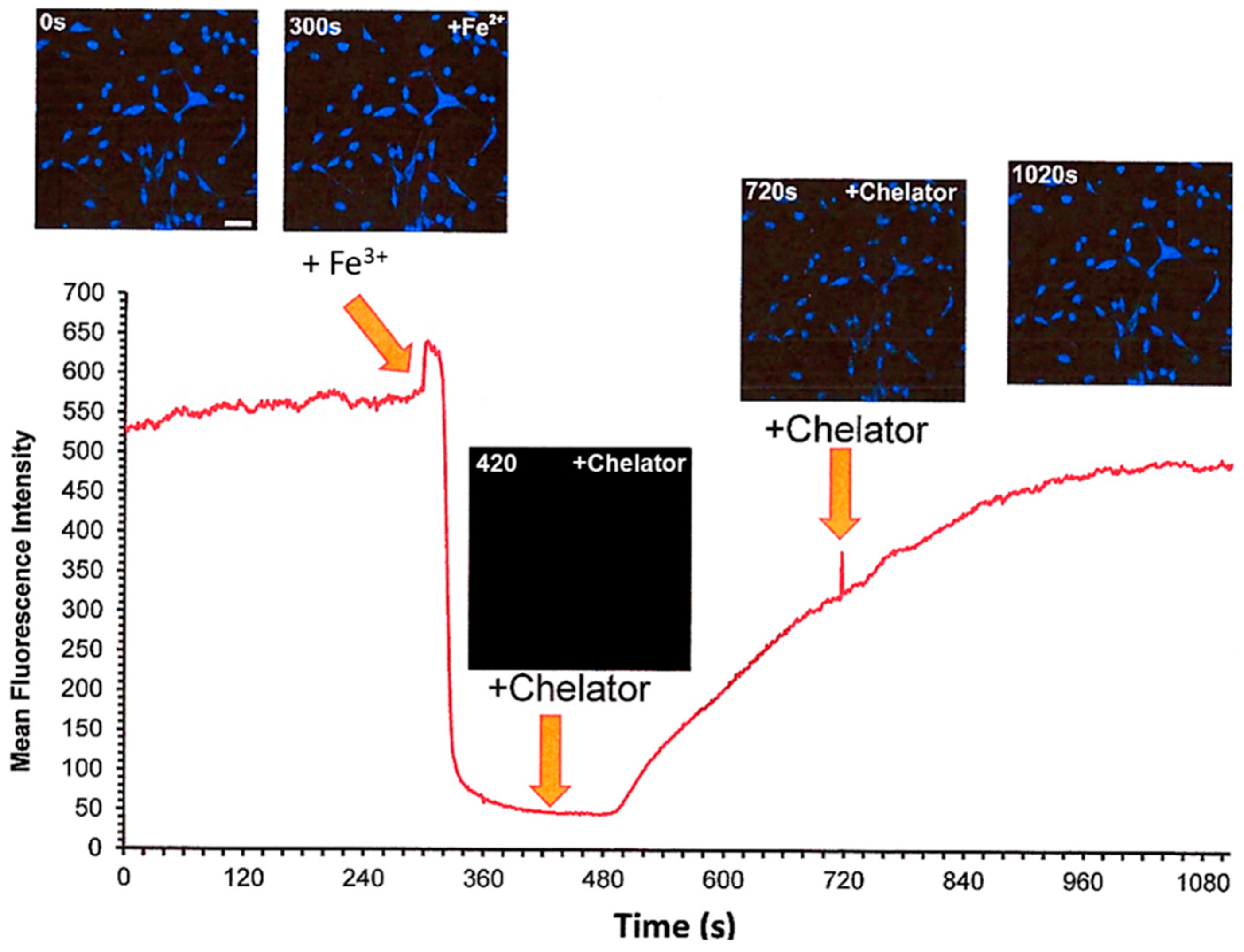
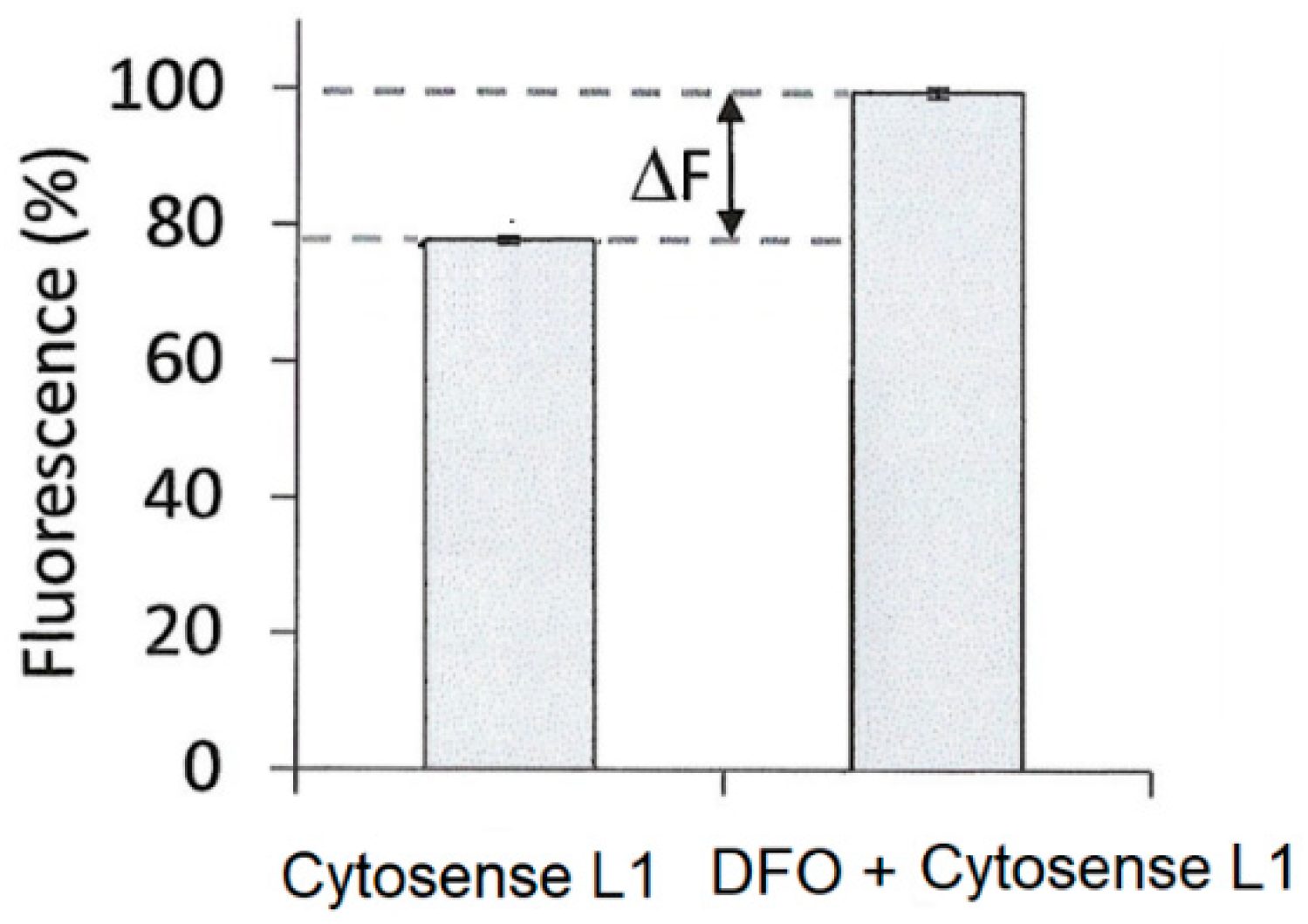
5. Iron-Sensitive Fluorescent Probes



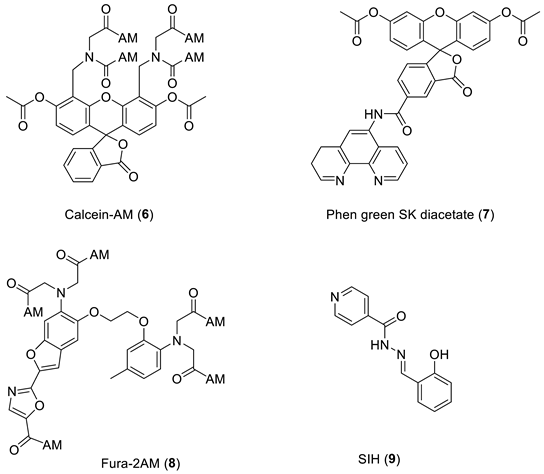


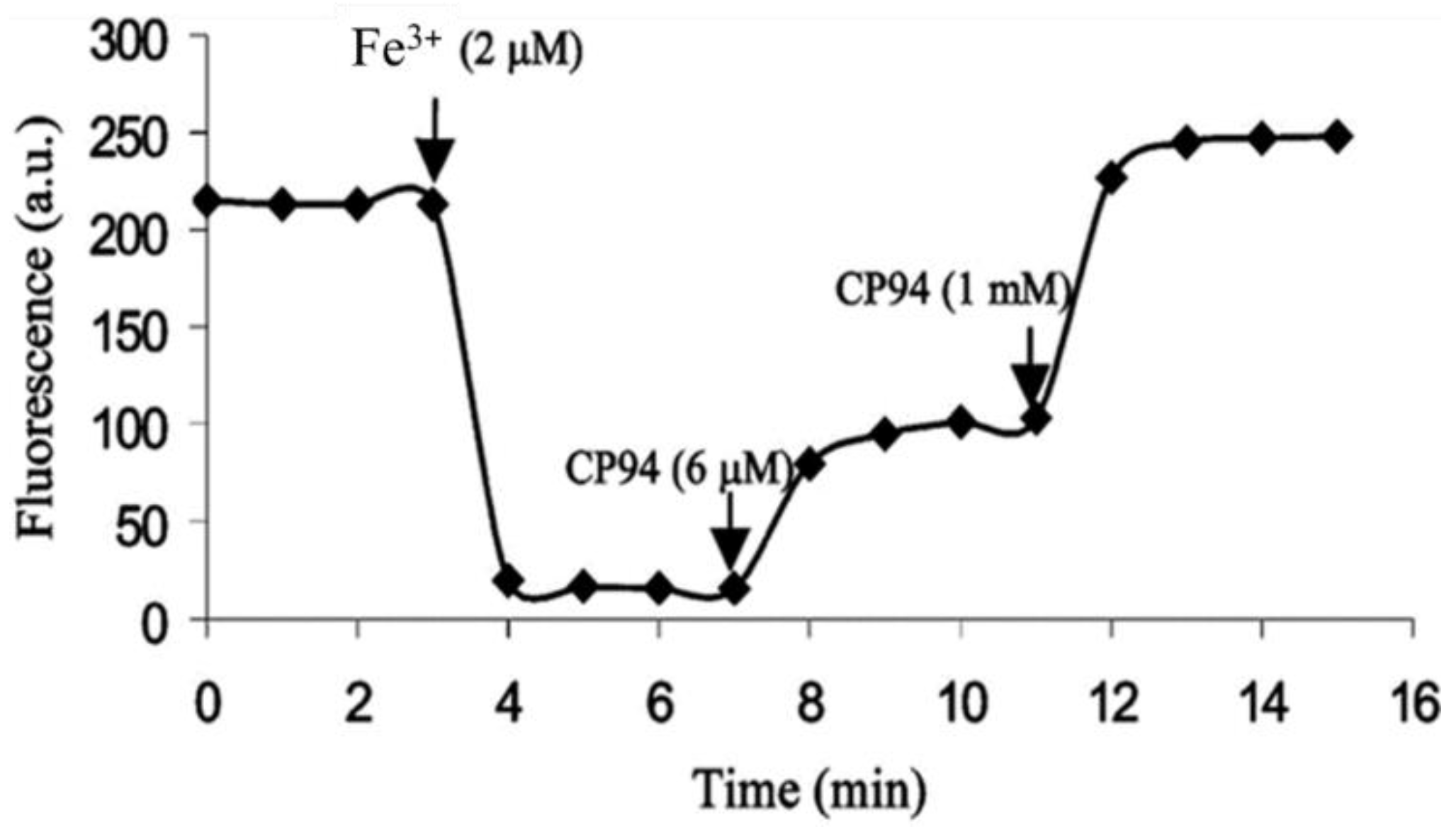


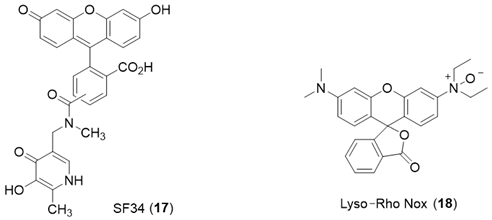
6. NIR Probes
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maret, W.; Wedd, A. Binding, Transport and Storage of Metal Ions in Biological Cells; Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Hider, R.C.; Kong, X. Iron speciation in the cytosol: An overview. Dalton Trans. 2013, 42, 3220–3229. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.P. Free manganese(II) and iron(II) cations can act as intracellular cell controls. FEBS Lett. 1982, 140, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Breuer, W.; Epsztejn, S.; Cabantchik, Z.I. Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron(II). J. Biol. Chem. 1995, 270, 24209–24215. [Google Scholar] [CrossRef]
- Petrat, F.; de Groot, H.; Sustmann, R.; Rauen, U. The chelatable iron pool in living cells: A methodically defined quantity. Biol. Chem. 2002, 383, 489–502. [Google Scholar] [CrossRef]
- Helm, L.; Merbach, A.E. Inorganic and bioinorganic solvent exchange mechanisms. Chem. Rev. 2005, 105, 1922–1959. [Google Scholar] [CrossRef]
- Koppenol, W.H. The centennial of the Fenton reaction. Free Rad. Biol. Med. 1993, 15, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Moursy, M.; Hider, R.C.; Cilibrizzi, A. The colorimetric detection of the hydroxyl radical. Int. J. Mol. Sci. 2023, 24, 4162. [Google Scholar] [CrossRef]
- Kühn, L. Iron regulatory proteins and their role in controlling iron metabolism. Metallomics 2015, 7, 232–243. [Google Scholar] [CrossRef]
- Hentze, M.W.; Kühn, L.C. Molecular control of vertebrate iron metabolism: In RNA based circuits operated by iron, nitric oxide and oxidative stress. Proc. Natl. Acad. Sci. USA 1996, 93, 8175–8182. [Google Scholar] [CrossRef]
- Simpson, R.J.; Mckie, A.T. Iron and oxygen sensing: A tale of 2 interacting elements? Metallomics 2015, 7, 223–231. [Google Scholar] [CrossRef]
- Miller, J.P.G.; Perkins, D.J. Model experiments for the study of iron transfer from transferrin to ferritin. Eur. J. Biochem. 1969, 10, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.; Pollack, S. Low-Mr iron isolated from guinea pig reticulocytes as AMP-Fe and ATP-Fe complexes. Biochem. J. 1989, 261, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Veiga, N.; Torres, J.; Mansell, D.; Freeman, S.; Dominguez, S.; Barker, C.J.; Diaz, A.; Kremer, C. “Chelatable iron pool”: Inositol 1,2,3-trisphosphate fulfils the conditions required to be a safe cellular iron ligand. J. Biol. Inorg. Chem. 2009, 14, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Morley, C.G.D.; Bezkorovainy, A. Identification of the iron chelate in hepatocyte cytosol. IRCS Med. Sci. 1983, 11, 1106–1107. [Google Scholar]
- Harris, D.C.; Aisen, P. Facilitation of Fe(II) autoxidation by Fe(III) complexing agents. Biochim. Biophys. Acta 1973, 329, 156–158. [Google Scholar] [CrossRef] [PubMed]
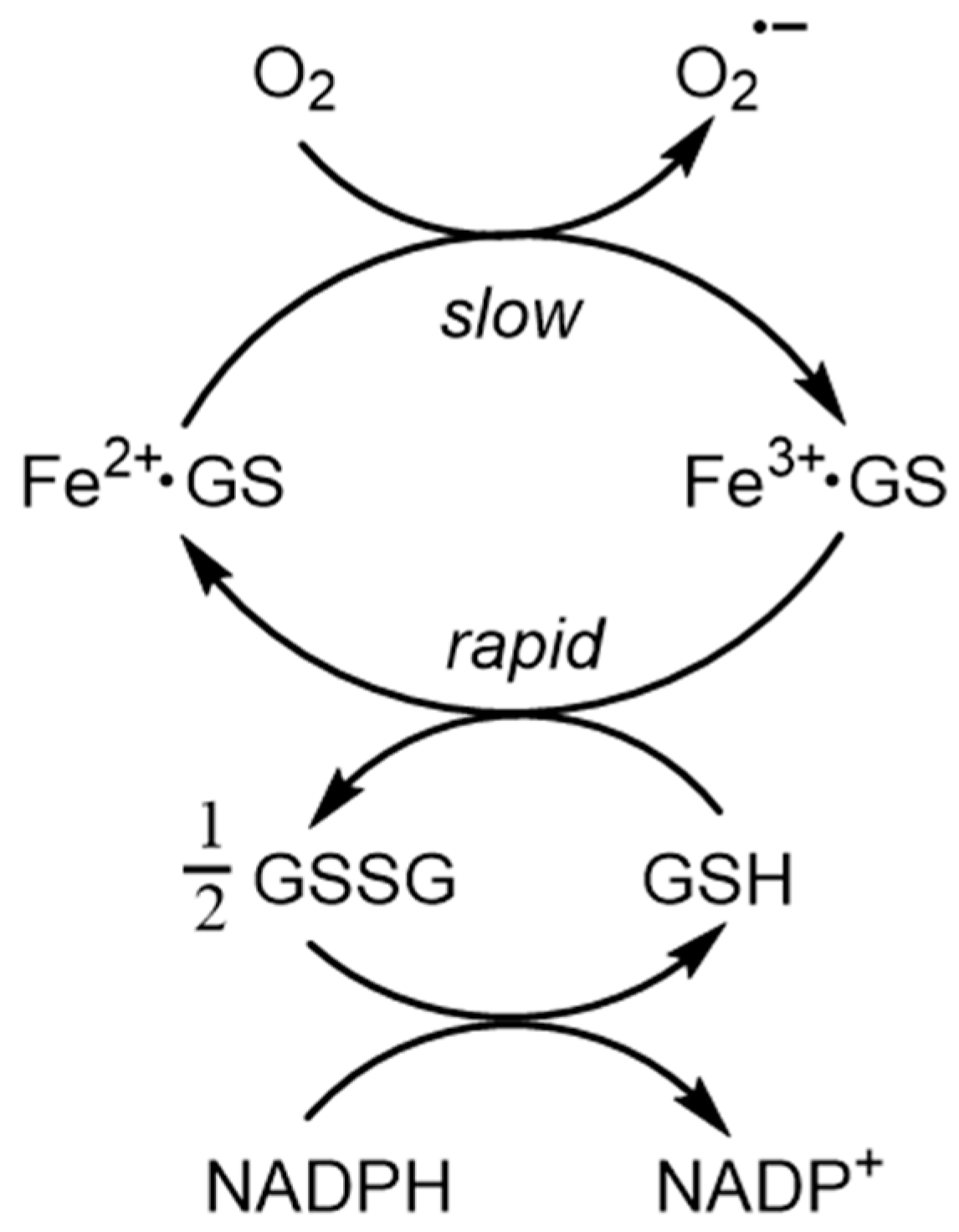
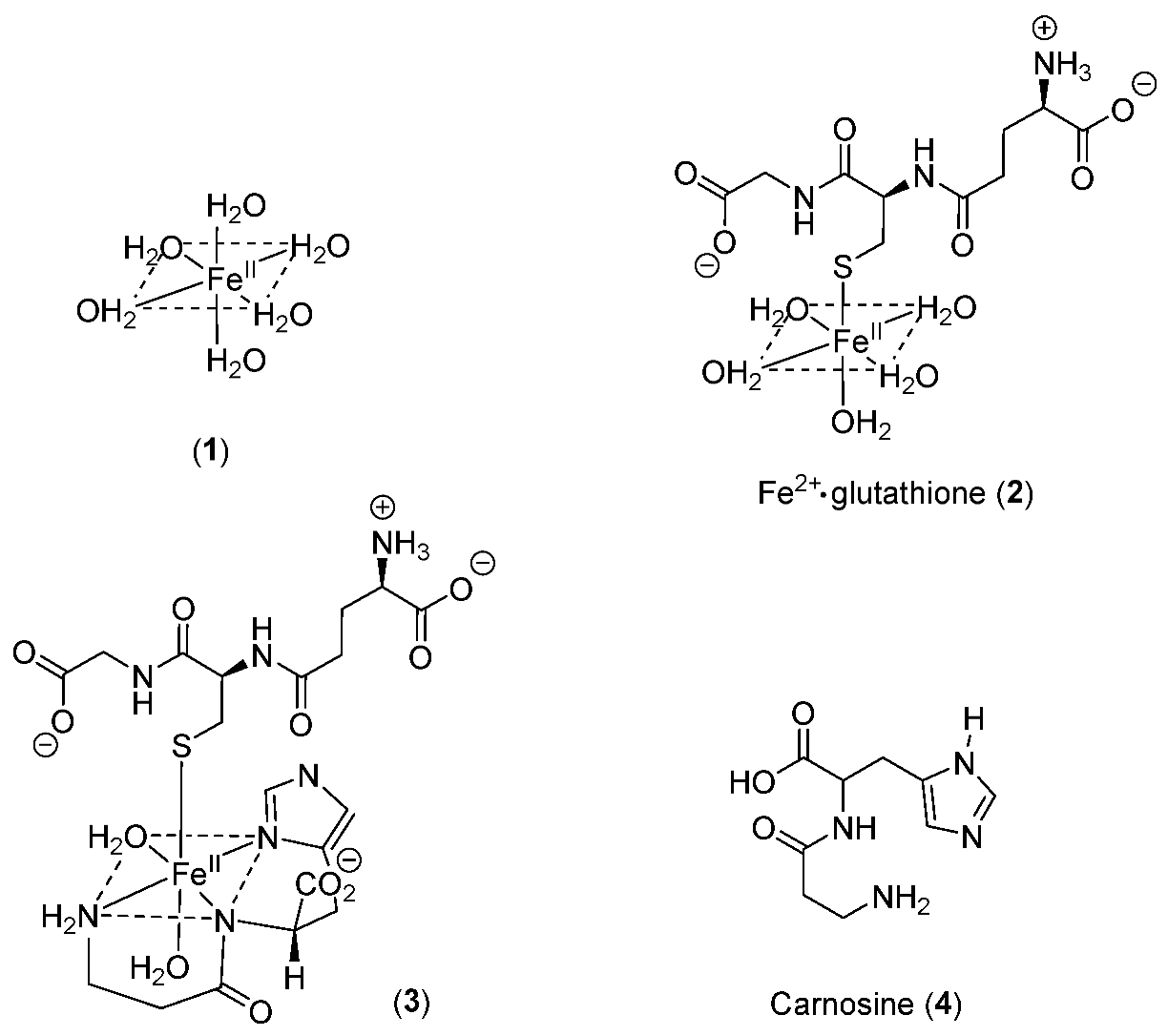
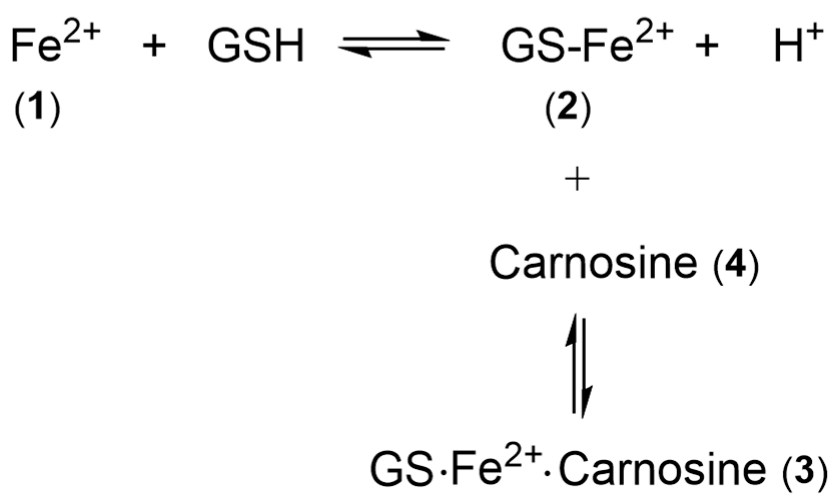
- Hider, R.C.; Kong, X.L. Glutathione: A key component of the cytoplasmic labile iron pool. Biometals 2011, 24, 1179–1187. [Google Scholar] [CrossRef]
- Kondo, T.; Dale, G.L.; Beutler, E. Thiol transport from human red blood cells. Methods Enzymol. 1995, 252, 72–82. [Google Scholar]
- Soboll, S.; Gründel, S.; Harris, J.; Kolb-Bachofen, V.; Ketterer, B.; Sies, H. The content of glutathione and glutathione S-transferases and the glutathione peroxidase activity in rat liver nuclei determined by a non-aqueous technique of cell fractionation. Biochem. J. 1995, 311, 889–894. [Google Scholar] [CrossRef]
- Hamed, M.Y.; Silver, J.; Wilson, M.T. Studies of the reactions of ferric iron with glutathione and some related thiols. Inorg. Chim. Acta 1983, 78, 1–11. [Google Scholar] [CrossRef]
- Fuhr, J.; Rabenstein, D. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. IX binding of cadmium, zinc, lead, and mercury by glutathione. J. Am. Chem. Soc. 1973, 95, 6944–6948. [Google Scholar] [CrossRef]
- Ba, L.A.; Doering, M.; Burkholz, T.; Jacob, C. Metal trafficking: From maintaining the metal homeostasis to future drug design. Metallomics 2009, 1, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.B.; Edsall, J.T. The association of divalent cations with glutathione. J. Am. Chem. Soc. 1959, 81, 4044–4047. [Google Scholar] [CrossRef]
- Baran, E.J. Metal complexes of carnosine. Biochemistry 2000, 65, 789–797. [Google Scholar] [PubMed]
- Boakye, A.A.; Zhang, D.; Guo, L.; Zheng, Y.; Hoetker, D.; Zhao, J.; Posa, D.K.; Ng, C.K.; Zheng, H.; Kumar, A.; et al. Carnosine supplementation enhances post ischemic hind limb revascularization. Front. Physiol. 2019, 10, 751. [Google Scholar] [CrossRef]
- Brown, C.E.; Antholine, W.E. Chelation chemistry of carnosine. Evidence that mixed complexes may occur in vivo. J. Chem. Phys. 1979, 83, 3314–3319. [Google Scholar] [CrossRef]
- Shaw, G.C.; Cope, J.J.; Li, L.; Corson, K.; Hersey, C.; Ackermann, G.E.; Gwynn, B.; Lambert, A.J.; Wingert, R.A.; Traver, D.; et al. Mitoferrin is essential for erythroid iron assimilation. Nature 2006, 440, 96–100. [Google Scholar] [CrossRef]
- Paradkar, P.N.; Zumbrennen, K.B.; Paw, B.H.; Ward, D.M.; Kaplan, J. Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol. Cell Biol. 2009, 29, 1007–1016. [Google Scholar] [CrossRef]
- Atkinson, A.; Winge, D.R. Metal acquisition of availability in the mitochondria. Chem. Rev. 2009, 109, 4708–4721. [Google Scholar] [CrossRef]
- Chen, Z.; Lash, L.H. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J. Pharmacol. Exp. Ther. 1998, 285, 608–618. [Google Scholar]
- Lash, L.H. Mitochondrial glutathione transport: Physiological, pathological and toxicological implications. Chem. Biol. Interact. 2006, 163, 54–67. [Google Scholar] [CrossRef]
- Bowman, E.J.; Ikuma, H.; Stein, H.J. Citric acid cycle activity in mitochondria isolated from mung bean hypocotyls. Plant Physiol. 1976, 58, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, R.A.; Hiltunen, J.K.; Hassinen, I.E. Mitochondrial membrane potential, transmembrane difference in the NAD+ redox potential and the equilibrium of the glutamate-aspartate translocase in the isolated perfused rat heart. Biochim. Biophys. Acta 1982, 681, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.M.; Jones, D.P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta 2008, 1780, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Lillig, C.H.; Berndt, C.; Holmgren, A. Glutaredoxin systems. Biochim. Biophys. Acta 2008, 1780, 1304–1317. [Google Scholar] [CrossRef]
- Wells, W.W.; Yang, Y.; Deits, T.L.; Gran, Z.R. Thioltransferases. Adv. Enzymol. Relat. Areas Mol. Biol. 1993, 66, 149–201. [Google Scholar]
- Mesecke, N.; Mittler, S.; Eckers, E.; Herrmann, J.M.; Depante, M. Two novel monothiol glutaredoxins from Saccharomyces cerevisiae provide further insight into iron-sulfur cluster binding, oligomerization, and enzymatic activity of glutaredoxins. Biochemistry 2008, 47, 1452–1463. [Google Scholar] [CrossRef]
- Ehrensberger, K.M.; Bird, A.J. Hammering out details: Regulating metal levels in eukaryotes. Trends Biochem. Sci. 2011, 36, 524–531. [Google Scholar] [CrossRef]
- Lill, R.; Hoffmann, B.; Molik, S.; Pierik, A.J.; Rielzschel, N.; Stchling, O.; Uzarska, M.A.; Webert, H.; Wilbrecht, C.; Muhlenhoff, U. The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim. Biophys. Acta 2012, 1823, 1491–1508. [Google Scholar] [CrossRef]
- Lill, R. Function and biogenesis of iron-sulphur proteins. Nature 2009, 460, 831–838. [Google Scholar] [CrossRef]
- Sipos, K.; Lange, H.; Fekete, Z.; Uilmann, P.; Lill, R.; Kispal, G. Maturation of cytosolic iron-sulfur proteins requires glutathione. J. Biol. Chem. 2002, 277, 26944–26949. [Google Scholar] [CrossRef]
- Sharma, A.K.; Pallesen, L.J.; Spang, R.J.; Walden, W.E. Cytosolic iron-sulfur cluster assembly (CIA) system: Factors, Mechanism, and relevance to cellular iorn regulation. J. Biol. Chem. 2010, 285, 26745–26751. [Google Scholar] [CrossRef] [PubMed]
- Mühlenhoff, U.; Molik, S.; Godoy, J.R.; Uzarska, M.A.; Richter, N.; Seubert, A.; Zhang, Y.; Stubbe, J.; Pierrel, F.; Herrers, E.; et al. Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab. 2010, 12, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Rouault, T.A. Human iron-sulfur cluster assembly, cellular iron homeostasis, and disease. Biochemistry 2010, 49, 4945–4956. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Li, J.; Chain, C.Y.; Pasquevich, G.A.; Pasquevich, A.F.; Cowan, J.A. Glutathione-complexes iron-sulfur clusters. Reaction intermediates and evidence for a template effect promoting assembly and stability. Chem. Commum. 2013, 49, 6313–6315. [Google Scholar] [CrossRef]
- Kürz, T.; Gustafsson, B.; Brunk, U.T. Intralysosomal iron chelation protects against oxidative stress-induced cellular damage. FEBS J. 2006, 273, 3106–3117. [Google Scholar] [CrossRef]
- Babitt, J.L.; Huang, F.W.; Xia, Y.; Sidis, Y.; Andrews, N.C.; Lin, H.Y. Modulation of bone morphogenic protein signaling in vivo regulates systemic iron balance. J. Clin. Investig. 2007, 117, 1933–1939. [Google Scholar] [CrossRef]
- Yu, P.B.; Hong, C.C.; Sachidanandan, C.; Babitt, J.L.; Deng, D.Y.; Hoyng, S.A.; Lin, H.Y.; Bloch, K.D.; Peterson, R.T. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol. 2008, 4, 33–41. [Google Scholar] [CrossRef]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martinez-Pastor, M.T. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef]
- Macara, I.G. Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 2001, 65, 570–594. [Google Scholar] [CrossRef]
- Yasuhura, N.; Takeda, E.; Inoue, H.; Kotera, I.; Yoneda, Y. Importin alpha/beta-mediated nuclear protein import is regulated in a cell cycle-dependent manner. Exp. Cell Res. 2004, 297, 285–293. [Google Scholar] [CrossRef]
- O’Keeffe, R.; Latunde-Dada, G.O.; Chen, Y.-L.; Kong, X.L.; Cilibrizzi, A.; Hider, R.C. Glutathione and the intracellular labile heme pool. Biometals 2021, 34, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Abbate, V.; Hider, R.C. Iron-sensitive fluorescent probes: Monitoring intracellular iron pools. Metallomics 2015, 7, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Cilibrizzi, A.; Abbate, V.; Chen, Y.-L.; Ma, Y.; Zhou, T.; Hider, R.C. Hydroxypyridinone journey into metal chelation. Chem. Rev. 2018, 118, 7657–7701. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Analysing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics 2015, 7, 202–211. [Google Scholar] [CrossRef]
- Zander, M. Fluorimetrie; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- Alhawsah, B.; Yan, B.; Aydin, Z.; Niu, X.; Guo, M. Highly selective fluorescent probe with an ideal pH profile for the rapid and unambiguous determination of subcellular labile iron (III) pools in human cells. Anal. Lett. 2022, 55, 1954–1970. [Google Scholar] [CrossRef]
- Tenopoulou, M.; Kurz, T.; Doulias, P.T.; Galaris, D.; Brunk, U.T. Does the calcein-AM method assay the total cellular ‘labile iron pool’ or only a fraction of it? Biochem. J. 2007, 403, 261–266. [Google Scholar] [CrossRef]
- Hirayama, T.; Okuda, K.; Nagasawa, H. A highly selective turn-on fluorescent probe for iron(II) to visualize labile iron in living cells. Chem. Sci. 2013, 4, 1250–1256. [Google Scholar] [CrossRef]
- Hirayama, T.; Tsuboi, H.; Niwa, M.; Miki, A.; Kadota, S.; Ikeshita, Y.; Okua, K.; Nagasawa, H. A universal fluorogenic switch for Fe(II) ion based on N-oxide chemistry permits the visualization of intracellular redox equilibrium shift towards labile iron in hypoxic tumor cells. Chem. Sci. 2017, 8, 4858–4866. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Hirayama, T.; Nagasawa, H. Chemical tools for detecting Fe ions. J. Clin. Biochem. Nutr. 2017, 60, 39–48. [Google Scholar] [CrossRef]
- Cabantchik, I.; Glickstein, H.; Milgram, P.; Breuer, W. A fluorescence assay for assessing chelation of intracellular iron in a membrane model system and in mammalian cells. Anal. Biochem. 1996, 233, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Glickstein, H.; El, R.B.; Shvartsman, M.; Cabantchik, I. Intracellular labile iron pools as direct targets of iron chelators: A fluorescence study of chelator action in living cells. Blood 2005, 106, 3242–3250. [Google Scholar] [CrossRef] [PubMed]
- Epsztejn, S.; Kakhlon, O.; Glickstein, H.; Breuer, W.; Cabantchik, Z.I. Fluorescence analysis of the labile iron pool of mammalian cells. Anal. Biochem. 1997, 248, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Serratrice, G.; Béguin, C.; Aman, E.S.; Pierre, J.L.; Fontecave, M.; Laulhère, J.P. Calcein as a fluorescent probe for ferric iron. Application to iron nutrition in plant cells. J. Biol. Chem. 1999, 274, 13375–13383. [Google Scholar] [CrossRef] [PubMed]
- Zanninelli, G.; Glickstein, H.; Breuer, W.; Milgram, P.; Brissot, P.; Hider, R.C.; Konijn, A.M.; Lipman, J.; Shanzer, A.; Cabantchik, Z.I. Chelation and Mobilization of Cellular Iron by Different Classes of Chelators. Mol. Pharmacol. 1997, 51, 842–852. [Google Scholar] [CrossRef]
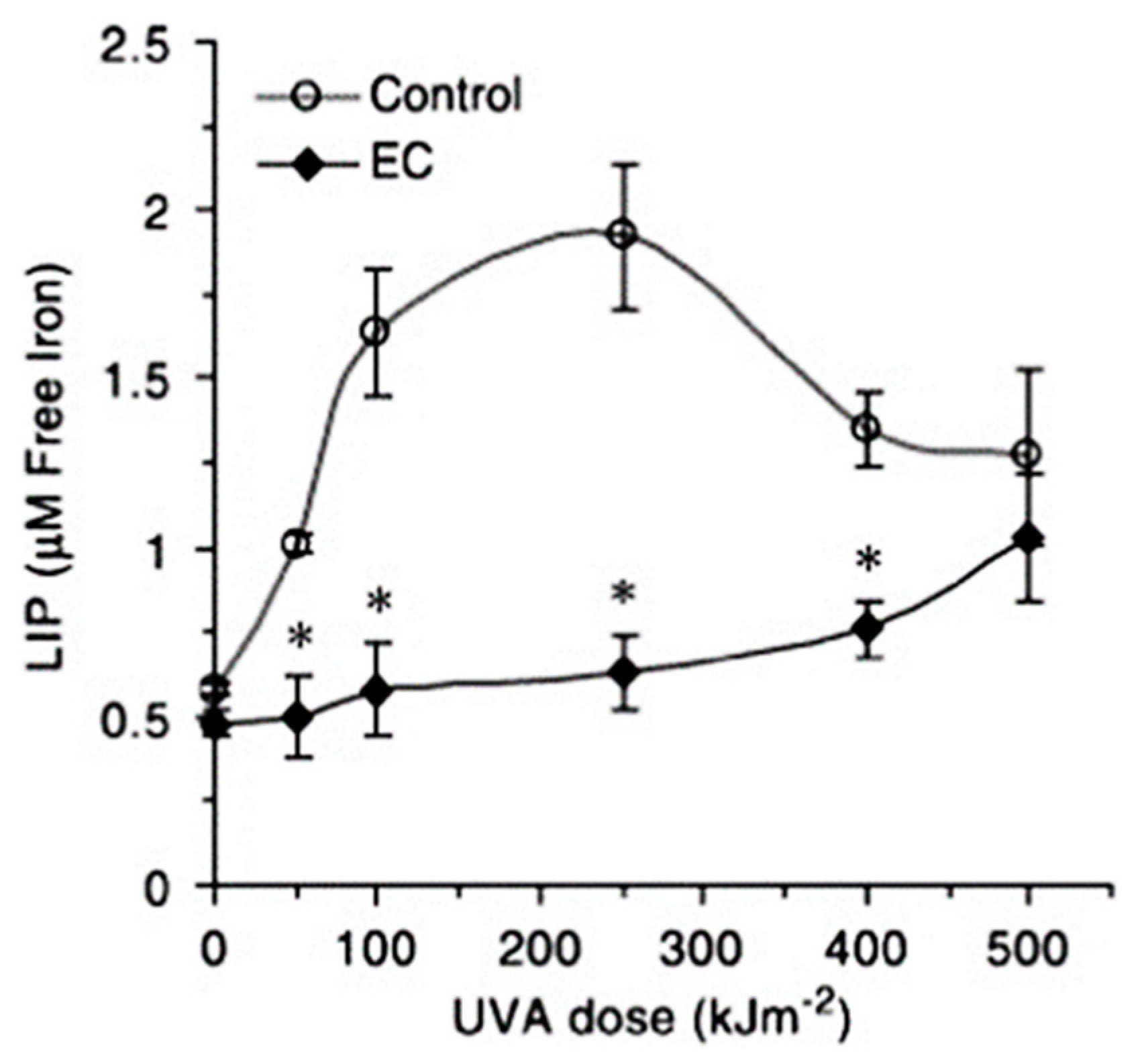
- Basu-Modak, S.; Ali, D.; Gordon, M.; Polte, T.; Yiakouvaki, A.; Pourzand, C.; Rice-Evans, C.; Tyrrell, R.M. Suppression of UVA-mediated release of labile iron by epicatechin—A link to lysosomal protection. Free Radical Biol. Med. 2006, 41, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Fisher, N.C.; Kasthuri, R.; Hand, C.C. Parasite maturation and host serum iron influence the labile iron pool of erythrocyte stage Plasmodium falciparum. Br. J. Haematol. 2013, 161, 262–269. [Google Scholar] [CrossRef]
- Petrat, F.; Rauen, U.; de Groot, H. Determination of the chelatable iron pool of isolated rat hepatocytes by digital fluorescence microscopy using the fluorescent probe, phen green SK. Hepatology 1999, 29, 1171–1179. [Google Scholar] [CrossRef]
- Petrat, F.; de Groot, H.; Rauen, U. Determination of the chelatable iron pool of single intact cells by laser scanning microscopy. Arch. Biochem. Biophys. 2000, 376, 74–81. [Google Scholar] [CrossRef]
- Ma, Y.M.; de Groot, H.; Liu, Z.; Hider, R.C.; Petrat, F. Chelation and determination of labile iron in primary hepatocytes by pyridinone fluorescent probes. Biochem. J. 2006, 395, 49–55. [Google Scholar] [CrossRef]
- Hider, R.C.; Ma, Y.; Reelfs, O.; Cilibrizzi, A.; Pourzand, C. A Novel Fluorescent Probe with High Selectivity and Sensitivity for Quantitation and Imaging of Cytosolic Labile Iron Pool. Abstract in European Iron Club Conference. 2022, p. 0101. Available online: https://researchportal.bath.ac.uk/en/publications/a-novel-fluorescent-probe-with-high-selectivity-and-sensitivity-f (accessed on 16 July 2022).
- Aron, A.T.; Loehr, M.O.; Bogena, J.; Chang, C.J. An endoperoxide reactivity-based FRET probe for ratiometric fluorescence imaging of labile iron pools in living cells. J. Am. Chem. Soc. 2016, 138, 14338–14346. [Google Scholar] [CrossRef]
- Spangler, B.; Morgan, C.W.; Fontaine, S.D.; Vander Wal, M.N.; Chang, C.J.; Wells, J.A.; Renslo, A.R. A reactivity-based probe of the intracellular labile ferrous iron pool. Nat. Chem. Biol. 2016, 12, 680–685. [Google Scholar] [CrossRef]
- Monfort, B.; Want, K.; Gervason, S.; D’Autréaux, B. Recent advances in the elucidation of Frataxin biochemical function open novel perspectives for the Treatment of Friedreich’s Ataxia. Front. Neurosci. 2022, 16, 838335. [Google Scholar] [CrossRef]
- Petrat, F.; Weisheit, D.; Lensen, M.; de Groot, H.; Sustmann, R.; Rauen, U. Selective determination of mitochondrial chelatable iron in viable cells with a new fluorescent sensor. Biochem. J. 2002, 362, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Shvartsman, M.; Fibach, E.; Cabantchik, Z.I. Transferrin-iron routing to the cytosol and mitochondria as studied by live and real-time fluorescence. Biochem. J. 2010, 429, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Cabantchik, Z.I. Labile iron in cells and body fluids: Physiology, pathology, and pharmacology. Front. Pharmacol. 2014, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Cilibrizzi, A.; Pourzand, C.; Abbate, V.; Reelfs, O.; Versari, L.; Floresta, G.; Hider, R. The synthesis and properties of mitochondrial targeted iron chelators. Biometals 2023, 36, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Reelfs, O.; Abbate, V.; Cilibrizzi, A.; Pook, M.A.; Hider, R.C.; Pourzand, C. The role of mitochondrial labile iron in Friedreich’s ataxia skin fibroblasts sensitivity to ultraviolet A. Metallomics 2019, 11, 656–665. [Google Scholar] [CrossRef]
- Rouault, T.A. Mitochondrial iron overload: Causes and consequences. Curr. Opin. Genet. Dev. 2016, 38, 31–37. [Google Scholar] [CrossRef]
- Hirayama, T.; Kadota, S.; Niwa, M.; Nagasawa, H. A mitochondria-targeted fluorescent probe for selective detection of mitochondrial labile Fe(II). Metallomics 2018, 10, 794–801. [Google Scholar] [CrossRef]
- Kholmukhamedov, A.; Li, L.; Lindsey, C.C.; Hu, J.; Nieminen, A.-L.; Takemoto, K.; Beeson, G.C.; Beneker, C.M.; McInnes, C.; Beeson, C.C.; et al. A new fluorescent sensor mitoferrofluor indicates the presence of chelatable iron in polarized and depolarized mitochondria. J. Biol. Chem. 2022, 298, 102336. [Google Scholar] [CrossRef] [PubMed]
- Kurz, T.; Terman, A.; Gustafsson, B.; Brunk, U.T. Lysosomes and oxidative stress in aging and apoptosis. Biochim. Biophys. Acta 2008, 1780, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Kidane, T.Z.; Sauble, E.; Linder, M.C. Release of iron from ferritin requires lysosomal activity. Am. J. Physiol. Cell Physiol. 2006, 291, C445–C455. [Google Scholar] [CrossRef] [PubMed]
- .Kurz, T.; Terman, A.; Gustafsson, B.; Brunk, U.T. Lysosomes in iron metabolism, ageing and apoptosis. Histochem. Cell. Biol. 2008, 129, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Petrat, F.; de Groot, H.; Rauen, U. Subcellular distribution of chelatable iron: A laser scanning microscopic study in isolated hepatocytes and liver endothelial cells. Biochem. J. 2001, 356, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Fakih, S.; Podinovskaia, M.; Kong, X.; Collins, H.L.; Schaible, U.E.; Hider, R.C. Targeting the Lysosome: Fluorescent Iron(III) Chelators To Selectively Monitor Endosomal/Lysosomal Labile Iron Pools. J. Med. Chem. 2008, 51, 4539–4552. [Google Scholar] [CrossRef]
- Fakih, S.; Podinovskaia, M.; Kong, X.; Schaible, U.E.; Collins, H.L.; Hider, R.C. Monitoring intracellular labile iron pools: A novel fluorescent iron(III) sensor as a potential non-invasive diagnosis tool. J. Pharm. Sci. 2009, 98, 2212–2226. [Google Scholar] [CrossRef]
- Hirayama, T.; Miki, A.; Nagasawa, H. Organelle-specific analysis of labile Fe(II) during ferroptosis by using a cocktail of various colour organelle-targeted fluorescent probes. Metallomics 2019, 11, 111–117. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, P.; Guo, W. Fluorescent probes for iron, heme, and related enzymes. Coord. Chem. Rev. 2021, 429, 213645. [Google Scholar] [CrossRef]
- Lu, M.; Wang, Y.; Li, Y.; Li, Z.; Xu, S.; Yao, C. A colorimetric and fluorescent chemosensor of Fe3+ based on an asymmetrical squarylium dye. J. Appl. Spectrosc. 2018, 85, 341–348. [Google Scholar] [CrossRef]
- Wu, D.; Chen, L.; Lee, W.; Ko, G.; Yin, J.; Yoon, J. Recent progress in the development of organic dye based near-infrared fluorescence probes for metal ions. Coord. Chem. Rev. 2018, 354, 74–97. [Google Scholar] [CrossRef]
- Zheng, X.; Cheng, W.; Ji, C.; Zhang, J.; Yin, M. Detection of metal ions in biological systems: A review. Rev. Anal. Chem. 2020, 39, 231–246. [Google Scholar] [CrossRef]
- Hirayama, T. Fluorescent probes for the detection of catalytic Fe(II) ion. Free Rad. Biol. Med. 2019, 133, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cong, T.; Liang, Q.; Li, Z.; Xu, S. Dual colorimetric and fluorescent chemosensor of Fe3+ and Cu2+ based on 2,5-bis[(4-carboxylic-piperidylamino)thiophenyl] -croconine. Tetrahedron 2015, 71, 5478–5483. [Google Scholar] [CrossRef]
- Qu, X.; Liu, Q.; Ji, X.; Chen, H.; Zhou, Z.; Shen, Z. Enhancing the Stokes’ shift of BODIPY dyes via through-bond energy transfer and its application for Fe3+-detection in live cell imaging. Chem. Commun. 2012, 48, 4600–4602. [Google Scholar] [CrossRef]
- Shen, B.-X.; Qian, Y. A novel triphenylamine-BODIPY dendron: Click synthesis, near-infrared emission and a multi-channel chemodosimeter for Hg2+ and Fe3+. J. Mater. Chem. B 2016, 4, 7549–7559. [Google Scholar] [CrossRef] [PubMed]
- Vijay, N.; Wu, S.P.; Velmathi, S. Turn on fluorescent chemosensor containing rhodamine B fluorophore for selective sensing and in vivo fluorescent imaging of Fe3+ ions in HeLa cell line and zebrafish. J. Photochem. Photobiol. A Chem. 2019, 384, 112060. [Google Scholar] [CrossRef]
- Dong, B.; Song, W.; Lu, Y.; Tian, M.; Kong, X.; Mehmood, A.H.; Lin, W. Live cell-specific fluorescent probe for the detection of labile Fe(II) and the evaluation of esterase activity in live animals. Sens. Actuators B Chem. 2020, 305, 127470. [Google Scholar] [CrossRef]
- Aron, A.T.; Heffern, M.C.; Lonergan, Z.R.; Vander Wal, M.N.; Blank, B.R.; Spangler, B.; Zhangd, Y.; Park, H.M.; Stahl, A.; Renslo, A.R.; et al. In vivo bioluminescence imaging of labile iron accumulation in a murine model of Acinetobacter baumannii infection. Proc. Natl. Acad. Sci. USA 2017, 114, 12669–12674. [Google Scholar] [CrossRef]
- Yao, Q.F.; Lü, B.Z.; Ji, C.D.; Cai, Y.; Yin, M.Z. Supramolecular host-guest system as ratiometric Fe3+ ion sensor based on water-soluble pillar[5]arene. ACS Appl. Mater. Interfaces 2017, 9, 36320–36326. [Google Scholar] [CrossRef]
- Wang, M.; Guo, L.; Cao, D. Porous organic polymer nanotubes as luminescent probe for highly selective and sensitive detection of Fe3+. Sci China Chem. 2017, 60, 1090–1097. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Liu, M.L.; Liu, Y. Selective detection of Fe3+ ions based on fluorescence MXene quantum dots via a mechanism integrating electron transfer and inner filter effect. Nanoscale 2020, 12, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, D.; Huang, Y.; Zhang, J.; Wang, Z.; Yang, D.; Qian, G. Photo-induced electron transfer in a metal–organic framework: A new approach towards a highly sensitive luminescent probe for Fe3+. Chem. Commun. 2019, 55, 11231. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.R.; Feng, S.M.; Gong, S.Y.; Xia, Q.F.; Feng, G.Q. In vivo imaging of Fe2+ using an easily obtained probe with a large stokes shift and bright strong lipid droplet-targetable near-infrared fluorescence. Sens. Actuators B Chem. 2020, 309, 127796. [Google Scholar] [CrossRef]
- Zhu, M.; Shi, C.; Xu, X.; Guo, Z.; Zhu, W. Near-infrared cyanine-based sensor for Fe3+ with high sensitivity: Its intracellular imaging application in colorectal cancer cells. RSC Adv. 2016, 6, 100759–100764. [Google Scholar] [CrossRef]
- Li, S.; Zhang, D.; Xie, X.; Ma, S.; Liu, Y.; Xu, Z.; Gao, Y.; Ye, Y. A novel solvent-dependently bifunctional NIR absorptive and fluorescent ratiometric probe for detecting Fe3+/Cu2+ and its application in bioimaging. Sens. Actuators B Chem. 2016, 224, 661–667. [Google Scholar] [CrossRef]
- Kang, H.; Han, M.; Xue, J.; Baek, Y.; Chang, J.O.; Hu, S.; Nam, H.Y.; Jo, M.J.; El Fakhri, G.; Hutchens, M.P.; et al. Renal clearable nanochelators for iron overload therapy. Nat. Commun. 2019, 10, 5134. [Google Scholar] [CrossRef]
 , sulfur;
, sulfur;  , iron;
, iron;  , oxygen). This figure is adapted from Figure 2 in Kühn [9].
, oxygen). This figure is adapted from Figure 2 in Kühn [9].
 , sulfur;
, sulfur;  , iron;
, iron;  , oxygen). This figure is adapted from Figure 2 in Kühn [9].
, oxygen). This figure is adapted from Figure 2 in Kühn [9].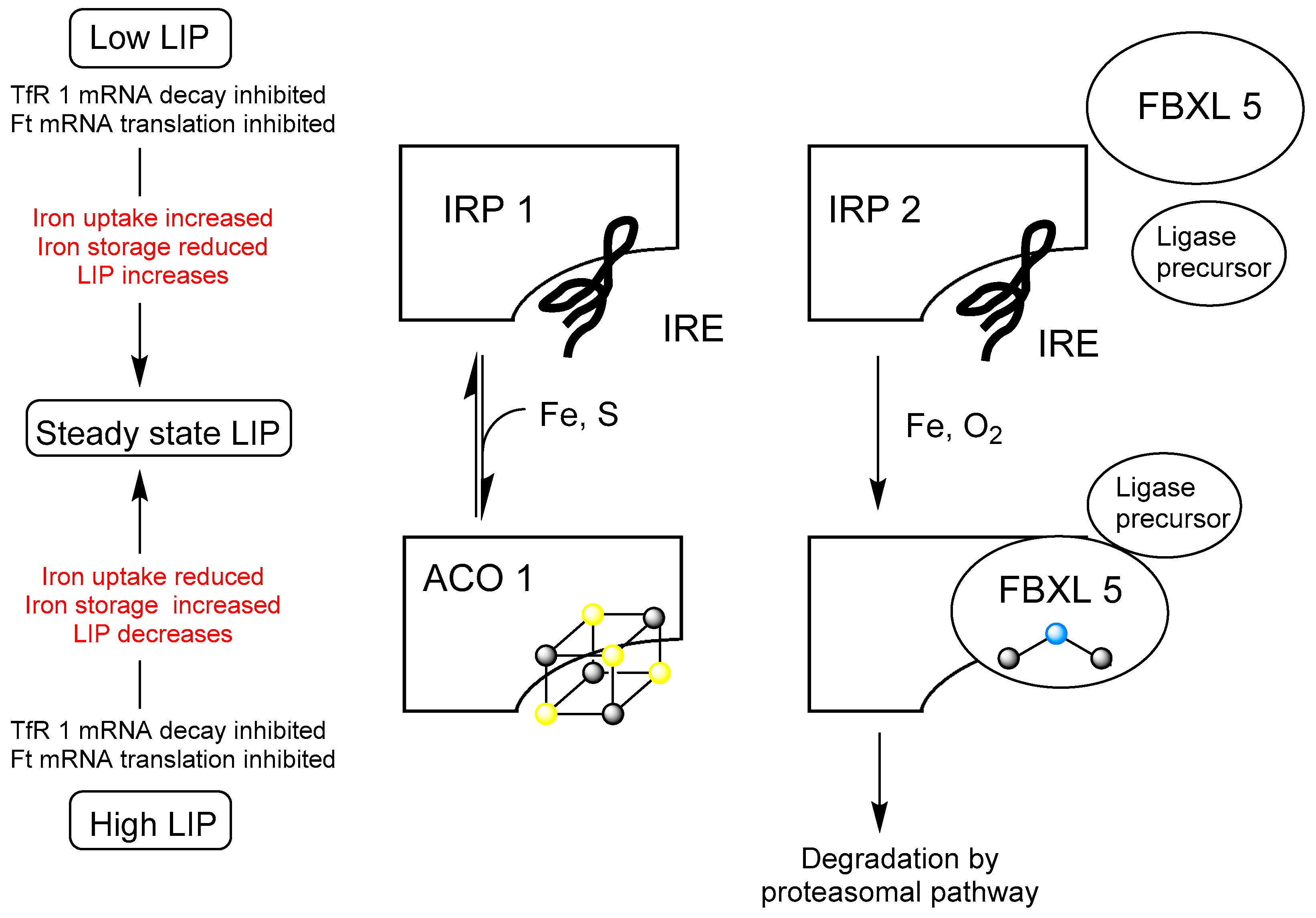




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hider, R.C.; Pourzand, C.; Ma, Y.; Cilibrizzi, A. Optical Imaging Opportunities to Inspect the Nature of Cytosolic Iron Pools. Molecules 2023, 28, 6467. https://doi.org/10.3390/molecules28186467
Hider RC, Pourzand C, Ma Y, Cilibrizzi A. Optical Imaging Opportunities to Inspect the Nature of Cytosolic Iron Pools. Molecules. 2023; 28(18):6467. https://doi.org/10.3390/molecules28186467
Chicago/Turabian StyleHider, Robert Charles, Charareh Pourzand, Yongmin Ma, and Agostino Cilibrizzi. 2023. "Optical Imaging Opportunities to Inspect the Nature of Cytosolic Iron Pools" Molecules 28, no. 18: 6467. https://doi.org/10.3390/molecules28186467




