Synthesis of Pyridin-1(2H)-ylacrylates and the Effects of Different Functional Groups on Their Fluorescence
Abstract
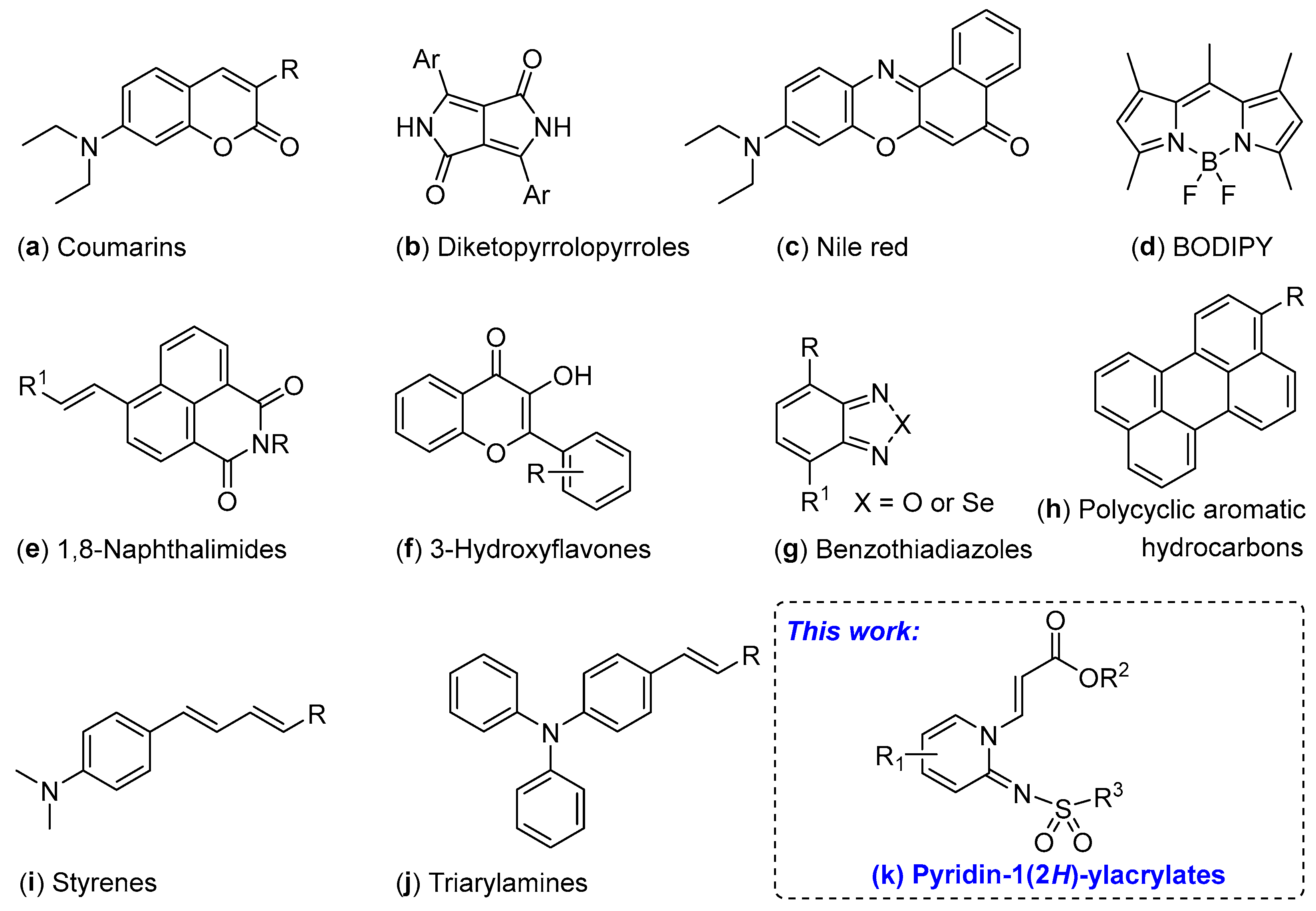
:1. Introduction
2. Results and Discussion
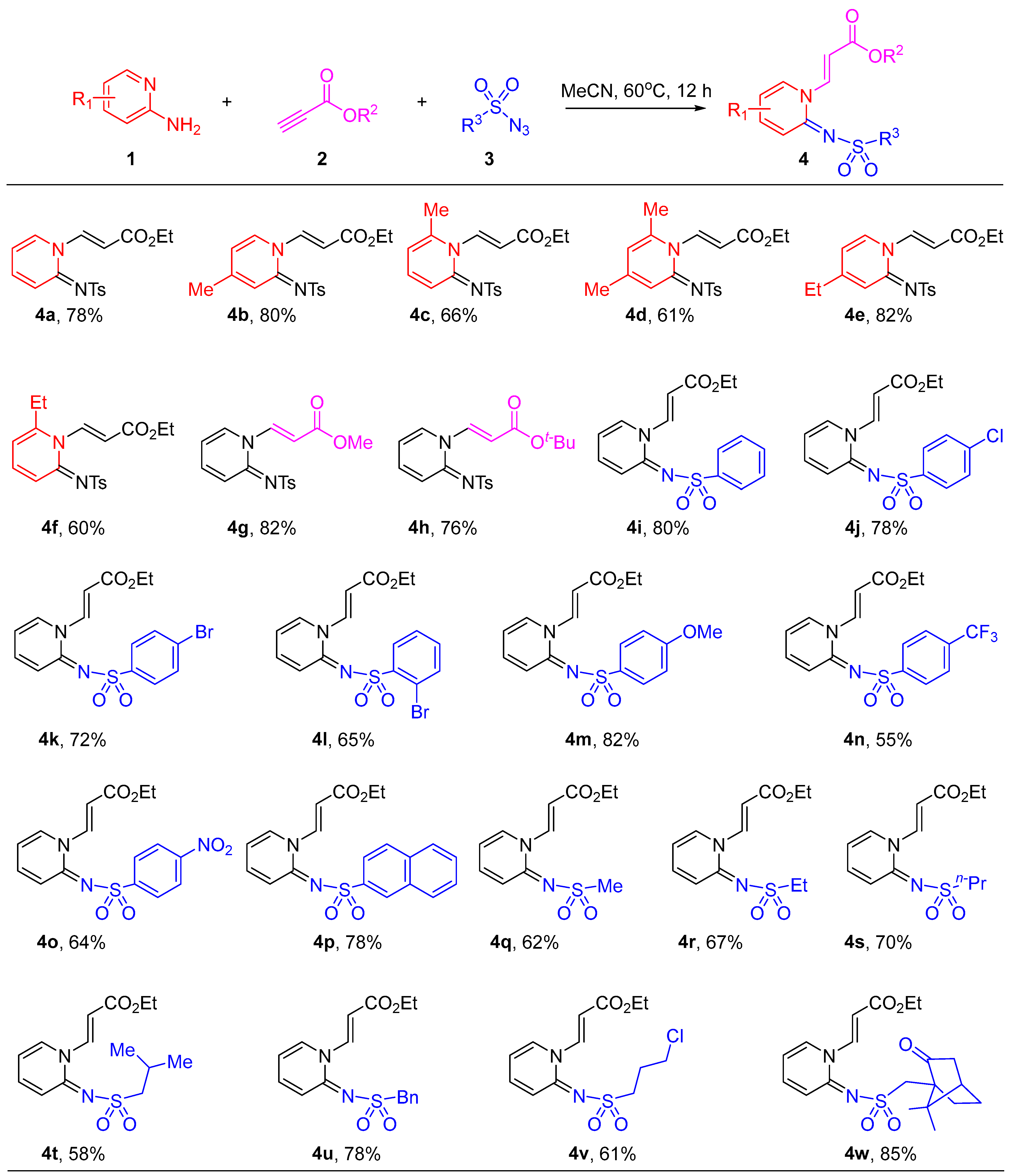
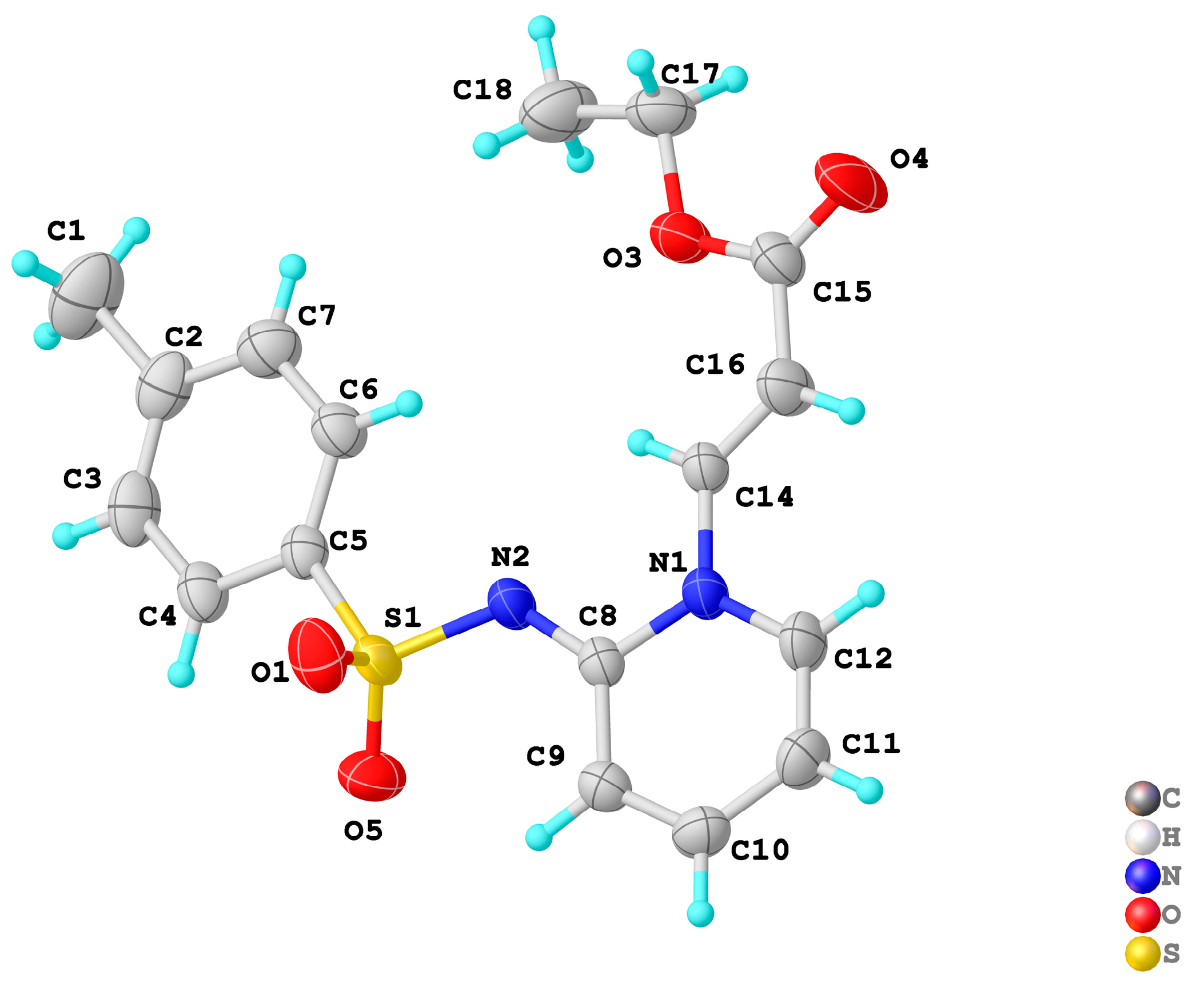
2.1. Synthesis of Pyridin-1(2H)-ylacrylate Derivatives
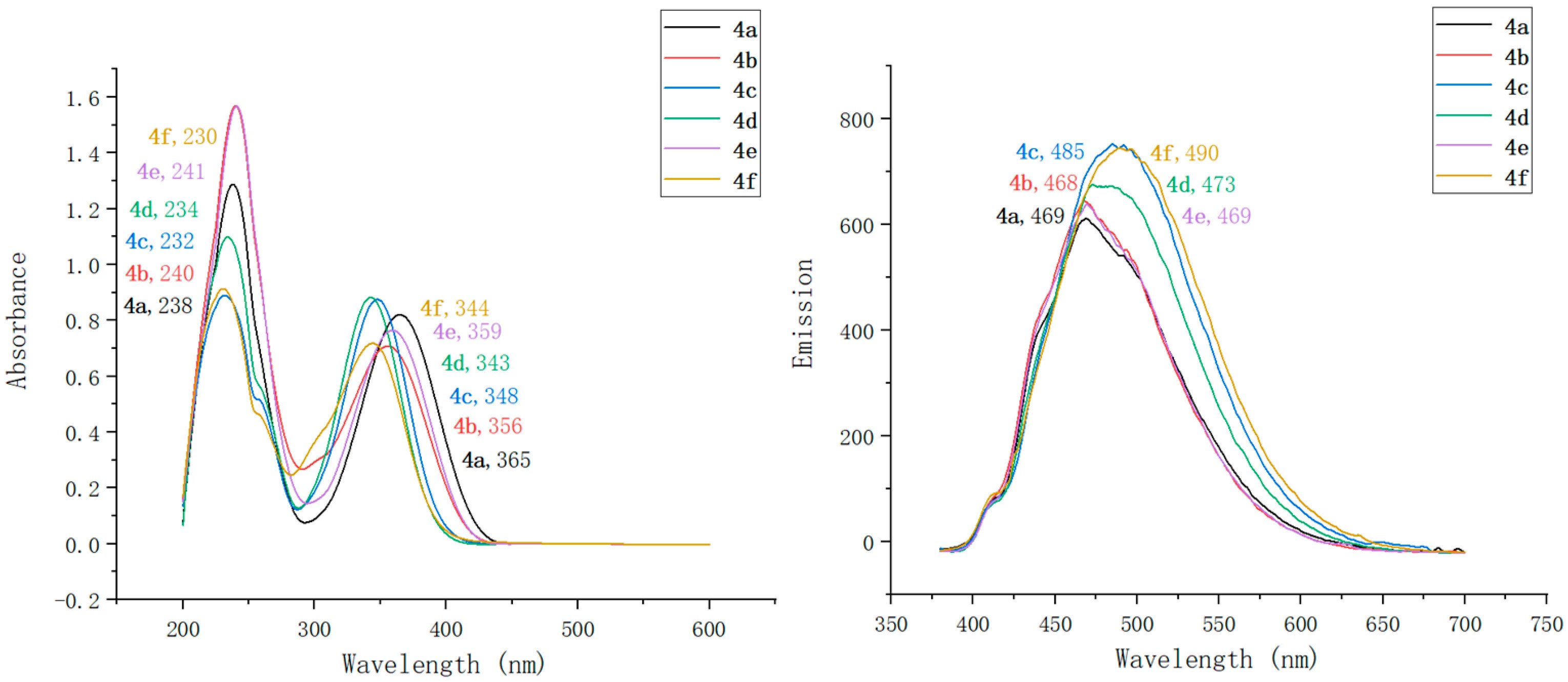
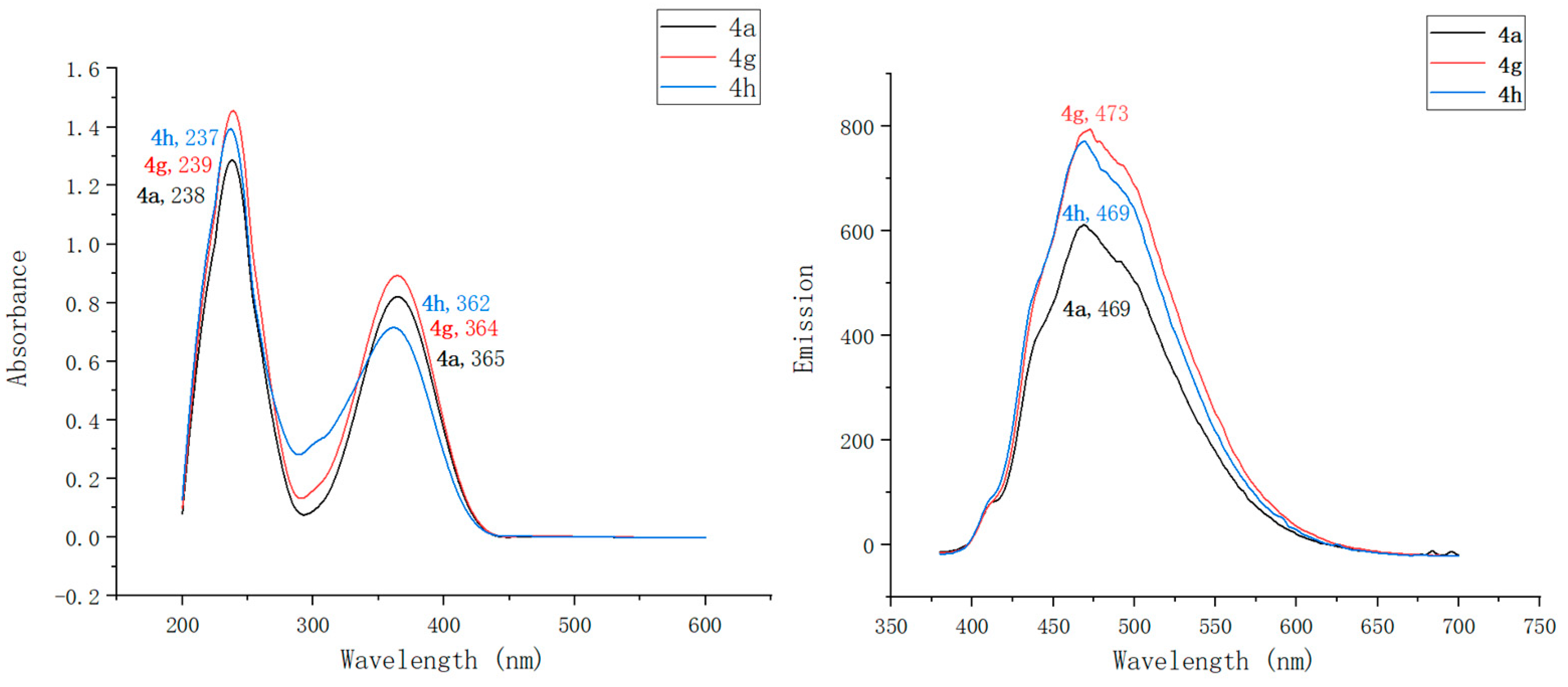
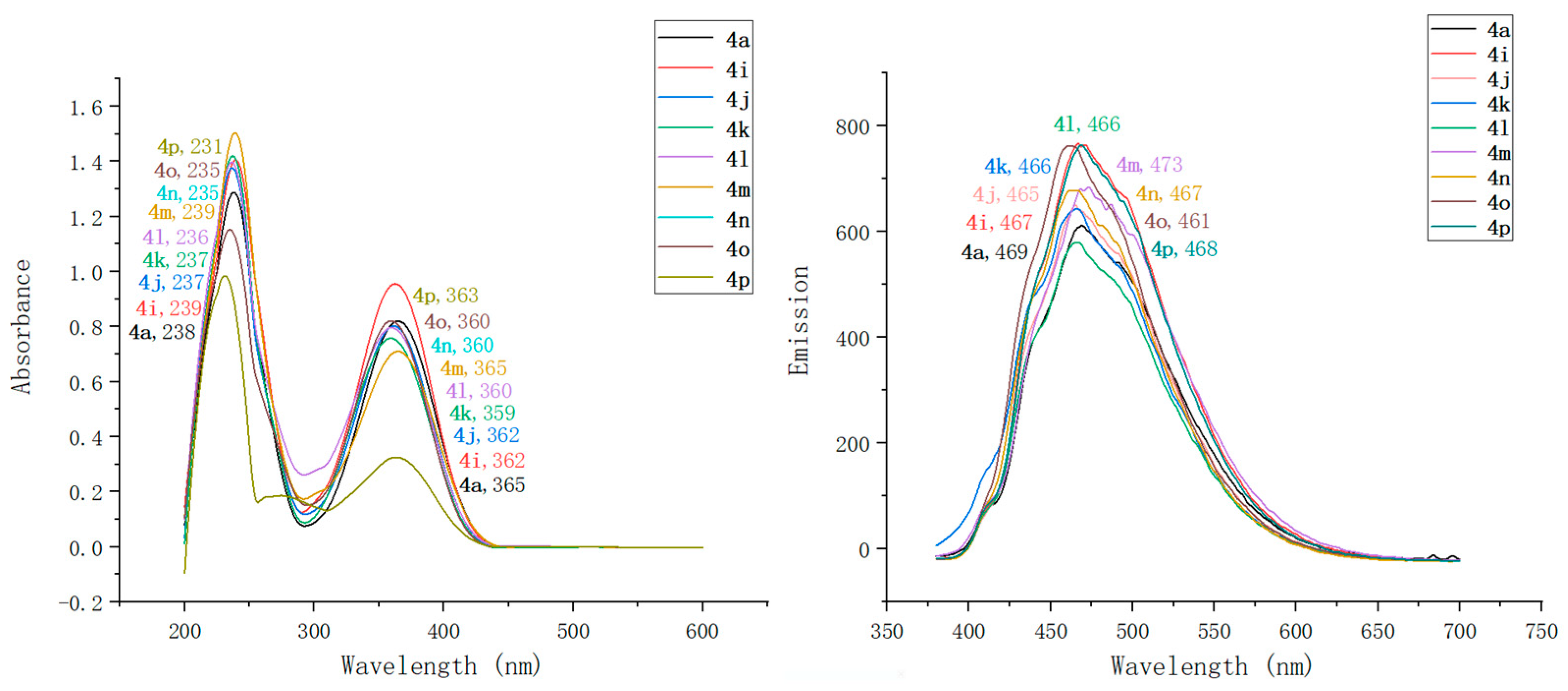
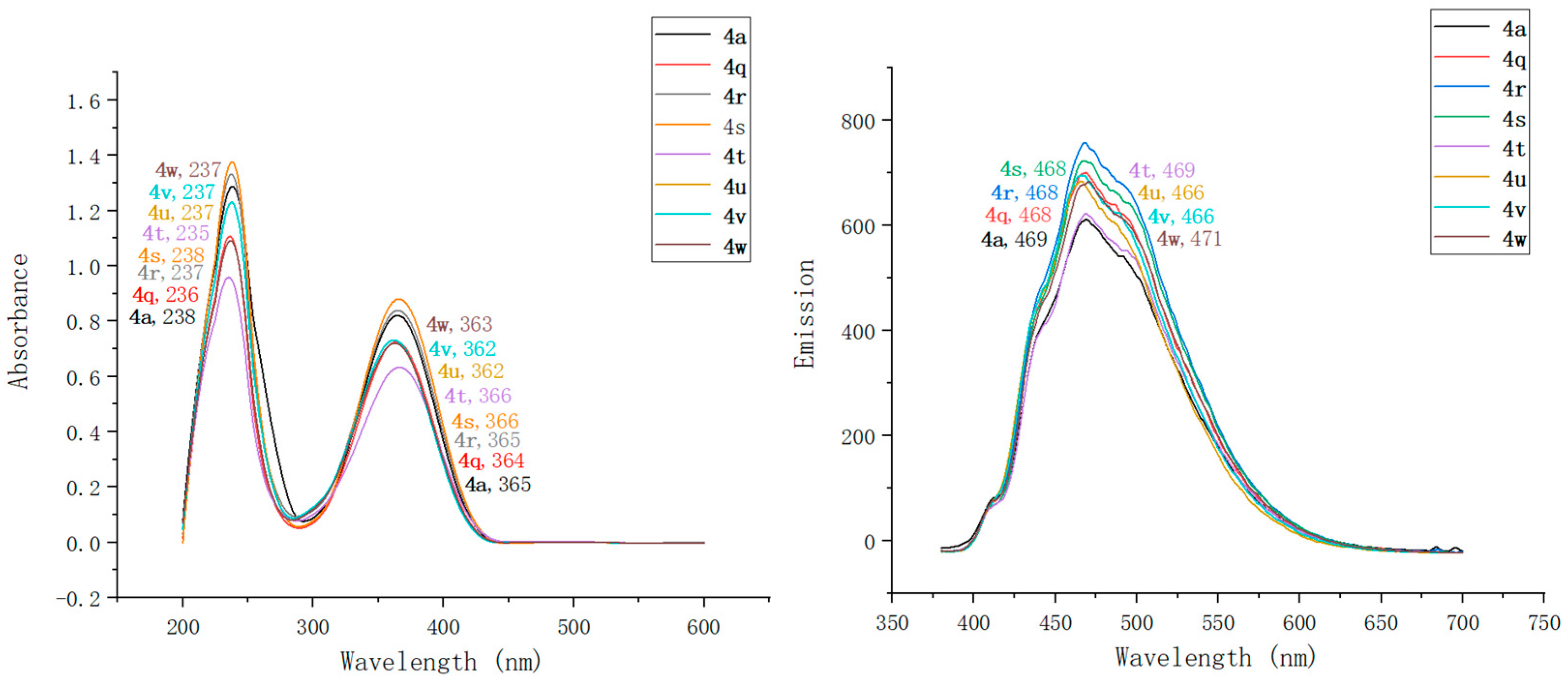
2.2. Optical Characterization of Pyridin-1(2H)-ylacrylate Derivatives
3. Materials and Methods
3.1. General Methods
3.2. General Procedure for the Synthesis of Pyridin-1(2H)-ylacrylates
3.3. UV–Vis and Fluorescence Measurements
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Miao, Y.; Yin, M. Recent progress on organic light-emitting diodes with phosphorescent ultrathin (<1nm) light-emitting layers. iScience 2022, 25, 103804. [Google Scholar] [PubMed]
- Sadhu, A.S.; Huang, Y.M.; Chen, L.Y.; Kuo, H.C.; Lin, C.C. Recent advances in colloidal quantum dots or perovskite quantum dots as a luminescent downshifting layer embedded on solar cells. Nanomaterials 2022, 12, 985. [Google Scholar] [CrossRef] [PubMed]
- Nimbalkar, M.; Yawalkar, M.; Mahajan, N.; Dhoble, S.J. Potential of luminescent materials in phototherapy. Photodiagnosis Photodyn. Ther. 2021, 33, 102082. [Google Scholar] [CrossRef] [PubMed]
- Mieog, J.S.D.; Achterberg, F.B.; Zlitni, A.; Hutteman, M.; Burggraaf, J.; Swijnenburg, R.J.; Gioux, S.; Vahrmeijer, A.L. Fundamentals and developments in fluorescence-guided cancer surgery. Nat. Rev. Clin. Oncol. 2022, 19, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.K.; Choi, A.W.; Law, W.H. Applications of luminescent inorganic and organometallic transition metal complexes as biomolecular and cellular probes. Dalton Trans. 2012, 41, 6021–6047. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Kim, J.; Lee, S.H.; Rho, W.Y.; Lee, J.H.; Jeong, D.H.; Jun, B.H. Luminescent nanomaterials (II). Adv. Exp. Med. Biol. 2021, 1309, 97–132. [Google Scholar]
- Tao, P.; Miao, Y.; Wang, H.; Xu, B.; Zhao, Q. High-performance organic electroluminescence: Design from organic light-emitting materials to devices. Chem. Rec. 2019, 19, 1531–1561. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, H.; Hu, W. Organic semiconductor single crystals for electronics and photonics. Adv. Mater. 2018, 30, 1801048. [Google Scholar] [CrossRef]
- Yuan, D.; Sharapov, V.; Liu, X.; Yu, L. Design of high-performance organic light-emitting transistors. ACS Omega 2020, 5, 68–74. [Google Scholar] [CrossRef]
- Wagh, S.B.; Maslivetc, V.A.; La Clair, J.J.; Kornienko, A. Lessons in organic fluorescent probe discovery. Chembiochem 2021, 22, 3109–3139. [Google Scholar] [CrossRef]
- Li, S.; Xiao, Y.; Chen, C.; Jia, L. Recent progress in organic small-molecule fluorescent probe detection of hydrogen peroxide. ACS Omega 2022, 7, 15267–15274. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, F.; Wang, Y.; Li, H.; Zhang, J.; Sun, Z.; Jiang, Y. Fluorescent organic small molecule probes for bioimaging and detection applications. Molecules 2022, 27, 8421. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, W.; Li, X.; Ma, H. Recent advances in fluorescent probes for lipid droplets. Chem. Commun. 2022, 58, 1495–1509. [Google Scholar] [CrossRef] [PubMed]
- Stennett, E.M.; Ciuba, M.A.; Levitus, M. Photophysical processes in single molecule organic fluorescent probes. Chem. Soc. Rev. 2014, 43, 1057–1075. [Google Scholar] [CrossRef]
- Wanderi, K.; Cui, Z. Organic fluorescent nanoprobes with NIR-IIb characteristics for deep learning. Exploration 2022, 2, 20210097. [Google Scholar] [CrossRef]
- Gao, P.; Pan, W.; Li, N.; Tang, B. Fluorescent probes for organelle-targeted bioactive species imaging. Chem. Sci. 2019, 10, 6035–6071. [Google Scholar] [CrossRef]
- Wang, K.-N.; Liu, L.-Y.; Mao, D.; Xu, S.; Tan, C.-P.; Cao, Q.; Mao, Z.-W.; Liu, B. A polarity-sensitive ratiometric fluorescence probe for monitoring changes in lipid droplets and nucleus during ferroptosis. Angew. Chem. Int. Ed. 2021, 60, 15095–15100. [Google Scholar] [CrossRef]
- Li, X.; Long, C.; Cui, Y.; Tao, F.; Yu, X.; Lin, W. Charge-dependent strategy enables a single fluorescent probe to study the interaction relationship between mitochondria and lipid droplets. ACS Sens. 2021, 6, 1595–1603. [Google Scholar] [CrossRef]
- Kaur, M.; Choi, D.H. Diketopyrrolopyrrole: Brilliant red pigment dye-based fluorescent probes and their applications. Chem. Soc. Rev. 2015, 44, 58–77. [Google Scholar] [CrossRef]
- Martinez, V.; Henary, M. Nile red and Nile blue: Applications and syntheses of structural analogues. Chem.-Eur. J. 2016, 22, 13764–13782. [Google Scholar] [CrossRef]
- Kolemen, S.; Akkaya, E.U. Reaction-based BODIPY probes for selective bio-imaging. Coord. Chem. Rev. 2018, 354, 121–134. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Zhu, T.; Du, W.; Yang, Z.; Cao, H.; Wang, S.; Tian, Y.; Zhang, Z.; Zhang, R.; et al. Revealing lipid droplets evolution at nanoscale under proteohormone stimulation by a BODIPY-hexylcarbazole derivative. Biosens. Bioelectron. 2021, 175, 112871. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, X.; Fang, Y.; Shi, W.; Li, X.; Chen, W.; Xian, M.; Ma, H. Reactive oxygen species-triggered off-on fluorescence donor for imaging hydrogen sulfide delivery in living cells. Chem. Sci. 2019, 10, 7690–7694. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, C.; Liu, W.; Qiao, Q.; Qi, H.; Zhou, W.; Xu, N.; Li, J.; Piao, H.; Tan, D.; et al. Stable super-resolution imaging of lipid droplet dynamics through a buffer strategy with a hydrogen-bond sensitive fluorogenic probe. Angew. Chem. Int. Ed. 2021, 60, 25104–25113. [Google Scholar] [CrossRef]
- Guo, L.; Tian, M.; Zhang, Z.; Lu, Q.; Liu, Z.; Niu, G.; Yu, X. Simultaneous two-color visualization of lipid droplets and endoplasmic reticulum and their interplay by single fluorescent probes in lambda mode. J. Am. Chem. Soc. 2021, 143, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.A.R.; Correa, J.R.; Andrade, L.P.d.; Assumpção, J.A.F.; Cintra, G.A.d.S.; Freitas-Junior, L.H.; Silva, W.A.d.; Oliveira, H.C.B.d.; Neto, B.A.D. From live cells to caenorhabditis elegans: Selective staining and quantification of lipid structures using a fluorescent hybrid benzothiadiazole derivative. ACS Omega 2018, 3, 3874–3881. [Google Scholar] [CrossRef]
- Medeiros, I.R.; Corrêa, J.R.; Barbosa, A.L.A.; Krüger, R.; Balaguez, R.A.; Lopes, T.O.; Oliveria, H.C.B.d.; Alves, D.; Neto, B.A.D. Fluorescent benzoselenadiazoles: Synthesis, characterization, and quantification of intracellular lipid droplets and multicellular model staining. J. Org. Chem. 2020, 85, 10561–10573. [Google Scholar] [CrossRef]
- Tatenaka, Y.; Kato, H.; Ishiyama, M.; Sasamoto, K.; Shiga, M.; Nishitoh, H.; Ueno, Y. Monitoring lipid droplet dynamics in living cells by using fluorescent probes. Biochemistry 2019, 58, 499–503. [Google Scholar] [CrossRef]
- Dong, B.; Song, W.; Lu, Y.; Sun, Y.; Lin, W. Revealing the viscosity changes in lipid droplets during ferroptosis by the real-time and in situ near-infrared imaging. ACS Sens. 2021, 6, 22–26. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, C.; Liang, X.; Liu, F.; Yan, X.; Liu, X.; Sun, P.; Zhang, H.; Wang, Y.; Lu, G. Stimulated emission depletion (STED) super-resolution imaging with an advanced organic fluorescent probe: Visualizing the cellular lipid droplets at the unprecedented nanoscale resolution. ACS Mater. Lett. 2021, 3, 516–524. [Google Scholar] [CrossRef]
- Fecková, M.; le Poul, P.; Robin-le Guen, F.; Roisnel, T.; Pytela, O.; Klikar, M.; Bureš, F.; Achelle, S. 2,4-Distyryl- and 2,4,6-tristyrylpyrimidines: Synthesis and photophysical properties. J. Org. Chem. 2018, 83, 11712–11726. [Google Scholar] [CrossRef]
- Hu, R.; Chen, B.; Wang, Z.; Qin, A.; Zhao, Z.; Lou, X.; Tang, B.Z. Intriguing “chameleon” fluorescent bioprobes for the visualization of lipid droplet-lysosome interplay. Biomaterials 2019, 203, 43–51. [Google Scholar] [CrossRef]
- Tan, P.; Zhuang, W.; Li, S.; Zhang, J.; Xu, H.; Yang, L.; Liao, Y.; Chen, M.; Wei, Q. A lipid droplet targeted fluorescent probe for high-efficiency image-guided photodynamic therapy of renal cell carcinoma. Chem. Commun. 2021, 57, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Li, Y.; Yang, W.; Zhuang, J.; Li, Y.; Li, N. Multifunctional pyrazoline based AIEgens: Real-time tracking and specific protein “fishing” of lipid droplets. Chem. Sci. 2019, 10, 9009–9016. [Google Scholar] [CrossRef]
- Taki, M.; Kajiwara, K.; Yamaguchi, E.; Sato, Y.; Yamaguchi, S. Fused thiophene-S,S-dioxide-based super-photostable fluorescent marker for lipid droplets. ACS Mater. Lett. 2021, 3, 42–49. [Google Scholar] [CrossRef]
- Liu, G.; Peng, G.; Dai, J.; Zhou, R.; Wang, C.; Yan, X.; Jia, X.; Liu, X.; Gao, Y.; Wang, L.; et al. STED Nanoscopy imaging of cellular lipid droplets employing a superior organic fluorescent probe. Anal. Chem. 2021, 93, 14784–14791. [Google Scholar] [CrossRef]
- Tydlitat, J.; Achelle, S.; Rodriguez-Lopez, J.; Pytela, O.; Mikysek, T.; Cabon, N.; Guen, F.R.-L.; Miklik, D.; Ruzickova, Z.; Bures, F. Photophysical properties of acid-responsive triphenylamine derivatives bearing pyridine fragments: Towards white light emission. Dye. Pigment. 2017, 146, 467–478. [Google Scholar] [CrossRef]
- Achelle, S.; Verbitskiy, E.V.; Fecková, M.; Bureš, F.; Barsella, A.; Robin-le Guen, F. V-Shaped Methylpyrimidinium Chromophores for Nonlinear Optics. ChemPlusChem 2021, 86, 758–762. [Google Scholar] [CrossRef]
- Li, J.; Tao, L.; Wang, Y.; Yao, Y.; Guo, Q. Heptazine-based π-conjugated materials for light-emitting. Front. Chem. 2021, 9, 717569. [Google Scholar] [CrossRef]
- Frath, D.; Massue, J.; Ulrich, G.; Ziessel, R. Luminescent materials: Locking π-conjugated and heterocyclic ligands with boron(III). Angew. Chem. Int. Ed. Engl. 2014, 53, 2290–2310. [Google Scholar] [CrossRef]
- Belyaev, A.; Chou, P.-T.; Koshevoy, L.O. Cationic organophosphorus chromophores: A diamond in the rough among ionic dyes. Chem.-Eur. J. 2021, 27, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Strianese, M.; Brenna, S.; Ardizzoia, G.A.; Guarnieri, D.; Lamberti, M.; D’Auria, I.; Pellecchia, C. Imidazo-pyridine-based zinc(II) complexes as fluorescent hydrogen sulfide probes. Dalton Trans. 2021, 50, 17075–17085. [Google Scholar] [CrossRef]
- Chaudhran, P.A.; Sharma, A. Progress in the development of imidazopyridine-based fluorescent probes for diverse applications. Crit. Rev. Anal. Chem. 2022, 1–18. [Google Scholar] [CrossRef]
- Bashmakova, N.V.; Shaydyuk, Y.O.; Dmytruk, A.M.; Świergosz, T.; Kachkovsky, O.D.; Belfield, K.D.; Bondar, M.V.; Kasprzyk, W. Nature of linear spectral properties and fast electronic relaxations in green fluorescent pyrrolo [3,4-c]pyridine derivative. Int. J. Mol. Sci. 2021, 22, 5592. [Google Scholar] [CrossRef] [PubMed]
- Fatykhov, R.F.; Sharapov, A.D.; Starnovskaya, E.S.; Shtaitz, Y.K.; Savchuk, M.I.; Kopchuk, D.S.; Nikonov, I.L.; Zyryanov, G.V.; Khalymbadzha, I.A.; Chupakhin, O.N. Coumarin-pyridine push-pull fluorophores: Synthesis and photophysical studies. Spectrochim Acta. A Mol. Biomol. Spectrosc. 2022, 267, 120499. [Google Scholar] [CrossRef] [PubMed]
- Soumya, T.V.; Ajmal, C.M.; Bahulayan, D. Synthesis of bioactive and fluorescent pyridine-triazole-coumarin peptidomimetics through sequential click-multicomponent reactions. Bioorg. Med. Chem. Lett. 2017, 27, 450–455. [Google Scholar] [CrossRef]
- Manohara, H.M.; Devaiah, C.T.; Hemavathi, B.; Ahipa, T.N. Synthesis, optical and electrochemical properties of new cyanopyridine derivatives. J. Lumin. 2019, 206, 284–291. [Google Scholar] [CrossRef]
- Li, D.; Li, J.; Liu, D.; Li, W.; Ko, C.-L.; Hung, W.-Y.; Duan, C. Highly efficient simple-structure sky-blue organic light-emitting diode using a bicarbazole/ cyanopyridine bipolar host. ACS Appl. Mater. Interfaces 2021, 13, 13459–13469. [Google Scholar] [CrossRef]
- Luo, X.; Yang, Z.; Zheng, J.; Liang, G.; Luo, H.; Yang, W. CuX dual catalysis: Construction of oxazolo [2,3-b][1,3]oxazines via a tandem CuAAC/ring cleavage/[4+2+3] annulation reaction. Org. Lett. 2022, 24, 7300–7304. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, Y.; Bu, Q.; Li, L.; Zhou, B.; Huang, Z. Tandem CuAAC/ring cleavage/[4 + 2] annulation reaction to synthesize dihydrooxazines and conversion to 2-aminopyrimidines. Org. Lett. 2022, 24, 457–461. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Lapis, A.A.M.; Mancilha, F.S.; Vasconcelos, L.B.; Thum, C.; Basso, L.A.; Santos, D.S.; Dupont, J. New sensitive fluorophores for selective DNA detection. Org. Lett. 2007, 9, 4001–4004. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Yuan, L.; Cao, Z.; Feng, J.; Feng, Y. Fluorescence enhancement of coumarin–quinoline by transition metal ions: Detection of paramagnetic Ni2+ and Co2+. Dye. Pigment. 2009, 83, 14–20. [Google Scholar] [CrossRef]
- Das, D.; Samanta, R. Iridium (III)-catalyzed regiocontrolled direct amidation of isoquinolones and pyridines. Adv. Synth. Catal. 2018, 360, 379–384. [Google Scholar] [CrossRef]
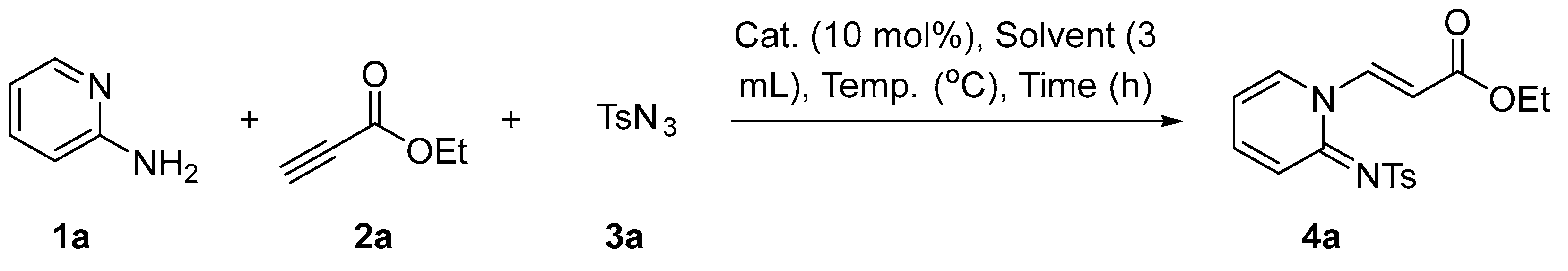 | |||||
|---|---|---|---|---|---|
| Entry | Cat. (10 mol%) | Solvent (10 mol%) | Temp. (°C) | Time (h) | Yield (%) b |
| 1 | Fe(C5H5)2 | DCE | 60 | 12 | 68 |
| 2 | FeCl3 | DCE | 60 | 12 | 18 |
| 3 | Fe(OAc)2 | DCE | 60 | 12 | 42 |
| 4 | Fe(acac)3 | DCE | 60 | 12 | 54 |
| 5 | [CpRhCl2]2 | DCE | 60 | 12 | 10 |
| 6 | Pd(OAc)2 | DCE | 60 | 12 | 23 |
| 7 | CuI | DCE | 60 | 12 | 0 |
| 8 | – | DCE | 60 | 12 | 70 |
| 9 | – | Toluene | 60 | 12 | 62 |
| 10 | – | CH2Cl2 | 60 | 12 | 45 |
| 11 | – | THF | 60 | 12 | 66 |
| 12 | – | MeCN | 60 | 12 | 78 |
| 13 | – | 1,4-Dioxane | 60 | 12 | 60 |
| 14 | – | DMF | 60 | 12 | 32 |
| 15 | − | DMSO | 60 | 12 | 30 |
| 16 | – | MeCN | 80 | 12 | 78 |
| 17 | – | MeCN | 100 | 12 | 70 |
| 18 | – | MeCN | 40 | 12 | 64 |
| 19 | – | MeCN | rt | 12 | 26 |
| 20 | – | MeCN | 60 | 16 | 78 |
| 21 | – | MeCN | 60 | 24 | 72 |
| 22 | – | MeCN | 60 | 10 | 76 |
| 23 | – | MeCN | 60 | 8 | 70 |
| Compound | λmaxAbs (nm) | ε b (mM−1 cm−1) | λmaxEm (nm) | Stokes Shift (nm) | ΦF c | Brightness d (mM−1 cm−1) |
|---|---|---|---|---|---|---|
| 4a | 238, 365 | 91 | 469 | 104 | 0.13 | 11.83 |
| 4b | 240, 356 | 95 | 468 | 112 | 0.17 | 16.15 |
| 4c | 232, 348 | 103 | 485 | 137 | 0.55 | 56.65 |
| 4d | 234, 343 | 114 | 473 | 130 | 0.31 | 35.34 |
| 4e | 241, 359 | 94 | 469 | 110 | 0.34 | 31.96 |
| 4f | 230, 344 | 97 | 490 | 146 | 0.61 | 59.17 |
| 4g | 239, 364 | 108 | 473 | 109 | 0.17 | 18.36 |
| 4h | 237, 362 | 95 | 469 | 107 | 0.15 | 14.25 |
| 4i | 239, 362 | 97 | 467 | 105 | 0.21 | 20.37 |
| 4j | 237, 362 | 149 | 465 | 103 | 0.27 | 40.23 |
| 4k | 237, 359 | 184 | 466 | 107 | 0.14 | 25.76 |
| 4l | 236, 360 | 151 | 466 | 106 | 0.08 | 12.08 |
| 4m | 239, 365 | 114 | 473 | 108 | 0.72 | 82.08 |
| 4n | 235, 360 | 119 | 467 | 107 | 0.04 | 4.76 |
| 4o | 235, 360 | 209 | 461 | 101 | 0.12 | 25.08 |
| 4p | 231, 363 | 235 | 468 | 105 | 0.31 | 72.85 |
| 4q | 236, 364 | 91 | 468 | 104 | 0.11 | 10.01 |
| 4r | 237, 365 | 95 | 468 | 103 | 0.17 | 16.15 |
| 4s | 238, 366 | 95 | 468 | 102 | 0.21 | 19.95 |
| 4t | 235, 366 | 119 | 469 | 103 | 0.18 | 21.42 |
| 4u | 237, 362 | 126 | 466 | 104 | 0.27 | 34.02 |
| 4v | 237, 362 | 131 | 466 | 104 | 0.43 | 56.33 |
| 4w | 237, 363 | 151 | 471 | 108 | 0.25 | 37.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Luo, D.; Li, G.; Luo, Q.; Banwell, M.G.; Chen, L. Synthesis of Pyridin-1(2H)-ylacrylates and the Effects of Different Functional Groups on Their Fluorescence. Molecules 2023, 28, 6511. https://doi.org/10.3390/molecules28186511
Yang W, Luo D, Li G, Luo Q, Banwell MG, Chen L. Synthesis of Pyridin-1(2H)-ylacrylates and the Effects of Different Functional Groups on Their Fluorescence. Molecules. 2023; 28(18):6511. https://doi.org/10.3390/molecules28186511
Chicago/Turabian StyleYang, Weiguang, Danyang Luo, Guanrong Li, Qiaoli Luo, Martin G. Banwell, and Lanmei Chen. 2023. "Synthesis of Pyridin-1(2H)-ylacrylates and the Effects of Different Functional Groups on Their Fluorescence" Molecules 28, no. 18: 6511. https://doi.org/10.3390/molecules28186511
APA StyleYang, W., Luo, D., Li, G., Luo, Q., Banwell, M. G., & Chen, L. (2023). Synthesis of Pyridin-1(2H)-ylacrylates and the Effects of Different Functional Groups on Their Fluorescence. Molecules, 28(18), 6511. https://doi.org/10.3390/molecules28186511






