In-Depth Analysis of the Mechanism of Astaxanthin Succinate Diester in Reducing Ulcerative Colitis in C57BL/6J Mice Based on Microbiota Informatics
Abstract
:1. Introduction
2. Results
2.1. Improvement Effect of Asta-SD on Inflammation Model of Zebrafish
2.2. Influence on Body Weight and Colon Histology
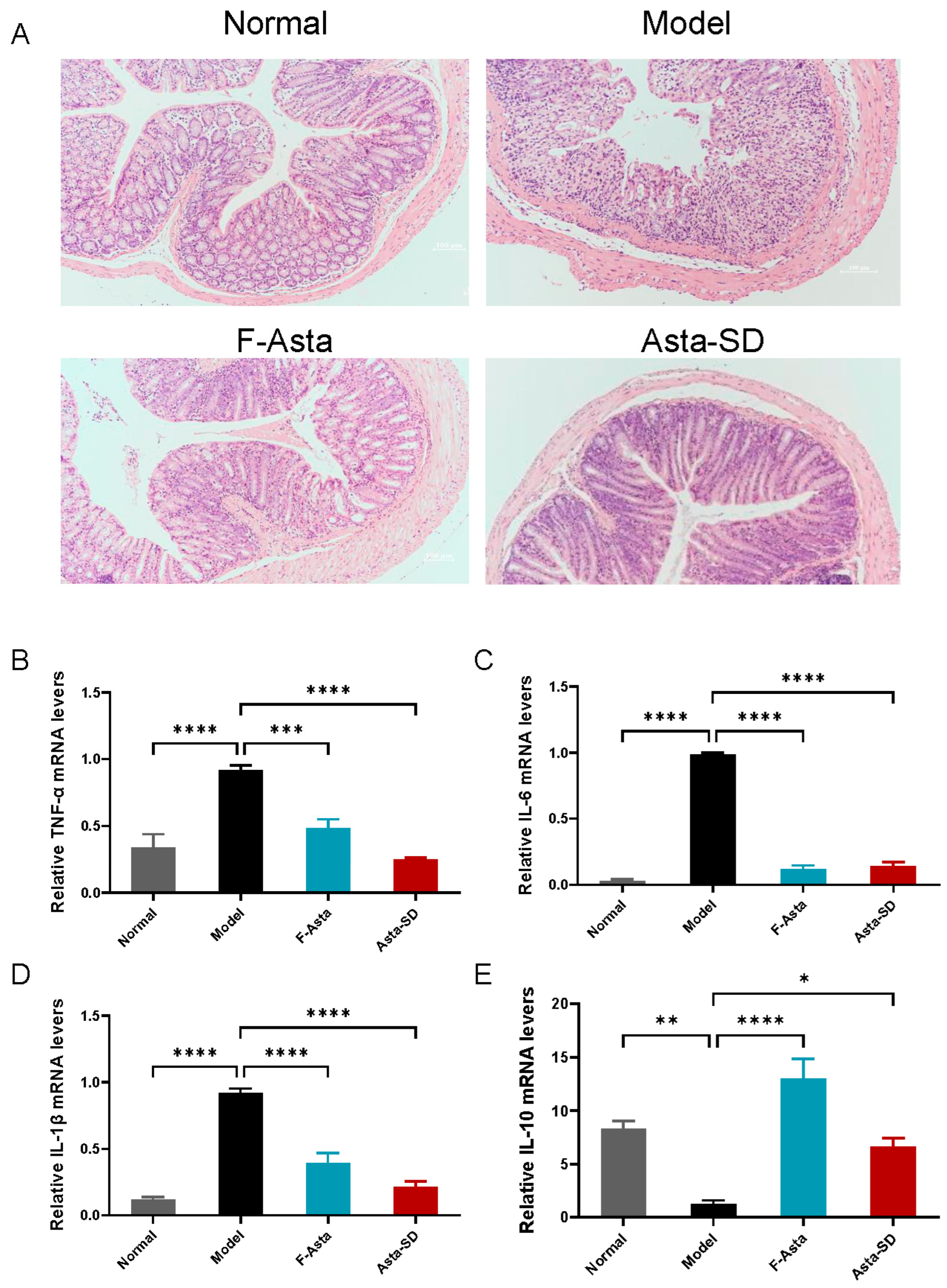
2.3. Pathological Analysis
2.4. Gene Expression of Inflammatory Factors in Colon Tissue
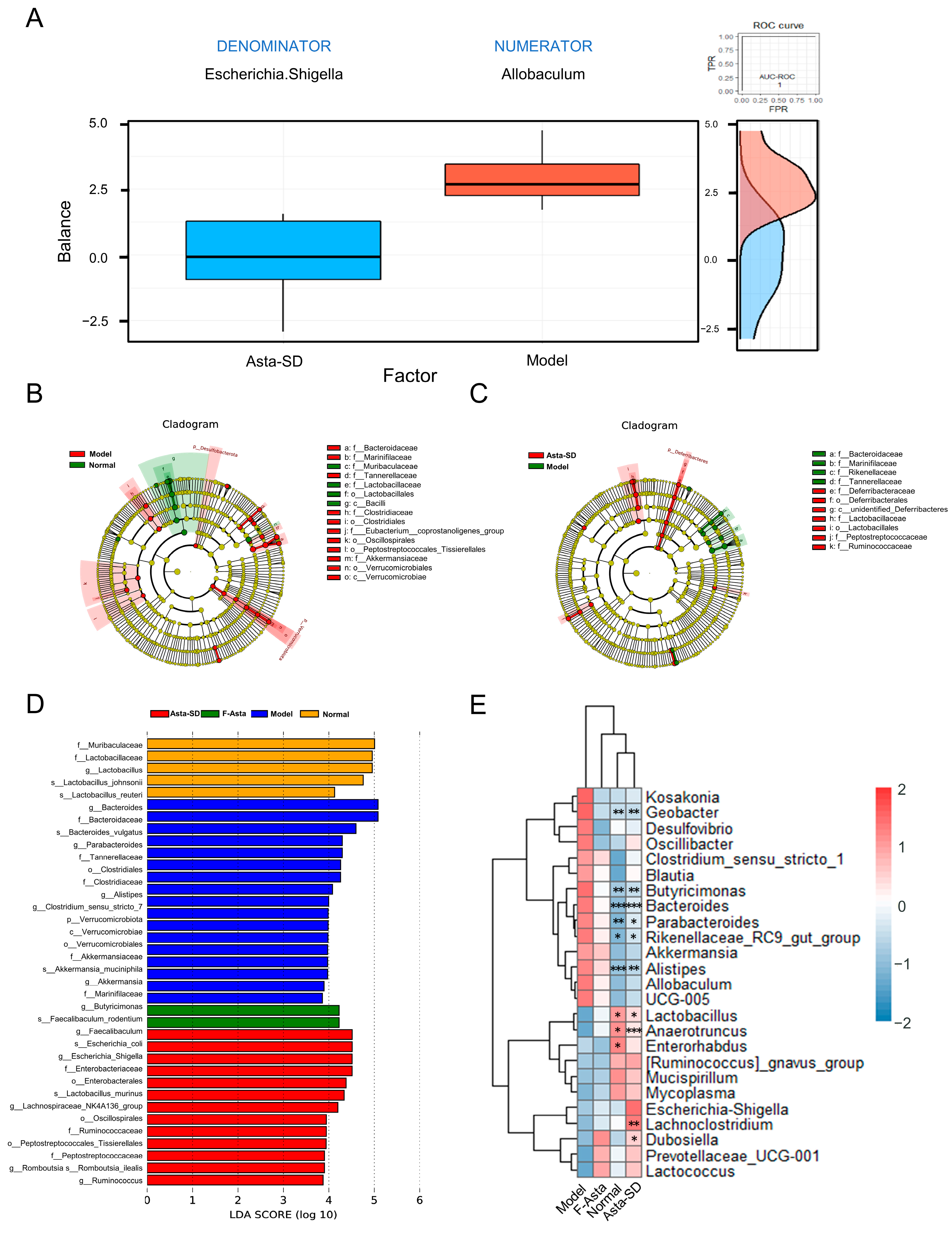
2.5. Asta-SD Regulates Intestinal Microbial Structure and Composition
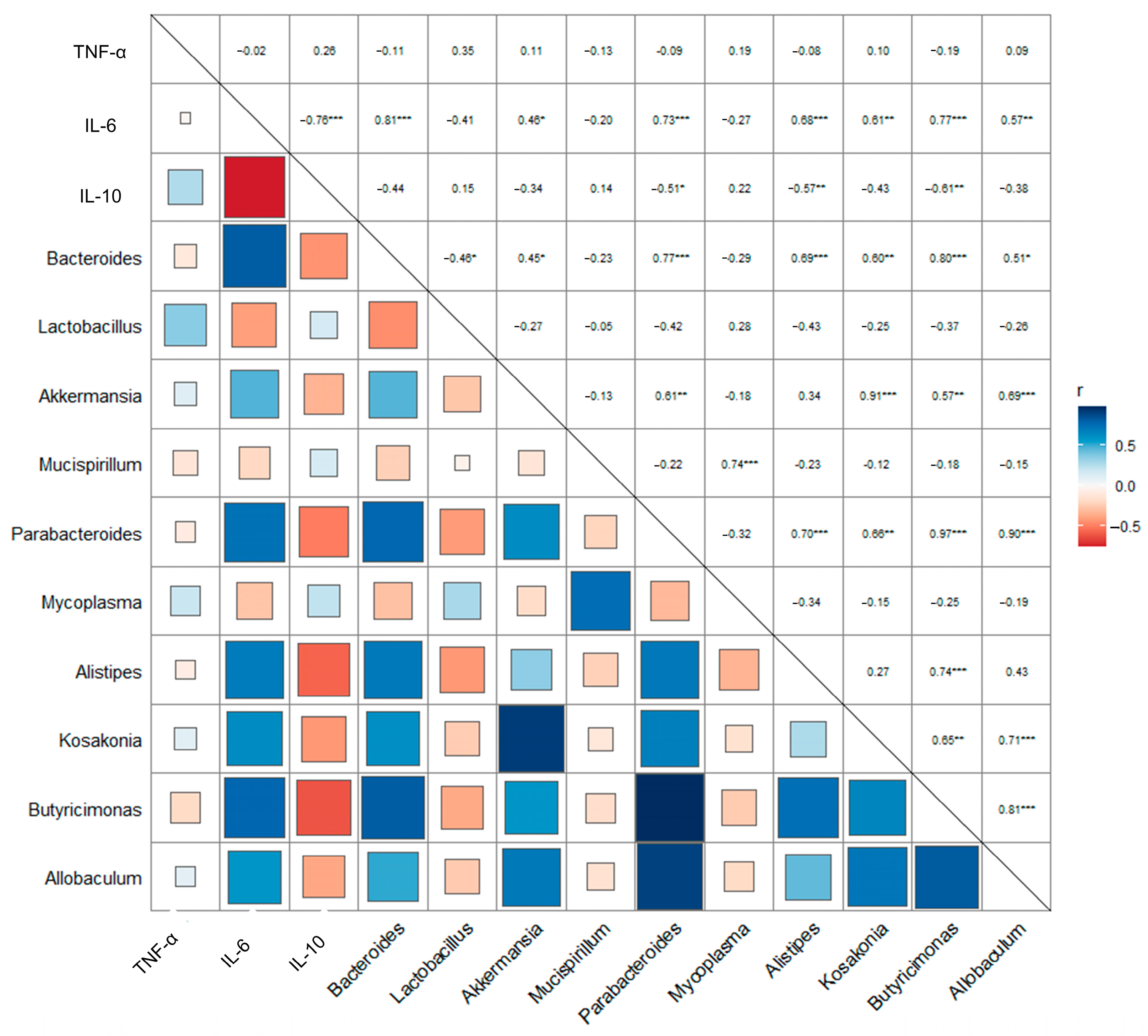
2.6. Asta-SD Affects the Correlation of the Intestinal Microbe and Anti-Inflammatory Factors in Mice with UC
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and Purification of Asta-SD
4.3. Intracellular Reactive Oxygen Species (ROS) in LPS-Stimulated Zebrafish
4.4. Single DSS Injury Zebrafish Model
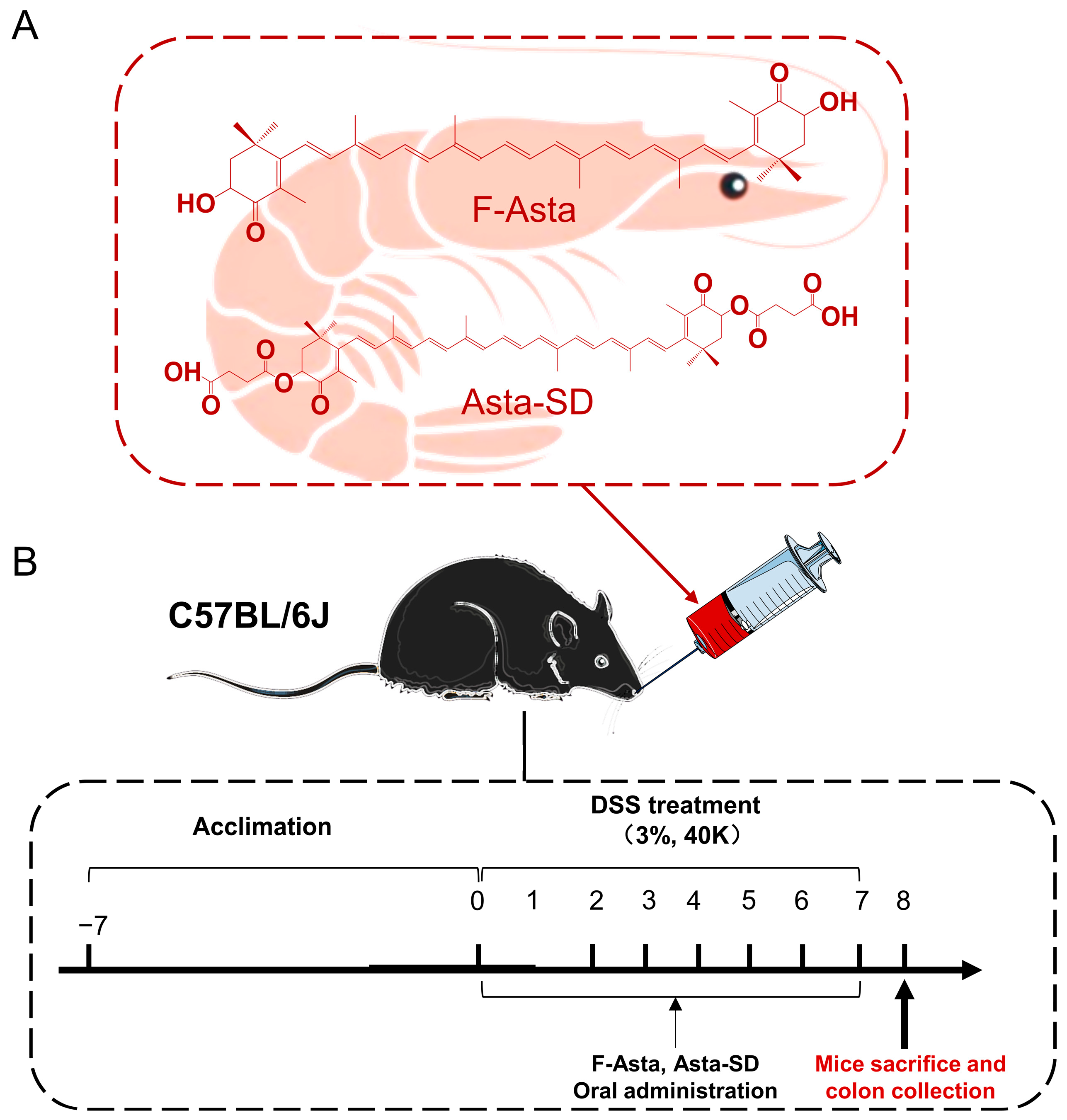
4.5. Animals and Treatments
4.6. Sample Collection
4.7. Evaluation of Disease Activity Index (DAI) Parameters and Colonic Damage
4.8. RT-qPCR Analysis of Inflammatory Cytokine Expression
4.9. Determination of Colon Histology
4.10. DNA Extraction, 16S rRNA Gene Sequencing, and Microbiota Data Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; Leleiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79–R97. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Yuan, B.; Wu, X.; Du, H.; Gu, M.; Han, Y.; Yang, W. Dietary intake of Pleurotus eryngii ameliorated dextran-sodium-sulfate-induced colitis in mice. Mol. Nutr. Food Res. 2019, 63, 1801265. [Google Scholar] [CrossRef]
- Ge, H.; Cai, Z.; Chai, J.; Liu, J.; Liu, B.; Yu, Y.; Liu, J.; Zhang, T. Egg White peptides ameliorate dextran sulfate sodium-induced acute colitis symptoms by inhibiting the production of pro-inflammatory cytokines and modulation of gut microbiota composition. Food Chem. 2021, 360, 129981. [Google Scholar] [CrossRef]
- Kanwal, S.; Joseph, T.P.; Aliya, S.; Song, S.; Saleem, M.Z.; Nisar, M.A.; Wang, Y.; Meyiah, A.; Ma, Y.; Xin, Y. Attenuation of DSS induced colitis by Dictyophora Indusiata Polysaccharide (DIP) via modulation of gut microbiota and inflammatory related signaling pathways. J. Funct. Foods 2020, 64, 103641. [Google Scholar] [CrossRef]
- De Almeida, C.V.; de Camargo, M.R.; Russo, E.; Amedei, A. Role of diet and gut microbiota on colorectal cancer immunomodulation. World J. Gastroenterol. 2019, 25, 151–162. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Hu, T.; Lin, Q.; Guo, T.; Yang, T.; Zhou, W.; Deng, X.; Yan, J.-K.; Luo, Y.; Ju, M.; Luo, F. Polysaccharide isolated from Phellinus linteus mycelia exerts anti-inflammatory effects via MAPK and PPAR signaling pathways. Carbohydr. Polym. 2018, 200, 487–497. [Google Scholar] [CrossRef]
- Oh, N.S.; Lee, J.Y.; Kim, Y.-T.; Kim, S.H.; Lee, J.-H. Cancer-protective effect of a synbiotic combination between Lactobacillus gasseri 505 and a Cudrania tricuspidata leaf extract on colitis-associated colorectal cancer. Gut Microbes 2020, 12, 1785803. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Varum, F.; Bravo, R.; Furrer, E.; Bojic, D.; Basit, A.W. Inflammatory bowel disease: Exploring gut pathophysiology for novel therapeutic targets. Transl. Res. 2016, 176, 38–68. [Google Scholar] [CrossRef]
- Schönbeck, C.; Madsen, T.L.; Peters, G.H.; Holm, R.; Loftsson, T. Soluble 1:1 complexes and insoluble 3:2 complexes—Understanding the phase-solubility diagram of hydrocortisone and γ-cyclodextrin. Int. J. Pharm. 2017, 531, 504–511. [Google Scholar] [CrossRef]
- Wang, H.; He, W.; Mahukpégo Dansou, D.; Zhang, H.; Dwi Nugroho, R.; Tang, C.; Guo, X.; Yu, Y.; Zhao, Q.; Qin, Y.; et al. Astaxanthin improved the storage stability of docosahexaenoic acid-enriched eggs by inhibiting oxidation of non-esterified poly-unsaturated fatty Acids. Food Chem. 2022, 381, 132256. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential anti-atherosclerotic properties of astaxanthin. Mar. Drugs 2016, 14, 35–48. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.; Wu, S.; Zheng, W.; Song, S.; Ai, C. Fabrication of astaxanthin-enriched colon-targeted alginate microspheres and its beneficial effect on dextran sulfate sodium-induced ulcerative colitis in mice. Int. J. Biol. Macromol. 2022, 205, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Nishida, A.; Ohno, M.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; Andoh, A. Astaxanthin, a xanthophyll carotenoid, prevents development of dextran sulphate sodium-induced murine colitis. J. Clin. Biochem. Nutr. 2019, 64, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Su, W.; Tie, S.; Cui, W.; Yu, X.; Zhang, L.; Hua, Z.; Tan, M. Orally deliverable sequence-targeted astaxanthin nanoparticles for colitis alleviation. Biomaterials 2023, 293, 121976. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Qiao, X.; Yang, L.; Zhang, T.; Zhou, Q.; Wang, Y.; Xu, J.; Xue, C. Synthesis, stability and bioavailability of astaxanthin succinate diester. J. Sci. Food Agric. 2018, 98, 3182–3189. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ko, C.-I.; Jee, Y.; Jeong, Y.; Kim, M.; Kim, J.-S.; Jeon, Y.-J. Anti-Inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar] [CrossRef]
- Handa, O.; Kokura, S.; Adachi, S.; Takagi, T.; Naito, Y.; Tanigawa, T.; Yoshida, N.; Yoshikawa, T. Methylparaben potentiates UV-induced damage of skin keratinocytes. Toxicology 2006, 227, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Levic, D.S.; Sumigray, K.D.; Bagwell, J.; Eroglu, O.; Block, C.L.; Eroglu, C.; Barry, R.; Lickwar, C.R.; Rawls, J.F.; et al. Lysosome-rich enterocytes mediate protein absorption in the vertebrate gut. Dev. Cell 2019, 51, 7–20.e6. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Zhang, B.; Lin, J.; Wang, S. Thrombospondin-1 aggravates colonic mucosal inflammatory injuries via promoting the differentiation of CD11c+ macrophages with lysosomal activity limited in colitis. Ann. Transl. Med. 2021, 9, 1738–1758. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.; Morrison, J.; Hsu, N.; Labrias, P.R.; Nayar, S.; Chen, E.; Villaverde, N.; Facey, J.A.; Boschetti, G.; Giri, M.; et al. Zebrafish modeling of intestinal injury, bacterial exposures and medications defines epithelial in vivo responses relevant to human inflammatory bowel disease. Dis. Model. Mech. 2019, 12, dmm037432. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef]
- Tessner, T.G.; Cohn, S.M.; Schloemann, S.; Stenson, W.F. Prostaglandins prevent decreased epithelial cell proliferation associated with dextran sodium sulfate injury in mice. Gastroenterology 1998, 115, 874–882. [Google Scholar] [CrossRef]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front. Microbiol. 2019, 10, 2259. [Google Scholar] [CrossRef]
- Barger, K.; Langsetmo, L.; Orwoll, E.S.; Lustgarten, M.S. Investigation of the diet-gut-muscle axis in the osteoporotic fractures in men study. J. Nutr. Health Aging 2020, 24, 445–452. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, Q.; Yousaf, L.; Xue, Y.; Shen, Q. Whole mung bean (Vigna radiata L.) supplementation prevents high-fat diet-induced obesity and disorders in a lipid profile and modulates gut microbiota in mice. Eur. J. Nutr. 2020, 59, 3617–3634. [Google Scholar] [CrossRef]
- Sireswar, S.; Dey, G.; Biswas, S. Influence of fruit-based beverages on efficacy of Lacticaseibacillus rhamnosus GG (Lactobacillus rhamnosus GG) against DSS-induced intestinal inflammation. Food Res. Int. 2021, 149, 110661–110674. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases. Biomed. Pharmacother. 2022, 145, 112179. [Google Scholar] [CrossRef]
- Leiba, J.; Özbilgiç, R.; Hernández, L.; Demou, M.; Lutfalla, G.; Yatime, L.; Nguyen-Chi, M. Molecular actors of inflammation and their signaling pathways: Mechanistic insights from zebrafish. Biology 2023, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, X.; Li, Q.; Sawaya, A.C.H.F.; Le Leu, R.K.; Conlon, M.A.; Wu, L.; Hu, F. Propolis from different geographic origins decreases intestinal inflammation and Bacteroides spp. populations in a model of DSS-induced colitis. Mol. Nutr. Food Res. 2018, 62, 1800080. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Hidaka, K.; Kanda, T.; Imaeda, H.; Shioya, M.; Inatomi, O.; Bamba, S.; Kitoh, K.; Sugimoto, M.; Andoh, A. Increased expression of interleukin-36, a member of the interleukin-1 cytokine family, in inflammatory bowel disease. Inflamm. Bowel Dis. 2016, 22, 303–314. [Google Scholar] [CrossRef]
- Cai, X.; Han, Y.; Gu, M.; Song, M.; Wu, X.; Li, Z.; Li, F.; Goulette, T.; Xiao, H. Dietary cranberry suppressed colonic inflammation and alleviated gut microbiota dysbiosis in dextran sodium sulfate-treated mice. Food Funct. 2019, 10, 6331–6341. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715–1731. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef]
- Palm, N.W.; de Zoete, M.R.; Cullen, T.W.; Barry, N.A.; Stefanowski, J.; Hao, L.; Degnan, P.H.; Hu, J.; Peter, I.; Zhang, W.; et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 2014, 158, 1000–1010. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Wang, X.; Wang, S.; Bi, D. The impact of lactobacillus plantarum on the gut microbiota of mice with DSS-induced colitis. BioMed Res. Int. 2019, 2019, 3921315. [Google Scholar] [CrossRef]
- Jiang, D.; Kang, A.; Yao, W.; Lou, J.; Zhang, Q.; Bao, B.; Cao, Y.; Yu, S.; Guo, S.; Zhang, Y.; et al. Euphorbia kansui fry-baked with vinegar modulates gut microbiota and reduces intestinal toxicity in rats. J. Ethnopharmacol. 2018, 226, 26–35. [Google Scholar] [CrossRef]
- Wen, X.; Wang, H.-G.; Zhang, M.-N.; Zhang, M.-H.; Wang, H.; Yang, X.-Z. Fecal microbiota transplantation ameliorates experimental colitis via gut microbiota and T-cell modulation. World J. Gastroenterol. 2021, 27, 2834–2849. [Google Scholar] [CrossRef]
- Hamamah, S.; Gheorghita, R.; Lobiuc, A.; Sirbu, I.-O.; Covasa, M. Fecal microbiota transplantation in non-communicable diseases: Recent advances and protocols. Front. Med. 2022, 9, 1060581. [Google Scholar] [CrossRef] [PubMed]
- Dziarski, R.; Park, S.Y.; Kashyap, D.R.; Dowd, S.E.; Gupta, D. Pglyrp-regulated gut microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii Enhance and Alistipes finegoldii attenuates colitis in mice. PLoS ONE 2016, 11, e0146162. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, R.S. Role of bacteria in Inflammatory Bowel Disease (IBD). Int. J. Infect. Dis. 2016, 45, 134. [Google Scholar] [CrossRef]
- Lucke, K.; Miehlke, S.; Jacobs, E.; Schuppler, M. Prevalence of Bacteroides and Prevotella spp. in ulcerative colitis. J. Med. Microbiol. 2006, 55, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Enemchukwu, C.U.; Ben-Faras, H.; Gialanella, P.; Szymczak, W.A.; Nosanchuk, J.D.; Madaline, T.F. Butyricimonas virosa bacteraemia and bowel disease: Case report and review. New Microbes New Infect. 2016, 13, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Ulger Toprak, N.; Bozan, T.; Birkan, Y.; Isbir, S.; Soyletir, G. Butyricimonas virosa: The first clinical case of bacteraemia. New Microbes New Infect. 2015, 4, 7–8. [Google Scholar] [CrossRef]
- Zhu, L.; Song, Y.; Liu, H.; Wu, M.; Gong, H.; Lan, H.; Zheng, X. Gut microbiota regulation and anti-inflammatory effect of β-carotene in dextran sulfate sodium-stimulated ulcerative colitis in rats. J. Food Sci. 2021, 86, 2118–2130. [Google Scholar] [CrossRef]
- Oehlers, S.H.; Flores, M.V.; Hall, C.J.; Okuda, K.S.; Sison, J.O.; Crosier, K.E.; Crosier, P.S. Chemically induced intestinal damage models in zebrafish larvae. Zebrafish 2013, 10, 184–193. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, X.; Gao, Q.; Yang, L.; Wang, X.; Wang, Z.; Li, Z.; Xu, J.; Xue, C. In-Depth Analysis of the Mechanism of Astaxanthin Succinate Diester in Reducing Ulcerative Colitis in C57BL/6J Mice Based on Microbiota Informatics. Molecules 2023, 28, 6513. https://doi.org/10.3390/molecules28186513
Qiao X, Gao Q, Yang L, Wang X, Wang Z, Li Z, Xu J, Xue C. In-Depth Analysis of the Mechanism of Astaxanthin Succinate Diester in Reducing Ulcerative Colitis in C57BL/6J Mice Based on Microbiota Informatics. Molecules. 2023; 28(18):6513. https://doi.org/10.3390/molecules28186513
Chicago/Turabian StyleQiao, Xing, Qun Gao, Lu Yang, Xiaoxu Wang, Zhigao Wang, Zhaojie Li, Jie Xu, and Changhu Xue. 2023. "In-Depth Analysis of the Mechanism of Astaxanthin Succinate Diester in Reducing Ulcerative Colitis in C57BL/6J Mice Based on Microbiota Informatics" Molecules 28, no. 18: 6513. https://doi.org/10.3390/molecules28186513
APA StyleQiao, X., Gao, Q., Yang, L., Wang, X., Wang, Z., Li, Z., Xu, J., & Xue, C. (2023). In-Depth Analysis of the Mechanism of Astaxanthin Succinate Diester in Reducing Ulcerative Colitis in C57BL/6J Mice Based on Microbiota Informatics. Molecules, 28(18), 6513. https://doi.org/10.3390/molecules28186513





