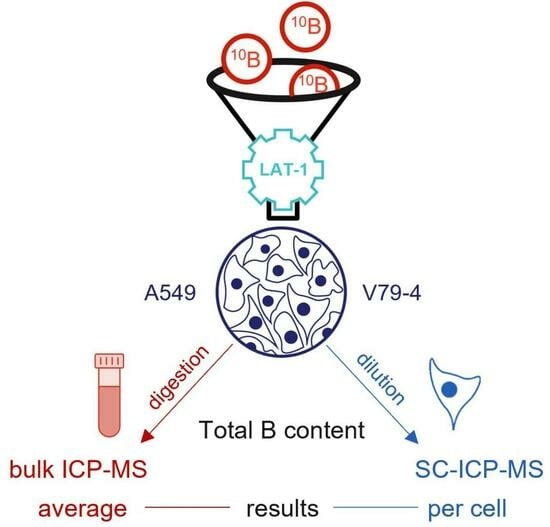
Investigation of the Impact of L-Phenylalanine and L-Tyrosine Pre-Treatment on the Uptake of 4-Borono-L-Phenylalanine in Cancerous and Normal Cells Using an Analytical Approach Based on SC-ICP-MS
Abstract
:1. Introduction
2. Results and Discussion
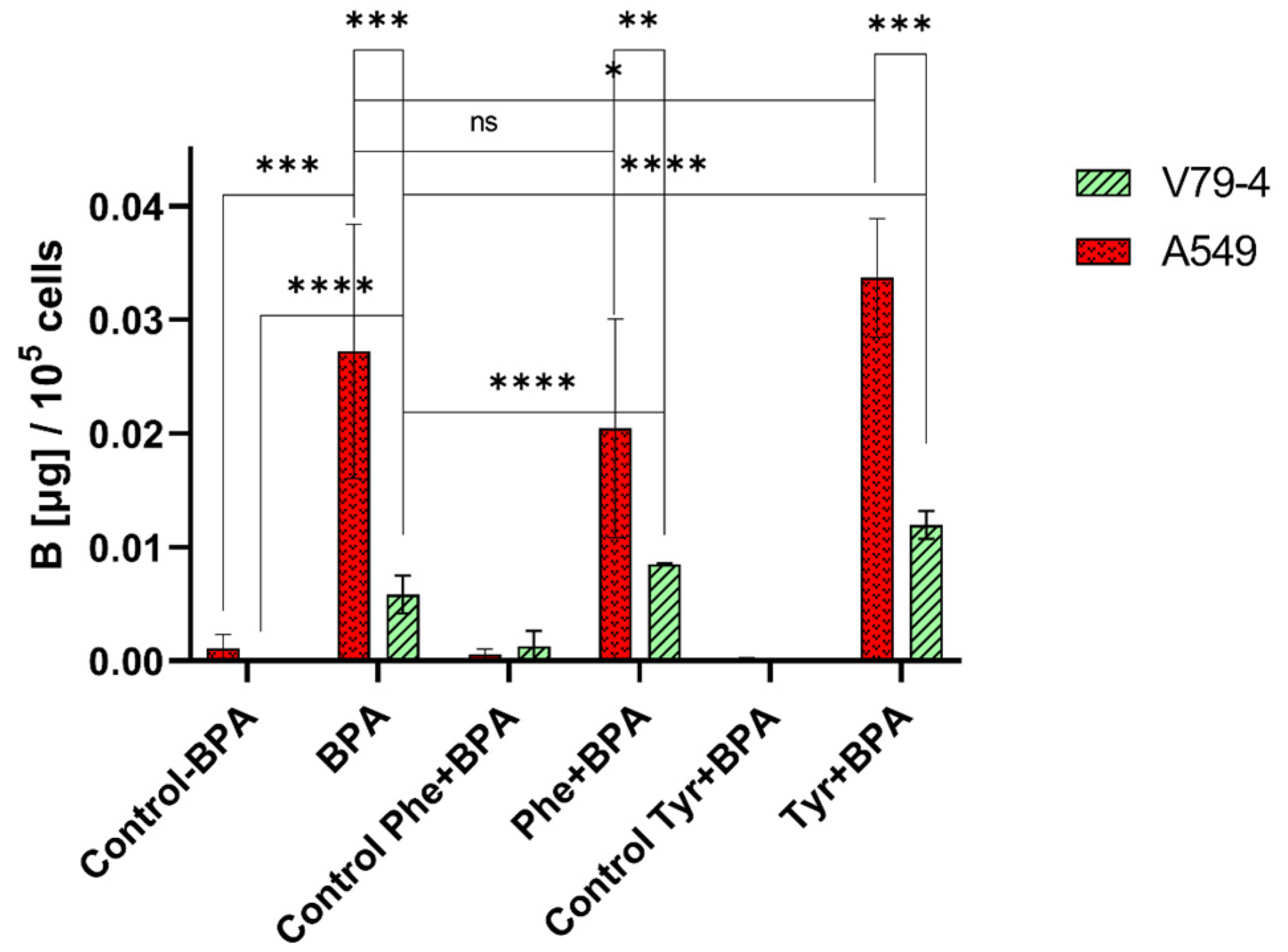
2.1. Influence of L-Phenylalanine and L-Tyrosine Pre-Treatment on Boron Concentration
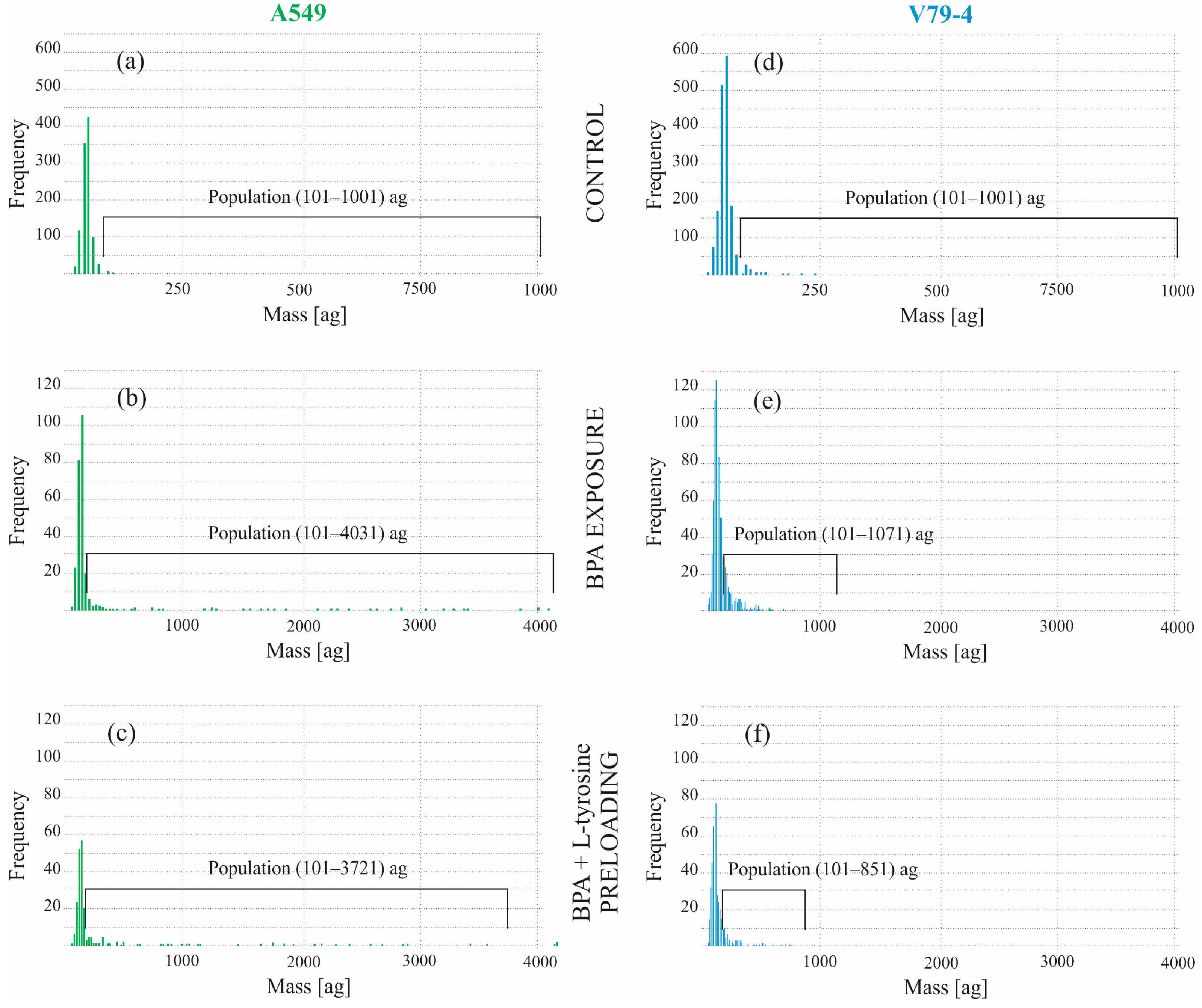
2.2. Boron Concentration in Individual Cells by SC-ICP-MS
| Percentage of the Highlighted Population in the Entire Sample [%] | Mean Mass [ag] * | |||
|---|---|---|---|---|
| Cell line | A549 | V79-4 | A549 | V79-4 |
| Control | 1.01 | 2.94 | 447 | 205 |
| BPA exposure | 39.45 | 38.72 | 719 | 165 |
| BPA + L-tyrosine preloading | 40.97 | 41.11 | 619 | 179 |
3. Materials and Methods
3.1. Cell Cultivation
3.2. Cell Pre-Treatment with L-Phenylalanine and L-Tyrosine
3.3. Cell Exposure to 4-Borono-L-Phenylalanine
3.4. Sample Preparation for ICP-MS and SC-ICP-MS Measurements
3.5. Instrumentation
3.6. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, S.; Zhang, Z.; Miao, L.; Li, Y. Boron Neutron Capture Therapy: Current Status and Challenges. Front. Oncol. 2022, 12, 788770. [Google Scholar] [CrossRef]
- Wongthai, P.; Hagiwara, K.; Miyoshi, Y.; Wiriyasermkul, P.; Wei, L.; Ohgaki, R.; Kato, I.; Hamase, K.; Nagamori, S.; Kanai, Y. Boronophenylalanine, a Boron Delivery Agent for Boron Neutron Capture Therapy, Is Transported by ATB 0,+, LAT 1 and LAT 2. Cancer Sci. 2015, 106, 279–286. [Google Scholar] [CrossRef]
- Seneviratne, D.; Advani, P.; Trifiletti, D.M.; Chumsri, S.; Beltran, C.J.; Bush, A.F.; Vallow, L.A. Exploring the Biological and Physical Basis of Boron Neutron Capture Therapy (BNCT) as a Promising Treatment Frontier in Breast Cancer. Cancers 2022, 14, 3009. [Google Scholar] [CrossRef]
- Kanai, Y. Amino Acid Transporter LAT1 (SLC7A5) as a Molecular Target for Cancer Diagnosis and Therapeutics. Pharmacol. Ther. 2022, 230, 107964. [Google Scholar] [CrossRef]
- Coghi, P.; Li, J.; Hosmane, N.S.; Zhu, Y. Next Generation of Boron Neutron Capture Therapy (BNCT) Agents for Cancer Treatment. Med. Res. Rev. 2023, 43, 1809–1830. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, T.; Inoue, Y.; Yao, Y.; Suzuki, M.; Kanamori, K.; Takemoto, H.; Matsui, M.; Tomoda, K.; Nishiyama, N. Poly(Vinyl Alcohol) Boosting Therapeutic Potential of p -Boronophenylalanine in Neutron Capture Therapy by Modulating Metabolism. Sci. Adv. 2020, 6, eaaz1722. [Google Scholar] [CrossRef] [PubMed]
- Detta, A.; Cruickshank, G.S. L-Amino Acid Transporter-1 and Boronophenylalanine-Based Boron Neutron Capture Therapy of Human Brain Tumors. Cancer Res. 2009, 69, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Wittig, A.; Sauerwein, W.A.; Coderre, J.A.; Coderre, J.A. Mechanisms of Transport of p -Borono-Phenylalanine through the Cell Membrane In Vitro. Radiat. Res. 2000, 153, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Capuani, S.; Gili, T.; Bozzali, M.; Russo, S.; Porcari, P.; Cametti, C.; D’Amore, E.; Colasanti, M.; Venturini, G.; Maraviglia, B.; et al. L-DOPA Preloading Increases the Uptake of Borophenylalanine in C6 Glioma Rat Model: A New Strategy to Improve BNCT Efficacy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Papaspyrou, M.; Feinendegen, L.E.; Müller-Gärtner, H.W. Preloading with L-Tyrosine Increases the Uptake of Boronophenylalanine in Mouse Melanoma Cells. Cancer Res. 1994, 54, 6311–6314. [Google Scholar] [PubMed]
- Wingelhofer, B.; Kreis, K.; Mairinger, S.; Muchitsch, V.; Stanek, J.; Wanek, T.; Langer, O.; Kuntner, C. Preloading with L-BPA, L-Tyrosine and L-DOPA Enhances the Uptake of [18F]FBPA in Human and Mouse Tumour Cell Lines. Appl. Radiat. Isot. 2016, 118, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Gielisch, M.; Moergel, M.; Al-Nawas, B.; Kämmerer, P.W. Does Trans-Stimulation of L-Tyrosine Lead to an Increase in Boron Uptake in Head and Neck Squamous Cell Carcinoma Cells? Appl. Sci. 2021, 11, 7286. [Google Scholar] [CrossRef]
- Grunewald, C.; Sauberer, M.; Filip, T.; Wanek, T.; Stanek, J.; Mairinger, S.; Rollet, S.; Kudejova, P.; Langer, O.; Schütz, C.; et al. On the Applicability of [18F]FBPA to Predict L-BPA Concentration after Amino Acid Preloading in HuH-7 Liver Tumor Model and the Implication for Liver Boron Neutron Capture Therapy. Nucl. Med. Biol. 2017, 44, 83–89. [Google Scholar] [CrossRef]
- Aldossari, S.; McMahon, G.; Lockyer, N.P.; Moore, K.L. Microdistribution and Quantification of the Boron Neutron Capture Therapy Drug BPA in Primary Cell Cultures of Human Glioblastoma Tumour by NanoSIMS. Analyst 2019, 144, 6214–6224. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Barth, R.F.; Huo, T.; Kabalka, G.W.; Shaikh, A.L.; Haider, S.A.; Chandra, S. Effects of L-DOPA Pre-Loading on the Uptake of Boronophenylalanine Using the F98 Glioma and B16 Melanoma Models. Appl. Radiat. Isot. 2014, 88, 69–73. [Google Scholar] [CrossRef]
- Watanabe, T.; Tanaka, H.; Fukutani, S.; Suzuki, M.; Hiraoka, M.; Ono, K. L-Phenylalanine Preloading Reduces the 10B(n, α)7Li Dose to the Normal Brain by Inhibiting the Uptake of Boronophenylalanine in Boron Neutron Capture Therapy for Brain Tumours. Cancer Lett. 2016, 370, 27–32. [Google Scholar] [CrossRef]
- Farías, R.O.; Bortolussi, S.; Menéndez, P.R.; González, S.J. Exploring Boron Neutron Capture Therapy for Non-Small Cell Lung Cancer. Phys. Med. 2014, 30, 888–897. [Google Scholar] [CrossRef]
- Quah, S.C. Boron Neutron Capture Therapy in the Treatment of Lung Cancer. J. Xiangya Med. 2018, 3, 29. [Google Scholar] [CrossRef]
- Nakagawa, F.; Kawashima, H.; Morita, T.; Nakamura, H. Water-Soluble Closo-Docecaborate-Containing Pteroyl Derivatives Targeting Folate Receptor-Positive Tumors for Boron Neutron Capture Therapy. Cells 2020, 9, 1615. [Google Scholar] [CrossRef]
- Wang, M.; Tong, Y.; Luo, Q.; Hu, S. Comparative Study on Neutron Irradiation Sensitization Effects of Nucleotide Borate Esters and Several Other Boron Agents. Radiat. Res. 2020, 193, 249. [Google Scholar] [CrossRef]
- Varol, M.; Benkli, K.; Koparal, A.T.; Bostancıoğlu, R.B. Design and Synthesis of Novel Organometallic Complexes Using Boronated Phenylalanine Derivatives as Potential Anticancer Agents. Drug Chem. Toxicol. 2019, 42, 436–443. [Google Scholar] [CrossRef]
- Ueda, H.; Suzuki, M.; Kuroda, R.; Tanaka, T.; Aoki, S. Design, Synthesis, and Biological Evaluation of Boron-Containing Macrocyclic Polyamines and Their Zinc(II) Complexes for Boron Neutron Capture Therapy. J. Med. Chem. 2021, 64, 8523–8544. [Google Scholar] [CrossRef]
- Kondo, N.; Hirano, F.; Temma, T. Evaluation of 3-Borono-l-Phenylalanine as a Water-Soluble Boron Neutron Capture Therapy Agent. Pharmaceutics 2022, 14, 1106. [Google Scholar] [CrossRef]
- Sahni, A.; Qian, Z.; Pei, D. Cell-Penetrating Peptides Escape the Endosome by Inducing Vesicle Budding and Collapse. ACS Chem. Biol. 2020, 15, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Linko, S.; Revitzer, H.; Zilliacus, R.; Kortesniemi, M.; Kouri, M.; Savolainen, S. Boron Detection from Blood Samples by ICP-AES and ICP-MS during Boron Neutron Capture Therapy. Scand. J. Clin. Lab. Inv. 2008, 68, 696–702. [Google Scholar] [CrossRef]
- Gibson, C.R.; Staubus, A.E.; Barth, R.F.; Yang, W.; Ferketich, A.K.; Moeschberger, M.M. Pharmacokinetics of Sodium Borocaptate: A Critical Assessment of Dosing Paradigms for Boron Neutron Capture Therapy. J. Neurooncol. 2003, 62, 157–169. [Google Scholar] [CrossRef]
- Basilico, F.; Sauerwein, W.; Pozzi, F.; Wittig, A.; Moss, R.; Mauri, P.L. Analysis Of10B Antitumoral Compounds by Means of Flow-Injection into ESI-MS/MS. J. Mass Spectrom. 2005, 40, 1546–1549. [Google Scholar] [CrossRef]
- Takagaki, M.; Mishima, Y. Boron-10 Quantitative Analysis of Neutron Capture Therapy on Malignant Melanoma by Spectrophotometric α-Track Reading. Int. J. Radiat. Appl. Instrum. Part D. Nucl. Tracks Radiat. Meas. 1990, 17, 531–535. [Google Scholar] [CrossRef]
- Zhu, Y.; Fazal, T. Application of Theranostic Technology in Boron Neutron Capture Therapy. In Frontiers in Boron-Based Medicinal Chemistry, 1st ed.; Zhu, Y., Ed.; World Scientific Publishing Co., Pte Ltd.: Singapore, 2023; pp. 90–114. ISBN 9789811267963. [Google Scholar]
- Ishiwata, K. 4-Borono-2-18F-Fluoro-l-Phenylalanine PET for Boron Neutron Capture Therapy-Oriented Diagnosis: Overview of a Quarter Century of Research. Ann. Nucl. Med. 2019, 33, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Vogl, J.; Rosner, M.; Deng, L.; Jin, Z. A Validated Analytical Procedure for Boron Isotope Analysis in Plants by MC-ICP-MS. Talanta 2019, 196, 389–394. [Google Scholar] [CrossRef]
- Adamska, A.; Rumijowska-Galewicz, A.; Ruszczynska, A.; Studzińska, M.; Jabłońska, A.; Paradowska, E.; Bulska, E.; Munier-Lehmann, H.; Dziadek, J.; Leśnikowski, Z.J.; et al. Anti-Mycobacterial Activity of Thymine Derivatives Bearing Boron Clusters. Eur. J. Med. Chem. 2016, 121, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Ma, R.; McLeod, C.W.; Wang, X.; Cox, A.G. Determination of Boron in Serum, Plasma and Urine by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Use of Mannitol-Ammonia as Diluent and for Eliminating Memory Effect. J. Anal. At. Spectrom. 2000, 15, 257–261. [Google Scholar] [CrossRef]
- Liu, T.; He, T.; Shi, Q.; Ni, Q. Rapid Determination of Boron in 61 Soil, Sediment, and Rock Reference Materials by ICP-MS. At. Spectrosc. 2019, 40, 55–62. [Google Scholar] [CrossRef]
- Egger, A.E.; Rappel, C.; Jakupec, M.A.; Hartinger, C.G.; Heffeter, P.; Keppler, B.K. Development of an Experimental Protocol for Uptake Studies of Metal Compounds in Adherent Tumor Cells. J. Anal. At. Spectrom. 2009, 24, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fan, Y.; Li, Q.; Sheng, R.; Si, H.; Fang, J.; Tong, L.; Tang, B. Simultaneous Single-Cell Analysis of Na+, K+, Ca2+, and Mg2+ in Neuron-Like PC-12 Cells in a Microfluidic System. Anal. Chem. 2017, 89, 4559–4565. [Google Scholar] [CrossRef]
- Watanabe, N.; Dickinson, D.A.; Krzywanski, D.M.; Iles, K.E.; Zhang, H.; Venglarik, C.J.; Forman, H.J. A549 Subclones Demonstrate Heterogeneity in Toxicological Sensitivity and Antioxidant Profile. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 283, L726–L736. [Google Scholar] [CrossRef]
- Ho, K.-S.; Chan, W.-T. Time-Resolved ICP-MS Measurement for Single-Cell Analysis and on-Line Cytometry. J. Anal. At. Spectrom. 2010, 25, 1114. [Google Scholar] [CrossRef]
- Galé, A.; Hofmann, L.; Lüdi, N.; Hungerbühler, M.N.; Kempf, C.; Heverhagen, J.T.; Von Tengg-Kobligk, H.; Broekmann, P.; Ruprecht, N. Beyond Single-Cell Analysis of Metallodrugs by ICP-MS: Targeting Cellular Substructures. Int. J. Mol. Sci. 2021, 22, 9468. [Google Scholar] [CrossRef]
- Taylor, A.; Barlow, N.; Day, M.P.; Hill, S.; Martin, N.; Patriarca, M. Atomic Spectrometry Update: Review of Advances in the Analysis of Clinical and Biological Materials, Foods and Beverages. J. Anal. At. Spectrom. 2019, 34, 426–459. [Google Scholar] [CrossRef]
- Da Silva, A.B.S.; Arruda, M.A.Z. Single-Cell ICP-MS to Address the Role of Trace Elements at a Cellular Level. J. Trace Elem. Med. Biol. 2023, 75, 127086. [Google Scholar] [CrossRef]
- Salisbury, T.; Arthur, S. The Regulation and Function of the L-Type Amino Acid Transporter 1 (LAT1) in Cancer. Int. J. Mol. Sci. 2018, 19, 2373. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Ahmad, T.; Barth, R.F.; Kabalka, G.W. Quantitative Evaluation of Boron Neutron Capture Therapy (BNCT) Drugs for Boron Delivery and Retention at Subcellular-Scale Resolution in Human Glioblastoma Cells with Imaging Secondary Ion Mass Spectrometry (SIMS). J. Microsc. 2014, 254, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Freshney, R.I. Transformation and Immortalization. In Culture of Animal Cells, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 279–297. ISBN 978-0-470-64936-7. [Google Scholar]
| Parameter | ICP-MS | SC-ICP-MS |
|---|---|---|
| Instrument | NexION 300D (PerkinElmer) | NexION 2000 (PerkinElmer) |
| Mass analyzer | Quadrupole | Quadrupole |
| Measurement Mode | Standard | Standard |
| Nebulizer | Quartz Mainhardt | HEN Mainhardt |
| Spray chamber | Quartz cyclonic | Quartz Asperon |
| Carrier gas | Ar | Ar |
| Nebulizer gas flow | 0.88 L min−1 | 0.40 L min−1 |
| Sample introduction | Manually, peristaltic pump | SC Micro DX, syringe pump |
| Sample Flow Rate | 0.2 mL min−1 | 10 μL min−1 |
| Dwell time | 100 ms | 50 μs |
| AMS gas flow | n/a | 0.7 L min−1 |
| Measured isotopes * | B-10, B-11 | B-10, B-11 |
| Transport Efficiency | n/a | 28% |
| Sample preparation | Digestion and dilution | Dilution with PBS to ~105 cells mL−1 |
| Analytical Parameters | Digestion + ICP-MS | Dilution + SC-ICP-MS |
| Calibration range | (10–100) μg L−1 in 1% HNO3 | (1–5) μg L−1 in PBS |
| Regression equation ** | y = 1526x + 157.13 | y = 0.8108x + 0.335 |
| Correlation coefficient, R2 | 0.9999 | 0.9999 |
| LOD/PDL | 20 ng kg−1 | 12 ag/cell |
| Recovery | 100–115% | n/a |
| Bin size | n/a | 10 ag |
| Threshold | n/a | 1.06–3.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balcer, E.; Giebułtowicz, J.; Sochacka, M.; Ruszczyńska, A.; Muszyńska, M.; Bulska, E. Investigation of the Impact of L-Phenylalanine and L-Tyrosine Pre-Treatment on the Uptake of 4-Borono-L-Phenylalanine in Cancerous and Normal Cells Using an Analytical Approach Based on SC-ICP-MS. Molecules 2023, 28, 6552. https://doi.org/10.3390/molecules28186552
Balcer E, Giebułtowicz J, Sochacka M, Ruszczyńska A, Muszyńska M, Bulska E. Investigation of the Impact of L-Phenylalanine and L-Tyrosine Pre-Treatment on the Uptake of 4-Borono-L-Phenylalanine in Cancerous and Normal Cells Using an Analytical Approach Based on SC-ICP-MS. Molecules. 2023; 28(18):6552. https://doi.org/10.3390/molecules28186552
Chicago/Turabian StyleBalcer, Emilia, Joanna Giebułtowicz, Małgorzata Sochacka, Anna Ruszczyńska, Magdalena Muszyńska, and Ewa Bulska. 2023. "Investigation of the Impact of L-Phenylalanine and L-Tyrosine Pre-Treatment on the Uptake of 4-Borono-L-Phenylalanine in Cancerous and Normal Cells Using an Analytical Approach Based on SC-ICP-MS" Molecules 28, no. 18: 6552. https://doi.org/10.3390/molecules28186552
APA StyleBalcer, E., Giebułtowicz, J., Sochacka, M., Ruszczyńska, A., Muszyńska, M., & Bulska, E. (2023). Investigation of the Impact of L-Phenylalanine and L-Tyrosine Pre-Treatment on the Uptake of 4-Borono-L-Phenylalanine in Cancerous and Normal Cells Using an Analytical Approach Based on SC-ICP-MS. Molecules, 28(18), 6552. https://doi.org/10.3390/molecules28186552