Making Every Single Puff Count—Simple and Sensitive E-Cigarette Aerosol Sampling for GCxIMS and GC-MS Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cooling Methods
2.2. Condensation Aids
2.3. Sampling Procedure
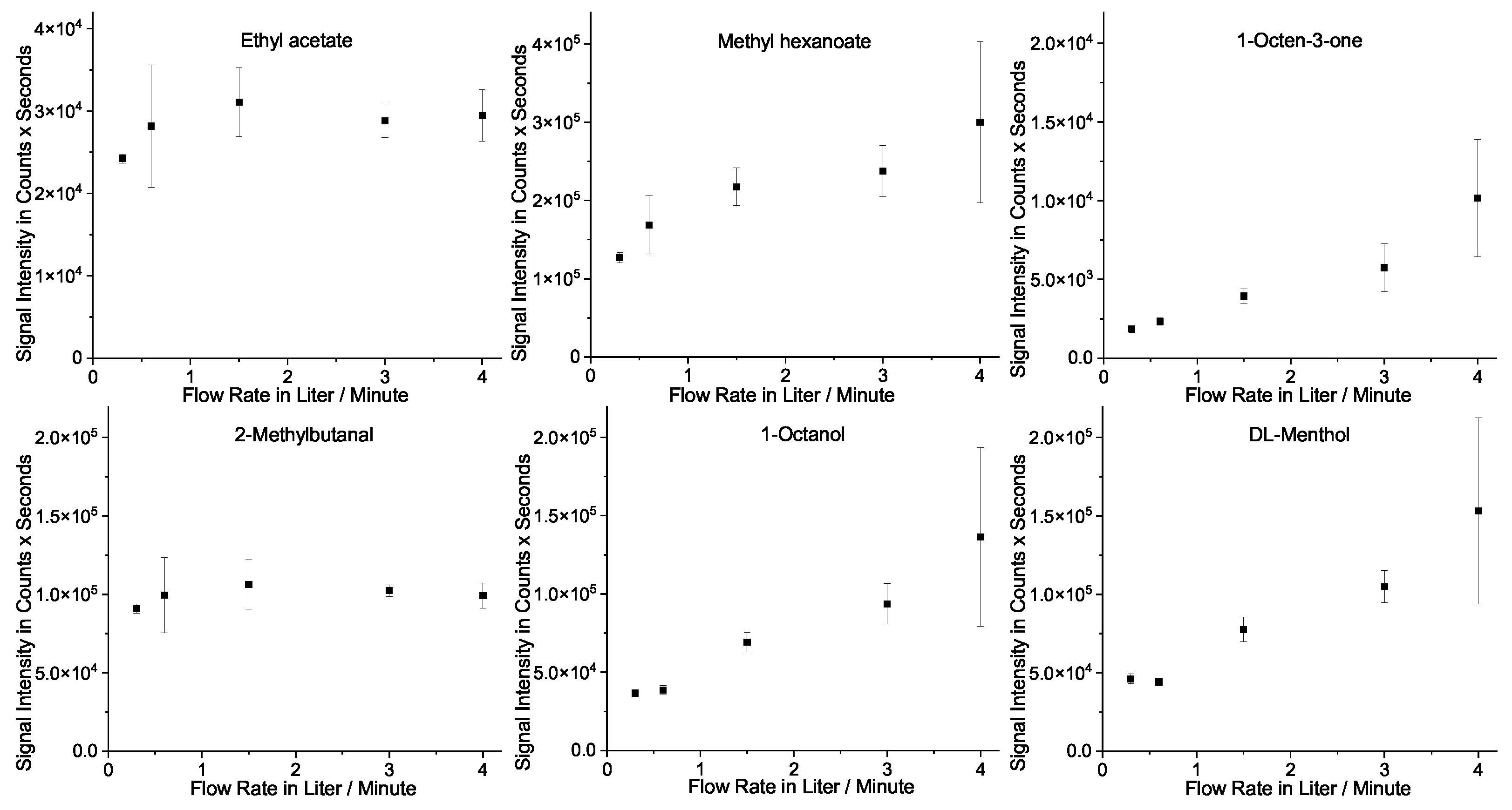
2.4. Puff Length and Flow Rate
2.5. Reproducibility of Measurements and the Usage of Internal Standards
2.6. Quantitative Analysis
2.7. Reactivity and Degradation
3. Materials and Methods
3.1. Samples
3.2. Aerosol Production and Sampling Setup
3.3. The Sampling Process
3.4. Analysis
3.5. Data Processing
3.6. Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| A | Analyte |
| AIP | Analyte ion peak |
| c | Concentration |
| CAS | Chemical Abstract Service |
| DT | Drift tube |
| EI | Electron ionization |
| eV | Electron Volts |
| g | Gram |
| GC | Gas chromatography |
| GL | Glycerol (1,2,3-propanetriol) |
| HS | Headspace |
| Hz | Hertz |
| IMS | Ion mobility spectrometry |
| ISTD | Internal Standard |
| K0 | Reduced mobility |
| L | Liter |
| m/z | Mass-to-charge ratio |
| MIBK | Methyl isobutyl ketone |
| min | Minute |
| MS | Mass spectrometry |
| NIST | National Institute of Standards and Technologies |
| Ohm | |
| PFA | Perfluoroalkoxy alkane |
| PG | Propylene glycol (1,2-propanediol) |
| PTFE | Polytetrafluoroethylene (Poly(1,1,2,2-tetrafluoroethylene) |
| R-APCI | Radioactive-atmospheric pressure chemical ionization |
| RE | Relative error |
| RIP | Reactant ion peak |
| RMSE | Root mean squared error |
| Rt | Retention time |
| s | Second |
| SPME | Solid phase micro extraction |
| TD | Thermal desorption |
| TIC | Total ion chromatogramm |
| VOC | Volatile organic compounds |
| W | Watt |
Appendix A

| Substance | Class | Manufacturer | Purity | CAS-Nr. | RtIMS/min | K0/Vs cm−2 | RtMS/min | Quantifier (Qualifier)/m/z |
|---|---|---|---|---|---|---|---|---|
| 2-Methylpropanal | Aldehyde | Alfa Aesar | 0.98 | 78-84-2 | 4.8 | 1.786, 1.630, 1.488 | 2.0 | 41.00 (43.00, 72.00) |
| Diacetyl | Ketone | Alfa Aesar | 0.99 | 431-03-8 | 5.2 | 1.683, 1.623 | 2.3 | 86.00 (–) |
| 2-Butanone | ISTD | Sigma-Aldrich | 0.997 | 78-93-3 | 5.3 | 1.851, 1.540 | 2.4 | 72.10 (57.00) |
| Ethyl acetate | Ester | Carl Roth | 0.999 | 141-78-6 | 5.6 | 1.786, 1.431 | 2.5 | 70.00 (61.00) |
| 2-Methylpropanol | Alcohol | Honeywell | 0.995 | 78-83-1 | 5.9 | 1.675, 1.484, 1.424 | 2.6 | 41.00 (42.00) |
| 2-Methylbutanal | Aldehyde | Alfa Aesar | 0.95 | 96-17-3 | 6.7 | 1.691, 1.53, 1.479, 1.437, 1.374 | 3.1 | 57.00 (41.00, 86.00) |
| 2-Pentanone | ISTD | Sigma-Aldrich | 0.995 | 107-87-9 | 5.8 | 1.728, 1.405 | 3.5 | 43.00 (86.10, 71.00) |
| Isoamyl alcohol | Alcohol | Alfa Aesar | 0.985 | 123-51-3 | 8.6 | 1.583, 1.465, 1.410, 1.293 | 4.6 | 70.00 (–) |
| Methyl isobutyl ketone | Ketone | Acros Organics | 0.995 | 108-10-1 | 8.9 | 1.650, 1.393, 1.283 | 4.8 | 100.00 (85.00) |
| 2,3-Hexanedione | Ketone | Alfa Aesar | 0.94 | 3848-24-6 | 10.2 | 1.704, 1.612, 1.571, 1.476, 1.286, 1.229 | 5.8 | 43.00 (71.00) |
| 2-Hexanone | ISTD | Sigma-Aldrich | 0.98 | 591-78-6 | 10.4 | 1.641, 1.371, 1.269 | 6.2 | 43.00 (58.00, 100.10) |
| Hexanal | Aldehyde | Alfa Aesar | 0.98 | 66-25-1 | 10.8 | 1.576, 1.430, 1.344, 1.226 | 6.6 | 56.00 (82.00, 72.00) |
| Butyl acetate | Ester | Acros Organics | 0.995 | 123-86-4 | 11.2 | 1.580, 1.326, 1.180 | 7.1 | 43.00 (73.00) |
| Furfural | Aldehyde | Acros Organics | 0.99 | 98-01-1 | 12.0 | 1.819, 1.475, 1.439 | 7.7 | 96.00 (38.95, 66.95) |
| Ethyl 2-methylbutanoate | Ester | Alfa Aesar | 0.98 | 7452-79-1 | 12.5 | 1.582, 1.306, 1.153 | 8.3 | 57.00 (102.10, 85.10) |
| (E)-2-Hexenal | Aldehyde | Acros Organics | 0.99 | 6728-26-3 | 12.6 | 1.646, 1.371, 1.259 | 8.4 | 55.00 (69.00, 83.10) |
| (E)-2-Hexenol | Alcohol | Carl Roth | 0.97 | 928-95-0 | 13.0 | 1.904, 1.439, 1.057 | 8.8 | 57.00 (41.00, 82.10) |
| 2-Heptanone | ISTD | Sigma-Aldrich | 0.99 | 110-43-0 | 13.9 | 1.567, 1.317, 1.167 | 9.7 | 43.00 (58.00, 71.05) |
| Ethyl pentanoate | Ester | Acros Organics | 0.99 | 539-82-2 | 14.2 | 1.568, 1.292, 1.138 | 10.1 | 88.05 (57.00, 70.00) |
| Methyl hexanoate | Ester | Acros Organics | 0.99 | 106-70-7 | 15.0 | 1.499, 1.289, 1.135 | 10.9 | 74.00 (43.00, 87.00) |
| (+)--Pinene | Terpene | Alfa Aesar | 0.98 | 7785-70-8 | 15.6 | 1.587, 1.146, 1.101 | 11.3 | 93.10 (77.00, 121.10) |
| Benzaldehyde | Aldehyde | Carl Roth | 0.995 | 100-52-7 | 16.5 | 1.692, 1.399, 1.306 | 12.2 | 106.05 (50.95, 77.00) |
| 1-Octen-3-one | Ketone | TCI | 0.95 | 4312-99-6 | 16.9 | 1.574, 1.287, 1.130 | 12.8 | 70.00 (55.00, 97.10) |
| Octanal | Aldehyde | Alfa Aesar | 0.98 | 124-13-0 | 17.3 | 1.326, 1.213, 1.019 | 13.6 | 43.00 (–) |
| (E,E)-2,4-Heptadienal | Aldehyde | TCI | 0.90 | 05.03.4313 | 17.7 | 1.454, 1.256, 1.119, 1.082 | 13.9 | 81.00 (53.00) |
| D(+)-Limonene | Terpene | Carl Roth | 0.95 | 5989-27-5 | 18.0 | 1.387, 1.289, 1.222, 1.048 | 14.6 | 68.00 (93.05, 121.10) |
| Octanol | Alcohol | Alfa Aesar | 0.99 | 111-87-5 | 18.8 | 1.640, 1.325, 1.180 | 15.9 | 56.00 (55.00, 69.00) |
| 2-Octanone | ISTD | Sigma-Aldrich | 0.98 | 111-13-7 | 20.0 | 1.585, 1.485, 1.155, 1.122, 1.097 | 13.2 | 58.00 (43.00, 71.00) |
| 2-Nonanone | ISTD | Sigma-Aldrich | 0.99 | 821-55-6 | 20.7 | 1.381, 1.199, 1.166, 1.010 | 16.6 | 58.00 (42.95, 71.00) |
| Linalool | Terpene | Carl Roth | pure | 78-70-6 | 21.0 | 1.588, 1.153, 1.121, 1.085 | 16.9 | 71.00 (93.10, 55.00) |
| L/D-Menthone | Terpene | Carl Roth | 0.80/0.16 | 89-80-5 | 23.0/23.3 | 1.489, 1.394, 1.225, 1.187, 1.03/1.446, 1.226, 1.188, 1.029 | 18.7/19.1 | 112.00/112.10 (69.00, 139.20/69.00, 55.00) |
| D/L-Menthol | Terpene | Carl Roth | 0.99 | 1490-04-6 | 23.6 | 1.572, 1.206, 1.171 | 19.3 | 81.10 (71.00, 95.10) |
| 2-Decanone | ISTD | Alfa Aesar | 0.97 | 693-54-9 | 24.0 | 1.310, 1.153, 1.119, 0.951 | 19.8 | 58.00 (43.00, 71.00) |
| Neral/Geranial | Terpene | Acros Organics | 0.44/0.51 | 5392-40-5 | 25.8/26.9 | 0.951, 1.850/1.397, 1.186, 1.149, 0.958 | 21.3/22.1 | 69.00/69.05 (41.00, 94.10/105.05, 163.05) |
References
- Peace, M.R.; Baird, T.R.; Smith, N.; Wolf, C.E.; Poklis, J.L.; Poklis, A. Concentration of Nicotine and Glycols in 27 Electronic Cigarette Formulations. J. Anal. Toxicol. 2016, 40, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.A.; Flora, J.W.; Melvin, M.S.; Avery, K.C.; Ballentine, R.M.; Brown, A.P.; McKinney, W.J. An evaluation of electronic cigarette formulations and aerosols for harmful and potentially harmful constituents (HPHCs) typically derived from combustion. Regul. Toxicol. Pharmacol. 2018, 95, 153–160. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, M.; Hastings, G.; Angus, K. Promotion of electronic cigarettes: Tobacco marketing reinvented? BMJ 2013, 347, f7473. [Google Scholar] [CrossRef] [PubMed]
- Pepper, J.K.; Ribisl, K.M.; Emery, S.L.; Brewer, N.T. Reasons for starting and stopping electronic cigarette use. Int. J. Environ. Res. Public Health 2014, 11, 10345–10361. [Google Scholar] [CrossRef]
- Rutten, L.J.F.; Blake, K.D.; Agunwamba, A.A.; Grana, R.A.; Wilson, P.M.; Ebbert, J.O.; Okamoto, J.; Leischow, S.J. Use of E-Cigarettes Among Current Smokers: Associations Among Reasons for Use, Quit Intentions, and Current Tobacco Use. Nicotine Tob. Res. 2015, 17, 1228–1234. [Google Scholar] [CrossRef]
- Eddingsaas, N.; Pagano, T.; Cummings, C.; Rahman, I.; Robinson, R.; Hensel, E. Qualitative Analysis of E-Liquid Emissions as a Function of Flavor Additives Using Two Aerosol Capture Methods. Int. J. Environ. Res. Public Health 2018, 15, 323. [Google Scholar] [CrossRef]
- Kosmider, L.; Sobczak, A.; Fik, M.; Knysak, J.; Zaciera, M.; Kurek, J.; Goniewicz, M.L. Carbonyl compounds in electronic cigarette vapors: Effects of nicotine solvent and battery output voltage. Nicotine Tob. Res. 2014, 16, 1319–1326. [Google Scholar] [CrossRef]
- Dai, J.; Kim, K.H.; Szulejko, J.E.; Jo, S.H. A simple method for the parallel quantification of nicotine and major solvent components in electronic cigarette liquids and vaped aerosols. Microchem. J. 2017, 133, 237–245. [Google Scholar] [CrossRef]
- Cirillo, S.; Vivarelli, F.; Turrini, E.; Fimognari, C.; Burattini, S.; Falcieri, E.; Rocchi, M.B.L.; Cardenia, V.; Rodriguez-Estrada, M.T.; Paolini, M.; et al. The Customizable E-cigarette Resistance Influences Toxicological Outcomes: Lung Degeneration, Inflammation, and Oxidative Stress-Induced in a Rat Model. Toxicol. Sci. 2019, 172, 132–145. [Google Scholar] [CrossRef]
- Cancelada, L.; Tang, X.; Russell, M.L.; Maddalena, R.L.; Litter, M.I.; Gundel, L.A.; Destaillats, H. Volatile aldehyde emissions from “sub-ohm” vaping devices. Environ. Res. 2021, 197, 111188. [Google Scholar] [CrossRef]
- Krüsemann, E.J.Z.; Pennings, J.L.A.; Cremers, J.W.J.M.; Bakker, F.; Boesveldt, S.; Talhout, R. GC-MS analysis of e-cigarette refill solutions: A comparison of flavoring composition between flavor categories. J. Pharm. Biomed. Anal. 2020, 188, 113364. [Google Scholar] [CrossRef] [PubMed]
- Aszyk, J.; Kubica, P.; Kot-Wasik, A.; Namieśnik, J.; Wasik, A. Comprehensive determination of flavouring additives and nicotine in e-cigarette refill solutions. Part I: Liquid chromatography-tandem mass spectrometry analysis. J. Chromatogr. A 2017, 1519, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Poklis, J.L.; Wolf, C.E.; Peace, M.R. Ethanol concentration in 56 refillable electronic cigarettes liquid formulations determined by headspace gas chromatography with flame ionization detector (HS-GC-FID). Drug Test. Anal. 2017, 9, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- LeBouf, R.F.; Burns, D.A.; Ranpara, A.; Attfield, K.; Zwack, L.; Stefaniak, A.B. Headspace analysis for screening of volatile organic compound profiles of electronic juice bulk material. Anal. Bioanal. Chem. 2018, 410, 5951–5960. [Google Scholar] [CrossRef] [PubMed]
- Palmisani, J.; Abenavoli, C.; Famele, M.; Di Gilio, A.; Palmieri, L.; de Gennaro, G.; Draisci, R. Chemical Characterization of Electronic Cigarette (e-cigs) Refill Liquids Prior to EU Tobacco Product Directive Adoption: Evaluation of BTEX Contamination by HS-SPME-GC-MS and Identification of Flavoring Additives by GC-MS-O. Atmosphere 2020, 11, 374. [Google Scholar] [CrossRef]
- Geiss, O.; Bianchi, I.; Barahona, F.; Barrero-Moreno, J. Characterisation of mainstream and passive vapours emitted by selected electronic cigarettes. Int. J. Hyg. Environ. Health 2015, 218, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Tayyarah, R.; Long, G.A. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul. Toxicol. Pharmacol. 2014, 70, 704–710. [Google Scholar] [CrossRef]
- Klager, S.; Vallarino, J.; MacNaughton, P.; Christiani, D.C.; Lu, Q.; Allen, J.G. Flavoring Chemicals and Aldehydes in E-Cigarette Emissions. Environ. Sci. Technol. 2017, 51, 10806–10813. [Google Scholar] [CrossRef]
- Allen, J.G.; Flanigan, S.S.; LeBlanc, M.; Vallarino, J.; MacNaughton, P.; Stewart, J.H.; Christiani, D.C. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ. Health Perspect. 2016, 124, 733–739. [Google Scholar] [CrossRef]
- Pankow, J.F.; Kim, K.; McWhirter, K.J.; Luo, W.; Escobedo, J.O.; Strongin, R.M.; Duell, A.K.; Peyton, D.H. Benzene formation in electronic cigarettes. PLoS ONE 2017, 12, e0173055. [Google Scholar] [CrossRef]
- Pellegrino, R.M.; Tinghino, B.; Mangiaracina, G.; Marani, A.; Vitali, M.; Protano, C.; Osborn, J.F.; Cattaruzza, M.S. Electronic cigarettes: An evaluation of exposure to chemicals and fine particulate matter (PM). Ann. Ig. 2012, 24, 279–288. [Google Scholar] [PubMed]
- Rawlinson, C.; Martin, S.; Frosina, J.; Wright, C. Chemical characterisation of aerosols emitted by electronic cigarettes using thermal desorption-gas chromatography-time of flight mass spectrometry. J. Chromatogr. A 2017, 1497, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, M.; Logue, J.M.; Montesinos, V.N.; Russell, M.L.; Litter, M.I.; Gundel, L.A.; Destaillats, H. Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals. Environ. Sci. Technol. 2016, 50, 9644–9651. [Google Scholar] [CrossRef] [PubMed]
- Peace, M.R.; Krakowiak, R.I.; Wolf, C.E.; Poklis, A.; Poklis, J.L. Identification of MDMB-FUBINACA in commercially available e-liquid formulations sold for use in electronic cigarettes. Forensic Sci. Int. 2017, 271, 92–97. [Google Scholar] [CrossRef]
- McAdam, K.; Waters, G.; Moldoveanu, S.C.; Margham, J.; Cunningham, A.; Vas, C.; Porter, A.; Digard, H. Diacetyl and Other Ketones in e-Cigarette Aerosols: Some Important Sources and Contributing Factors. Front. Chem. 2021, 9, 742538. [Google Scholar] [CrossRef] [PubMed]
- Noël, J.C.; Ruzsanyi, V.; Rainer, M.; Bonn, G. Investigation of the evaporation behavior of aroma compounds in e-cigarettes. Anal. Bioanal. Chem. 2019, 411, 3029–3035. [Google Scholar] [CrossRef]
- Trehy, M.L.; Ye, W.; Hadwiger, M.E.; Moore, T.W.; Allgire, J.F.; Woodruff, J.T.; Ahadi, S.S.; Black, J.C.; Westenberger, B.J. Analysis of electronic cigarette cartridges, refill solutions and smoke for nicotine and nicotine related impurities. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1442–1458. [Google Scholar] [CrossRef]
- McAuley, T.R.; Hopke, P.K.; Zhao, J.; Babaian, S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal. Toxicol. 2012, 24, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Kuma, T.; Gawron, M.; Knysak, J.; Kosmider, L. Nicotine levels in electronic cigarettes. Nicotine Tob. Res. 2013, 15, 158–166. [Google Scholar] [CrossRef]
- Ho, S.S.H.; Ho, K.F.; Liu, W.D.; Lee, S.C.; Dai, W.T.; Cao, J.J.; Ip, H. Unsuitability of using the DNPH-coated solid sorbent cartridge for determination of airborne unsaturated carbonyls. Atmos. Environ. 2011, 45, 261–265. [Google Scholar] [CrossRef]
- Flora, J.W.; Wilkinson, C.T.; Wilkinson, J.W.; Lipowicz, P.J.; Skapars, J.A.; Anderson, A.; Miller, J.H. Method for the Determination of Carbonyl Compounds in E-Cigarette Aerosols. J. Chromatogr. Sci. 2017, 55, 142–148. [Google Scholar] [CrossRef]
- Dai, J.; Kim, K.H.; Szulejko, J.E.; Jo, S.H.; Kwon, K.; Choi, D.W. Quantification of nicotine and major solvents in retail electronic cigarette fluids and vaped aerosols. Microchem. J. 2018, 140, 262–268. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Voudris, V.; Poulas, K. E-cigarettes generate high levels of aldehydes only in ‘dry puff’ conditions. Addiction 2015, 110, 1352–1356. [Google Scholar] [CrossRef]
- Smets, J.; Baeyens, F.; Chaumont, M.; Adriaens, K.; van Gucht, D. When Less is More: Vaping Low-Nicotine vs. High-Nicotine E-Liquid is Compensated by Increased Wattage and Higher Liquid Consumption. Int. J. Environ. Res. Public Health 2019, 16, 723. [Google Scholar] [CrossRef]
- Li, Y.; Burns, A.E.; Tran, L.N.; Abellar, K.A.; Poindexter, M.; Li, X.; Madl, A.K.; Pinkerton, K.E.; Nguyen, T.B. Impact of e-Liquid Composition, Coil Temperature, and Puff Topography on the Aerosol Chemistry of Electronic Cigarettes. Chem. Res. Toxicol. 2021, 34, 1640–1654. [Google Scholar] [CrossRef]
- Louw, S. Recent trends in the chromatographic analysis of volatile flavor and fragrance compounds: Annual review 2020. Anal. Sci. Adv. 2021, 2, 157–170. [Google Scholar] [CrossRef]
- Behar, R.Z.; Davis, B.; Wang, Y.; Bahl, V.; Lin, S.; Talbot, P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol. Vitr. 2014, 28, 198–208. [Google Scholar] [CrossRef]
- Logue, J.M.; Sleiman, M.; Montesinos, V.N.; Russell, M.L.; Litter, M.I.; Benowitz, N.L.; Gundel, L.A.; Destaillats, H. Emissions from Electronic Cigarettes: Assessing Vapers’ Intake of Toxic Compounds, Secondhand Exposures, and the Associated Health Impacts. Environ. Sci. Technol. 2017, 51, 9271–9279. [Google Scholar] [CrossRef] [PubMed]
- St Helen, G.; Liakoni, E.; Nardone, N.; Addo, N.; Jacob, P.; Benowitz, N.L. Comparison of Systemic Exposure to Toxic and/or Carcinogenic Volatile Organic Compounds (VOC) during Vaping, Smoking, and Abstention. Cancer Prev. Res. 2020, 13, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, N.; Birkenmeier, M.; Sanders, D.; Rohn, S.; Weller, P. Resolution-optimized headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS) for non-targeted olive oil profiling. Anal. Bioanal. Chem. 2017, 409, 3933–3942. [Google Scholar] [CrossRef]
- Eiceman, G.A. Ion-mobility spectrometry as fast monitor of chemical composition. TrAC Trends Anal. Chem. 2002, 21, 259–275. [Google Scholar] [CrossRef]
- Cumeras, R.; Figueras, E.; Davis, C.E.; Baumbach, J.I.; Gràcia, I. Review on ion mobility spectrometry. Part 1: Current instrumentation. Analyst 2015, 140, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Vautz, W.; Schwarz, L.; Hariharan, C.B.; Schilling, M. Ion characterisation by comparison of ion mobility spectrometry and mass spectrometry data. Int. J. Ion Mobil. Spec. 2010, 13, 121–129. [Google Scholar] [CrossRef]
- Snyder, A.P.; Harden, C.S.; Brittain, A.H.; Kim, M.G.; Arnold, N.S.; Meuzelaar, H.L.C. Portable hand-held gas chromatography/ion mobility spectrometry device. Anal. Chem. 1993, 65, 299–306. [Google Scholar] [CrossRef]
- Ewing, R.G.; Eiceman, G.A.; Stone, J.A. Proton-bound cluster ions in ion mobility spectrometry. Int. J. Mass Spectrom. 1999, 193, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.B.; Brokenshire, J.L. Hand-held ion mobility spectrometers. TrAC Trends Anal. Chem. 1994, 13, 275–280. [Google Scholar] [CrossRef]
- Ewing, R.G.; Atkinson, D.A.; Eiceman, G.A.; Ewing, G.J. A critical review of ion mobility spectrometry for the detection of explosives and explosive related compounds. Talanta 2001, 54, 515–529. [Google Scholar] [CrossRef]
- Cottingham, K. Product Review: Ion mobility spectrometry rediscovered. Anal. Chem. 2003, 75, 435-A–439-A. [Google Scholar] [CrossRef]
- Mäkinen, M.A.; Anttalainen, O.A.; Sillanpää, M.E.T. Ion mobility spectrometry and its applications in detection of chemical warfare agents. Anal. Chem. 2010, 82, 9594–9600. [Google Scholar] [CrossRef]
- Márquez-Sillero, I.; Aguilera-Herrador, E.; Cárdenas, S.; Valcárcel, M. Ion-mobility spectrometry for environmental analysis. TrAC Trends Anal. Chem. 2011, 30, 677–690. [Google Scholar] [CrossRef]
- Holopainen, S.; Luukkonen, V.; Nousiainen, M.; Sillanpää, M.E.T. Determination of chlorophenols in water by headspace solid phase microextraction ion mobility spectrometry (HS-SPME-IMS). Talanta 2013, 114, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Haley, L.V.; Romeskie, J.M. GC-IMS: A technology for many applications. In Enforcement and Security Technologies; DePersia, A.T., Pennella, J.J., Eds.; SPIE: Bellingham, WA, USA, 1998; pp. 375–383. [Google Scholar] [CrossRef]
- Cook, G.W.; LaPuma, P.T.; Hook, G.L.; Eckenrode, B.A. Using gas chromatography with ion mobility spectrometry to resolve explosive compounds in the presence of interferents. J. Forensic Sci. 2010, 55, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Kwan, C.; Snyder, A.P.; Erickson, R.P.; Smith, P.A.; Maswadeh, W.M.; Ayhan, B.; Jensen, J.L.; Jensen, J.O.; Tripathi, A. Chemical Agent Detection Using GC-IMS: A Comparative Study. IEEE Sens. J. 2010, 10, 451–460. [Google Scholar] [CrossRef]
- Vera, L.; Companioni, E.; Meacham, A.; Gygax, H. Real Time Monitoring of VOC and Odours Based on GC-IMS at Wastewater Treatment Plants. Chem. Eng. Trans. 2016, 54, 74–84. [Google Scholar] [CrossRef]
- Jurado-Campos, N.; Martín-Gómez, A.; Saavedra, D.; Arce, L. Usage considerations for headspace-gas chromatography-ion mobility spectrometry as a suitable technique for qualitative analysis in a routine lab. J. Chromatogr. A 2021, 1640, 461937. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Martín-Gómez, A.; Jurado-Campos, N.; Garrido-Delgado, R.; Arce, C.; Arce, L. Target vs spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace-gas chromatography-ion mobility spectrometry. Food Chem. 2018, 246, 65–73. [Google Scholar] [CrossRef]
- Gerhardt, N.; Birkenmeier, M.; Schwolow, S.; Rohn, S.; Weller, P. Volatile-Compound Fingerprinting by Headspace-Gas-Chromatography Ion-Mobility Spectrometry (HS-GC-IMS) as a Benchtop Alternative to 1H NMR Profiling for Assessment of the Authenticity of Honey. Anal. Chem. 2018, 90, 1777–1785. [Google Scholar] [CrossRef]
- Valli, E.; Panni, F.; Casadei, E.; Barbieri, S.; Cevoli, C.; Bendini, A.; García-González, D.L.; Gallina Toschi, T. An HS-GC-IMS Method for the Quality Classification of Virgin Olive Oils as Screening Support for the Panel Test. Foods 2020, 9, 657. [Google Scholar] [CrossRef]
- Piotr Konieczka, P.; Aliaño-González, M.J.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Characterization of Arabica and Robusta Coffees by Ion Mobility Sum Spectrum. Sensors 2020, 20, 3123. [Google Scholar] [CrossRef]
- Budzyńska, E.; Sielemann, S.; Puton, J.; Surminski, A.L.R.M. Analysis of e-liquids for electronic cigarettes using GC-IMS/MS with headspace sampling. Talanta 2020, 209, 120594. [Google Scholar] [CrossRef]
- Augustini, A.L.R.M.; Sielemann, S.; Telgheder, U. Strategy for the identification of flavor compounds in e-liquids by correlating the analysis of GCxIMS and GC-MS. Talanta 2021, 230, 122318. [Google Scholar] [CrossRef]
- Augustini, A.L.R.M.; Sielemann, S.; Telgheder, U. Quantitation of Flavor Compounds in Refill Solutions for Electronic Cigarettes Using HS-GCxIMS and Internal Standards. Molecules 2022, 27, 8067. [Google Scholar] [CrossRef] [PubMed]
- Kanu, A.B.; Hill, H.H. Ion mobility spectrometry detection for gas chromatography. J. Chromatogr. A 2008, 1177, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Hewitt, C. Thermal desorption efficiencies for different adsorbate/adsorbent systems typically used in air monitoring programmes. Chemosphere 1993, 27, 695–705. [Google Scholar] [CrossRef]
- Flora, J.W.; Meruva, N.; Huang, C.B.; Wilkinson, C.T.; Ballentine, R.M.; Smith, D.C.; Werley, M.S.; McKinney, W.J. Characterization of potential impurities and degradation products in electronic cigarette formulations and aerosols. Regul. Toxicol. Pharmacol. 2016, 74, 1–11. [Google Scholar] [CrossRef]
- Hua, M.; Yip, H.; Talbot, P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tob. Control 2013, 22, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Talih, S.; Balhas, Z.; Eissenberg, T.; Salman, R.; Karaoghlanian, N.V.; El Hellani, A.; Baalbaki, R.; Saliba, N.A.; Shihadeh, A.L. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: Measurements and model predictions. Nicotine Tob. Res. 2015, 17, 150–157. [Google Scholar] [CrossRef]
- Geiss, O.; Bianchi, I.; Barrero-Moreno, J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int. J. Hyg. Environ. Health 2016, 219, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Taudte, R.V.; Nizio, K.; Herok, G.; Cranfield, C.; Shimmon, R. Headspace analysis of E-cigarette fluids using comprehensive two dimensional GC×GC-TOF-MS reveals the presence of volatile and toxic compounds. J. Pharm. Biomed. Anal. 2021, 196, 113930. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Jabba, S.V.; DeWinter, T.M.; Mendizabal, M.; Anastas, P.T.; Jordt, S.E.; Zimmerman, J.B. Formation of flavorant-propylene Glycol Adducts With Novel Toxicological Properties in Chemically Unstable E-Cigarette Liquids. Nicotine Tob. Res. 2019, 21, 1248–1258. [Google Scholar] [CrossRef]
- van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Benkwitz, F.; Sarmadi, B.; Kilmartin, P.A. Validation Study on the Simultaneous Quantitation of Multiple Wine Aroma Compounds with Static Headspace-Gas Chromatography-Ion Mobility Spectrometry. J. Agric. Food Chem. 2021, 69, 15020–15035. [Google Scholar] [CrossRef] [PubMed]
- Brendel, R.; Schwolow, S.; Rohn, S.; Weller, P. Comparison of PLSR, MCR-ALS and Kernel-PLSR for the quantification of allergenic fragrance compounds in complex cosmetic products based on nonlinear 2D GC-IMS data. Chemom. Intell. Lab. Syst. 2020, 205, 104128. [Google Scholar] [CrossRef]







| Label | Name | Rt (MS) | Rt (IMS) | ||
|---|---|---|---|---|---|
| /min | /min | /Vs/cm2 | /Vs/cm2 | ||
| 1 | 2-Butanoneistd | 2.34 | 5.28 | 1.85 | 1.55 |
| 2 | Ethyl acetateref | 2.49 | 5.59 | 1.79 | 1.44 |
| 3 | 2-Pentanoneistd | 3.52 | 7.28 | 1.75 | 1.40 |
| PG | Propylene glycolmatrix | 4.67 | 9.13 | 1.77 | 1.53 |
| 4 | 2-Hexanoneistd | 6.22 | 10.42 | 1.64 | 1.28 |
| 5 | Allyloxy-2-propanollib | 8.14 | 12.35 | 1.70 | 1.28 |
| 6 | Ethyl 2-methylbutanoateref | 8.28 | 12.47 | 1.59 | 1.16 |
| 7 | Ethyl 3-methylbutanoateref | 8.39 | 12.57 | 1.56 | 1.16 |
| 8 | Ethyl 2-allyloxypropanoatelib | 8.73 | 12.95 | 1.54 | 1.27 |
| 9 | 2-Heptanoneistd | 9.69 | 13.87 | 1.54 | 1.18 |
| 10 | -Pineneref | 11.32 | 15.60 | 1.60 | |
| 11 | Benzaldehyderef | 12.22 | 16.55 | 1.54 | 1.33 |
| 12 | 2-Octanoneistd | 13.23 | 20.63 | 1.46 | 1.09 |
| 13 | -Pineneref | 14.45 | 18.33 | 1.60 | |
| 14 | o-Cymeneref | 14.46 | 18.58 | 1.60 | |
| 15 | Limoneneref | 14.61 | 20.42 | 1.59 | 1.16/1.12 |
| 16 | Benzyl alcoholref | 14.69 | 18.87 | 1.49 | 1.07 |
| 17 | Ethyl levulatelib | 15.58 | 19.70 | 1.62 | |
| 18 | Diethyl malonatelib | 15.88 | 19.90 | 1.55 | |
| 19 | 2-Nonanoneistd | 16.61 | 20.63 | 1.39 | 1.00 |
| 20 | Pentyl butanoatelib | 16.65 | 20.67 | 1.39 | 0.98 |
| 21 | Linaloolref | 16.88 | 20.95 | 1.59 | 1.16/1.13/1.09 |
| 22 | Iso-amyl isovaleratelib | 17.05 | 21.10 | 1.35 | 0.94 |
| 23 | Menthoneref | 18.74 | 22.92 | 1.49 | 1.04 |
| 24 | Menthoneref | 18.74 | 23.28 | 1.45 | 1.03 |
| 25 | 2-Decanoneistd | 19.79 | 23.97 | 1.32 | 0.96 |
| 26 | Methyl salicylatelib | 20.00 | 24.27 | 1.62 | |
| 27 | Linalyl acetatelib | 21.66 | 26.12 | 1.59 | |
| 28 | Ethyl 3-(2-methyl-1.3-dioxan-2-yl)propanoatelib | 22.62 | 27.55 | 1.44 | 1.00 |
| 29 | Ethyl 3-(2.4-dimethyl-1.3-dioxolan-2-yl)propanoatelib | 22.82 | 27.77 | 1.39 |
| GCxIMS | GC-MS | ||||||
|---|---|---|---|---|---|---|---|
| Name | Range | RMSE | RE | r2 | RMSE | RE | r2 |
| /µg/g | /µg/g | /% | /µg/g | /% | |||
| 2-Methylpropanal a | 0.18–8.1 | 0.53 | 11.45 | 0.973 | 0.51 | 5.43 | 0.992 |
| Diacetyl a | 0.24–11.0 | 0.1 | 1.68 | 0.999 | 0.29 | 2.29 | 0.999 |
| Ethyl acetate | 0.21–19.6 | 0.36 | 3.6 | 0.997 | 0.71 | 7.08 | 0.990 |
| Isobutanol | 0.19–17.6 | 0.23 | 2.57 | 0.999 | 0.45 | 4.93 | 0.995 |
| 2-Methylbutanal | 0.19–18.0 | 0.67 | 7.21 | 0.990 | 0.08 | 0.86 | 1.000 |
| Isoamyl alcohol b | 0.20–18.2 | - | - | - | 0.67 | 7.16 | 0.990 |
| Methyl isobutyl ketone b | 0.19–17.9 | - | - | - | 0.11 | 1.21 | 1.000 |
| 2,3-Hexanedione b | 0.22–20.6 | - | - | - | 0.36 | 3.38 | 0.998 |
| Hexanal | 0.20–18.7 | 1.39 | 14.33 | 0.960 | 0.11 | 1.14 | 1.000 |
| Butyl acetate | 0.22–20.0 | 0.58 | 5.62 | 0.994 | 0.14 | 1.36 | 1.000 |
| Furfural | 0.28–26.2 | 0.92 | 6.78 | 0.991 | 0.46 | 3.39 | 0.998 |
| Ethyl 2-methyl butanoate | 0.20–18.3 | 0.02 | 0.2 | 1.000 | 0.20 | 2.13 | 0.999 |
| (E)-2-Hexenal | 0.14–13.4 | 0.45 | 6.6 | 0.991 | 0.05 | 0.70 | 1.000 |
| (E)-2-Hexen-1-ol | 0.20–18.7 | 0.64 | 6.64 | 0.991 | 0.44 | 4.60 | 0.996 |
| Ethyl pentanoate | 0.21–19.5 | 0.5 | 5.01 | 0.995 | 0.24 | 2.44 | 0.999 |
| Methyl hexanoate | 0.21–19.7 | 0.63 | 6.27 | 0.992 | 0.25 | 2.53 | 0.999 |
| (+)--Pinene | 0.21–19.2 | 1.3 | 13.13 | 0.966 | 0.29 | 3.00 | 0.998 |
| Benzaldehyde | 0.26–23.7 | 0.62 | 5.13 | 0.995 | 0.37 | 3.03 | 0.998 |
| 1-Octen-3-one | 0.20–18.9 | 0.67 | 6.88 | 0.991 | 0.20 | 2.02 | 0.999 |
| Octanal | 0.20–18.7 | 1.00 | 10.36 | 0.979 | 0.11 | 1.20 | 1.000 |
| (E,E)-2,4-Heptadienal | 0.19–17.4 | 0.86 | 9.59 | 0.982 | 0.31 | 3.43 | 0.998 |
| D(+)-Limonene | 0.20–18.9 | 1.54 | 15.71 | 0.952 | 0.47 | 4.86 | 0.995 |
| 1-Octanol | 0.20–18.9 | 0.31 | 3.23 | 0.998 | 0.41 | 4.19 | 0.997 |
| Linalool | 0.21–19.1 | 0.63 | 6.45 | 0.992 | 0.38 | 3.87 | 0.997 |
| L-Menthone a | 0.18–16.5 | 0.25 | 2.99 | 0.998 | 0.20 | 2.35 | 0.999 |
| D-Menthone a | 0.04–3.4 | 0.06 | 3.65 | 0.997 | 0.06 | 3.23 | 0.998 |
| D/L-Menthol | 0.19–18.0 | 0.23 | 2.47 | 0.999 | 0.41 | 4.41 | 0.996 |
| Neral a | 0.47–9.3 | 0.53 | 9.81 | 0.975 | 0.30 | 5.62 | 0.992 |
| Geranial a | 0.12–10.9 | 1.51 | 23.51 | 0.868 | 0.35 | 5.52 | 0.992 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augustini, A.L.R.M.; Borg, C.; Sielemann, S.; Telgheder, U. Making Every Single Puff Count—Simple and Sensitive E-Cigarette Aerosol Sampling for GCxIMS and GC-MS Analysis. Molecules 2023, 28, 6574. https://doi.org/10.3390/molecules28186574
Augustini ALRM, Borg C, Sielemann S, Telgheder U. Making Every Single Puff Count—Simple and Sensitive E-Cigarette Aerosol Sampling for GCxIMS and GC-MS Analysis. Molecules. 2023; 28(18):6574. https://doi.org/10.3390/molecules28186574
Chicago/Turabian StyleAugustini, Alexander L. R. M., Christopher Borg, Stefanie Sielemann, and Ursula Telgheder. 2023. "Making Every Single Puff Count—Simple and Sensitive E-Cigarette Aerosol Sampling for GCxIMS and GC-MS Analysis" Molecules 28, no. 18: 6574. https://doi.org/10.3390/molecules28186574






