Solvent-Controlled Morphology of Zinc–Cobalt Bimetallic Sulfides for Supercapacitors
Abstract
:1. Introduction
2. Results and Discussion
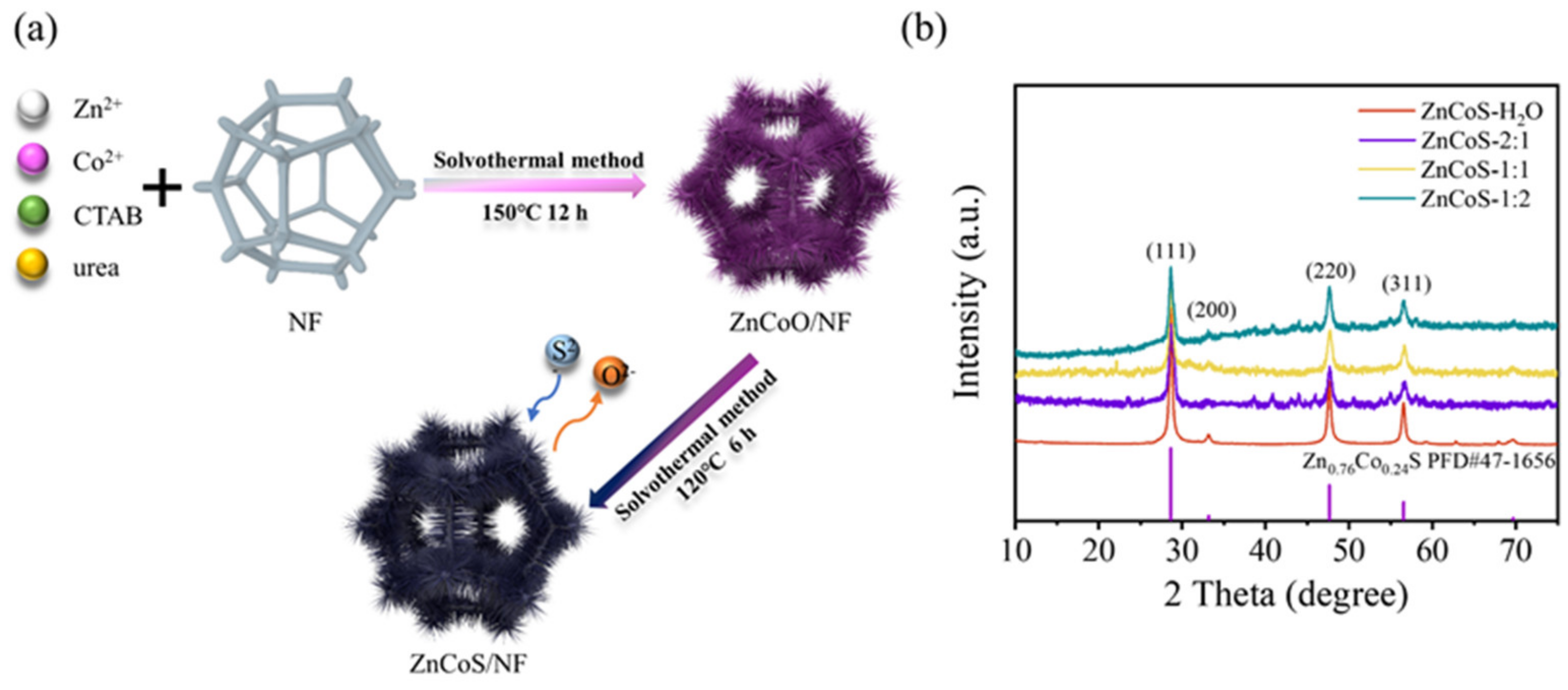
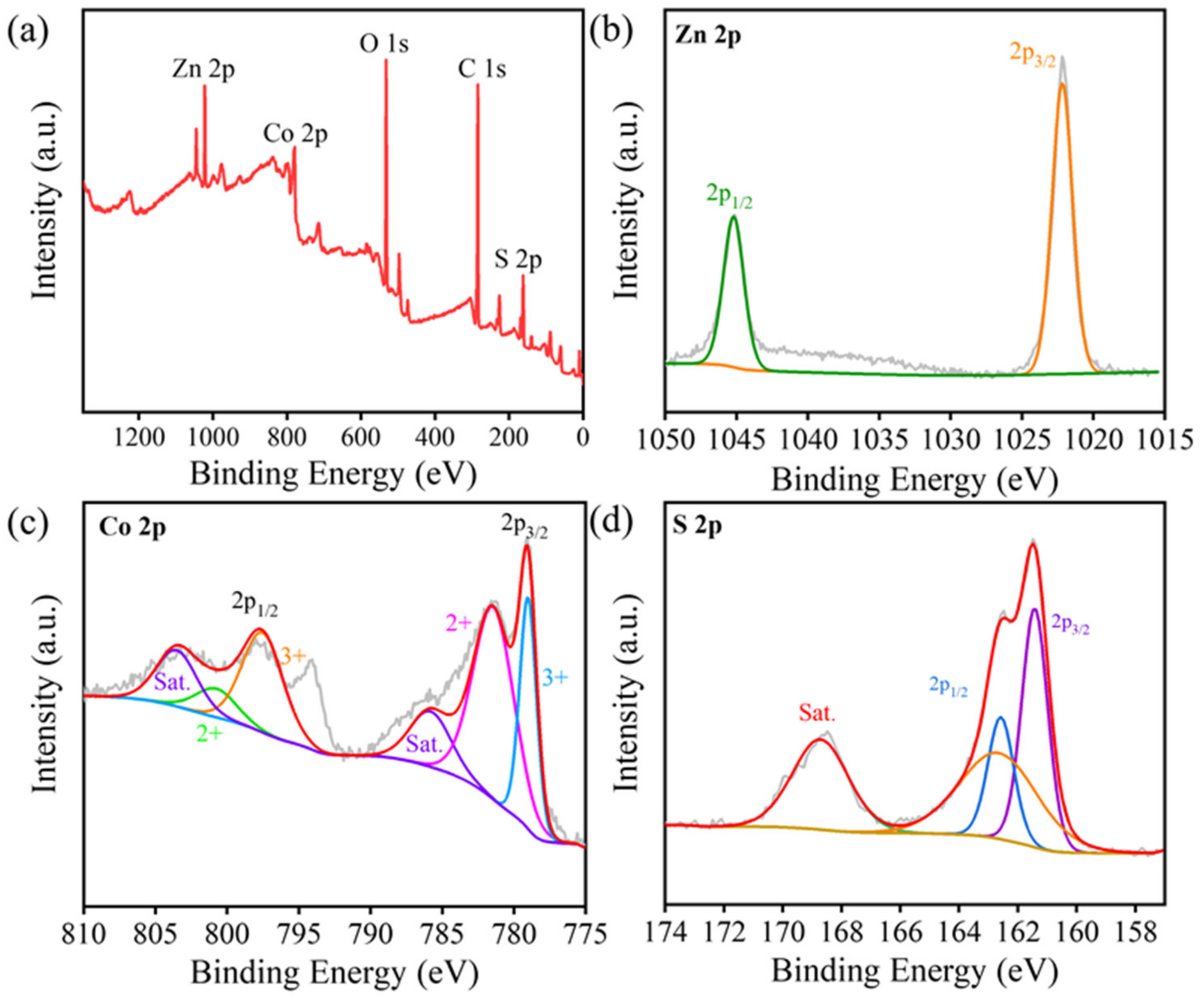
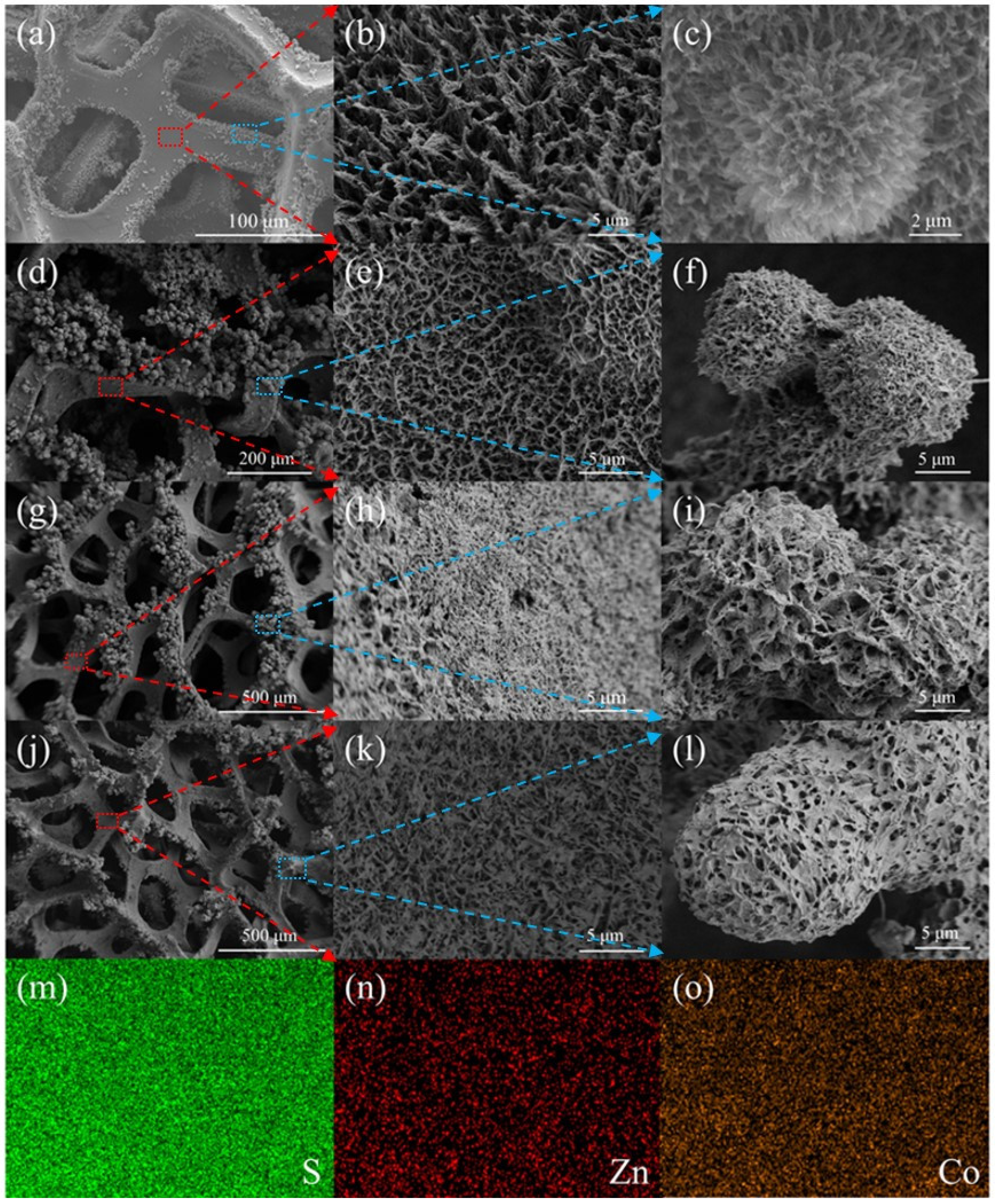
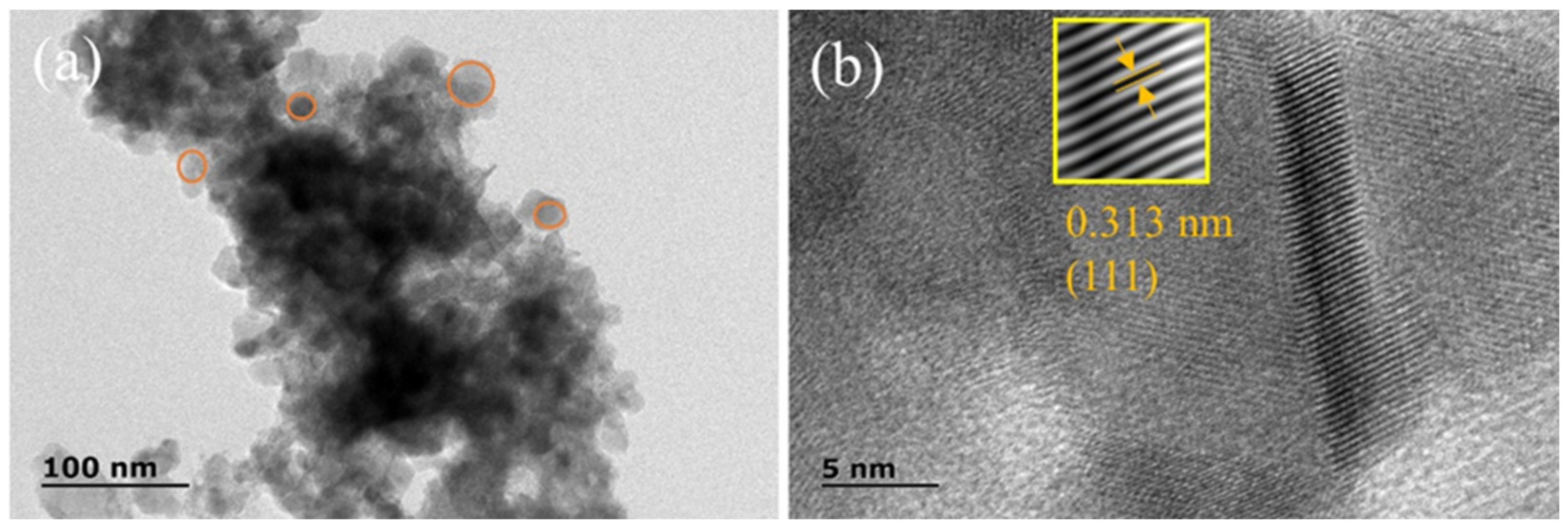
2.1. Morphology and Structure Analysis
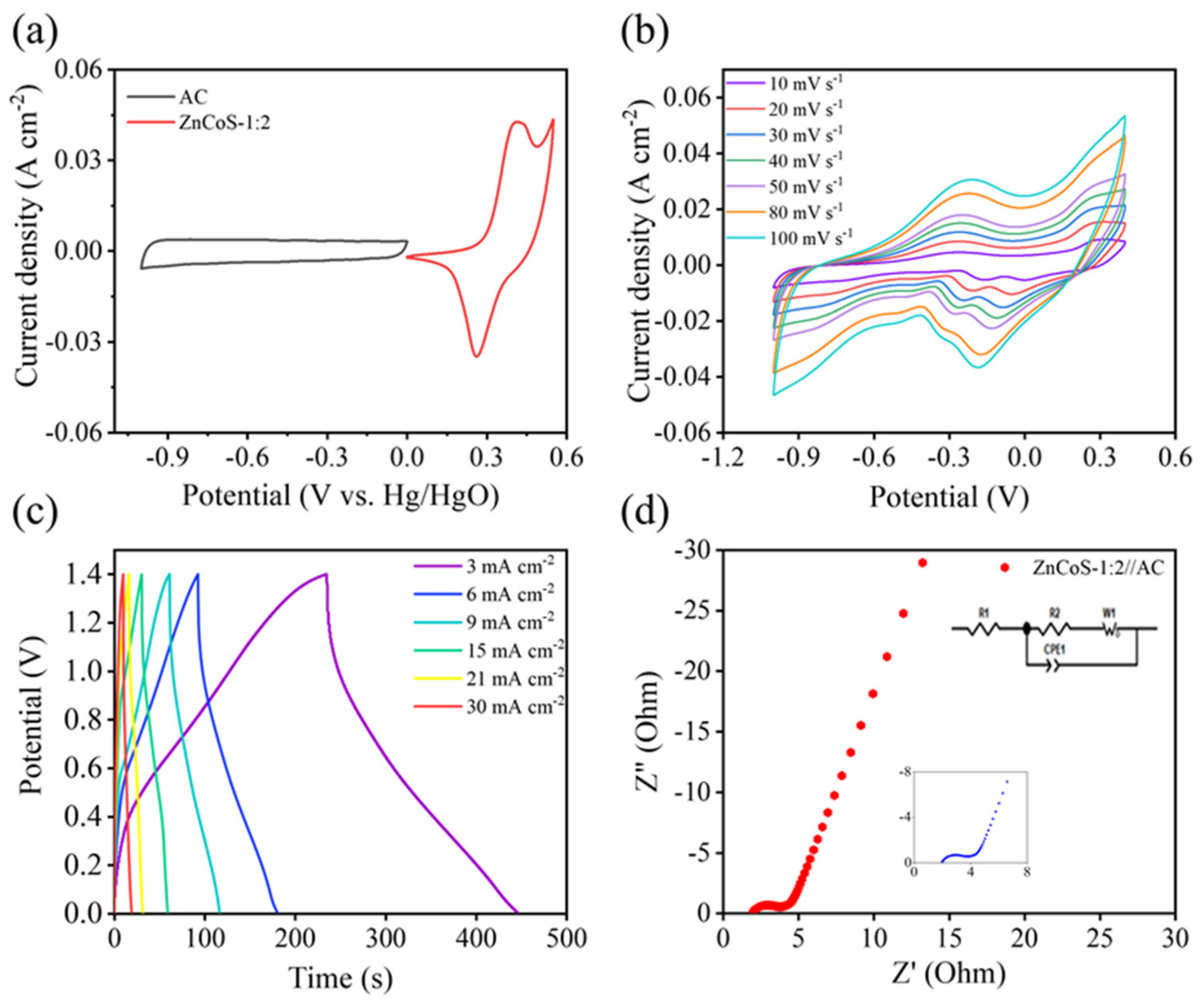
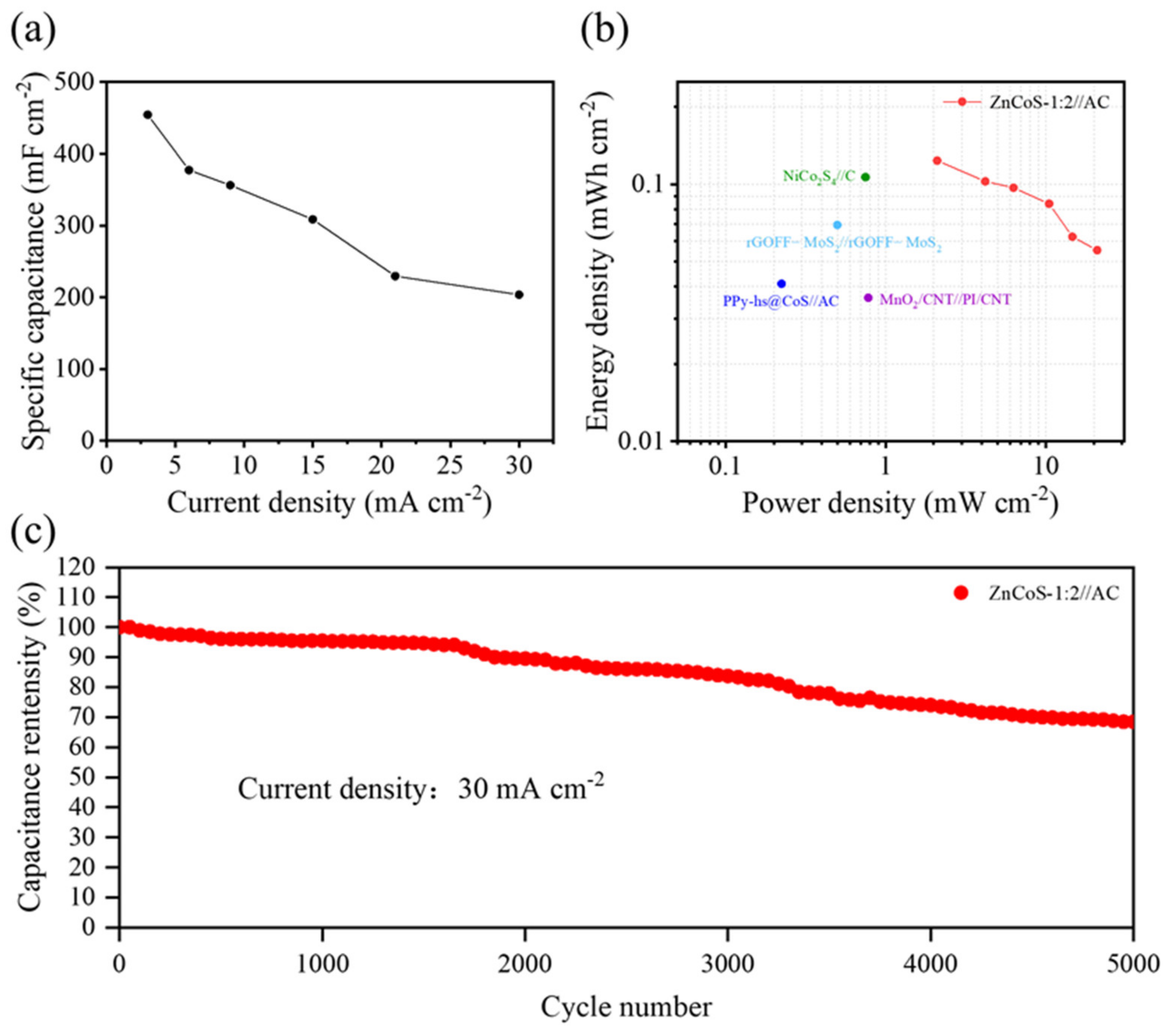
2.2. Electrochemical Characterization
3. Experimental
3.1. Chemicals and Materials
3.2. Material Preparation
3.3. Material Characterization
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yin, Y.; Liu, Q.; Zhao, Y.; Chen, T.; Wang, J.; Gui, L.; Lu, C. Recent Progress and Future Directions of Biomass-Derived Hierarchical Porous Carbon: Designing, Preparation, and Supercapacitor Applications. Energy Fuels 2023, 37, 3523–3554. [Google Scholar] [CrossRef]
- Roy, B.K.; Tahmid, I.; Rashid, T.U. Chitosan-based materials for supercapacitor applications: A review. J. Mater. Chem. A 2021, 9, 17592–17642. [Google Scholar] [CrossRef]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Sowjanya, V.; Dhananjaya, M.; Reddy, N.R.; Jyothi, N.; Jung, J.H.; Joo, S.W. CoNi bimetallic engineering on porous carbon for high energy density hybrid supercapacitors. J. Alloys Compd. 2023, 947, 169464. [Google Scholar] [CrossRef]
- Ouyang, Y.; Xia, X.; Ye, H.; Wang, L.; Jiao, X.; Lei, W.; Hao, Q. Three-Dimensional Hierarchical Structure ZnO@C@NiO on Carbon Cloth for Asymmetric Supercapacitor with Enhanced Cycle Stability. ACS Appl. Mater. Interfaces 2018, 10, 3549–3561. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, Z.; Ye, M.; Shen, J. Nanoconfined Space: Revisiting the Charge Storage Mechanism of Electric Double Layer Capacitors. ACS Appl. Mater. Interfaces 2022, 14, 37259–37269. [Google Scholar] [CrossRef]
- Yusran, Y.; Li, H.; Guan, X.; Li, D.; Tang, L.; Xue, M.; Zhuang, Z.; Yan, Y.; Valtchev, V.; Qiu, S.; et al. Exfoliated Mesoporous 2D Covalent Organic Frameworks for High-Rate Electrochemical Double-Layer Capacitors. Adv. Mater. 2020, 32, 1907289. [Google Scholar] [CrossRef]
- Zhu, X. Recent advances of transition metal oxides and chalcogenides in pseudo-capacitors and hybrid capacitors: A review of structures, synthetic strategies, and mechanism studies. J. Energy Storage 2022, 49, 104148. [Google Scholar] [CrossRef]
- Chen, T.; Li, M.; Song, S.; Kim, P.; Bae, J. Biotemplate preparation of multilayered TiC nanoflakes for high performance symmetric supercapacitor. Nano Energy 2020, 71, 104549. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Li, S.; Si, P.; Ci, L. High-performance hybrid supercapacitor enabled by advantageous heterojunction boosted starfish-like ZnCo-S electrode. J. Alloys Compd. 2022, 928, 166997. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, K.; Chen, H.; Liu, H.; Yang, L.; Chen, Y.; Li, H. ZIF-67 derived in-situ grown N–Co3S4-GN/CNT interlinked conductive networks for high-performance especially cycling stable supercapacitors. Carbon 2022, 194, 10–22. [Google Scholar] [CrossRef]
- He, W.; Wang, C.; Li, H.; Deng, X.; Xu, X.; Zhai, T. Ultrathin and Porous Ni3S2/CoNi2S4 3D-Network Structure for Superhigh Energy Density Asymmetric Supercapacitors. Adv. Energy Mater. 2017, 7, 1700983. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Z.; Liang, S.; Qin, H.; Zhao, X.; Chen, L.; Wang, H.; Chen, S. Two-dimensional porous zinc cobalt sulfide nanosheet arrays with superior electrochemical performance for supercapatteries. J. Mater. Sci. Technol. 2021, 89, 199–208. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Wu, Q. Facile synthesis of CoS porous nanoflake for high performance supercapacitor electrode materials. J. Energy Storage 2019, 23, 511–514. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, S.; Zhang, J.; Wang, J.; She, W.; Wang, K.; Xia, X.; Yang, B.; Meng, X. Facile solid−phase synthesis of layered NiS/rGO nanocomposite for high−performance hybrid supercapacitor. J. Power Sources 2021, 514, 230590. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.; Sun, Y.; Zhang, X.; Tang, L.; Zhang, X. Double-shell CuS nanocages as advanced supercapacitor electrode materials. J. Power Sources 2017, 355, 31–35. [Google Scholar] [CrossRef]
- Mohan, V.V.; Manuraj, M.; Anjana, P.M.; Rakhi, R.B. WS2 Nanoflowers as Efficient Electrode Materials for Supercapacitors. Energy Technol. 2022, 10, 2100976. [Google Scholar] [CrossRef]
- Teli, A.M.; Beknalkar, S.A.; Mane, S.M.; Bhat, T.S.; Kamble, B.B.; Patil, S.B.; Sadale, S.B.; Shin, J.C. Electrodeposited crumpled MoS2 nanoflakes for asymmetric supercapacitor. Ceram. Int. 2022, 48, 29002–29010. [Google Scholar] [CrossRef]
- Huang, T.; Song, X.; Chen, X.; Chen, X.; Sun, F.F.; Su, Q.F.; Li, L.; Tan, Z. Carbon coated nickel–cobalt bimetallic sulfides hollow dodecahedrons for a supercapacitor with enhanced electrochemical performance. New J. Chem. 2018, 42, 5128–5134. [Google Scholar] [CrossRef]
- Cao, J.; Hu, Y.; Zhu, Y.; Cao, H.; Fan, M.; Huang, C.; Shu, K.; He, M.; Chen, H.C. Synthesis of mesoporous nickel-cobalt-manganese sulfides as electroactive materials for hybrid supercapacitors. Chem. Eng. J. 2021, 405, 126928. [Google Scholar] [CrossRef]
- Yi, T.-F.; Pan, J.-J.; Wei, T.-T.; Li, Y.; Cao, G. NiCo2S4-based nanocomposites for energy storage in supercapacitors and batteries. Nano Today 2020, 33, 100894. [Google Scholar] [CrossRef]
- Yi, T.-F.; Chang, H.; Wei, T.-T.; Qi, S.-Y.; Li, Y.; Zhu, Y.-R. Approaching high-performance electrode materials of ZnCo2S4 nanoparticle wrapped carbon nanotubes for supercapacitors. J. Mater. 2021, 7, 563–576. [Google Scholar] [CrossRef]
- Singh, A.; Ojha, S.K.; Singh, M.; Ojha, A.K. Controlled synthesis of NiCo2S4@NiCo2O4 core@Shell nanostructured arrays decorated over the rGO sheets for high-performance asymmetric supercapacitor. Electrochim. Acta 2020, 349, 136349. [Google Scholar] [CrossRef]
- Chen, P.; Yang, C.; Gao, P.; Chen, X.; Cheng, Y.-J.; Liu, J.; Guo, K. Distinctive Formation of Bifunctional ZnCoS-rGO 3D Hollow Microsphere Flowers with Excellent Energy Storage Performances. Chem. Mater. 2022, 34, 5896–5911. [Google Scholar] [CrossRef]
- Xu, J.-M.; Wang, X.-C.; Cheng, J.-P. Supercapacitive Performances of Ternary CuCo2S4 Sulfides. ACS Omega 2020, 5, 1305–1311. [Google Scholar] [CrossRef]
- Vignesh, A.; Vajeeston, P.; Pannipara, M. Bimetallic metal-organic framework derived 3D hierarchical NiO/Co3O4/C hollow microspheres on biodegradable garbage bag for sensitive, selective, and flexible enzyme-free electrochemical glucose detection. Chem. Eng. J. 2022, 430, 133157. [Google Scholar] [CrossRef]
- Veerakumar, P.; Sangili, A.; Manavalan, S.; Thanasekaran, P.; Lin, K.-C. Research Progress on Porous Carbon Supported Metal/Metal Oxide Nanomaterials for Supercapacitor Electrode Applications. Ind. Eng. Chem. Res. 2020, 59, 6347–6374. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, L. Review on recent advances in nanostructured transition-metal-sulfide-based electrode materials for cathode materials of asymmetric supercapacitors. Chem. Eng. J. 2022, 430, 132745. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, H.; Wu, N.; Pan, Z.; Li, C.; Chen, Y.; Cao, Y.; Yang, W. Trimesic acid-modified. J. Alloys Compd. 2023, 934, 167779. [Google Scholar] [CrossRef]
- Wu, D.; Xie, X.; Ma, Y.; Zhang, J.; Hou, C.; Sun, X.; Yang, X.; Zhang, Y.; Kimura, H.; Du, W. Morphology controlled hierarchical NiS/carbon hexahedrons derived from nitrilotriacetic acid-assembly strategy for high-performance hybrid supercapacitors. Chem. Eng. J. 2022, 433, 133673. [Google Scholar] [CrossRef]
- Zou, J.; Xu, J.; Wu, H.; Huang, J.; Zeng, X.; Zhao, F. Regulating the flower-like NiCo2S4/Zn0.76Co0.24S heterojunction through microwave heating for supercapacitors with superior cycling performance. J. Energy Storage 2023, 58, 106439. [Google Scholar] [CrossRef]
- Ye, Y.; Luo, Y.; Lou, J.; Chen, X.; Cheng, Y.-J.; Xia, J.; Li, Y.; Guo, K. Hollow Spherical NiCo2S4@N-CNT Composites with High Energy Density for All-Solid-State Supercapacitors. ACS Appl. Energy Mater. 2023, 6, 6742–6751. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, F.; Lavernia, E.J. On the analysis of grain size in bulk nanocrystalline materials via X-ray diffraction. Metall. Mater. Trans. A 2003, 34, 1349–1355. [Google Scholar] [CrossRef]
- Li, S.; Hua, M.; Yang, Y.; Zheng, X.; Huang, W.; Si, P.; Ci, L.; Lou, J. Phosphorous-doped bimetallic sulfides embedded in heteroatom-doped carbon nanoarrays for flexible all-solid-state supercapacitors. Sci. China Mater. 2021, 64, 2439–2453. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, F.; He, X.; Chen, B.; Li, G. Efficient overall water splitting over a Mo(IV)-doped Co3O4/NC electrocatalyst. Int. J. Hydrog. Energy 2021, 46, 20905–20918. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H. Epitaxial ZnCoS nanodendritics grown along 3-D carbonaceous scaffolds for high-performance hybrid supercapacitors. J. Alloys Compd. 2022, 905, 164250. [Google Scholar] [CrossRef]
- Haripriya, M.; Ashok, A.M.; Hussain, S.; Sivasubramanian, R. Nanostructured MnCo2O4 as a high-performance electrode for supercapacitor application. Ionics 2021, 27, 325–337. [Google Scholar] [CrossRef]
- Wu, X.; Yu, X.; Zhang, Z.; Liu, H.; Ling, S.; Liu, X.; Lian, C.; Xu, J. Anisotropic ZnS Nanoclusters/Ordered Macro-Microporous Carbon Superstructure for Fibrous Supercapacitor toward Commercial-Level Energy Density. Adv. Funct. Mater. 2023, 2300329. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Y.; Huang, K.; Chen, Y.; Guo, H. CMK-3/NiCo2S4 nanostructures for high performance asymmetric supercapacitors. Mater. Chem. Phys. 2018, 220, 270–277. [Google Scholar] [CrossRef]
- Ma, J.; Xia, J.; Liang, Z.; Chen, X.; Du, Y.; Yan, C.-H. Layered Double Hydroxide Hollowcages with Adjustable Layer Spacing for High Performance Hybrid Supercapacitor. Small 2021, 17, 2104423. [Google Scholar] [CrossRef]
- Jia, H.; Fan, J.; Fan, Y.; Feng, C.; Jin, H.; Cai, Y.; Liu, M.-C. Cation substituted Ni3S2 nanosheets wrapped Zn0.76Co0.24S nanowire arrays prepared with in-situ oxidative etching strategy for high performance solid-state asymmetric supercapacitors. J. Energy Storage 2022, 46, 103870. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Y.; Lu, C.-X.; Zhang, Y.-Y.; Zhang, X.-H.; Liu, Y.-Y. A high energy density fiber-shaped supercapacitor based on zinc-cobalt bimetallic oxide nanowire forests on carbon nanotube fibers. New Carbon Mater. 2019, 34, 559–568. [Google Scholar] [CrossRef]
- Kong, W.; Lu, C.; Zhang, W.; Pu, J.; Wang, Z. Homogeneous core–shell NiCo2S4 nanostructures supported on nickel foam for supercapacitors. J. Mater. Chem. A 2015, 3, 12452–12460. [Google Scholar] [CrossRef]
- El-Hout, S.I.; Mohamed, S.G.; Gaber, A.; Attia, S.Y.; Shawky, A.; El-Sheikh, S.M. High electrochemical performance of rGO anchored CuS nanospheres for supercapacitor applications. J. Energy Storage 2021, 34, 102001. [Google Scholar] [CrossRef]
- Shao, Q.; Tang, J.; Lin, Y.; Li, J.; Qin, F.; Zhang, K.; Yuan, J.; Qin, L. Ionic liquid modified graphene for supercapacitors with high rate capability. Electrochim. Acta 2015, 176, 1441–1446. [Google Scholar] [CrossRef]
- Shao, Q.; Tang, J.; Lin, Y.; Li, J.; Qin, F.; Yuan, J.; Qin, L. Carbon nanotube spaced graphene aerogels with enhanced capacitance in aqueous and ionic liquid electrolytes. J. Power Sources 2015, 278, 751–759. [Google Scholar] [CrossRef]
- Javed, M.S.; Han, X.; Hu, C.; Zhou, M.; Huang, Z.; Tang, X.; Gu, X. Tracking Pseudocapacitive Contribution to Superior Energy Storage of MnS Nanoparticles Grown on Carbon Textile. ACS Appl. Mater. Interfaces 2016, 8, 24621–24628. [Google Scholar] [CrossRef]
- Liu, S.; Yin, Y.; Ni, D.; Hui, K.S.; Hui, K.N.; Lee, S.; Ouyang, C.-Y.; Jun, S.C. Phosphorous-containing oxygen-deficient cobalt molybdate as an advanced electrode material for supercapacitors. Energy Storage Mater. 2019, 19, 186–196. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, H.; Wu, X. Bi-interface induced multi-active MCo2O4@ MCo2S4@ PPy (M = Ni, Zn) sandwich structure for energy storage and electrocatalysis. Nano Energy 2019, 57, 363–370. [Google Scholar] [CrossRef]
- Guan, T.; Cheng, Z.; Li, Z.; Gao, L.; Yan, K.; Shen, L.; Bao, N. Hydrothermal-Assisted In Situ Growth of Vertically Aligned MoS2 Nanosheets on Reduced Graphene Oxide Fiber Fabrics toward High-Performance Flexible Supercapacitors. Ind. Eng. Chem. Res. 2022, 61, 3840–3849. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, J.; Yang, J.; Liu, W.; Qiao, F.; Lian, J.; Li, G.; Wang, T.; Zhang, J.; Wu, L. Hierarchical polypyrrole@ cobalt sulfide-based flexible on-chip microsupercapacitors with ultrahigh energy density for self-charging system. Nano Res. 2023, 16, 555–563. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, Y.; Wang, L.; Sheng, P.; Peng, H. Fiber-based MnO2/carbon nanotube/polyimide asymmetric supercapacitor. Carbon 2017, 125, 595–604. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, X.; Tang, X.; Liang, L.; Liu, X.; Zhang, X.; Ma, X.; Liu, G.; Li, C.; Cao, N.; Shao, Q. Solvent-Controlled Morphology of Zinc–Cobalt Bimetallic Sulfides for Supercapacitors. Molecules 2023, 28, 6578. https://doi.org/10.3390/molecules28186578
Zang X, Tang X, Liang L, Liu X, Zhang X, Ma X, Liu G, Li C, Cao N, Shao Q. Solvent-Controlled Morphology of Zinc–Cobalt Bimetallic Sulfides for Supercapacitors. Molecules. 2023; 28(18):6578. https://doi.org/10.3390/molecules28186578
Chicago/Turabian StyleZang, Xiaobei, Xiaoqi Tang, Liheng Liang, Xuhui Liu, Xiaobin Zhang, Xingdong Ma, Guoshun Liu, Chao Li, Ning Cao, and Qingguo Shao. 2023. "Solvent-Controlled Morphology of Zinc–Cobalt Bimetallic Sulfides for Supercapacitors" Molecules 28, no. 18: 6578. https://doi.org/10.3390/molecules28186578






