Insights into the Relationship between the Microstructure and the Catalytic Behavior of Fe2(MoO4)3 during the Ethanolysis of Naomaohu Coal
Abstract
:1. Introduction
2. Results and Discussion
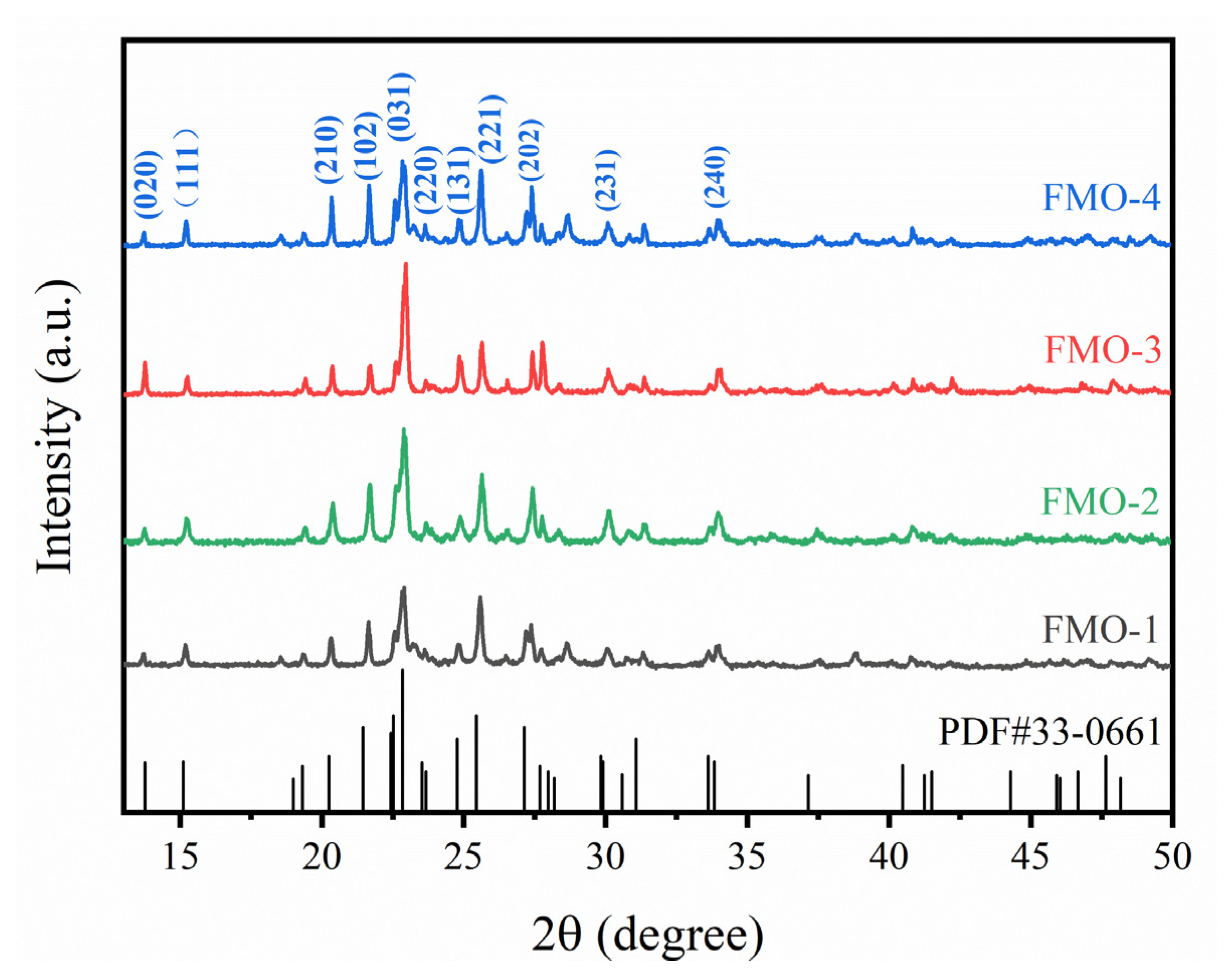
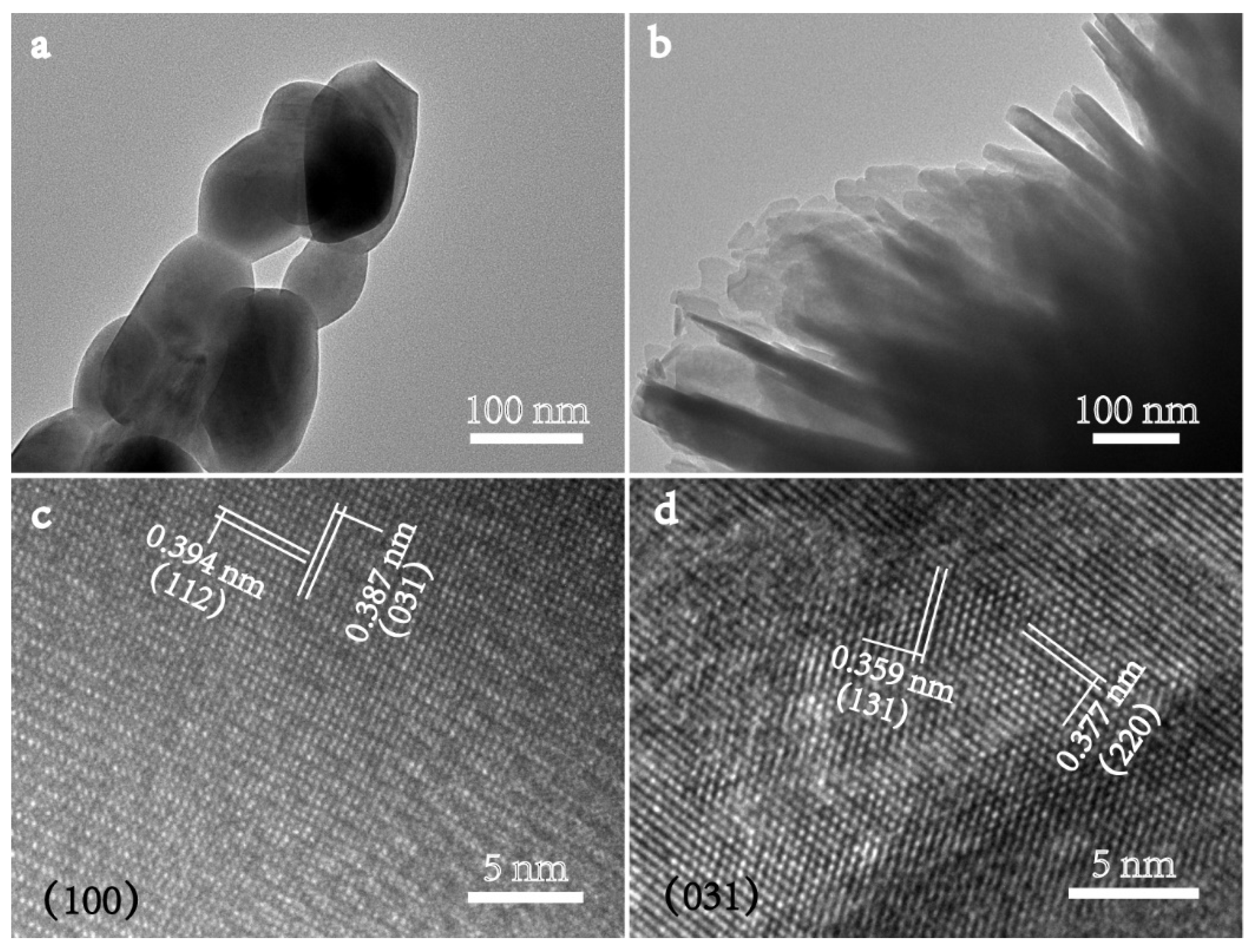
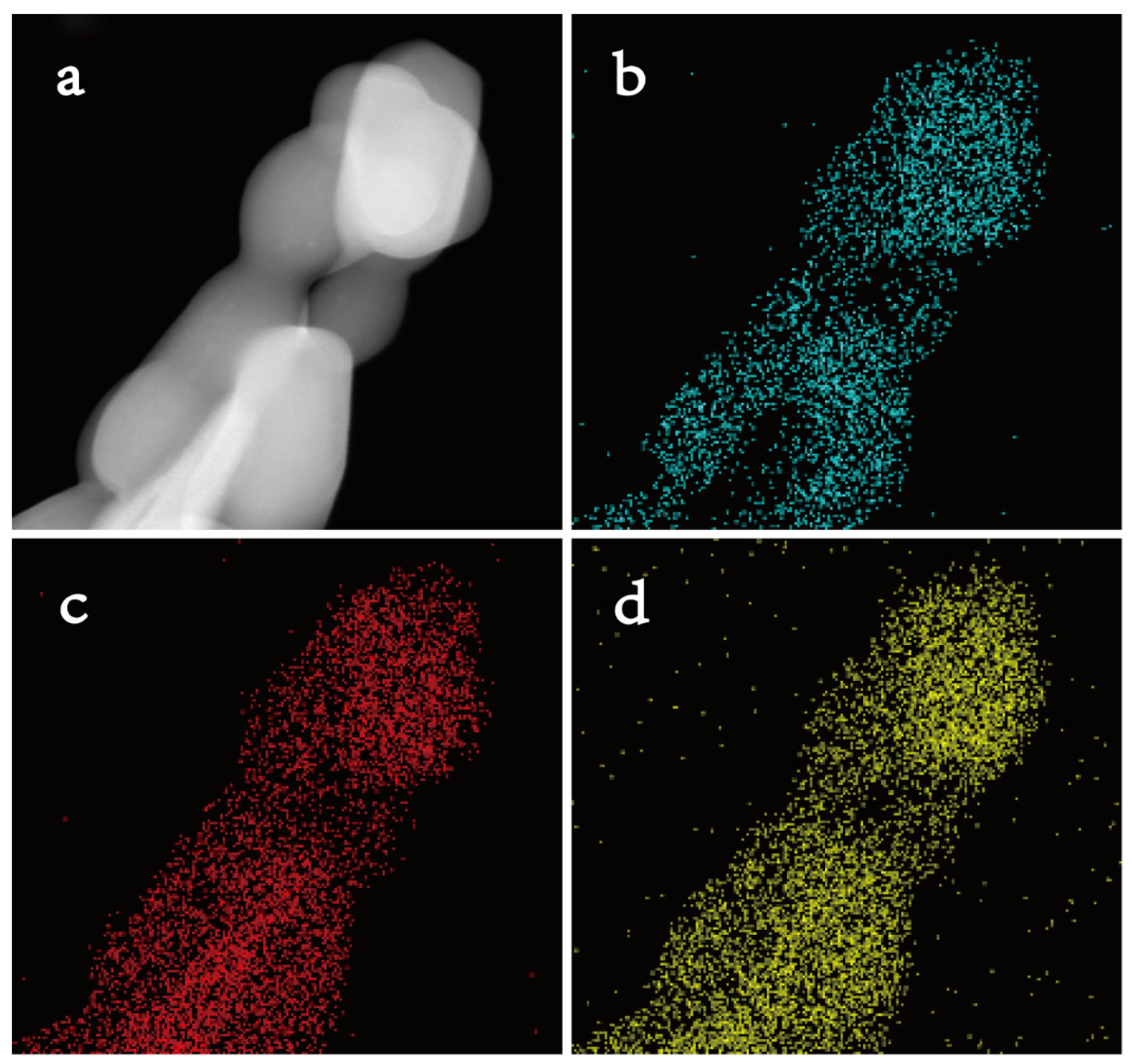
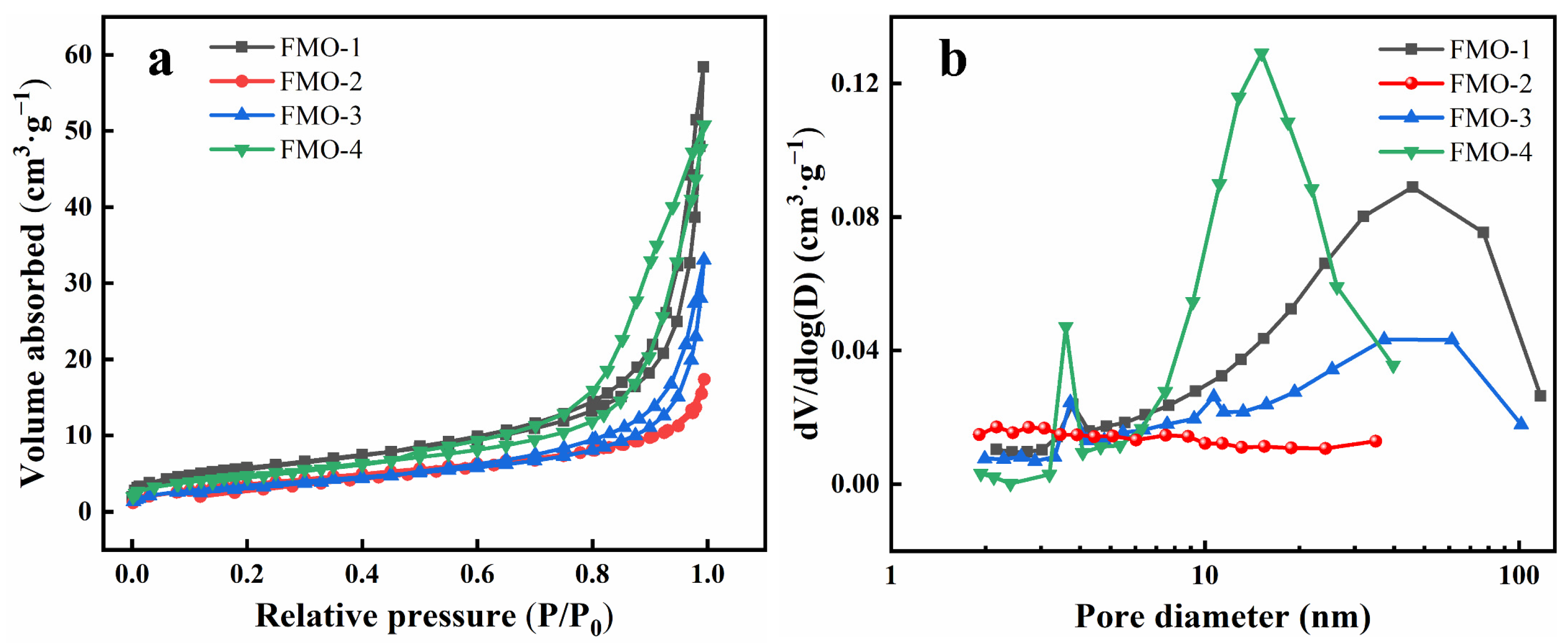
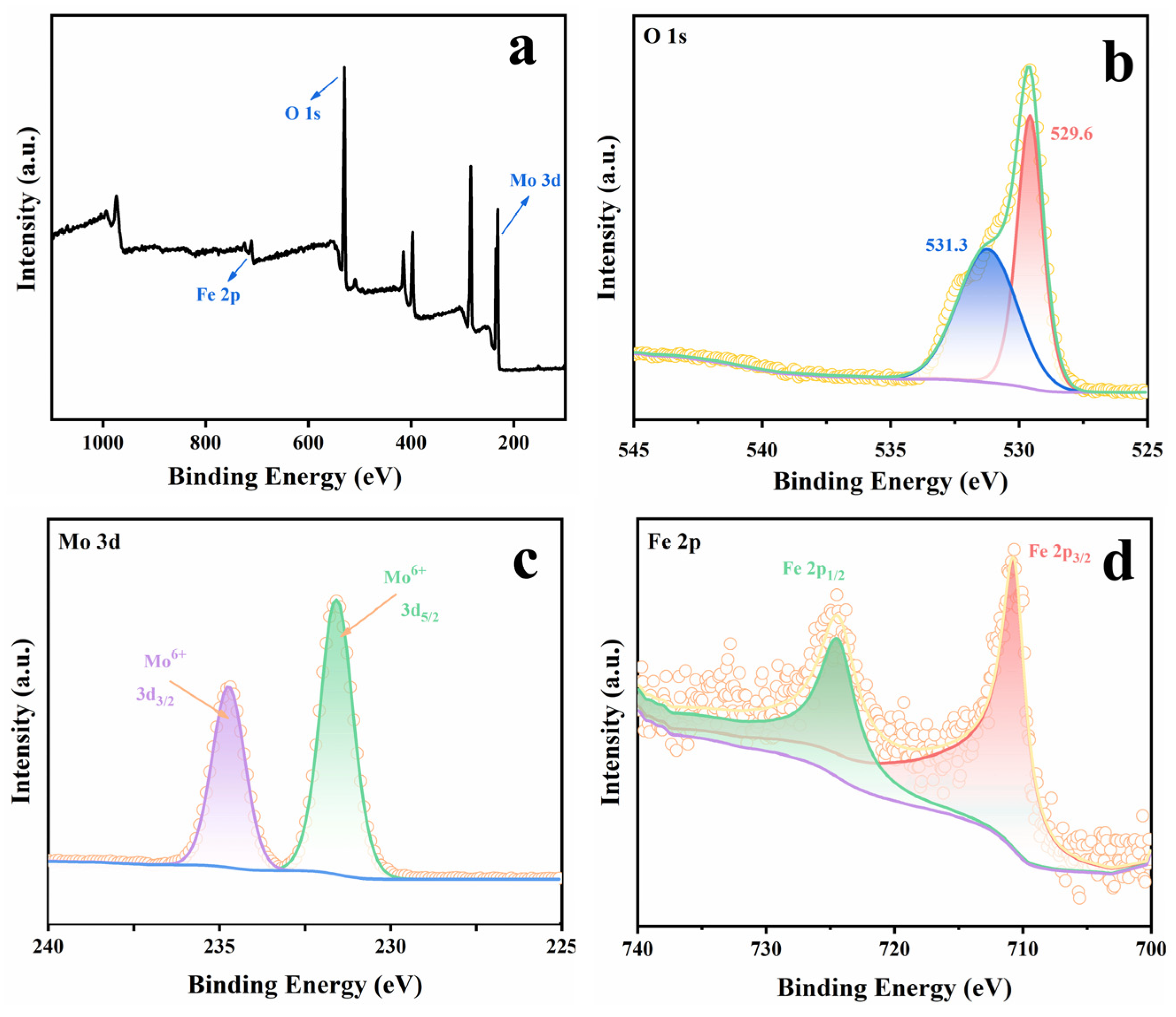
2.1. Catalyst Characterizations
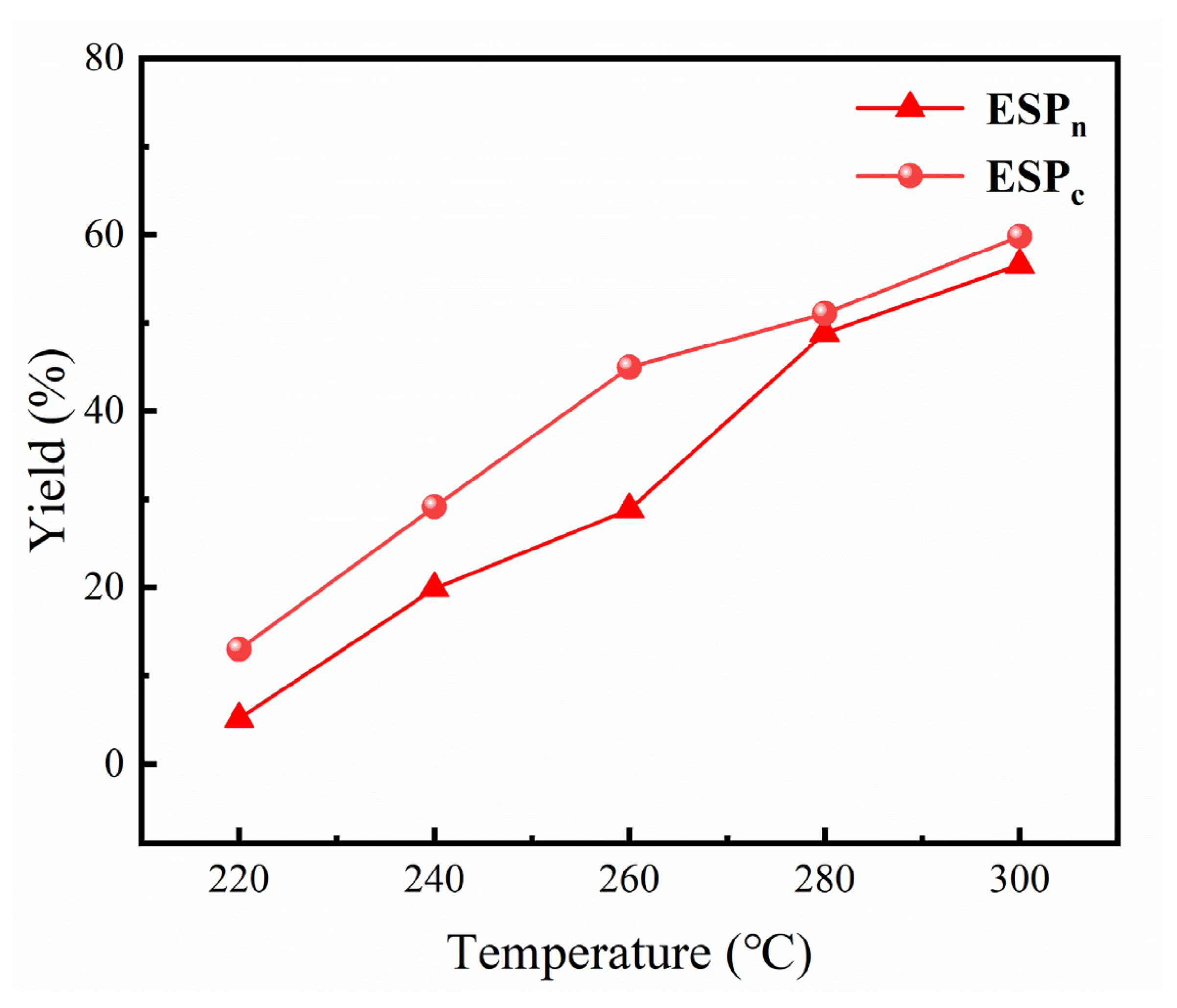
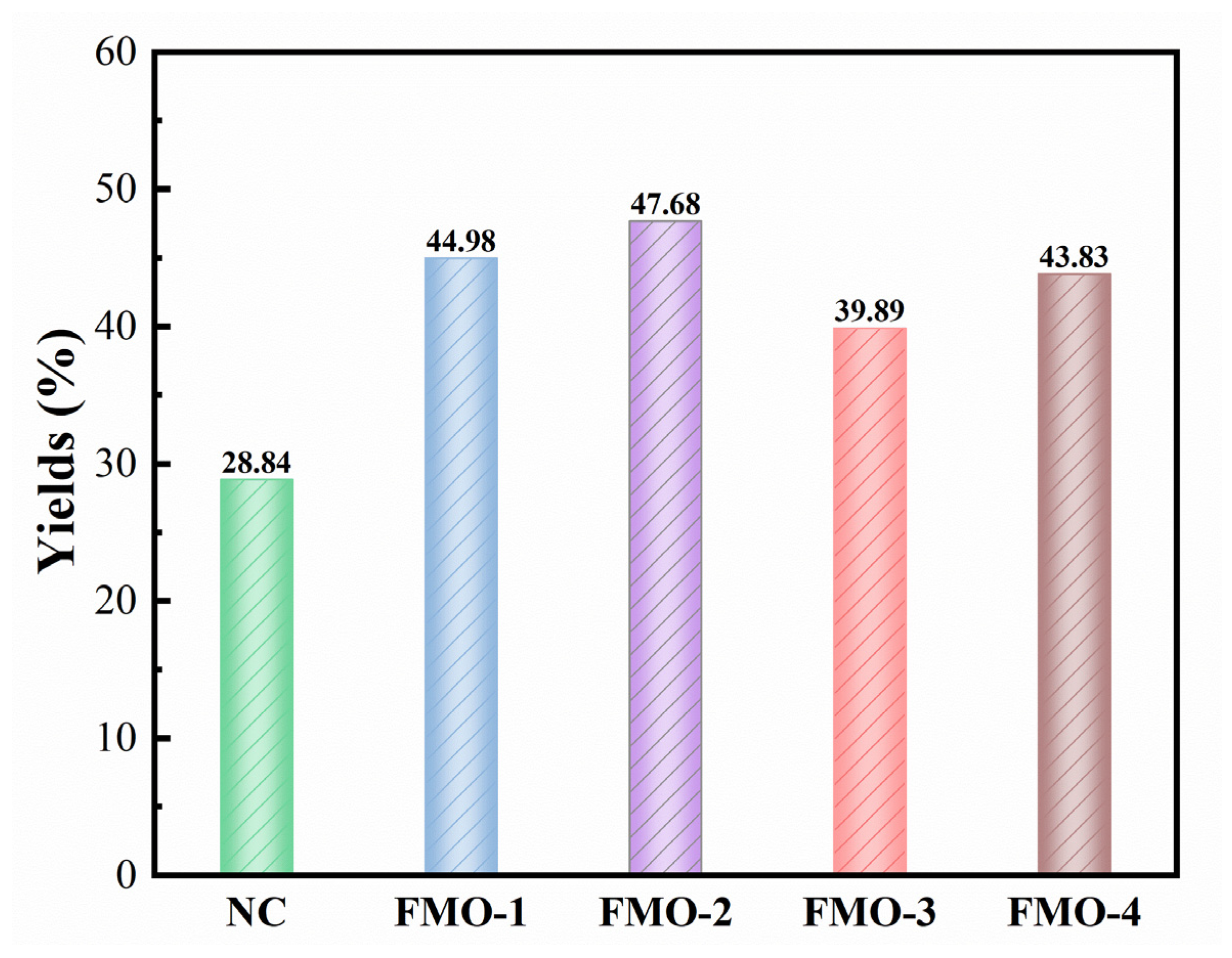
2.2. ESP Yields of Ethanolysis
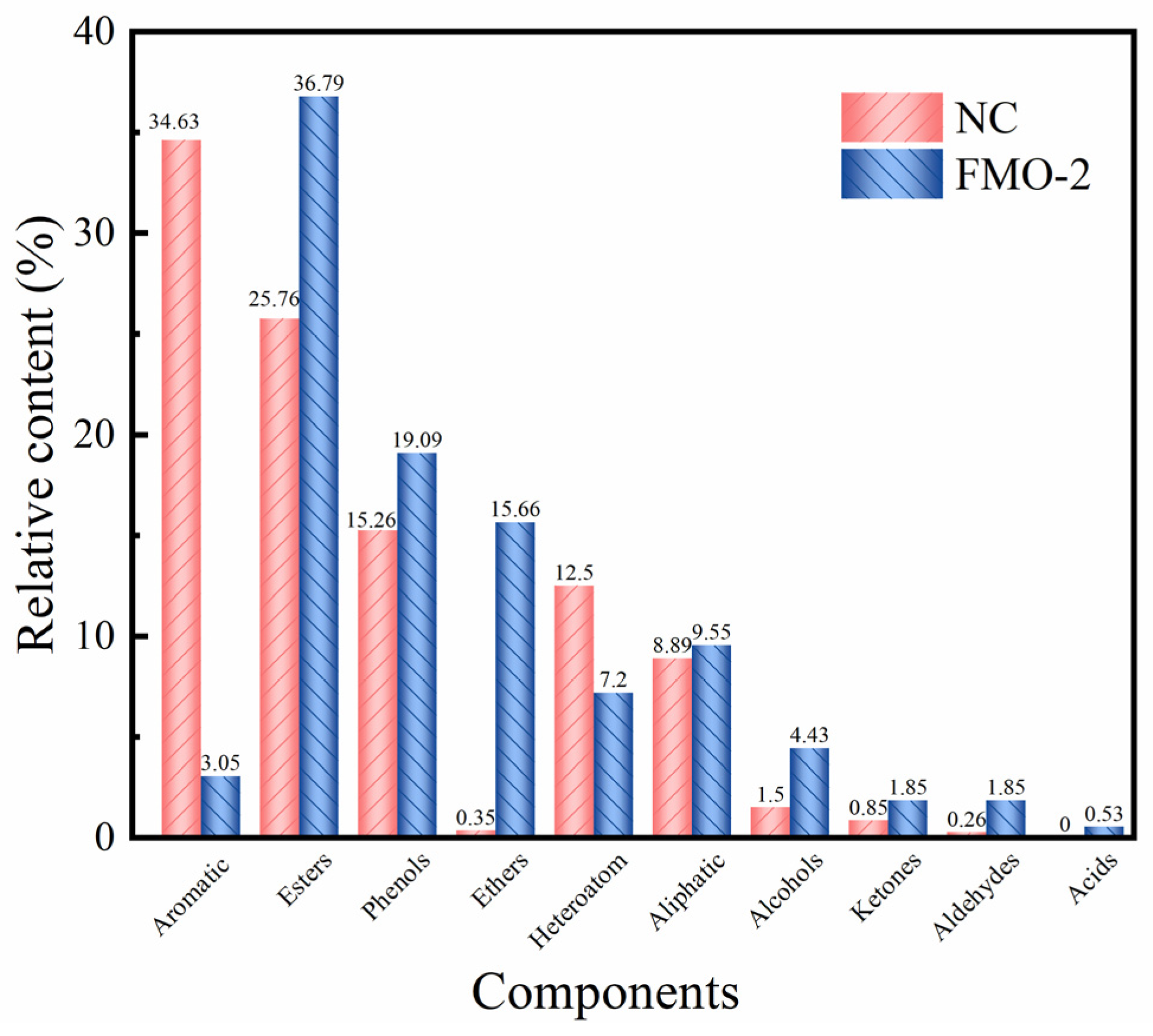
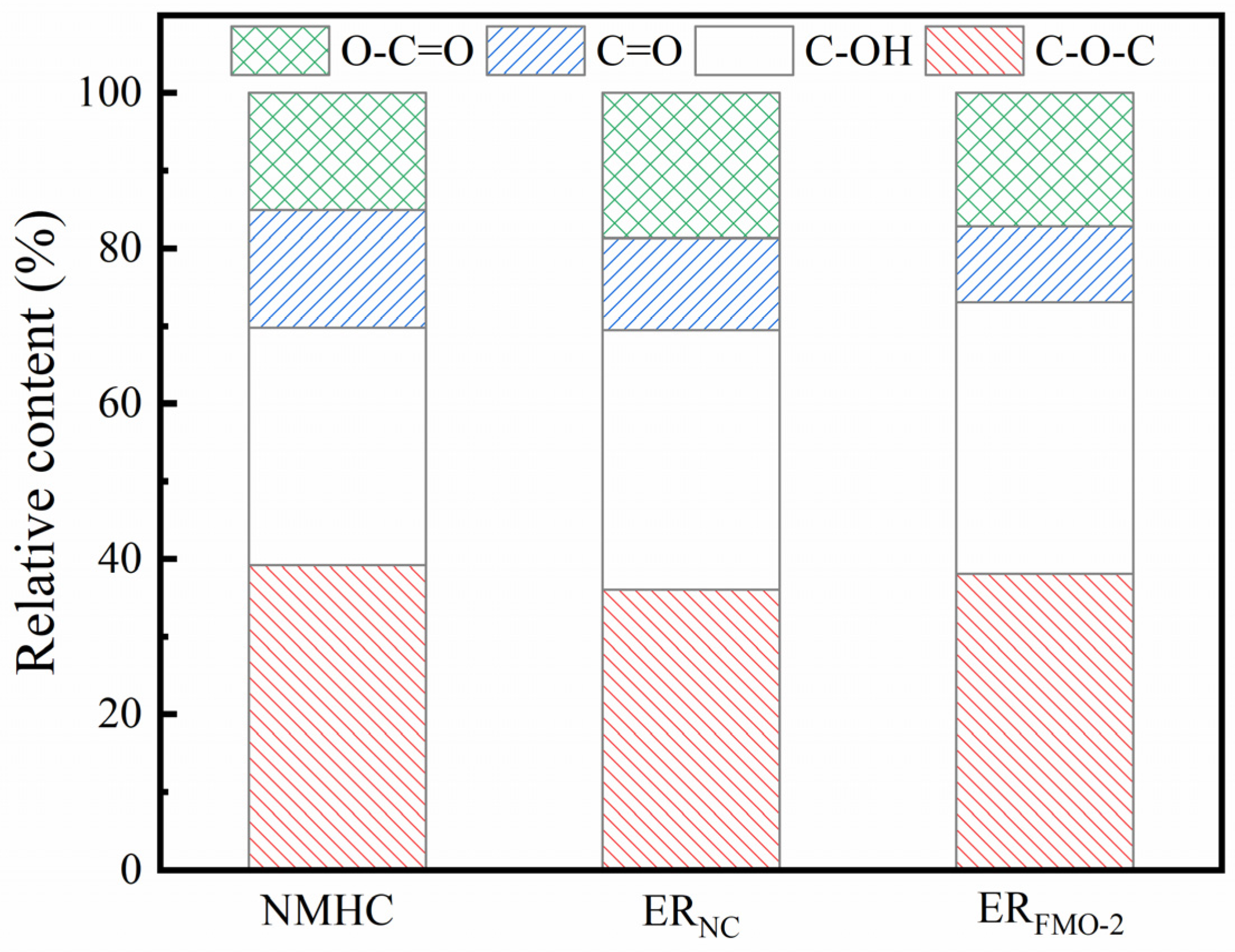
2.3. Characterizations of ESP and Ethanolysis Residues (ER)
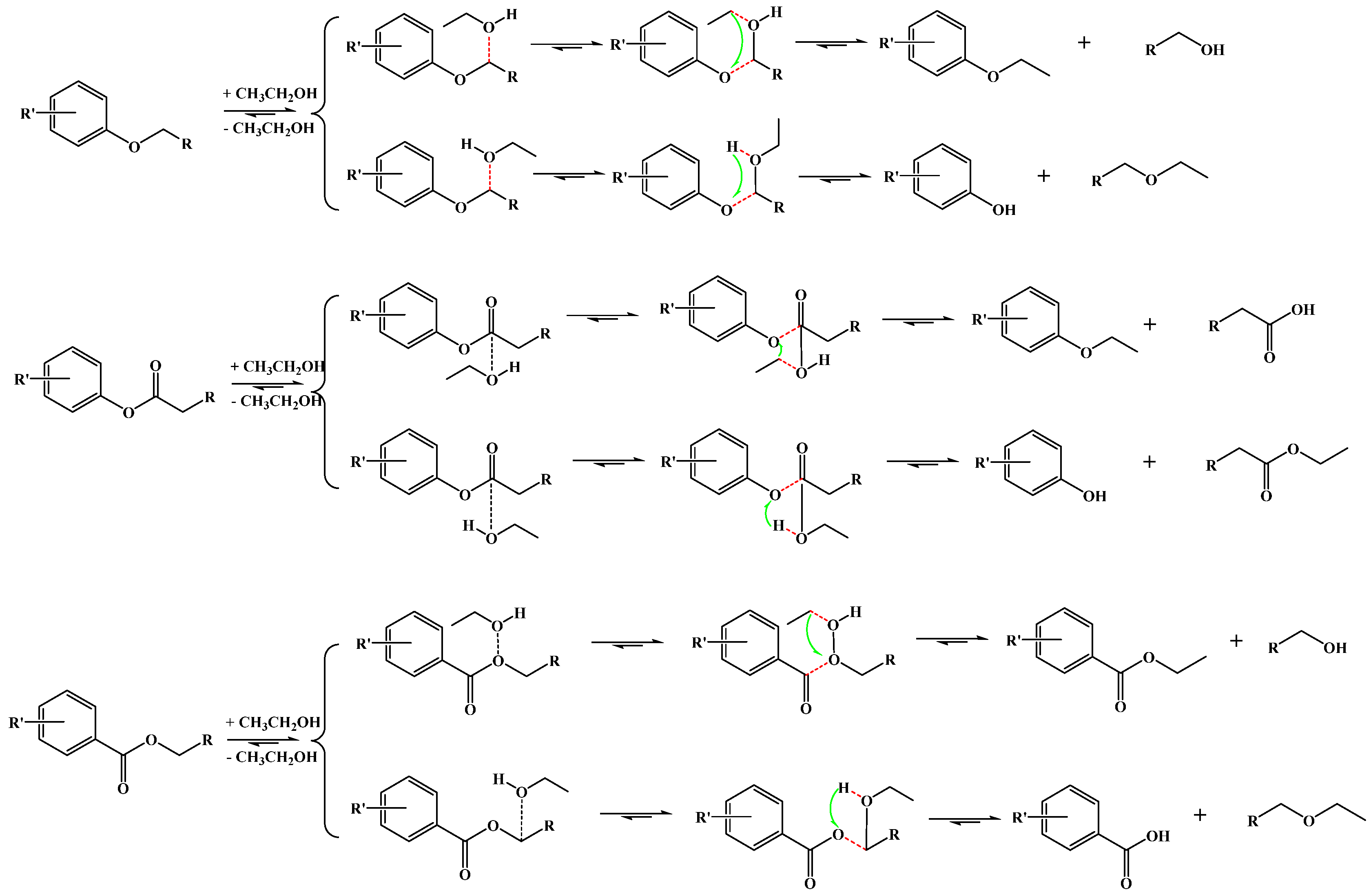
2.4. Catalytic Activity in the Cleaving of BOB
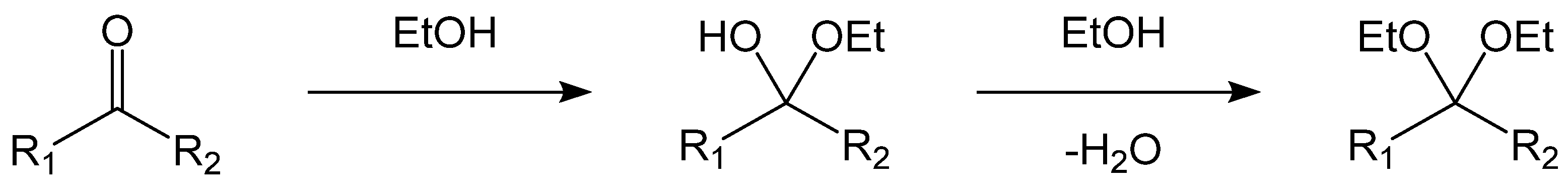
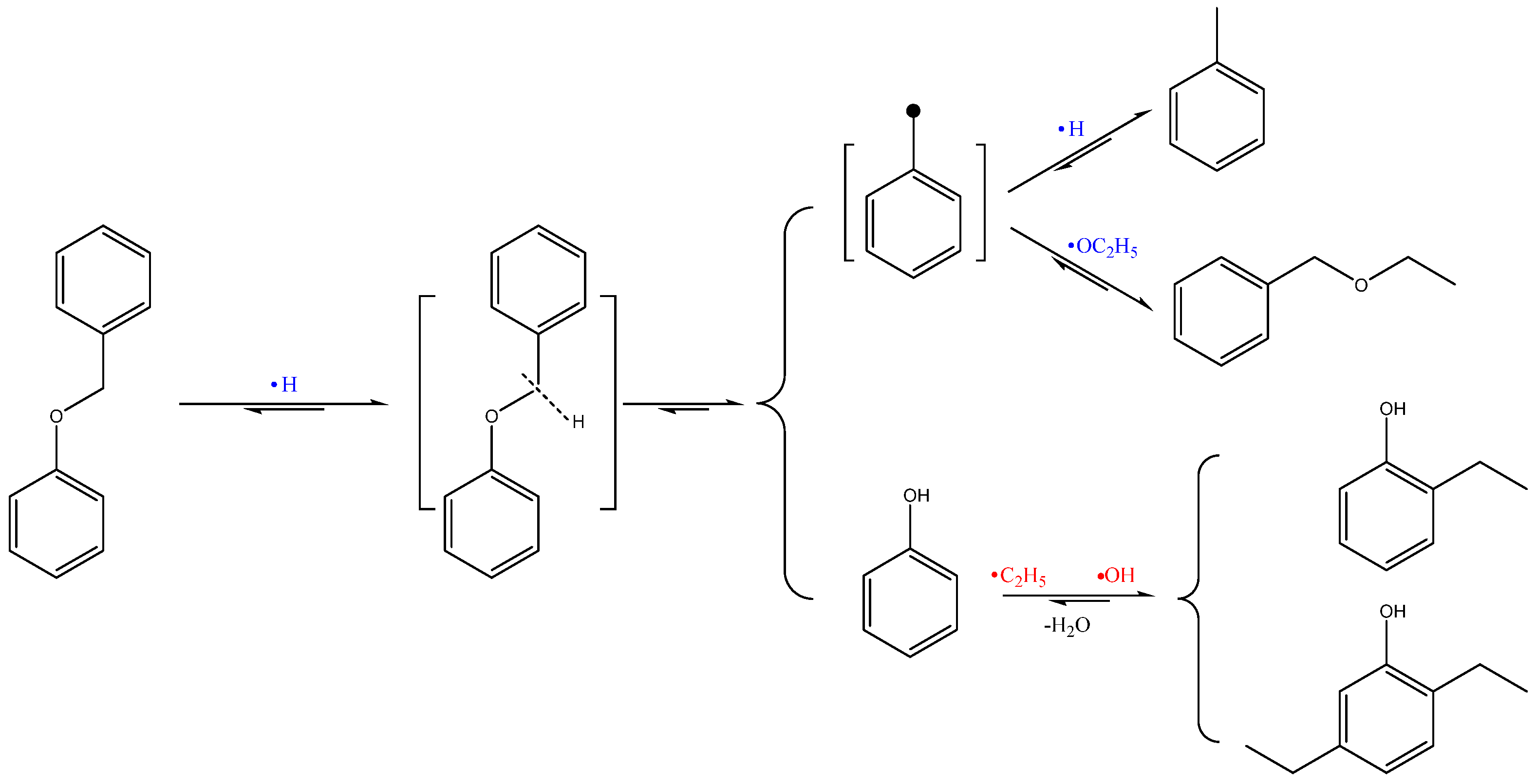
2.5. Possible Mechanisms of NMHC Catalytic Ethanolysis
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the Fe2(MoO4)3 Samples
3.3. Ethanolysis
3.4. Catalytic Ethanolysis of BOB
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, G.H.; Li, Y.J.; Bai, J.J.; Gao, Y.; Kang, Y.H.; Wang, A.M.; Lu, C.Y.; Bai, H.C.; Zong, Z.M.; Wei, X.Y. Advances in mild degradation and directional upgrading of lignites: From feature identification to value-added utilization. J. Anal. Appl. Pyrolysis 2022, 163, 105477. [Google Scholar] [CrossRef]
- Liu, F.J.; Wei, X.Y.; Fan, M.; Zong, Z.M. Separation and structural characterization of the value-added chemicals from mild degradation of lignites: A review. Appl. Energy 2016, 170, 415–436. [Google Scholar] [CrossRef]
- Shui, H.; Yao, J.; Wu, H.; Li, Z.; Yan, J.; Wang, Z.; Ren, S.; Lei, Z.; Chunbao Xu, C. Study on catalytic thermal dissolution of lignite and hydro-liquefaction of its soluble fraction for potential jet fuel. Fuel 2022, 315, 123237. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, J.-b.; Yang, H.-y.; Liu, Q. Chemical Composition and Structural Characteristics of Oil Shales and Their Kerogens Using Fourier Transform Infrared (FTIR) Spectroscopy and Solid-State 13C Nuclear Magnetic Resonance (NMR). Energy Fuels 2016, 30, 6271–6280. [Google Scholar] [CrossRef]
- Li, S.; Du, F.F.; Ma, Z.H.; Zhang, L.H.; Liu, Z.Q.; Zhao, T.S.; Wei, X.Y.; Xu, M.L.; Cong, X.S. Insight into the Compositional Features of Organic Matter in Xilinguole Lignite through Two Mass Spectrometers. ACS Omega 2022, 7, 46384–46390. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Wei, X.Y.; Yan, H.L.; Shi, D.L.; Liu, F.J.; Li, P.; Fan, X.; Zhao, Y.P.; Zong, Z.M. Structural Features of Extraction Residues from Supercritical Methanolysis of Two Chinese Lignites. Energy Fuels 2013, 27, 4632–4638. [Google Scholar] [CrossRef]
- Wu, F.P.; Qin, S.; Zhao, Y.P.; Qiu, L.L.; Xiao, J.; Bai, Y.H.; Liu, F.J.; Cao, J.P.; Wei, X.Y. Correlations of composition and structural characteristics between pyrolysis tar and soluble organic species of Baoqing lignite. J. Anal. Appl. Pyrolysis 2023, 171, 105954. [Google Scholar] [CrossRef]
- Liu, M.; Lei, Z.; Cao, X.; Yan, J.; Shui, H.; Wang, Z.; Hu, J.; Hong, M. Construction of Macromolecules of depolymerized Lignite. ACS Omega 2023, 8, 22820–22826. [Google Scholar] [CrossRef]
- Yu, X.Y.; Wei, X.Y.; Li, Z.K.; Zhang, D.D.; Zong, Z.M. Two-step depolymerization of Zhaotong lignite in ethanol. Fuel 2017, 196, 391–397. [Google Scholar] [CrossRef]
- Lu, H.Y.; Wei, X.Y.; Yu, R.; Peng, Y.L.; Qi, X.Z.; Qie, L.M.; Wei, Q.; Lv, J.; Zong, Z.M.; Zhao, W.; et al. Sequential Thermal Dissolution of Huolinguole Lignite in Methanol and Ethanol. Energy Fuels 2011, 25, 2741–2745. [Google Scholar] [CrossRef]
- Schobert, H.H.; Song, C. Chemicals and materials from coal in the 21st century. Fuel 2002, 81, 15–32. [Google Scholar] [CrossRef]
- Chen, B.; Wei, X.Y.; Zong, Z.M.; Yang, Z.S.; Qing, Y.; Liu, C. Difference in chemical composition of supercritical methanolysis products between two lignites. App. Energy 2011, 88, 4570–4576. [Google Scholar] [CrossRef]
- Li, Z.K.; Wei, X.Y.; Yan, H.L.; Yu, X.Y.; Zong, Z.M. Characterization of soluble portions from thermal dissolution of Zhaotong lignite in cyclohexane and methanol. Fuel Process. Technol. 2016, 151, 131–138. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, X.Y.; Dong, X.; Liao, J.J.; Zhao, Y.P.; Wei, X.Y.; Ma, F.Y.; Nulahong, A. Structural insights of four thermal dissolution products of Dongming lignite by using in-source collision-activated dissociation mass spectrometry. Fuel 2018, 230, 78–82. [Google Scholar] [CrossRef]
- Liu, Q.; Hou, Y.; Wu, W.; Wang, Q.; Ren, S.; Liu, Q. New insight into the chemical structures of Huadian kerogen with supercritical ethanolysis: Cleavage of weak bonds to small molecular compounds. Fuel Process. Technol. 2018, 176, 138–145. [Google Scholar] [CrossRef]
- Li, Z.K.; Cheng, J.Y.; Yan, H.L.; Lei, Z.P.; Yan, J.C.; Ren, S.B.; Wang, Z.C.; Kang, S.G.; Shui, H.F. Ethanol and formic acid as synergistic solvents for converting lignite to platform chemicals. Fuel 2021, 293, 120491. [Google Scholar] [CrossRef]
- Kang, Y.H.; Chen, T.; Gao, J.; Li, F.; Hu, L.; Liu, G.H.; Lu, C.Y.; Li, Y.J.; Wei, X.Y.; Ma, Y.J.; et al. Comprehensive investigation of the mechanisms for pyrolyzing macromolecular networks in Hecaogou subbituminous coal by comparing the ethanolysis and flash pyrolysis. Fuel 2022, 324, 124619. [Google Scholar] [CrossRef]
- Zheng, J.J.; Liu, F.J.; Sun, B.K.; Li, Y.H.; Meng, B.; Yu, Y.M.; Zhao, Y.P.; Cao, J.P.; Wei, X.Y.; Lu, Y.; et al. Efficient Two-Step Utilization of Organic Matter in Shengli Lignite for Producing Chemicals and Supercapacitor Electrode Materials. Energy Fuels 2023, 37, 3166–3177. [Google Scholar] [CrossRef]
- Li, Z.K.; Wei, X.Y.; Yan, H.L.; Yu, X.Y.; Zong, Z.M. Insight into the chemical complexity of ethanolysis products from extraction residue of Zhaotong lignite. Fuel 2016, 174, 287–295. [Google Scholar] [CrossRef]
- Liang, S.; Hou, Y.; Wu, W.; Li, L.; Ren, S. New Insights into the Primary Reaction Products of Naomaohu Coal via Breaking Weak Bonds with Supercritical Ethanolysis. Energy Fuels 2019, 33, 6294–6301. [Google Scholar] [CrossRef]
- Hu, X.B.; Xu, H.; Mo, W.L.; Fan, X.; Guo, W.C.; Guo, J.; Niu, J.M.; Mi, H.Y.; Ma, Y.Y.; Wei, X.Y. Effect of sequential thermal dissolution on the structure and pyrolysis characteristics of Naomaohu lignite. Fuel 2023, 331, 125930. [Google Scholar] [CrossRef]
- Yang, W.Q.; Mao, K.M.; Mo, W.L.; Ma, F.Y.; Wei, X.Y.; Fan, X.; Ren, T.Z. Mechanism analysis of methanol alcoholysis of Naomaohu lignite extraction residue based on model compound reaction path. J. Fuel Chem. Technol. 2022, 50, 396–407. [Google Scholar] [CrossRef]
- Liu, F.J.; Wei, X.Y.; Li, W.T.; Gui, J.; Li, P.; Wang, Y.G.; Xie, R.L.; Zong, Z.M. Methanolysis of extraction residue from Xianfeng lignite with NaOH and product characterizations with different spectrometries. Fuel Process. Technol. 2015, 136, 8–16. [Google Scholar] [CrossRef]
- Li, X.K.; Zong, Z.M.; Chen, Y.F.; Yang, Z.; Liu, G.H.; Liu, F.J.; Wei, X.Y.; Wang, B.J.; Ma, F.Y.; Liu, J.M. Catalytic hydroconversion of Yinggemajianfeng lignite over difunctional Ni-Mg2Si/γ-Al2O3. Fuel 2019, 249, 496–502. [Google Scholar] [CrossRef]
- Liu, G.H.; Zong, Z.M.; Liu, F.J.; Meng, X.L.; Zhang, Y.Y.; Wang, S.K.; Li, S.; Zhu, C.; Wei, X.Y.; Ma, F.Y.; et al. Deep hydroconversion of ethanol-soluble portion from the ethanolysis of Dahuangshan lignite to clean liquid fuel over a mordenite supported nickel catalyst. J. Anal. Appl. Pyrolysis 2019, 139, 13–21. [Google Scholar] [CrossRef]
- Liu, G.H.; Zhang, Z.W.; Li, X.L.; Kang, Y.H.; Lu, C.Y.; Chen, J.Z.; Liu, F.J.; Bai, H.C.; Wei, X.Y. Catalytic Degradation and Directional Upgrading of Zhunnan Lignite: Double Constraint of Active Hydrogen and Effective Acquisition of Derived Arenes over Nickel Ferrite. Energy Fuels 2021, 35, 19943–19952. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Liang, L.; Guan, G.; Huang, W. Catalytic depolymerization of coal char over iron-based catalyst: Potential method for producing high value-added chemicals. Fuel 2017, 210, 329–333. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, F.J.; Guo, J.P.; Yin, F.; Gao, S.S.; Lu, Y.; Song, R.; Yu, Y.M.; Zheng, J.J.; Zhao, Y.P.; et al. Green and highly selective hydrogenation of lignin-derived aromatics at low temperature over Ru-Fe bimetallic nanoparticles supported on porous nitrogen-doped carbon. Fuel Process. Technol. 2023, 247, 107754. [Google Scholar] [CrossRef]
- Kong, Q.Q.; Bai, X.; Wei, X.Y.; Dilixiati, Y.; Yin, F.; Zhao, J.; Fan, Z.C.; Li, Z.; An, M.; Li, L.; et al. Specific cleavage of >CH-O-bonds in benzyloxybenzene and poplar lignin over nickel supported on a nitrogen-doped carbon material. Fuel Process. Technol. 2023, 248, 107813. [Google Scholar] [CrossRef]
- Li, Z.K.; Wang, H.T.; Yan, H.L.; Yan, J.C.; Lei, Z.P.; Ren, S.B.; Wang, Z.C.; Kang, S.G.; Shui, H.F. Catalytic ethanolysis of Xilinguole lignite over layered and mesoporous metal oxide composites to platform chemicals. Fuel 2021, 287, 119560. [Google Scholar] [CrossRef]
- Du, F.; Zhang, Z.; Liu, Z.; Cong, X.; Ma, Z.; Zhao, T.; Li, S. Selective catalytic ethanolysis of C-O bonds in lignite-related model compounds and Xilinguole lignite over difunctional Ni/HZSM-5 in H2-free system. Fuel Process. Technol. 2023, 241, 107590. [Google Scholar] [CrossRef]
- Xie, J.; Lu, H.; Shu, G.; Li, K.; Zhang, X.; Wang, H.; Yue, W.; Gao, S.; Chen, Y. The relationship between the microstructures and catalytic behaviors of iron–oxygen precursors during direct coal liquefaction. Chin. J. Catal. 2018, 39, 857–866. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Ma, H.; Yang, C.; Pan, Z.; Qu, W.; Tian, Z. Layer-structure adjustable MoS2 catalysts for the slurry-phase hydrogenation of polycyclic aromatic hydrocarbons. J. Energy Chem. 2021, 63, 294–304. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Teng, Y.; Zhang, M.; Deng, Z.P.; Xu, Y.M.; Huo, L.H.; Gao, S. Cooperative modulation of Fe2(MoO4)3 microstructure derived from absorbent cotton for enhanced gas-sensing performance. Sens. Actuators B Chem. 2021, 329, 129126. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Yu, R.; Wang, D. Construction of Multishelled Binary Metal Oxides via Coabsorption of Positive and Negative Ions as a Superior Cathode for Sodium-Ion Batteries. J. Am. Chem. Soc. 2018, 140, 17114–17119. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, C.A.; Zhu, T.; Lu, Q.; Li, Y.; Che, D. Simultaneous removal of NOx and SO2 from coal-fired flue gas based on the catalytic decomposition of H2O2 over Fe2(MoO4)3. Chem. Eng. J. 2019, 371, 486–499. [Google Scholar] [CrossRef]
- Zhou, Y.; Sadia Traore, A.; Peron, D.V.; Barrios, A.J.; Chernyak, S.A.; Corda, M.; Safonova, O.V.; Iulian Dugulan, A.; Ersen, O.; Virginie, M.; et al. Promotion effects of alkali metals on iron molybdate catalysts for CO2 catalytic hydrogenation. J. Energy Chem. 2023, 85, 291–300. [Google Scholar] [CrossRef]
- Hidalgo, G.; Tonelli, M.; Burel, L.; Aouine, M.; Millet, J.M.M. Microwave-assisted hydrothermal synthesis, characterization and catalytic performance of Fe2(MoO4)3 in the selective oxidation of propene. Catal. Today 2021, 363, 36–44. [Google Scholar] [CrossRef]
- Malik, M.I.; Abatzoglou, N.; Achouri, I.E. Methanol to Formaldehyde: An Overview of Surface Studies and Performance of an Iron Molybdate Catalyst. Catalysts 2021, 11, 893. [Google Scholar] [CrossRef]
- Lin, Z.; Xu, M.; Fu, P.; Deng, Q. Crystal plane control of 3D iron molybdate and the facet effect on gas sensing performances. Sens. Actuators B Chem. 2018, 254, 755–762. [Google Scholar] [CrossRef]
- Kong, J.; Zhao, R.; Bai, Y.; Li, G.; Zhang, C.; Li, F. Study on the formation of phenols during coal flash pyrolysis using pyrolysis-GC/MS. Fuel Process. Technol. 2014, 127, 41–46. [Google Scholar] [CrossRef]
- Li, B.; Yuan, Z.; Schmidt, J.; Xu, C. New foaming formulations for production of bio-phenol formaldehyde foams using raw kraft lignin. Eur. Polym. J. 2019, 111, 1–10. [Google Scholar] [CrossRef]
- Wu, F.P.; Zhao, Y.P.; Fu, Z.P.; Qiu, L.L.; Xiao, J.; Li, J.; Liu, F.J.; Cao, J.P. Catalytic transfer hydrogenolysis mechanism of benzyl phenyl ether over NiCu/Al2O3 using isopropanol as hydrogen source. Fuel Process. Technol. 2023, 250, 107874. [Google Scholar] [CrossRef]


| Retention Time/s | Name | Molecular Formula | Relative Content/(wt%) | |
|---|---|---|---|---|
| ESPNC | ESPFMO-2 | |||
| 983.3 | methyl 2-hydroxy-2-methylbutanoate | C6H12O3 | 1.51 | |
| 1268.7 | ethyl 3-methylpentanoate | C8H16O2 | 0.27 | |
| 1407.5 | ethyl hex-2-enoate | C8H14O2 | 1.04 | |
| 1429 | ethyl hexanoate | C8H16O2 | 0.16 | 1.17 |
| 1430.5 | isobutyl methacrylate | C8H14O2 | 0.24 | |
| 1442.9 | ethyl (E)-hex-3-enoate | C8H14O2 | 0.48 | 0.44 |
| 1444 | ethyl hex-4-enoate | C8H14O2 | 0.51 | |
| 1448.8 | prenyl isobutyrate | C9H16O2 | 0.18 | |
| 1542.8 | γ-Caprolactone | C6H10O2 | 0.45 | |
| 1600 | ethyl hex-2-enoate | C8H14O2 | 1.01 | 6.52 |
| 1730.4 | 4-methyloxan-2-one | C6H10O2 | 2.16 | |
| 1804.9 | ethyl hept-6-enoate | C9H16O2 | 0.20 | |
| 1859.5 | ethyl heptanoate | C9H18O2 | 0.69 | 0.93 |
| 1929.2 | ethyl (R)-2-(hydroxymethyl)butanoate | C7H14O3 | 0.33 | |
| 2026.3 | ethyl octanoate | C10H20O2 | 0.25 | |
| 2147.8 | diethyl succinate | C8H14O4 | 1.15 | 0.29 |
| 2153.7 | ethyl benzoate | C9H10O2 | 0.47 | |
| 2238.5 | ethyl oct-7-enoate | C10H18O2 | 0.19 | 0.39 |
| 2272.2 | ethyl (Z)-oct-3-enoate | C10H18O2 | 0.81 | |
| 2291.3 | ethyl octanoate | C10H20O2 | 1.98 | 1.75 |
| 2479.2 | ethyl 2-octenoate | C10H18O2 | 0.06 | |
| 2575.3 | diethyl glutarate | C9H16O4 | 0.15 | |
| 2615 | ethyl 3-methylbenzoate | C10H12O2 | 0.25 | 0.28 |
| 2661.8 | ethyl non-8-enoate | C11H20O2 | 0.39 | 0.20 |
| 2709 | ethyl nonanoate | C11H22O2 | 3.16 | 1.76 |
| 3109 | ethyl decanoate | C12H24O2 | 2.05 | 1.88 |
| 3402.7 | ethyl 3-hydroxybenzoate | C9H10O3 | 0.19 | |
| 3486.9 | ethyl undecanoate | C13H26O2 | 2.22 | |
| 3672.5 | ethyl dodecanoate | C14H28O2 | 1.89 | 1.89 |
| 3714.5 | 4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one | C11H16O2 | 0.20 | |
| 4430.7 | diethyl decanedioate | C14H26O4 | 0.08 | |
| 5020.9 | ethyl (E)-hept-3-enoate | C9H16O2 | 0.24 | |
| 5657.1 | ethyl undecanoate | C13H26O2 | 0.22 | 0.15 |
| 6621.4 | ethyl 8-methylnonanoate | C12H24O2 | 3.45 | |
| 7050.2 | ethyl icosanoate | C22H44O2 | 5.43 | 7.84 |
| 7449.3 | ethyl docosanoate | C24H48O2 | 3.01 | |
| Retention Time/s | Name | Molecular Formula | Relative Content/(wt%) | |
|---|---|---|---|---|
| ESPNC | ESPFMO-2 | |||
| 1320.5 | phenol | C6H6O | 2.89 | 2.56 |
| 1636 | o-cresol | C7H8O | 0.77 | 0.78 |
| 1720 | p-cresol | C7H8O | 1.65 | 1.55 |
| 1870.5 | 2,6-dimethylphenol | C8H10O | 0.37 | 0.12 |
| 1998.1 | 2-ethylphenol | C8H10O | 2.54 | 2.12 |
| 2046.7 | 2,4-dimethylphenol | C8H10O | 0.50 | |
| 2054.3 | 2,5-dimethylphenol | C8H10O | 0.37 | |
| 2055.2 | 2,3-dimethylphenol | C8H10O | 0.40 | |
| 2063.8 | 2-ethoxyphenol | C8H10O2 | 0.19 | |
| 2119.2 | 4-ethylphenol | C8H10O | 1.30 | |
| 2128.8 | 3-ethylphenol | C8H10O | 0.30 | |
| 2172.2 | 2,6-dimethylphenol | C8H10O | 0.06 | |
| 2206.3 | 2-ethyl-6-methylphenol | C9H12O | 3.17 | 4.60 |
| 2233.9 | 2,4-dimethylphenol | C8H10O | 0.07 | |
| 2298.9 | 2,4,6-trimethylphenol | C9H12O | 0.47 | 0.29 |
| 2362.6 | 2-propylphenol | C9H12O | 0.13 | 0.15 |
| 2453.1 | 3-ethyl-5-methylphenol | C9H12O | 0.64 | |
| 2512.5 | 5-isopropyl-2-methylphenol | C10H14O | 0.71 | 1.02 |
| 2516.5 | 4-isopropylphenol | C9H12O | 0.22 | |
| 2608.1 | 2-ethyl-4,5-dimethylphenol | C10H14O | 0.74 | 0.33 |
| 2740.2 | 2,5-diethylphenol | C10H14O | 0.36 | 0.84 |
| 2786.7 | 2,6-dimethylbenzene-1,4-diol | C8H10O2 | 0.09 | |
| 2925.5 | 4-butylphenol | C10H14O | 0.15 | |
| 2947.2 | 2-ethyl-5-propylphenol | C11H16O | 0.20 | |
| 3186.8 | 2-(tert-butyl)-4-ethylphenol | C12H18O | 0.29 | |
| 3458.6 | 2,6-diisopropylphenol | C12H18O | 0.20 | 0.11 |
| 3476.7 | 2-isopropyl-5-methylbenzene-1,4-diol | C13H26O2 | 0.22 | |
| 3477.3 | 4-methoxy-2,3,6-trimethylphenol | C10H14O2 | 0.31 | |
| 3612.2 | 3-(tert-butyl)-4-methoxyphenol | C11H16O2 | 0.08 | 0.42 |
| 3714.7 | 2-(tert-butyl)-4-methoxyphenol | C11H16O2 | 0.07 | |
| Temperature (°C) | Conversion (%) | Selectivity (%) | ||||
|---|---|---|---|---|---|---|
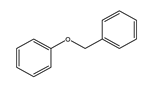 | 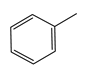 | 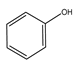 |  |  | 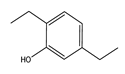 | |
| 220 | 46.77 | 45.80 | 34.14 | 16.21 | 3.84 | 0 |
| 240 | 92.39 | 45.27 | 22.35 | 10.52 | 18.35 | 3.51 |
| 260 | 100 | 43.59 | 11.64 | 6.30 | 25.00 | 13.47 |
| 280 | 100 | 41.68 | 8.95 | 6.19 | 24.47 | 18.71 |
| Proximate Analysis (wt%) | Ultimate Analysis (wt%, daf) | H/C | |||||
|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | C | H | N | Odiff | |
| 7.34 | 4.73 | 49.86 | 63.38 | 4.63 | 0.82 | 26.79 | 0.8766 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Sun, X.; Tang, Y.; Zhang, Y.; Liu, J.; Zhou, X.; Li, X.; Liu, L. Insights into the Relationship between the Microstructure and the Catalytic Behavior of Fe2(MoO4)3 during the Ethanolysis of Naomaohu Coal. Molecules 2023, 28, 6595. https://doi.org/10.3390/molecules28186595
Liu T, Sun X, Tang Y, Zhang Y, Liu J, Zhou X, Li X, Liu L. Insights into the Relationship between the Microstructure and the Catalytic Behavior of Fe2(MoO4)3 during the Ethanolysis of Naomaohu Coal. Molecules. 2023; 28(18):6595. https://doi.org/10.3390/molecules28186595
Chicago/Turabian StyleLiu, Ting, Xuesong Sun, Yakun Tang, Yue Zhang, Jingmei Liu, Xiaodong Zhou, Xiaohui Li, and Lang Liu. 2023. "Insights into the Relationship between the Microstructure and the Catalytic Behavior of Fe2(MoO4)3 during the Ethanolysis of Naomaohu Coal" Molecules 28, no. 18: 6595. https://doi.org/10.3390/molecules28186595
APA StyleLiu, T., Sun, X., Tang, Y., Zhang, Y., Liu, J., Zhou, X., Li, X., & Liu, L. (2023). Insights into the Relationship between the Microstructure and the Catalytic Behavior of Fe2(MoO4)3 during the Ethanolysis of Naomaohu Coal. Molecules, 28(18), 6595. https://doi.org/10.3390/molecules28186595






