Development and Application of a Chemical Ionization Focusing Integrated Ionization Source TOFMS for Online Detection of OVOCs in the Atmosphere
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
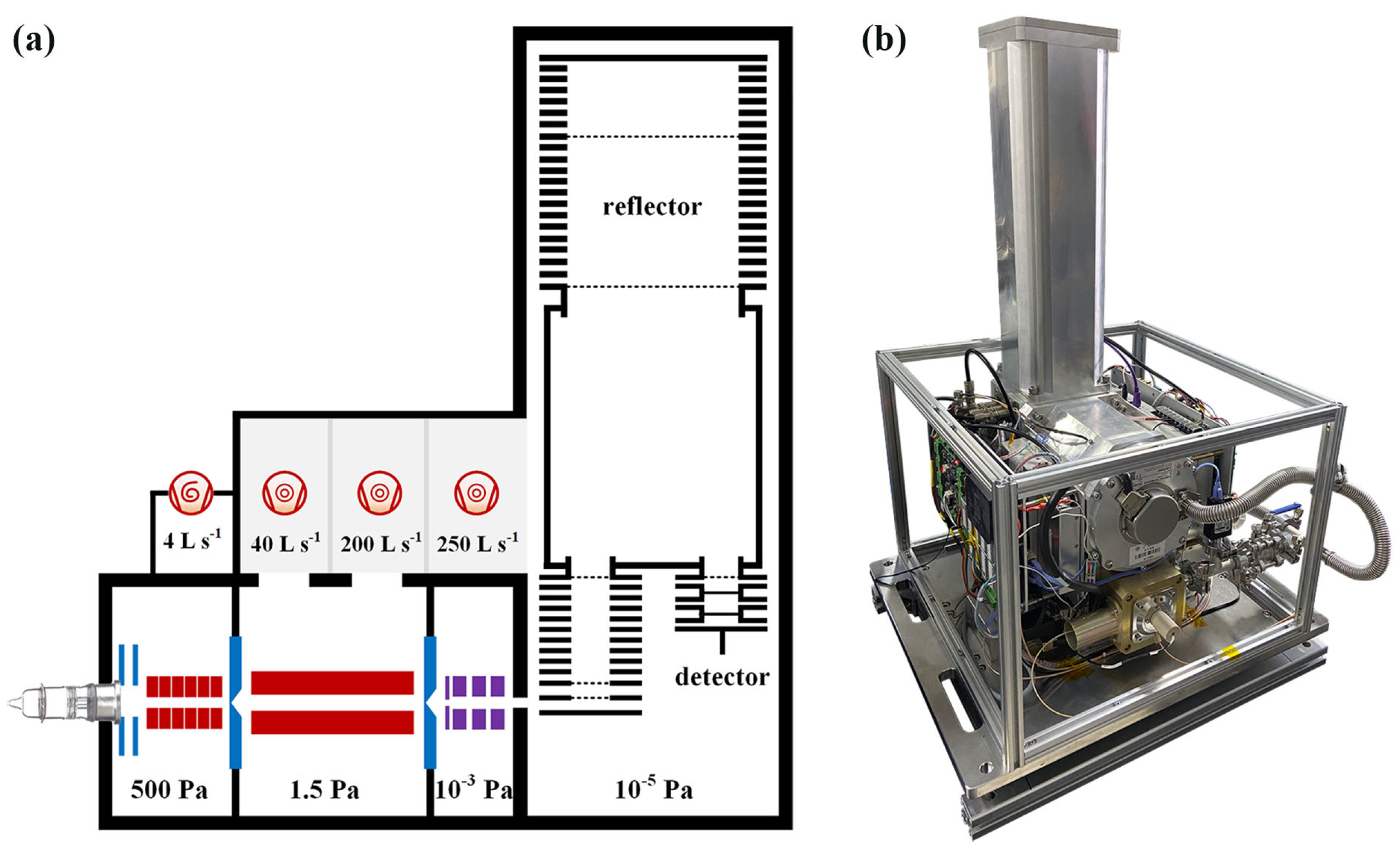
2.2. Instrument and Analytical Process
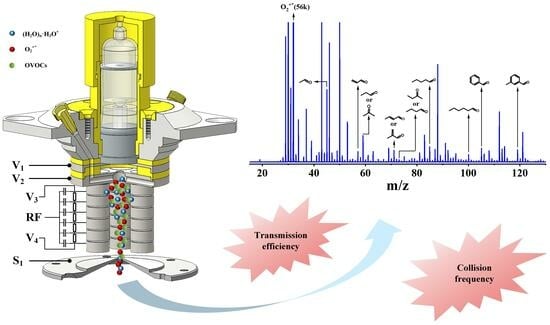
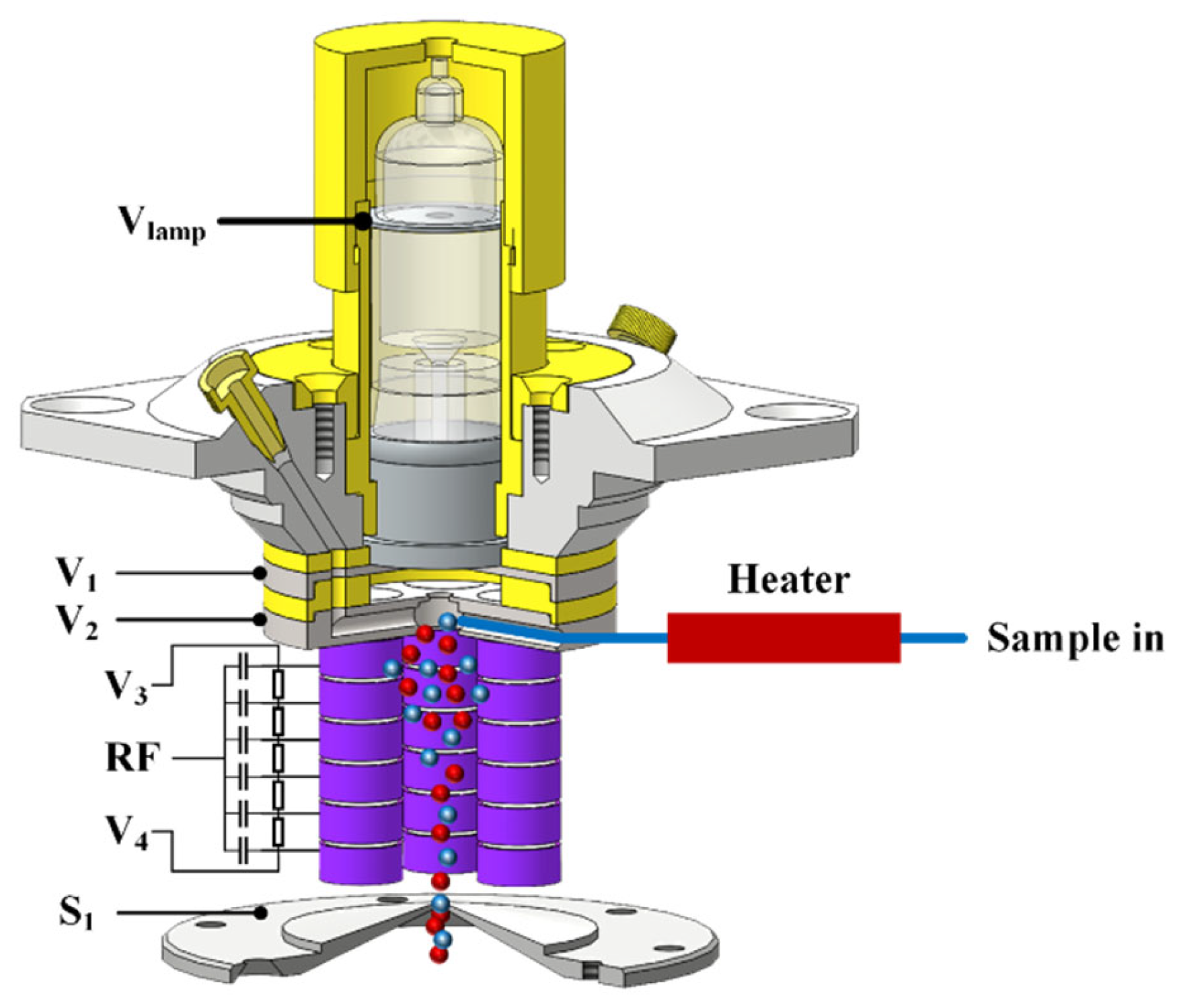
2.2.1. Design of the Chemical Ionization Focusing Integrated Ionization Source
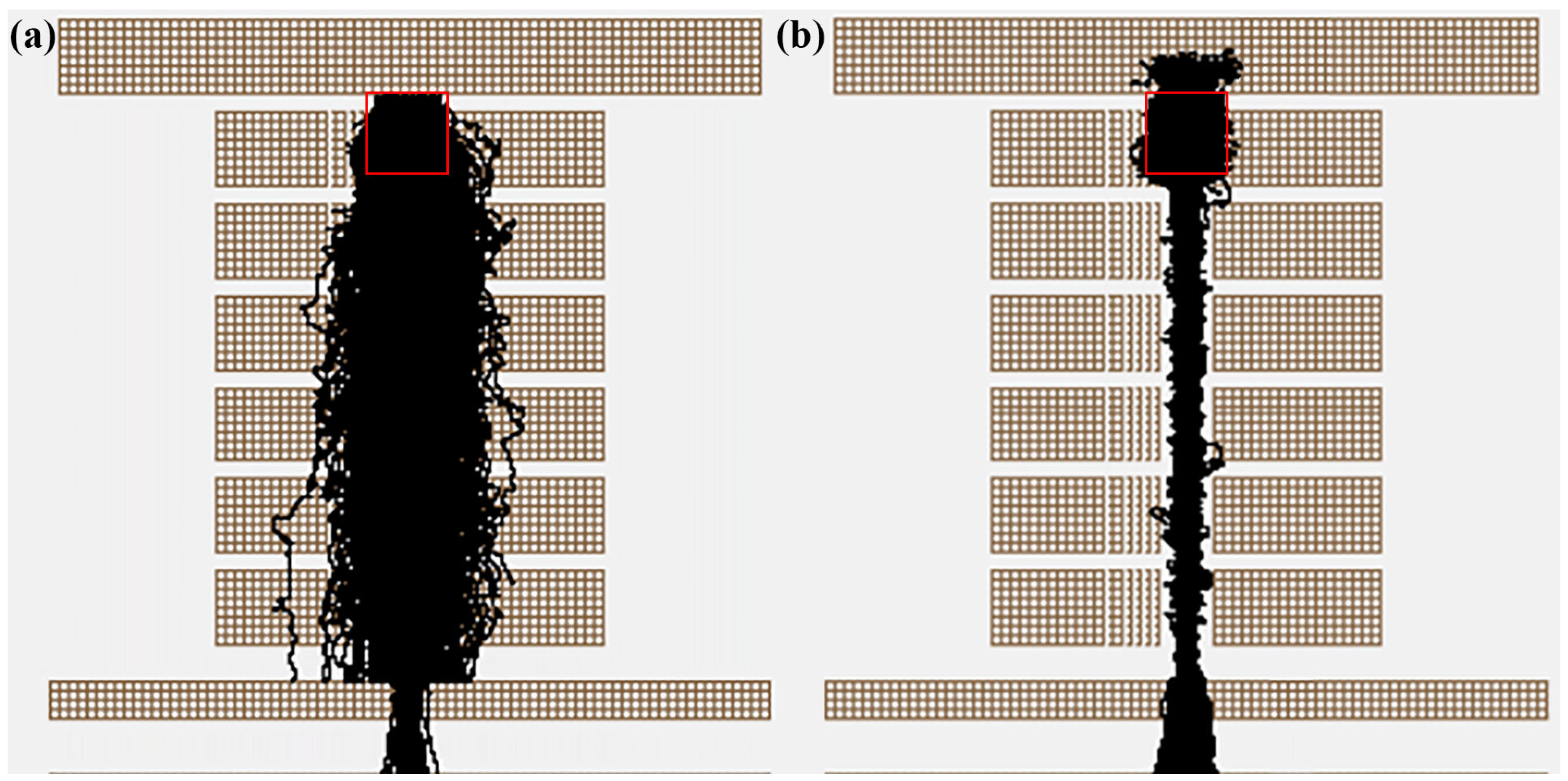
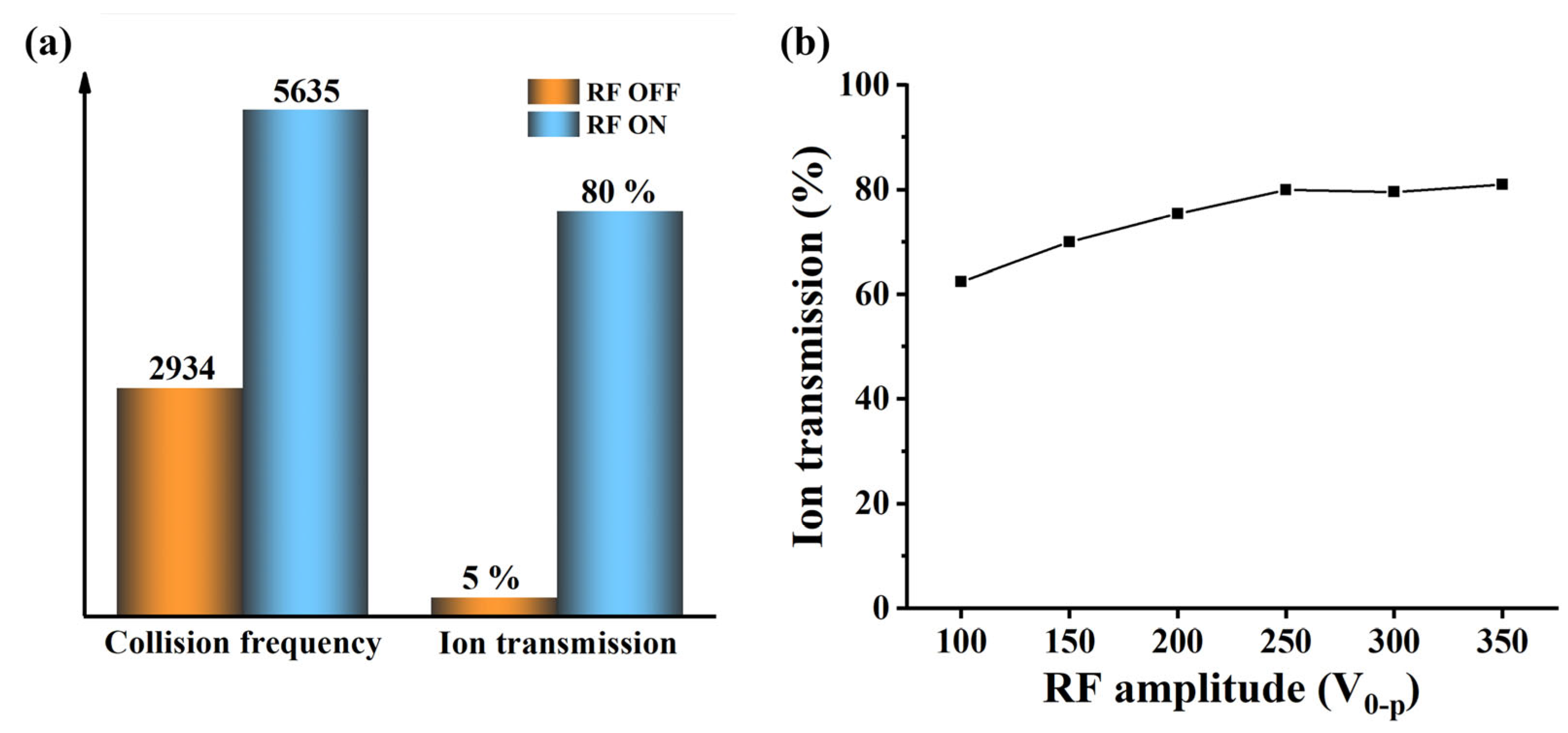
2.2.2. Ion Transmission System
2.2.3. TOF Mass Analyzer
2.2.4. Analytical Process
3. Results and Discussion
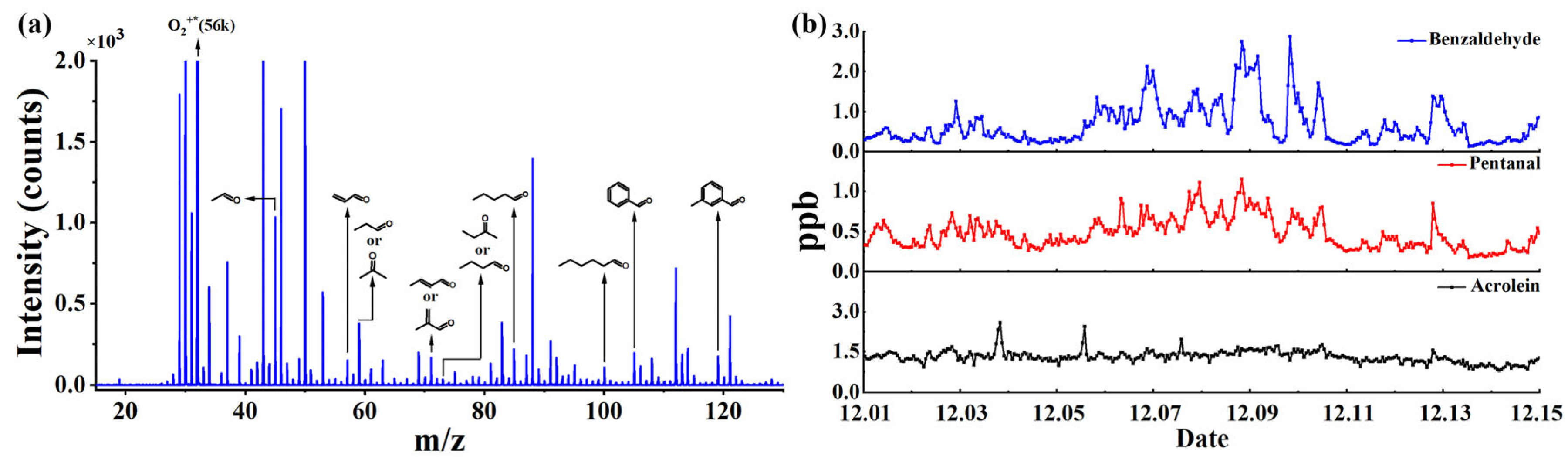
3.1. Enhanced Sensitivity and Soft Ionization for Samples with the CIFI Ionization Source
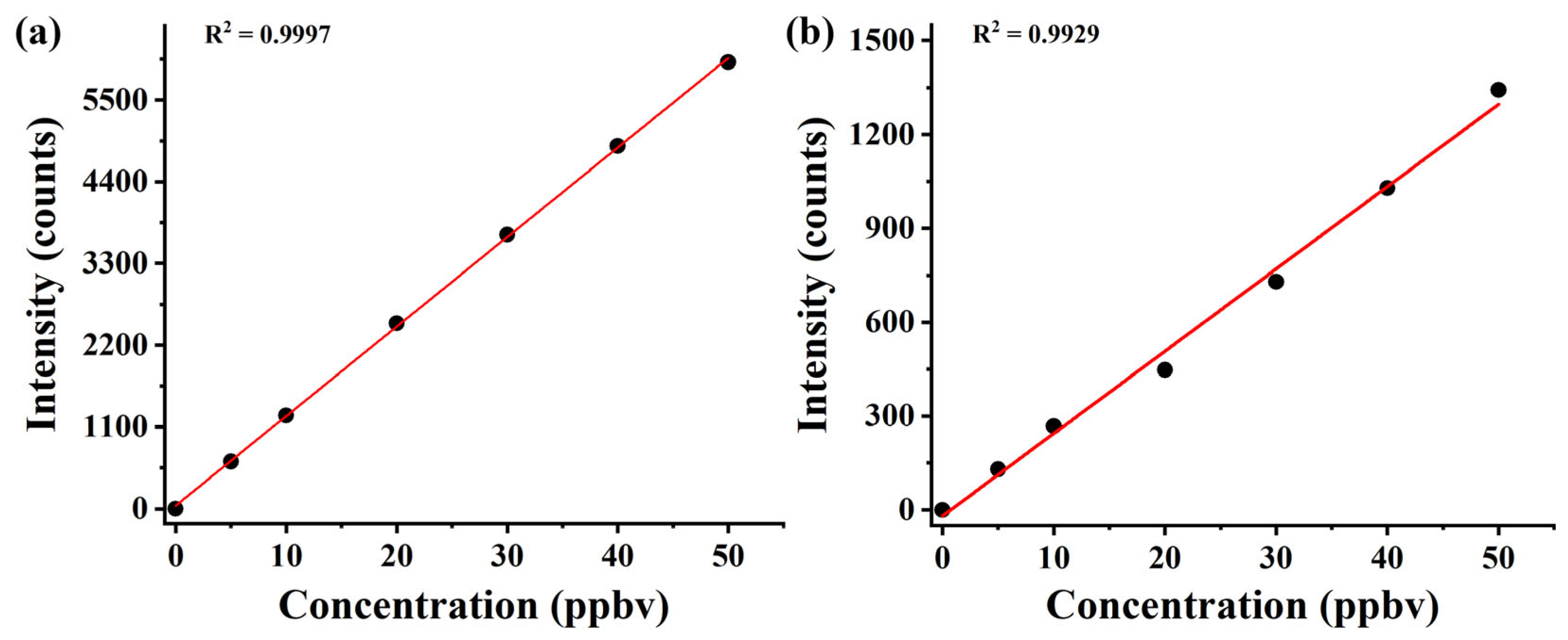
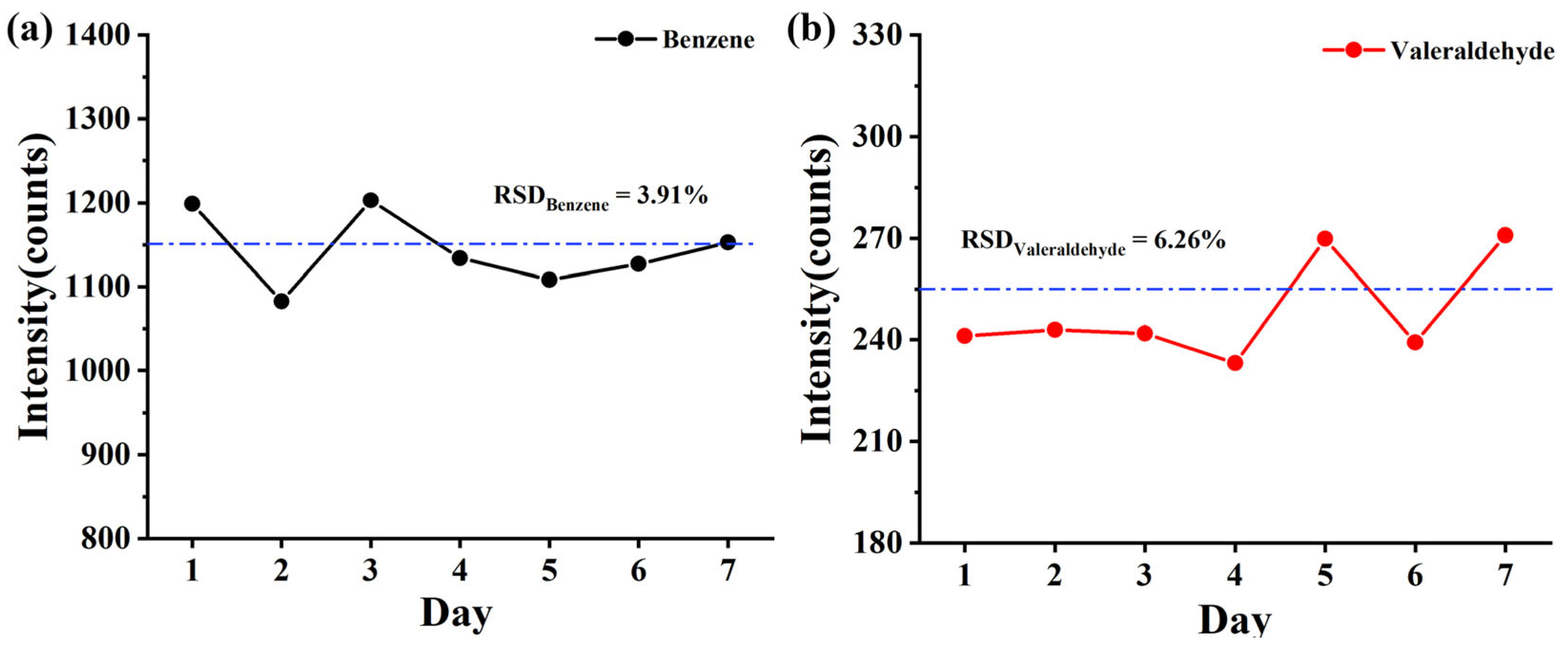
3.2. Linear Dynamic Range, Sensitivity, and Repeatability
3.3. Application in Measurement of OVOCs in the Atmosphere
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nier, A.O. Some Reflections on the Early Days of Mass Spectrometry at the University of Minnesota. Int. J. Mass Spectrom. Ion Process. 1990, 100, 1–13. [Google Scholar] [CrossRef]
- Buiarelli, F.; Canepari, S.; Di Filippo, P.; Perrino, C.; Pomata, D.; Riccardi, C.; Speziale, R. Extraction and Analysis of Fungal Spore Biomarkers in Atmospheric Bioaerosol by HPLC–MS–MS and GC–MS. Talanta 2013, 105, 142–151. [Google Scholar] [CrossRef]
- Bertram, T.H.; Kimmel, J.R.; Crisp, T.A.; Ryder, O.S.; Yatavelli, R.L.N.; Thornton, J.A.; Cubison, M.J.; Gonin, M.; Worsnop, D.R. A Field-Deployable, Chemical Ionization Time-of-Flight Mass Spectrometer. Atmos. Meas. Technol. 2011, 4, 1471–1479. [Google Scholar] [CrossRef]
- Adam, T.; Zimmermann, R. Determination of Single Photon Ionization Cross Sections for Quantitative Analysis of Complex Organic Mixtures. Anal. Bioanal. Chem. 2007, 389, 1941–1951. [Google Scholar] [CrossRef]
- Dorfner, R.; Ferge, T.; Yeretzian, C.; Kettrup, A.; Zimmermann, R. Laser Mass Spectrometry as On-Line Sensor for Industrial Process Analysis: Process Control of Coffee Roasting. Anal. Chem. 2004, 76, 1386–1402. [Google Scholar] [CrossRef]
- Mühlberger, F.; Zimmermann, R.; Kettrup, A. A Mobile Mass Spectrometer for Comprehensive On-Line Analysis of Trace and Bulk Components of Complex Gas Mixtures: Parallel Application of the Laser-Based Ionization Methods VUV Single- Photon Ionization, Resonant Multiphoton Ioniza- Tion, and Laser-Induced Electron Impact Ionization. Anal. Chem. 2001, 73, 3590–3604. [Google Scholar] [CrossRef]
- Breitenlechner, M.; Novak, G.A.; Neuman, J.A.; Rollins, A.W.; Veres, P.R. A Versatile Vacuum Ultraviolet Ion Source for Reduced Pressure Bipolar Chemical Ionization Mass Spectrometry. Atmos. Meas. Technol. 2022, 15, 1159–1169. [Google Scholar] [CrossRef]
- Mühlberger, F.; Saraji-Bozorgzad, M.; Gonin, M.; Fuhrer, K.; Zimmermann, R. Compact Ultrafast Orthogonal Acceleration Time-of-Flight Mass Spectrometer for On-Line Gas Analysis by Electron Impact Ionization and Soft Single Photon Ionization Using an Electron Beam Pumped Rare Gas Excimer Lamp as VUV-Light Source. Anal. Chem. 2007, 79, 8118–8124. [Google Scholar] [CrossRef]
- Mühlberger, F.; Streibel, T.; Wieser, J.; Ulrich, A.; Zimmermann, R. Single Photon Ionization Time-of-Flight Mass Spectrometry with a Pulsed Electron Beam Pumped Excimer VUV Lamp for On-Line Gas Analysis: Setup and First Results on Cigarette Smoke and Human Breath. Anal. Chem. 2005, 77, 7408–7414. [Google Scholar] [CrossRef]
- Kuribayashi, S.; Yamakoshi, H.; Danno, M.; Sakai, S.; Tsuruga, S.; Futami, H.; Morii, S. VUV Single-Photon Ionization Ion Trap Time-of-Flight Mass Spectrometer for On-Line, Real-Time Monitoring of Chlorinated Organic Compounds in Waste Incineration Flue Gas. Anal. Chem. 2005, 77, 1007–1012. [Google Scholar] [CrossRef]
- Wu, Q.; Hua, L.; Hou, K.; Cui, H.; Chen, W.; Chen, P.; Wang, W.; Li, J.; Li, H. Vacuum Ultraviolet Lamp Based Magnetic Field Enhanced Photoelectron Ionization and Single Photon Ionization Source for Online Time-of-Flight Mass Spectrometry. Anal. Chem. 2011, 83, 8992–8998. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, J.; Qiu, K.; Liu, C.; Qi, F.; Pan, Y. A Novel Vacuum Ultraviolet Light Source Assembly with Aluminum-Coated Electrodes for Enhancing the Ionization Efficiency of Photoionization Mass Spectrometry. Rev. Sci. Instrum. 2014, 85, 046110. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, H.; Shu, J.; Ma, P.; Zhang, P.; Huang, J.; Li, Z.; Xu, C. Vacuum-Ultraviolet-Excited and CH2Cl2/H2O-Amplified Ionization-Coupled Mass Spectrometry for Oxygenated Organics Analysis. Anal. Chem. 2018, 90, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, Q.; Hua, L.; Chen, P.; Hu, F.; Wan, N.; Li, H. Highly Selective and Sensitive Online Measurement of Trace Exhaled HCN by Acetone-Assisted Negative Photoionization Time-of-Flight Mass Spectrometry with in-Source CID. Anal. Chim. Acta 2020, 1111, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Liu, R.; Dong, F.; Liu, B.; Hou, K. Vacuum Ultraviolet Photoionization On-Line Mass Spectrometry: Instrumentation Developments and Applications. TrAC Trends Anal. Chem. 2022, 149, 116542. [Google Scholar] [CrossRef]
- Ye, C.; Yuan, B.; Lin, Y.; Wang, Z.; Hu, W.; Li, T.; Chen, W.; Wu, C.; Wang, C.; Huang, S.; et al. Chemical Characterization of Oxygenated Organic Compounds in the Gas Phase and Particle Phase Using Iodide CIMS with FIGAERO in Urban Air. Atmos. Chem. Phys. 2021, 21, 8455–8478. [Google Scholar] [CrossRef]
- Ding, L.; Sudakov, M.; Kumashiro, S. A Simulation Study of the Digital Ion Trap Mass Spectrometer. Int. J. Mass Spectrom. 2002, 221, 117–138. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, S.; Wu, S.; Che, X.; Yang, Y.; Gu, J.; Tan, W.; Ruan, D.; Xiu, G.; Fu, Q. Stationary Monitoring and Source Apportionment of VOCs in a Chemical Industrial Park by Combining Rapid Direct-Inlet MSs with a GC-FID/MS. Sci. Total Environ. 2021, 795, 148639. [Google Scholar] [CrossRef]
- Jochims, H.W.; Lohr, W.; Baumgärtel, H. Photoionization Mass Spectrometry Studies of Deuterated Acetaldehydes CH3CDO and CD3CHO. Chem. Phys. Lett. 1978, 54, 594–596. [Google Scholar] [CrossRef]
- Ohno, K.; Okamura, K.; Yamakado, H.; Hoshino, S.; Takami, T.; Yamauchi, M. Penning Ionization of HCHO, CH2CH2, and CH2CHCHO by Collision with He(23S) Metastable Atoms. J. Phys. Chem. 1995, 99, 14247–14253. [Google Scholar] [CrossRef]
- Tam, W.-C.; Yee, D.; Brion, C.E. Photoelectron Spectra of Some Aldehydes and Ketones. J. Electron Spectrosc. Relat. Phenom. 1974, 4, 77–80. [Google Scholar] [CrossRef]
- El-Sherbini, T.M.; Allam, S.H.; Migahed, M.D.; Dawoud, A.M. Mass Spectrometric Investigation of Aliphatic Aldehydes. Z. Für Naturforschung A 1981, 36, 1334–1337. [Google Scholar] [CrossRef]
- Dam, H.V.; Oskam, A. He(I) and He(II) Photoelectron Spectra of Some Substituted Ethylenes. J. Electron Spectrosc. Relat. Phenom. 1978, 13, 273–290. [Google Scholar] [CrossRef]
- Masclet, P.; Mouvier, G. Étude Par Spectrométrie Photoélectronique d’aldéhydes et de Cétones Éthyléniques Conjugués. J. Electron Spectrosc. Relat. Phenom. 1978, 14, 77–97. [Google Scholar] [CrossRef]
- McAdoo, D.J.; Hudson, C.E. The Decompositions of Metastable [C4H8O]+ Ions and the [C4H8O]+ Potential Surface. Org. Mass Spectrom. 1983, 18, 466–473. [Google Scholar] [CrossRef]
- Traeger, J.C.; McAdoo, D.J. Decomposition Thresholds and Associated Translational Energy Releases for Eight C4H8O+. Isomers. Int. J. Mass Spectrom. Ion Process. 1986, 68, 35–48. [Google Scholar] [CrossRef]
- Holmes, J.L.; Fingas, M.; Lossing, F.P. Towards a General Scheme for Estimating the Heats of Formation of Organic Ions in the Gas Phase. Part, I. Odd-Electron Cations. Can. J. Chem. 1981, 59, 80–93. [Google Scholar] [CrossRef]
- Ashmore, F.S.; Burgess, A.R. Photoelectron Spectra of the Unbranched C5–C7 Alkenes, Aldehydes and Ketones. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1978, 74, 734–742. [Google Scholar] [CrossRef]
- McLoughlin, R.G.; Traeger, J.C. A Photoionization Study of Some Benzoyl Compounds—Thermochemistry of [C7H5O]+ Formation. Org. Mass Spectrom. 1979, 14, 434–438. [Google Scholar] [CrossRef]


| Ion Source | Parameters |
|---|---|
| vacuum ultraviolet lamp | 1100 V; 0.55 mA |
| repulsion electrode (V1) | 30 V |
| extraction electrode (V2) | 25 V |
| voltage at the front end of the segmented quadrupole (V3) | 20 V |
| the segmented quadrupole RF frequency and magnitude | 2.1 MHz; 300 VP-P |
| voltage at the back end of the segmented quadrupole (V4) | 15 V |
| Ion Transmission System | Parameters |
| the first differential vacuum orifice (S1) | 13 V |
| RF-only quadrupole RF frequency, magnitude, and DC float | 1.8 MHz; 280 VP-P; 8 V |
| the second differential vacuum orifice | 5 V |
| the ion lens | −20 V; −50 V |
| TOF Mass Analyzer | Parameters |
| slit | 2 × 8 mm |
| modulator | 25 kHz frequency, ±480 V pulse voltage |
| accelerator | 62.8 mm in length; −3800 V |
| drift region | 468 mm in length |
| reflector | reflector 1, −200 V; reflector 2, 1020 V |
| microchannel plate detector | −4000 V |
| Compound (m/z) | Sensitivity (cps ppbv−1) | LOD (pptv) |
|---|---|---|
| Benzene (78) | 127.5 | 23.5 |
| Pentanal (85) | 25.4 | 118.1 |
| Compounds | Molecular Formula | Characteristic Ions | Linear Range (ppbv) | LOD (pptv) | PTRMS [18] LOD/0.1 s (pptv) | SPIMS [18] LOD/5 s (ppbv) | IE (eV) |
|---|---|---|---|---|---|---|---|
| Acetaldehyde | C2H4O | m/z = 45; [M + H]+ | 2–40 | 6 | 138 | -- | 10.22 [19] |
| Acrolein | C3H4O | m/z = 57; [M + H]+ | 2–40 | 45 | 27 | 22.5 | 10.10 [20] |
| Acetone | C3H6O | m/z = 59; [M + H]+ | 2–40 | 17 | 22 | 3.62 | 9.71 [21] |
| Propionaldehyde | C3H6O | -- | -- | 9.82 [22] | |||
| Crotonaldehyde | C4H6O | m/z = 71; [M + H]+ | 2–40 | 36 | 12 | 2.6 | 9.73 [23] |
| Methacrolein | C4H6O | -- | -- | 9.92 [24] | |||
| 2-Butanone | C4H8O | m/z = 73; [M + H]+ | 2–40 | 200 | -- | -- | 9.70 [25] |
| Butanal | C4H8O | -- | -- | 9.83 [26] | |||
| Pentanal | C5H10O | m/z = 85; [M−H]+ | 2–40 | 44 | 56 | 22.6 | 9.74 [27] |
| Hexanal | C6H12O | m/z = 100; M+* | 2–40 | 57 | 34 | 22 | 9.64 [28] |
| Benzaldehyde | C7H6O | m/z = 105; [M−H]+ | 2–40 | 31 | -- | -- | 9.49 [29] |
| m-Tolualdehyde | C8H8O | m/z = 119; [M−H]+ | 2–40 | 42 | -- | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Guo, Y.; Li, M.; Li, J.; Yang, D.; Hou, K. Development and Application of a Chemical Ionization Focusing Integrated Ionization Source TOFMS for Online Detection of OVOCs in the Atmosphere. Molecules 2023, 28, 6600. https://doi.org/10.3390/molecules28186600
Liu R, Guo Y, Li M, Li J, Yang D, Hou K. Development and Application of a Chemical Ionization Focusing Integrated Ionization Source TOFMS for Online Detection of OVOCs in the Atmosphere. Molecules. 2023; 28(18):6600. https://doi.org/10.3390/molecules28186600
Chicago/Turabian StyleLiu, Ruidong, Yingzhe Guo, Mei Li, Jing Li, Dong Yang, and Keyong Hou. 2023. "Development and Application of a Chemical Ionization Focusing Integrated Ionization Source TOFMS for Online Detection of OVOCs in the Atmosphere" Molecules 28, no. 18: 6600. https://doi.org/10.3390/molecules28186600