Design, Synthesis, Antitumour Evaluation, and In Silico Studies of Pyrazolo-[1,5-c]quinazolinone Derivatives Targeting Potential Cyclin-Dependent Kinases
Abstract
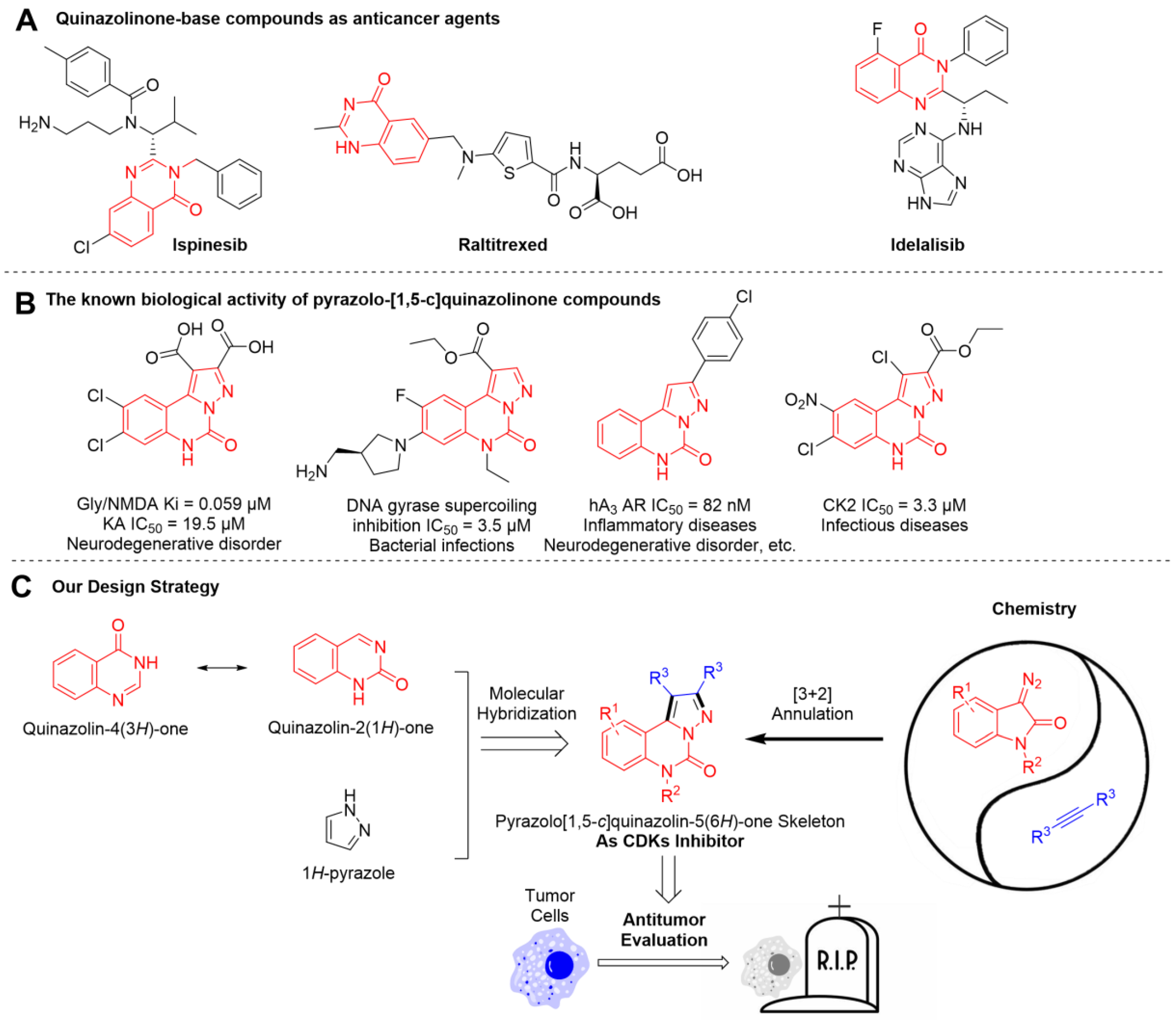
1. Introduction
2. Results and Discussion
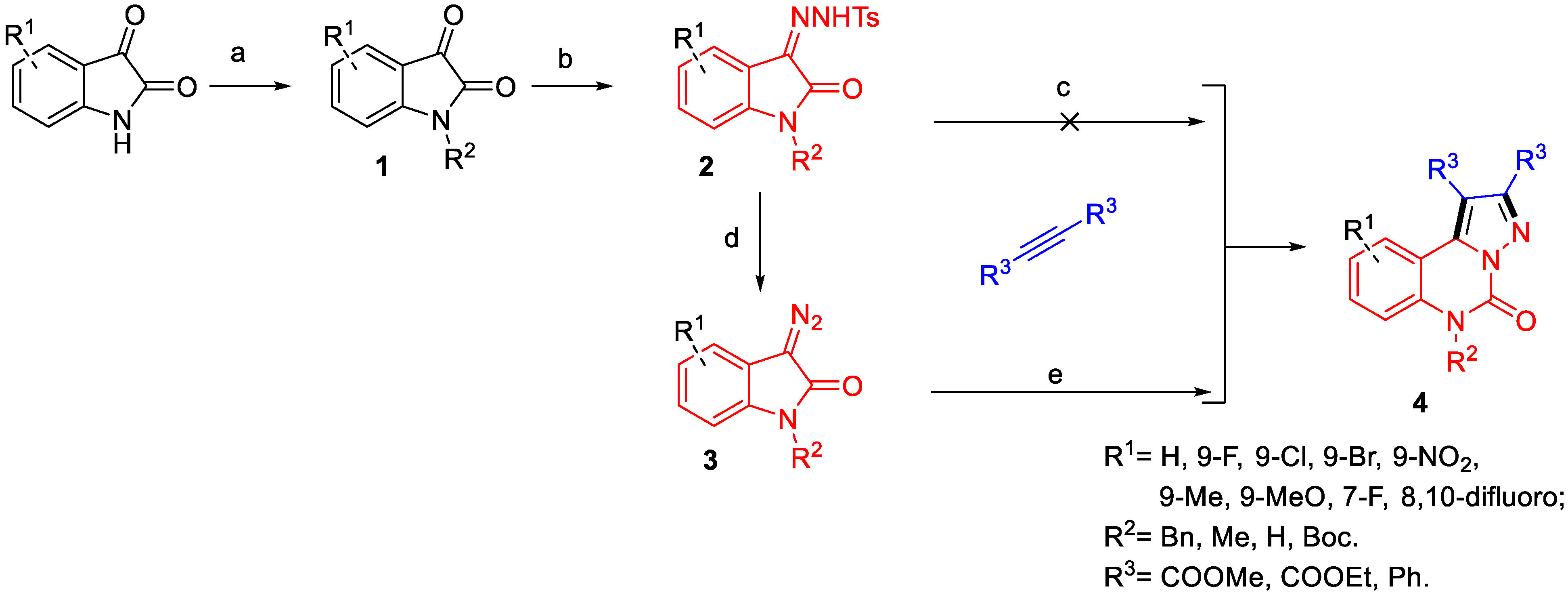
2.1. Chemistry Efforts of the Synthesised Compounds
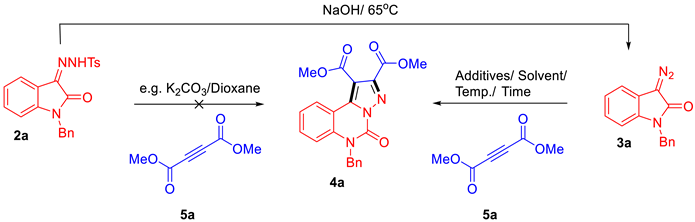
2.1.1. Design and Optimisation of Synthesis
2.1.2. Scope of the Reaction
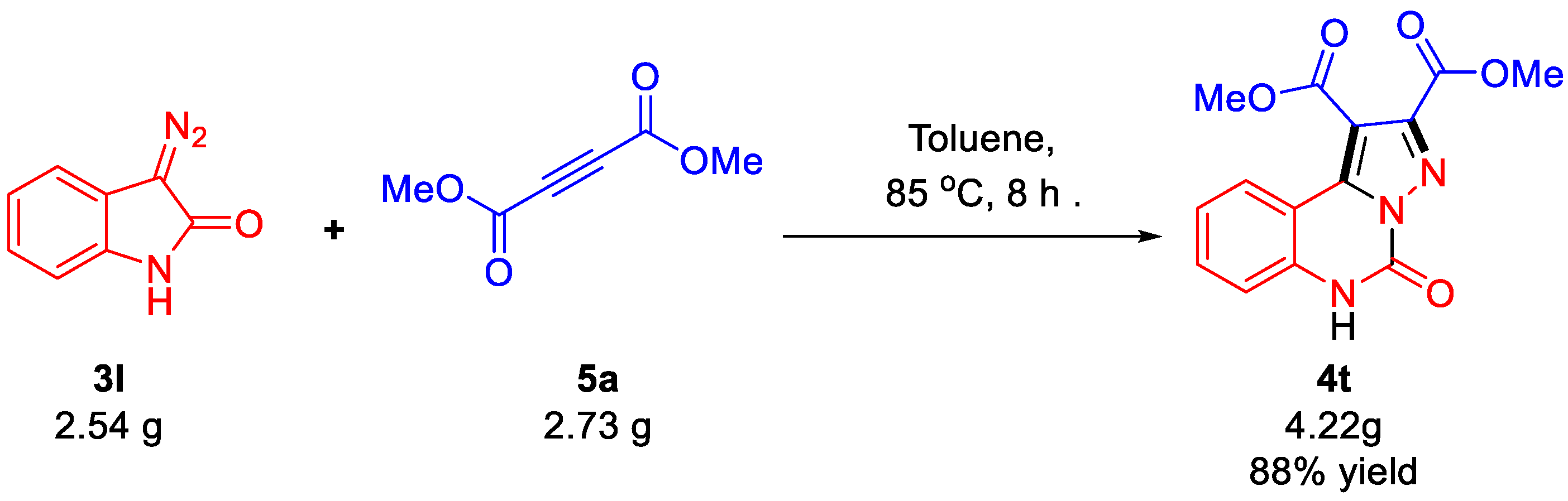
2.1.3. Scale-Up and Green Chemistry Applications
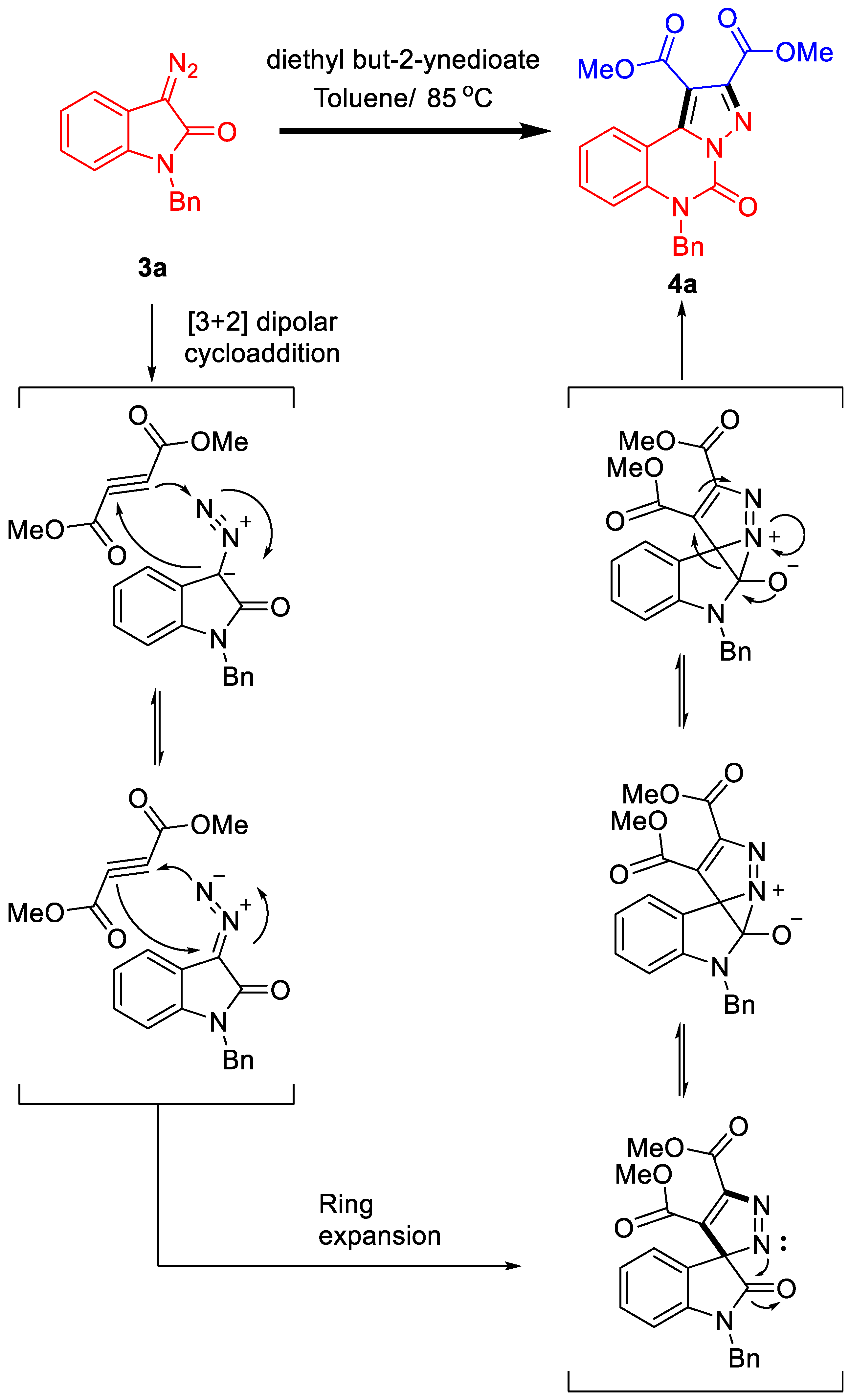
2.1.4. Plausible Mechanism of the Reaction
2.2. Biological Studies
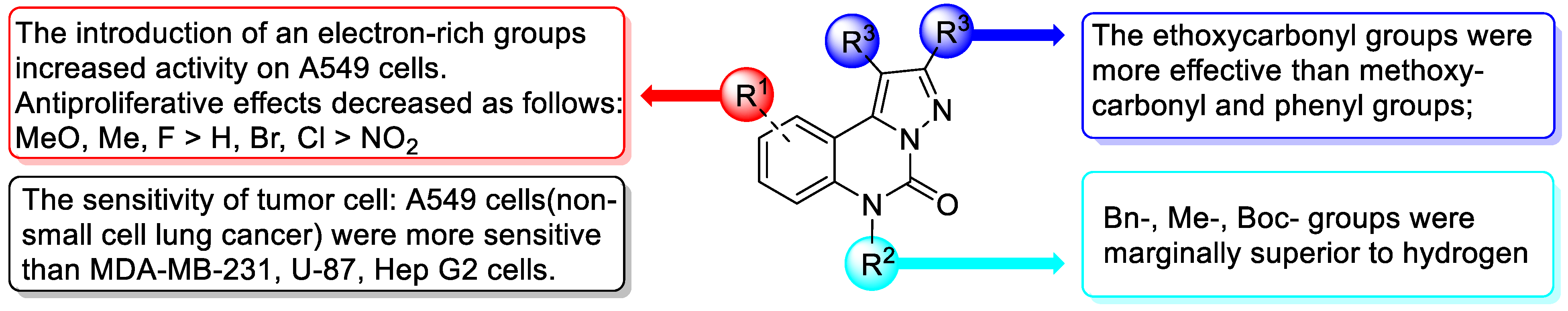
2.2.1. In Vitro Cytotoxicity Evaluation and Structure–Activity Relationship (SAR)
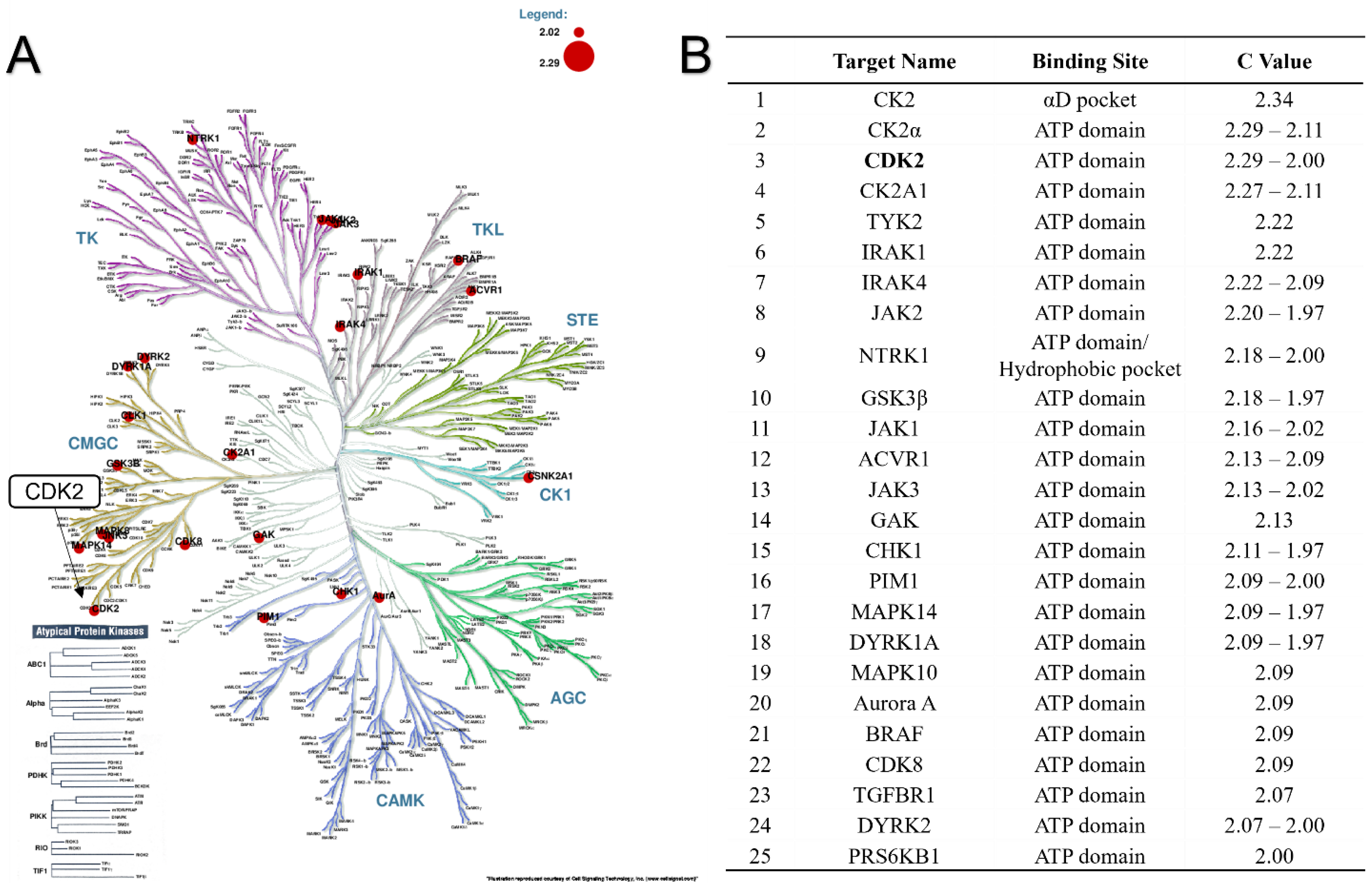
2.2.2. In Silico Studies of Biological Target and Molecular Modelling
2.2.3. CDK2/7/9 Enzyme Activity Assay
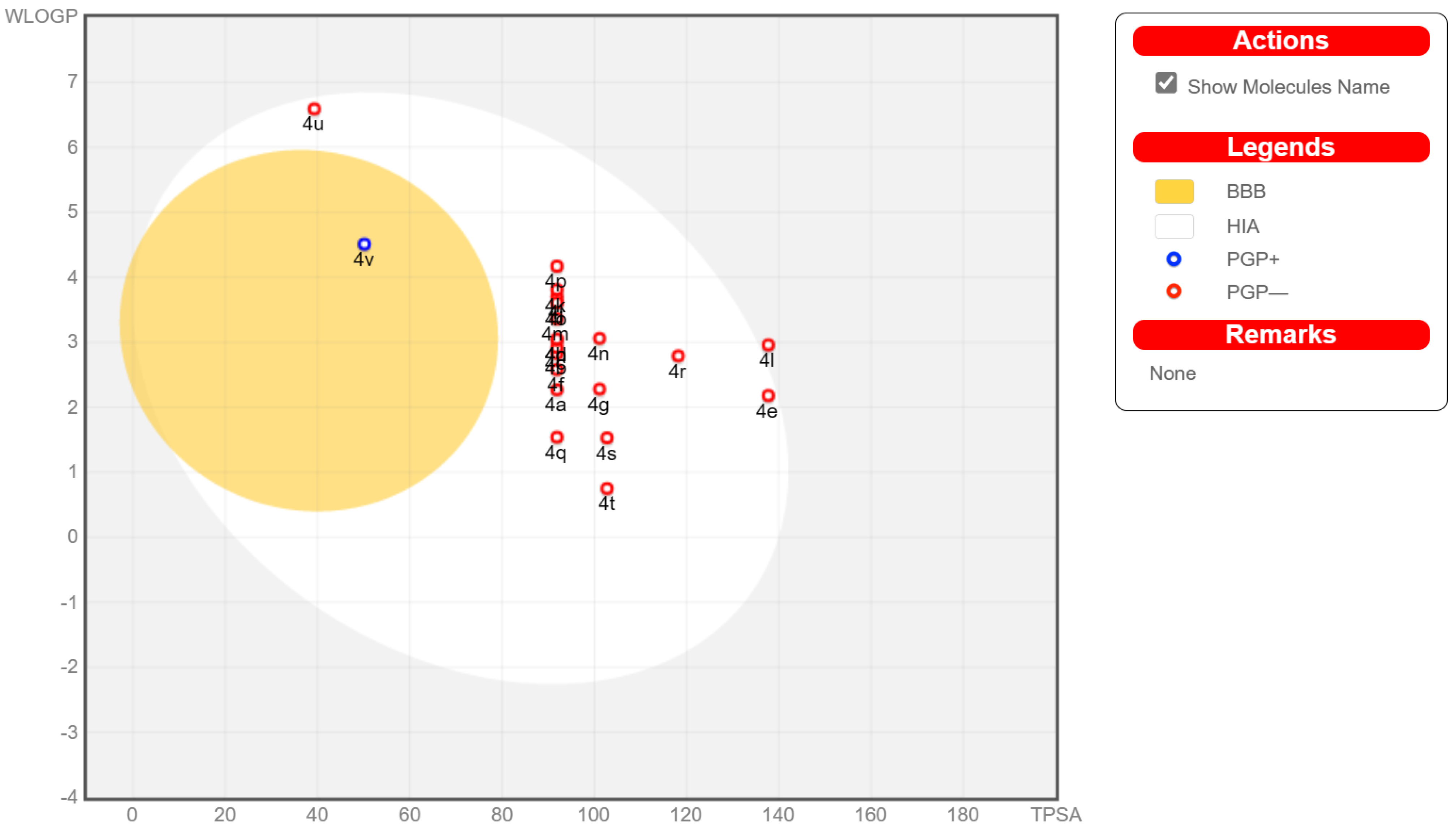
2.3. Drug-Likeness Studies In Silico
2.3.1. Pharmacokinetic and Drug-Likeness Prediction
2.3.2. Toxicity Risk Assessment
3. Conclusions
4. Experimental Section
4.1. General Methods and Materials
4.1.1. General Procedure for the Synthesis of Isatin-Derived Tosylhydrazone 2 and the Diazo Isatin 3
4.1.2. General Procedure for the Synthesis of 4
4.1.3. Characterisation of Products 4a–4v
4.2. Biology and In Silico Study Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Haider, K.; Das, S.; Joseph, A.; Yar, M.S. An appraisal of anticancer activity with structure–activity relationship of quinazoline and quinazolinone analogues through EGFR and VEGFR inhibition: A review. Drug Dev. Res. 2022, 83, 859–890. [Google Scholar] [CrossRef] [PubMed]
- Rezaeinasab, R.; Jafari, E.; Khodarahmi, G. Quinazolinone-based hybrids with diverse biological activities: A mini-review. J. Res. Med. Sci. 2022, 27, 68. [Google Scholar] [CrossRef]
- Nowar, R.M.; Osman, E.E.A.; Abou-Seri, S.M.; El Moghazy, S.M.; El Ella, D.A.A. Design, synthesis and biological evaluation of some novel quinazolinone derivatives as potent apoptotic inducers. Future Med. Chem. 2018, 10, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, C.; Tang, L.; Liu, Z. Discovery of novel quinazolinone derivatives as high potent and selective PI3Kδ and PI3Kδ/γ inhibitors. Eur. J. Med. Chem. 2018, 151, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Resende, D.I.S.P.; Kijjoa, A.; Silva, A.M.S.; Pina, A.; Fernández-Marcelo, T.; Vasconcelos, M.H.; Sousa, E.; Pinto, M.M.M. Antitumor Activity of Quinazolinone Alkaloids Inspired by Marine Natural Products. Mar. Drugs 2018, 16, 261. [Google Scholar] [CrossRef]
- Sun, J.; Fang, Z.-Y.; Tao, Y.-N.; Zhang, Y.-H.; Zhang, Y.; Sun, H.-Y.; Zhou, Y.; Wu, Y.-F. Design, Synthesis and Antitumor Activity of FAK/PLK1 Dual Inhibitors with Quinazolinone as the Skeleton. Chem. Biodivers. 2023, 20, e202300146. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Gan, Y.; Fu, Y.; Liu, J.; Li, W.; Zou, X.; Zhou, Z.; Wang, Z.; Ouyang, G.; Yan, L. Design, synthesis and in vitro biological evaluation of quinazolinone derivatives as EGFR inhibitors for antitumor treatment. J. Enzyme Inhib. Med. Chem. 2020, 35, 555–564. [Google Scholar] [CrossRef]
- Osman, E.O.; Emam, S.H.; Sonousi, A.; Kandil, M.M.; Abdou, A.M.; Hassan, R.A. Design, synthesis, anticancer, and antibacterial evaluation of some quinazolinone-based derivatives as DHFR inhibitors. Drug Dev. Res. 2023, 84, 888–906. [Google Scholar] [CrossRef]
- Wenthur, C.J.; Morrison, R.D.; Daniels, J.S.; Conn, P.J.; Lindsley, C.W. Synthesis and SAR of substituted pyrazolo[1,5-a]quinazolines as dual mGlu2/mGlu3 NAMs. Bioorg. Med. Chem. Lett. 2014, 24, 2693–2698. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Purcell, J.W.; Davis, J.; Reddy, M.; Martin, S.; Samayoa, K.; Vo, H.; Thomsen, K.; Bean, P.; Kuo, W.L.; Ziyad, S.; et al. Activity of the Kinesin Spindle Protein Inhibitor Ispinesib (SB-715992) in Models of Breast Cancer. Clin. Cancer Res. 2010, 16, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Shahin, R.; Aljamal, S. Kinesin spindle protein inhibitors in cancer: From high throughput screening to novel therapeutic strategies. Future Sci. OA 2022, 8, FSO778. [Google Scholar] [CrossRef] [PubMed]
- Chamariya, R.; Suvarna, V. Role of KSP Inhibitors as Anti-Cancer Therapeutics: An Update. Anticancer. Agents Med. Chem. 2022, 22, 2517–2538. [Google Scholar] [CrossRef] [PubMed]
- Batra, A.; Rigo, R.; Hannouf, M.B.; Cheung, W.Y. Real-world Safety and Efficacy of Raltitrexed in Patients With Metastatic Colorectal Cancer. Clin. Color. Cancer 2021, 20, E75–E81. [Google Scholar] [CrossRef] [PubMed]
- Avallone, A.; Gennaro, E.D.; Silvestro, L.; Iaffaioli, V.R.; Budillon, A. Targeting thymidylate synthase in colorectal cancer: Critical re-evaluation and emerging therapeutic role of raltitrexed. Expert Opin. Drug Saf. 2014, 13, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Lannutti, B.J.; Meadows, S.A.; Herman, S.E.M.; Kashishian, A.; Steiner, B.; Johnson, A.J.; Byrd, J.C.; Tyner, J.W.; Loriaux, M.M.; Deininger, M.; et al. CAL-101, a p110δ selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 2011, 117, 591–594. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Perry, M.W.D.; Brown, J.R.; André, F.; Okkenhaug, K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 2021, 20, 741–769. [Google Scholar] [CrossRef]
- Bennani, F.E.; Doudach, L.; Cherrah, Y.; Ramli, Y.; Karrouchi, K.; Ansar, M.H.; Faouzi, M.E.A. Overview of recent developments of pyrazole derivatives as an anticancer agent in different cell line. Bioorg. Chem. 2020, 97, 103470. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.M.; Hassan, A.M.A.; Elkaeed, E.B.; Alesawy, M.S.; Al-Karmalawy, A.A. Design, synthesis, and SAR studies of novel 4-methoxyphenyl pyrazole and pyrimidine derivatives as potential dual tyrosine kinase inhibitors targeting both EGFR and VEGFR-2. Bioorg. Chem. 2022, 123, 105770. [Google Scholar] [CrossRef] [PubMed]
- Varano, F.; Catarzi, D.; Colotta, V.; Calabri, F.R.; Lenzi, O.; Filacchioni, G.; Galli, A.; Costagli, C.; Deflorian, F.; Moro, S. 1-Substituted pyrazolo[1,5-c]quinazolines as novel Gly/NMDA receptor antagonists: Synthesis, biological evaluation, and molecular modeling study. Biorg. Med. Chem. 2005, 13, 5536–5549. [Google Scholar] [CrossRef] [PubMed]
- Varano, F.; Catarzi, D.; Colotta, V.; Filacchioni, G.; Galli, A.; Costagli, C.; Carlà, V. Synthesis and Biological Evaluation of a New Set of Pyrazolo[1,5-c]quinazoline-2-carboxylates as Novel Excitatory Amino Acid Antagonists. J. Med. Chem. 2002, 45, 1035–1044. [Google Scholar] [CrossRef]
- Catarzi, D.; Colotta, V.; Varano, F.; Poli, D.; Squarcialupi, L.; Filacchioni, G.; Varani, K.; Vincenzi, F.; Borea, P.A.; Ben, D.D.; et al. Pyrazolo[1,5-c]quinazoline derivatives and their simplified analogues as adenosine receptor antagonists: Synthesis, structure–affinity relationships and molecular modeling studies. Biorg. Med. Chem. 2013, 21, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.L.; Chheda, P.R.; Lentz, S.R.C.; Held, H.A.; Groves, N.P.; Hiasa, H.; Kerns, R.J. Identification of an ethyl 5,6-dihydropyrazolo[1,5-c]quinazoline-1-carboxylate as a catalytic inhibitor of DNA gyrase. Biorg. Med. Chem. 2020, 28, 115439. [Google Scholar] [CrossRef] [PubMed]
- Moro, S.; Varano, F.; Cozza, G.; Pagano, A.M.; Zagotto, G.; Chilin, A.; Guiotto, A.; Catarzi, D.; Calotta, V.; Pinna, A.L. Pyrazoloquinazoline Tricyclic System as Novel Scaffold to Design New Kinase CK2 Inhibitors. Lett. Drug Des. Discov. 2006, 3, 281–284. [Google Scholar] [CrossRef]
- Jacobson, K.; Gao, Z.G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef]
- Garg, M.; Chauhan, M.; Singh, P.K.; Alex, J.M.; Kumar, R. Pyrazoloquinazolines: Synthetic strategies and bioactivities. Eur. J. Med. Chem. 2015, 97, 444–461. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Paliwal, H.; Thareja, S.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.-H.; et al. Concept of Hybrid Drugs and Recent Advancements in Anticancer Hybrids. Pharmaceuticals 2022, 15, 1071. [Google Scholar] [CrossRef]
- Hawash, M.; Ergun, S.G.; Kahraman, D.C.; Olgac, A.; Hamel, E.; Cetin-Atalay, R.; Baytas, S.N. Novel indole-pyrazole hybrids as potential tubulin-targeting agents; Synthesis, antiproliferative evaluation, and molecular modeling studies. J. Mol. Struct. 2023, 1285, 135477. [Google Scholar] [CrossRef] [PubMed]
- Hawash, M.; Kahraman, D.C.; Ergun, S.G.; Cetin-Atalay, R.; Baytas, S.N. Synthesis of novel indole-isoxazole hybrids and evaluation of their cytotoxic activities on hepatocellular carcinoma cell lines. BMC Chem. 2021, 15, 66. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.; Wang, X.; Huang, J.; Li, S.; Liu, C.; Dong, R.; Zhu, G.; Duan, C.; Jiang, F.; et al. Discovery of coumarin derivatives as potent and selective cyclin-dependent kinase 9 (CDK9) inhibitors with high antitumour activity. Eur. J. Med. Chem. 2020, 200, 112424. [Google Scholar] [CrossRef]
- Chen, Q.; Li, K.; Lu, T.; Zhou, Q. Phosphine-catalyzed domino reactions of alkynyl ketones with sulfonylhydrazones: Construction of diverse pyrazoloquinazoline derivatives. RSC Adv. 2016, 6, 24792–24796. [Google Scholar] [CrossRef]
- Ramu, G.; Krishna, N.H.; Pawar, G.; Sastry, K.N.V.; Nanubolu, J.B.; Babu, B.N. Solvent-Controlled, Tunable Domino Reaction of 3-Ylideneoxindoles with in Situ-Generated α-Aryldiazomethanes: A Facile Access to 3-Spirocyclopropyl-2-oxindole and Pyrazoloquinazolinone Scaffolds. ACS Omega 2018, 3, 12349–12360. [Google Scholar] [CrossRef] [PubMed]
- Ramu, G.; Tangella, Y.; Ambala, S.; Babu, B.N. Regioselective Ring Expansion of 3-Ylideneoxindoles with Tosyldiazomethane (TsDAM): A Metal-Free and Greener Approach for the Synthesis of Pyrazolo-[1,5-c]quinazolines. J. Org. Chem. 2020, 85, 5370–5378. [Google Scholar] [CrossRef] [PubMed]
- Han, W.-Y.; Wang, J.-S.; Zhao, J.; Long, L.; Cui, B.-D.; Wan, N.-W.; Chen, Y.-Z. A Protocol for the Synthesis of CF2H-Containing Pyrazolo[1,5-c]quinazolines from 3-Ylideneoxindoles and in Situ Generated CF2HCHN2. J. Org. Chem. 2018, 83, 6556–6565. [Google Scholar] [CrossRef]
- Barluenga, J.; Tomás-Gamasa, M.; Aznar, F.; Valdés, C. Metal-free carbon–carbon bond-forming reductive coupling between boronic acids and tosylhydrazones. Nat. Chem. 2009, 1, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Kurma, S.H.; Sridhar, B.; Bhimapaka, C.R. Direct Access for the Regio- and Stereoselective Synthesis of N-Alkenylpyrazoles and Chromenopyrazoles. J. Org. Chem. 2021, 86, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.-Z.; Liu, Y.-F.; Cao, J.-Q.; Wang, X.-D.; Zhang, X.-S.; Zhao, C.; Zhao, Y.-Q. Novel 25-hydroxyprotopanaxadiol derivatives incorporating chloroacetyl chloride and their anti-tumor evaluation. Bioorg. Med. Chem. Lett. 2014, 24, 5390–5394. [Google Scholar] [CrossRef]
- Shen, Z.; Yan, Y.-H.; Yang, S.; Zhu, S.; Yuan, Y.; Qiu, Z.; Jia, H.; Wang, R.; Li, G.-B.; Li, H. ProfKin: A comprehensive web server for structure-based kinase profiling. Eur. J. Med. Chem. 2021, 225, 113772. [Google Scholar] [CrossRef]
- Zhang, J.W.; Gan, Y.C.; Li, H.Z.; Yin, J.; He, X.; Lin, L.M.; Xu, S.L.; Fang, Z.P.; Kim, B.W.; Gao, L.N.; et al. Inhibition of the CDK2 and Cyclin A complex leads to autophagic degradation of CDK2 in cancer cells. Nat. Commun. 2022, 13, 2835. [Google Scholar] [CrossRef]
- Tadesse, S.; Anshabo, A.T.; Portman, N.; Lim, E.; Tilley, W.; Caldon, C.E.; Wang, S. Targeting CDK2 in cancer: Challenges and opportunities for therapy. Drug Discov. Today 2020, 25, 406–413. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Huang, J.; Liu, C.; Li, H.; Wang, C.; Hong, Q.; Lei, Y.; Xia, J.; Yu, Z.; et al. Discovery of 2H-benzo[b][1,4]oxazin-3(4H)-one derivatives as potent and selective CDK9 inhibitors that enable transient target engagement for the treatment of hematologic malignancies. Eur. J. Med. Chem. 2022, 238, 114461. [Google Scholar] [CrossRef]
- Wang, X.; Yu, C.; Wang, C.; Ma, Y.; Wang, T.; Li, Y.; Huang, Z.; Zhou, M.; Sun, P.; Zheng, J.; et al. Novel cyclin-dependent kinase 9 (CDK9) inhibitor with suppression of cancer stemness activity against non-small-cell lung cancer. Eur. J. Med. Chem. 2019, 181, 111535. [Google Scholar] [CrossRef] [PubMed]
- Lemke, J.; von Karstedt, S.; El Hay, M.A.; Conti, A.; Arce, F.; Montinaro, A.; Papenfuss, K.; El-Bahrawy, M.A.; Walczak, H. Selective CDK9 inhibition overcomes TRAIL resistance by concomitant suppression of cFlip and Mcl-1. Cell Death Differ. 2014, 21, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Sander, T. OSIRIS Property Explorer. Available online: https://www.organic-chemistry.org/prog/peo/ (accessed on 20 May 2023).
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Çapan, İ.; Hawash, M.; Jaradat, N.; Sert, Y.; Servi, R.; Koca, İ. Design, synthesis, molecular docking and biological evaluation of new carbazole derivatives as anticancer, and antioxidant agents. BMC Chem. 2023, 17, 60. [Google Scholar] [CrossRef]
- Hawash, M.; Qaoud, M.T.; Jaradat, N.; Abdallah, S.; Issa, S.; Adnan, N.; Hoshya, M.; Sobuh, S.; Hawash, Z. Anticancer Activity of Thiophene Carboxamide Derivatives as CA-4 Biomimetics: Synthesis, Biological Potency, 3D Spheroid Model, and Molecular Dynamics Simulation. Biomimetics 2022, 7, 247. [Google Scholar] [CrossRef]
- Liu, J.; Mallick, S.; Xie, Y.; Grassin, C.; Lucas, B.; Scholermann, B.; Pahl, A.; Scheel, R.; Strohmann, C.; Protzel, C.; et al. Morphological Profiling Identifies the Motor Protein Eg5 as Cellular Target of Spirooxindoles. Angew. Chem. Int. Ed. 2023, 62, e202301955. [Google Scholar] [CrossRef]



 | ||||||
|---|---|---|---|---|---|---|
| Entry | 2a/3a | Additives | Solvent | Temp. (oC) | Time (hour) | Yield (%) |
| 1 | 2a | K2CO3 b | Dioxane | 105 | 24 | - c |
| 2 | 2a | Cs2CO3 b | Dioxane | 105 | 24 | - c |
| 3 | 2a | NaOH b | Dioxane | 105 | 24 | - c |
| 4 | 2a | Bu3P d | DCM | 45 | 48 | - c |
| 5 | 3a | - | Dioxane | 105 | 4 | 83% |
| 6 e | 3a | - | Dioxane | 105 | 4 | 86% |
| 7 | 3a | HCl f | Dioxane | 105 | 4 | 80% |
| 8 | 3a | NaOH g | Dioxane | 95 | 4 | Trace |
| 9 | 3a | - | DME | 90 | 4 | 56% |
| 10 | 3a | - | DMF | 65 | 12 | Trace |
| 11 | 3a | - | EtOH | 85 | 2 | 51% |
| 12 | 3a | - | CH3CN | 85 | 12 | Trace |
| 13 | 3a | - | DCE | 85 | 8 | 85% |
| 14 | 3a | - | THF | 65 | 6 | 88% |
| 15 e | 3a | - | THF | 45 | 8 | 86% |
| 16 | 3a | - | Toluene | 110 | 2 | 90% |
| 17 e | 3a | - | Toluene | 85 | 4 | 96% |
| Entry | Solvent (v/v) b | Temp. (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | H2O | 85 | 8 | Trace |
| 2 | H2O/acetone (2:1) | 60 | 12 | 66 |
| 3 | H2O/THF (2:1) | 65 | 12 | 86 |
| 4 | H2O/DME (2:1) | 85 | 12 | 63 |
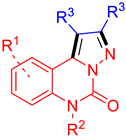 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Comp. | R1 | R2 | R3 | A549 IC50 (μM) | MDA-MB-231 IC50 (μM) | tPSA (Å2) | ClogP | MW (kg/mol) |
| 4a | H | Bn | COOMe | 63.3 | >100 | 88.5 | 2.28 | 391 |
| 4b | 9-F | Bn | COOMe | 99.4 | >100 | 88.5 | 2.42 | 409 |
| 4c | 9-Cl | Bn | COOMe | 99.4 | >100 | 88.5 | 2.99 | 426 |
| 4d | 9-Br | Bn | COOMe | >100 | 85.8 | 88.5 | 3.14 | 470 |
| 4e | 9-NO2 | Bn | COOMe | >100 | >100 | 140.3 | 2.02 | 436 |
| 4f | 9-Me | Bn | COOMe | 40.4 | 70.8 | 88.5 | 2.78 | 405 |
| 4g | 9-OMe | Bn | COOMe | 48.0 | 51.0 | 97.7 | 2.28 | 421 |
| 4h | H | Bn | COOEt | 40.0 | >100 | 88.5 | 3.34 | 419 |
| 4i | 9-F | Bn | COOEt | 17.0 | 75.3 | 88.5 | 3.48 | 437 |
| 4j | 9-Cl | Bn | COOEt | 36.7 | 65.8 | 88.5 | 4.05 | 453 |
| 4k | 9-Br | Bn | COOEt | 40.9 | 71.3 | 88.5 | 4.20 | 497 |
| 4l | 9-NO2 | Bn | COOEt | >100 | >100 | 140.3 | 3.08 | 464 |
| 4m | 9-Me | Bn | COOEt | 14.2 | >100 | 88.5 | 3.84 | 433 |
| 4n | 9-OMe | Bn | COOEt | 18.1 | 26.2 | 97.7 | 3.34 | 449 |
| 4o | 7-F | Bn | COOEt | 50.1 | >100 | 88.5 | 3.48 | 437 |
| 4p | 8,10-2F | Bn | COOEt | 31.9 | 75.3 | 88.5 | 3.62 | 455 |
| 4q | H | Me | COOEt | 31.0 | 93.0 | 88.5 | 1.57 | 343 |
| 4r | H | Boc | COOEt | 37.3 | 74.2 | 114.8 | 2.50 | 429 |
| 4s | H | H | COOEt | 56.2 | >100 | 97.3 | 1.00 | 329 |
| 4t | H | H | COOMe | 49.6 | >100 | 97.3 | −0.06 | 301 |
| 4u | 9-F | Bn | Ph | 46.4 | >100 | 35.9 | 6.39 | 445 |
| 4v | H | H | Ph | 44.7 | 22.5 | 44.7 | 3.91 | 337 |
| 5-Fu | 21.2 | 24.6 b | 58.2 | −0.58 | 130 | |||
| Comp. | CDK9 | CDK2 | CDK7 | |
|---|---|---|---|---|
| IC50 (μM) | At 1.0 μM % of Activity | At 1.0 μM % of Activity | At 1.0 μM % of Activity | |
| 4h | n.d. | 87.1% ± 0.2% | 87.7% ± 5.2% | 100.4% ± 1.8% |
| 4n | 9.8 | 75.0% ± 2.2% | 90.0% ± 9.9% | 102.7% ± 6.2% |
| 4t | 4.7 | 68.5% ± 0.2% | 97.8% ± 1.6% | 102.4% ± 4.5% |
| Pharmaco-Kinetic Aspects | Drug-Likeness (#Violations) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comp. | GI | BBB | P-gp | 2D6 | 3A4 | Lipinski | Ghose | Veber | Egan | Muegge | BS | PAINS |
| 4a | High | No | No | No | No | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4b | High | No | No | No | No | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4c | High | No | No | No | No | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4d | High | No | No | No | No | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4e | High | No | No | No | Yes | 1 | 0 | 0 | 1 | 0 | 0.55 | 0 |
| 4f | High | No | No | No | No | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4g | High | No | No | No | Yes | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4h | High | No | No | No | Yes | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4i | High | No | No | No | Yes | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4j | High | No | No | No | Yes | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4k | High | No | No | No | Yes | 0 | 1 | 0 | 0 | 0 | 0.55 | 0 |
| 4l | Low | No | No | No | Yes | 1 | 0 | 0 | 1 | 0 | 0.55 | 0 |
| 4m | High | No | No | No | Yes | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4n | High | No | No | No | Yes | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4o | High | No | No | No | Yes | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4p | High | No | No | No | Yes | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4q | High | No | No | No | No | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4r | High | No | No | No | Yes | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4s | High | No | No | No | No | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4t | High | No | No | No | No | 0 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| 4u | High | No | No | No | No | 1 | 2 | 0 | 1 | 1 | 0.55 | 0 |
| 4v | High | Yes | Yes | No | No | 1 | 0 | 0 | 0 | 0 | 0.55 | 0 |
| Comp. | Chronic Toxicity Risks a (OSIRIS) | Acute Oral Toxicity b (ProTox-II) | ||||
|---|---|---|---|---|---|---|
| MUT | TUM | IRRIT | RE | LD50 (mg/kg) | Toxicity Class (1–6) | |
| 4a |  |  |  |  | 564 | 4 |
| 4b |  |  |  |  | 564 | 4 |
| 4c |  |  |  |  | 1140 | 4 |
| 4d |  |  |  |  | 564 | 4 |
| 4e |  |  |  |  | 564 | 4 |
| 4f |  |  |  |  | 564 | 4 |
| 4g |  |  |  |  | 564 | 4 |
| 4h |  |  |  |  | 564 | 4 |
| 4i |  |  |  |  | 540 | 4 |
| 4j |  |  |  |  | 564 | 4 |
| 4k |  |  |  |  | 564 | 4 |
| 4l |  |  |  |  | 564 | 4 |
| 4m |  |  |  |  | 540 | 4 |
| 4n |  |  |  |  | 564 | 4 |
| 4o |  |  |  |  | 540 | 4 |
| 4p |  |  |  |  | 540 | 4 |
| 4q |  |  |  |  | 564 | 4 |
| 4r |  |  |  |  | 300 | 3 |
| 4s |  |  |  |  | 564 | 4 |
| 4t |  |  |  |  | 564 | 4 |
| 4u |  |  |  |  | 1000 | 4 |
| 4v |  |  |  |  | 1000 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, D.; Yang, C.; Li, X.; Liu, D.; Wang, Y.; Wang, X.; Zhang, X.; Tan, Y.; Zhang, Y.; Li, Y.; et al. Design, Synthesis, Antitumour Evaluation, and In Silico Studies of Pyrazolo-[1,5-c]quinazolinone Derivatives Targeting Potential Cyclin-Dependent Kinases. Molecules 2023, 28, 6606. https://doi.org/10.3390/molecules28186606
Zheng D, Yang C, Li X, Liu D, Wang Y, Wang X, Zhang X, Tan Y, Zhang Y, Li Y, et al. Design, Synthesis, Antitumour Evaluation, and In Silico Studies of Pyrazolo-[1,5-c]quinazolinone Derivatives Targeting Potential Cyclin-Dependent Kinases. Molecules. 2023; 28(18):6606. https://doi.org/10.3390/molecules28186606
Chicago/Turabian StyleZheng, Danyang, Chenqi Yang, Xiaogang Li, Dong Liu, Yan Wang, Xuesong Wang, Xueying Zhang, Yinfeng Tan, Yuchen Zhang, Youbin Li, and et al. 2023. "Design, Synthesis, Antitumour Evaluation, and In Silico Studies of Pyrazolo-[1,5-c]quinazolinone Derivatives Targeting Potential Cyclin-Dependent Kinases" Molecules 28, no. 18: 6606. https://doi.org/10.3390/molecules28186606
APA StyleZheng, D., Yang, C., Li, X., Liu, D., Wang, Y., Wang, X., Zhang, X., Tan, Y., Zhang, Y., Li, Y., & Xu, J. (2023). Design, Synthesis, Antitumour Evaluation, and In Silico Studies of Pyrazolo-[1,5-c]quinazolinone Derivatives Targeting Potential Cyclin-Dependent Kinases. Molecules, 28(18), 6606. https://doi.org/10.3390/molecules28186606






