Impact of Lactation Stage on the Metabolite Composition of Bovine Milk
Abstract
:1. Introduction
2. Results
2.1. Gross Compositional Analysis
2.2. Metabolomic Data Analysis
2.2.1. 1H-NMR Data Analysis
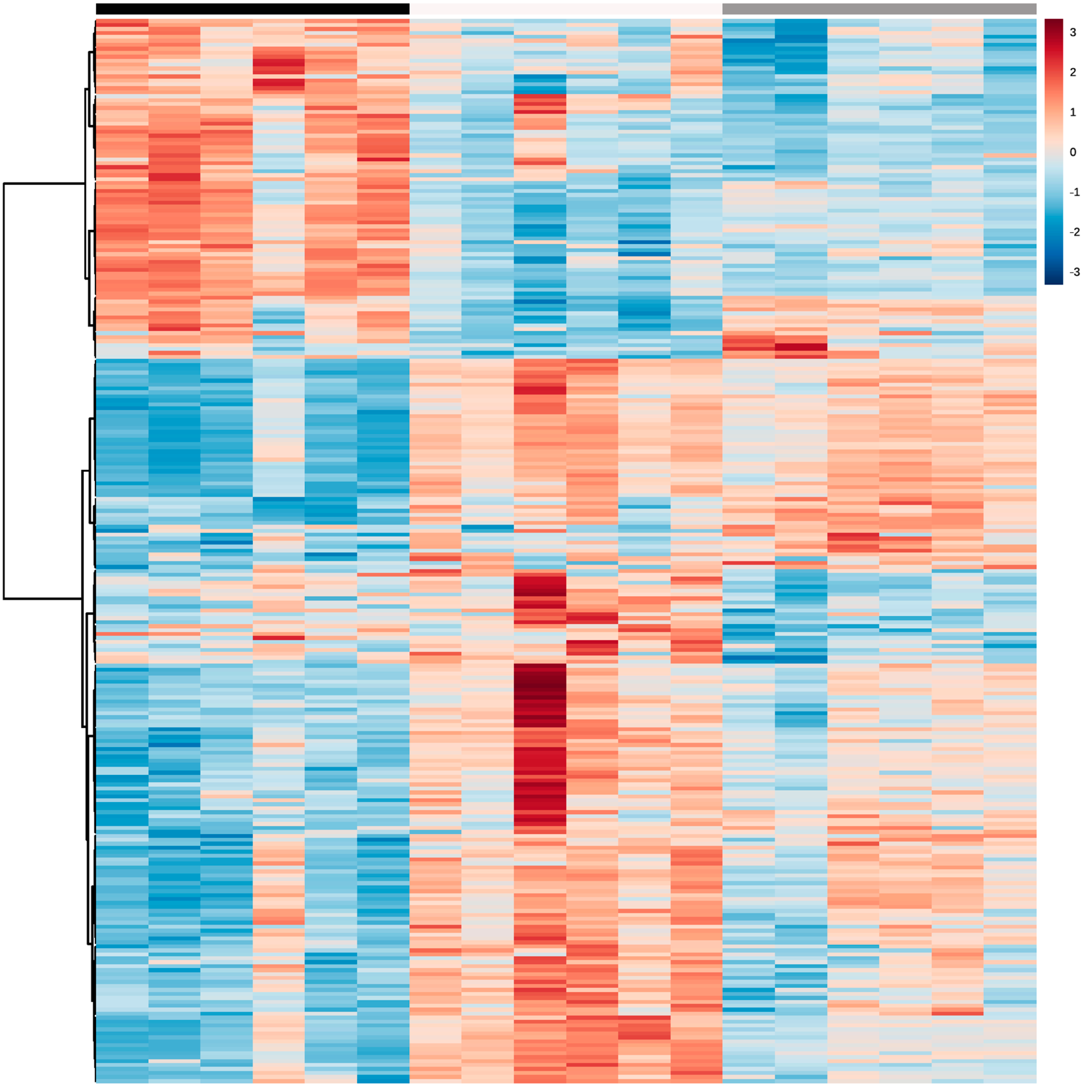
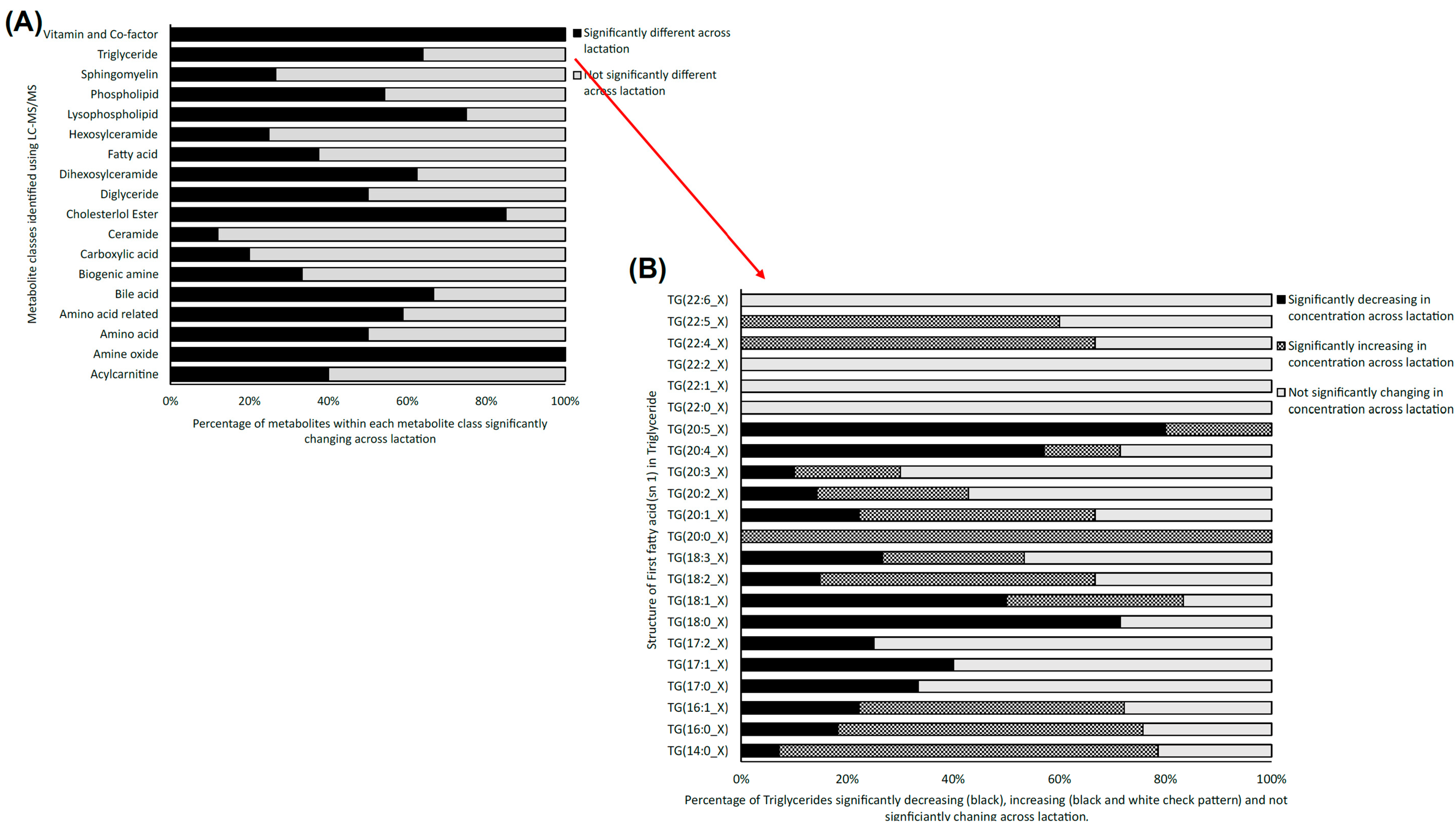
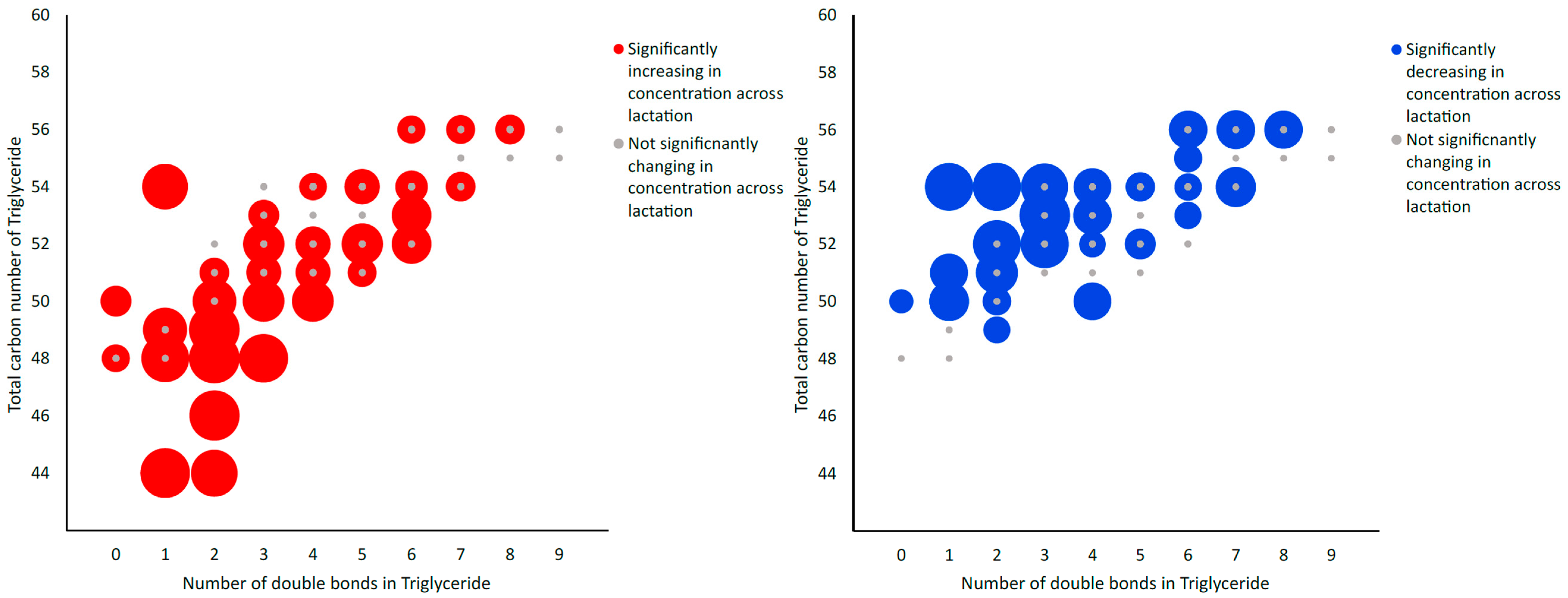
2.2.2. LC-MS/MS Data Analysis
3. Discussion
4. Materials and Methods
4.1. Milk Powder Samples
4.2. Experimental Design
4.3. Gross Compositional Analysis
4.4. Metabolomic Analysis
4.4.1. 1H-NMR Sample Preparation and Analysis
4.4.2. LC-MS/MS Sample Preparation and Analysis
Data Processing and Metabolite Quantification
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- FAO. OECD-FAO Agricultural Outlook 2019–2028; Paris/Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; pp. 180–189. [Google Scholar]
- O’Callaghan, T.; Sugrue, I.; Hill, C.; Ross, R.P.; Stanton, C. Chapter 7—Nutritional Aspects of Raw Milk; Biology; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- MacDonald, J.M.; Law, J.; Mosheim, R. Consolidation in U.S. Dairy Farming; United States Department of Agriculture: Washington, DC, USA, 2020.
- Barkema, H.W.; von Keyserlingk, M.A.; Kastelic, J.P.; Lam, T.J.G.M.; Luby, C.; Roy, J.P.; LeBlanc, S.J.; Keefe, G.P.; Kelton, D.F. Invited review: Changes in the dairy industry affecting dairy cattle health and welfare. J. Dairy Sci. 2015, 98, 7426–7445. [Google Scholar] [CrossRef]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Dairy Chemistry and Biochemistry, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- O’Brien, B.; Guinee, T.P. Milk|seasonal effects on processing properties of cows’ milk. Encycl. Dairy Sci. 2011, 589–606. [Google Scholar] [CrossRef]
- Alothman, M.; Hogan, S.A.; Hennessy, D.; Dillon, P.; Kilcawley, K.N.; O’Donovan, M.; Tobin, J.; Fenelon, M.A.; O’Callaghan, T.F. The “Grass-Fed” Milk Story: Understanding the Impact of Pasture Feeding on the Composition and Quality of Bovine Milk. Foods 2019, 8, 350. [Google Scholar] [CrossRef]
- Auldist, M.J.; Walsh, B.J.; Thomson, N.A. Seasonal and lactational influences on bovine milk composition in New Zealand. J. Dairy Res. 1998, 65, 401–411. [Google Scholar] [CrossRef]
- Mac Gibbon, A.; Taylor, M.; Fox, P.F.; McSweeney, P.L.H. Advanced Dairy Chemistry Volume 2 Lipids; Springer: New York, NY, USA, 2006; Volume 2, pp. XXV, 801. [Google Scholar]
- Kelsey, J.A.; Corl, B.A.; Collier, R.J.; Bauman, D.E. The Effect of Breed, Parity, and Stage of Lactation on Conjugated Linoleic Acid (CLA) in Milk Fat from Dairy Cows. J. Dairy Sci. 2003, 86, 2588–2597. [Google Scholar] [CrossRef]
- Gulati, A.; Galvin, N.; Lewis, E.; Hennessy, D.; O’Donovan, M. McManus, J.J.; Fenelon, M.A.; Guinee, T.P. Outdoor grazing of dairy cows on pasture versus indoor feeding on total mixed ration: Effects on gross composition and mineral content of milk during lactation. J. Dairy Sci. 2018, 101, 2710–2723. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ye, A.; Singh, H. Seasonal variations in composition, properties, and heat-induced changes in bovine milk in a seasonal calving system. J. Dairy Sci. 2019, 102, 7747–7759. [Google Scholar] [CrossRef]
- Timlin, M.; Tobin, J.T.; Brodkorb, A.; Murphy, E.G.; Dillon, P.H.D.; O’Donovan, M.; Pierce, K.M.; O’Callaghan, T.F. The Impact of Seasonality in Pasture-Based Production Systems on Milk Composition and Functionality. Foods 2021, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, T.F.; Hennessy, D.; McAuliffe, S.; Kilcawley, K.N.; O’Donovan, M.; Dillon, P.; Ross, R.P.; Stanton, C. Effect of pasture versus indoor feeding systems on raw milk composition and quality over an entire lactation. J. Dairy Sci. 2016, 99, 9424–9440. [Google Scholar] [CrossRef] [PubMed]
- Sundekilde, U.K.; Larsen, L.B.; Bertram, H.C. NMR-Based Milk Metabolomics. Metabolites 2013, 3, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.S.; Almstetter, M.F.; Schlamberger, G.; Nürnberger, N.; Dettmer, K.; Oefner, P.J.; Meyer, H.; Wiedemann, S.; Gronwald, W. Nuclear magnetic resonance and mass spectrometry-based milk metabolomics in dairy cows during early and late lactation. J. Dairy Sci. 2010, 93, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.; Ray, P.R.; Bhattacharyya, M. A critical review on metabolomic analysis of milk and milk products. Int. J. Dairy Technol. 2020, 74, 17–31. [Google Scholar] [CrossRef]
- Foroutan, A.C.; Guo, A.; Vazquez-Fresno, R.; Lipfert, M.; Zhang, L.; Zheng, J.; Badran, H.; Budinski, Z.; Mandal, R.; Ametaj, B.N.; et al. Chemical Composition of Commercial Cow’s Milk. J. Agric. Food Chem. 2019, 67, 4897–4914. [Google Scholar] [CrossRef] [PubMed]
- Tomassinia, A.; Curoneb, G.; Solè, M.; Capuani, G.; Sciubba, F.; Conta, G.; Miccheli, A.; Vigo, D. NMR-based metabolomics to evaluate the milk composition from Friesian and Autochthonous cows of Northern Italy at different lactation times. Nat. Prod. Res. 2018, 33, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, T.F.; O’Donovan, M.; Murphy, J.P.; Sugrue, K.; Mannion, D.; McCarthy, W.P.; Timlin, M.; Kilcawley, K.N.; Hickey, R.M.; Tobin, J.T. Evolution of the bovine milk fatty acid profile—From colostrum to milk five days post parturition. Int. Dairy J. 2020, 104, 104655. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; O’Donovan, M.; Murphy, J.P.; Sugrue, K.; Tobin, J.T.; McNamara, A.E.; Yin, X.; Sundaramoorthy, G.; Brennan, L. The bovine colostrum and milk metabolome at the onset of lactation as determined by 1H-NMR. Int. Dairy J. 2021, 113, 104881. [Google Scholar] [CrossRef]
- Magan, J.B.; O’Callaghan, T.F.; Zheng, J.; Zhang, L.; Mandal, R.; Hennessy, D.; Fenelon, M.A.; Wishart, D.S.; Kelly, A.L.; McCarthy, N.A. Impact of Bovine Diet on Metabolomic Profile of Skim Milk and Whey Protein Ingredients. Metabolites 2019, 9, 305. [Google Scholar] [CrossRef]
- Zhu, D.; Kebede, B.; McComb, K.; Hayman, A.; Chen, G.; Frew, R. Milk biomarkers in relation to inherent and external factors based on metabolomics. Trends Food Sci. Technol. 2021, 109, 51–64. [Google Scholar] [CrossRef]
- Stoop, W.M.; Bovenhuis, H.; Heck, J.M.; van Arendonk, J.A. Effect of lactation stage and energy status on milk fat composition of Holstein-Friesian cows. J. Dairy Sci. 2009, 92, 1469–1478. [Google Scholar] [CrossRef]
- Xu, W.; Van Knegsel, A.; Saccenti, E.; van Hoeij, R.; Kemp, B.; Vervoort, J. Metabolomics of Milk Reflects a Negative Energy Balance in Cows. J. Proteome Res. 2020, 19, 2942–2949. [Google Scholar] [CrossRef]
- Venkat, M.; Chia, L.W.; Lambers, T.T. Milk polar lipids composition and functionality: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 1–45. [Google Scholar] [CrossRef]
- Lee, H.; Padhi, E.; Hasegawa, Y.; Larke, J.; Parenti, M.; Wang, A.; Hernell, O.; Lönnerdal, B.; Slupsky, C. Compositional Dynamics of the Milk Fat Globule and Its Role in Infant Development. Front. Pediatr. 2018, 6, 313. [Google Scholar] [CrossRef]
- Lu, J.; Pickova, J.; Vázquez-Gutiérrez, J.L.; Langton, M. Influence of seasonal variation and ultra high temperature processing on lipid profile and fat globule structure of Swedish cow milk. Food Chem. 2018, 239, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Mesilati-Stahy, R.; Argov-Argaman, N. The relationship between size and lipid composition of the bovine milk fat globule is modulated by lactation stage. Food Chem. 2014, 145, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Bitman, J.; Wood, D.L. Changes in Milk Fat Phospholipids During Lactation. J. Dairy Sci. 1990, 73, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Pappenheim, S.; Yener, S.; Goselink, R.; Quintanilla-Carvajal, M.X.; van Valenberg, H.J.F.; Hettinga, K. Bovine milk fatty acid and triacylglycerol composition and structure differ between early and late lactation influencing milk fat solid fat content. Int. Dairy J. 2022, 131, 105370. [Google Scholar] [CrossRef]
- Garnsworthy, P.C.; Masson, L.L.; Lock, A.L.; Mottram, T.T. Variation of Milk Citrate with Stage of Lactation and De Novo Fatty Acid Synthesis in Dairy Cows. J. Dairy Sci. 2006, 89, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Vervoort, J.; Saccenti, E.; van Hoeij, R.; Kemp, B.; van Knegsel, A. Milk Metabolomics Data Reveal the Energy Balance of Individual Dairy Cows in Early Lactation. Sci. Rep. 2018, 8, 15828. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, M.; Larsen, L.B.; Sørensen, J.; Poulsen, N.A. Natural variations of citrate and calcium in milk and their effects on milk processing properties. J. Dairy Sci. 2019, 102, 6830–6841. [Google Scholar] [CrossRef]
- Artegoitia, V.M.; Middleton, J.L.; Harte, F.M.; Campagna, S.R.; De Veth, M.J. Choline and Choline Metabolite Patterns and Associations in Blood and Milk during Lactation in Dairy Cows. PLoS ONE 2014, 9, e103412. [Google Scholar] [CrossRef]
| Milk Component | Early Lactation | Mid Lactation | Late Lactation | |
|---|---|---|---|---|
| Fat | Fat content (g/100 g) | 26.2 (0.6) | 25.9 (0.1) | 25.3 (0.1) |
| SAFA (g/100 g) | 16.0 (0.4) | 16.3 (0.6) | 16.0 (0.1) | |
| MUFA (g/100 g) | 7.5 (0.3) | 6.4 (0.4) | 6.5 (0.1) | |
| PUFA (g/100 g) | 0.7 (0.0) | 0.7 (0.2) | 0.5 (0.0) | |
| TFA (g/100 g) | 0.9 (0.0) | 1.3 (0.0) | 1.3 (0.0) | |
| Sum of Omega-3 FA (g/100 g) | 0.2 (0.0) | 0.2 (0.0) | 0.2 (0.0) | |
| Sum of Omega-6 FFA (g/100 g) | 0.5 (0.0) | 0.5 (0.2) | 0.3 (0.0) | |
| Minerals | Ca (mg/kg) | 9715.0 (35.4) | 8755.0 (134.4) | 8395.0 (275.8) |
| CU (mg/kg) | 0.7 (0.0) | 0.4 (0.0) | 0.2 (0.0) | |
| Fe (mg/kg) | 1.7 (0.2) | 1.4 (0.1) | 1.6 (0.0) | |
| Mg (mg/kg) | 969.5 (24.8) | 862.0 (60.8) | 898.5 (12.0) | |
| Mn (mg/kg) | 0.2 (0.0) | 0.2 (0.0) | 0.2 (0.0) | |
| MO (mg/kg) | 0.3 (0.0) | 0.3 (0.0) | 0.3 (0.0) | |
| P (mg/kg) | 8935.0 (120.2) | 8305.0 (417.2) | 7465.0 (176.8) | |
| K (mg/kg) | 11,750.0 (70.7) | 11,200.0 (707.1) | 10,000.0 (424.3) | |
| Na (mg/kg) | 3085.0 (35.4) | 2670.0 (127.3) | 2830.0 (14.1) | |
| Zn (mg/kg) | 33.7 (0.1) | 28.6 (0.0) | 27.3 (0.4) | |
| Vitamins | Vitamin B12 (µg/100 g) | 2.0 (0.0) | 1.6 (0.3) | 1.7 (0.1) |
| Vitamin C (mg/kg) | 62.6 (1.7) | 83.1 (7.1) | 84.5 (2.1) | |
| Amino acids | Aspartic Acid (g/100 g) | 2.4 (0.7) | 1.9 (0.1) | 1.9 (0.0) |
| Serine (g/100 g) | 1.4 (0.0) | 1.4 (0.1) | 1.4 (0.0) | |
| Glutamic Acid (g/100 g) | 4.9 (0.2) | 5.1 (0.2) | 5.1 (0.0) | |
| Glycine (g/100 g) | 0.8 (0.0) | 0.8 (0.0) | 0.9 (0.0) | |
| Histidine (g/100 g) | 0.6 (0.0) | 0.6 (0.0) | 0.6 (0.0) | |
| Arginine (g/100 g) | 0.8 (0.0) | 0.8 (0.0) | 0.9 (0.0) | |
| Threonine (g/100 g) | 1.0 (0.0) | 1.1 (0.1) | 1.0 (0.0) | |
| Alanine (g/100 g) | 0.8 (0.0) | 0.8 (0.0) | 0.8 (0.0) | |
| Proline (g/100 g) | 2.1 (0.1) | 2.2 (0.1) | 2.3 (0.0) | |
| Cystine (g/100 g) | 0.2 (0.0) | 0.2 (0.0) | 0.2 (0.0) | |
| Tyrosine (g/100 g) | 1.1 (0.0) | 1.1 (0.0) | 1.1 (0.0) | |
| Valine (g/100 g) | 1.1 (0.1) | 1.3 (0.1) | 1.3 (0.1) | |
| Methionine (g/100 g) | 0.5 (0.0) | 0.5 (0.0) | 0.5 (0.0) | |
| Lysine (g/100 g) | 2.0 (0.1) | 2.1 (0.1) | 2.1 (0.0) | |
| Isoleucine (g/100 g) | 0.9 (0.1) | 1.0 (0.1) | 1.1 (0.1) | |
| Leucine (g/100 g) | 2.1 (0.1) | 2.3 (0.1) | 2.3 (0.1) | |
| Phenylalanine (g/100 g) | 1.1 (0.0) | 1.2 (0.1) | 1.2 (0.0) | |
| Compound | Early Lactation | Mid Lactation | Late Lactation | p-Value | FDR |
|---|---|---|---|---|---|
| Creatinine-phosphate | 0.17 (0.03) a | 0.11 (0.01) b | 0.1 (0.003) b | <0.001 | 0.001 |
| O-Phosphocholine | 0.38 (0.09) a | 0.07 (0.02) b | 0.03 (0.01) b | <0.001 | <0.001 |
| N-Acetylglucosamine | 0.27 (0.07) | 0.33 (0.1) | 0.24 (0.09) | 0.369 | 0.532 |
| Creatine | 0.66 (0.06) a | 0.51 (0.6) b | 0.50 (0.03) b | <0.001 | 0.003 |
| Citrate | 6.44 (0.49) a | 5.11 (0.75) b | 4.71 (0.45) b | <0.001 | 0.001 |
| Choline | 0.21 (0.02) a | 0.31 (0.04) b | 0.34 (0.02) c | <0.001 | <0.001 |
| Alanine | 0.03 (0.003) | 0.03 (0.01) | 0.03 (0.004) | 0.44 | 0.565 |
| Dimethylamine | 0.14 (0.02) a | 0.12 (0.02) b | 0.12 (0.02) b | 0.003 | 0.012 |
| Glutamate | 0.27 (0.03) a | 0.25 (0.04) | 0.22 (0.02) b | 0.007 | 0.023 |
| 3-Hydroxybutyrate | 0.04 (0.01) a | 0.03 (0.01) b | 0.03 (0.01) b | 0.007 | 0.023 |
| Acetone | 0.09 (0.02) | 0.09 (0.03) | 0.1 (0.04) | 0.848 | 0.848 |
| Glucose-1-phosphate | 0.14 (0.03) a | 0.03 (0.01) b | 0.04 (0.05) b | <0.001 | <0.001 |
| Butyrate | 0.04 (0.01) | 0.04 (0.01) | 0.05 (0.01) | 0.388 | 0.533 |
| 2-Oxoglutarate | 0.12 (0.02) | 0.12 (0.02) | 0.12 (0.01) | 0.764 | 0.802 |
| Isovalerate | 0.02 (0.01) | 0.01 (0.01) | 0.03 (0.01) | 0.139 | 0.278 |
| Glucose-6-phosphate | 0.47 (0.18) | 0.3 (0.09) | 0.37 (0.18) | 0.154 | 0.295 |
| Glycerophosphocholine | 7.00 (1.18) a | 6.63 (0.66) b | 6.12 (0.40) b | 0.002 | 0.012 |
| Hippurate | 0.15 (0.03) b | 0.19 (0.02) a | 0.17 (0.02) b | 0.009 | 0.025 |
| Isoleucine | 0.00 (0.001) | 0.00 (0.002) | 0.01 (0.001) | 0.305 | 0.463 |
| Dimethyl Sulfone | 0.03 (0.004) b | 0.04 (0.003) a | 0.03 (0.003) b | <0.001 | 0.002 |
| Valerate | 0.16 (0.01) | 0. 02 (0.004) | 0.03 (0.01) | 0.111 | 0.232 |
| Fumarate | 0.02 (0.002) | 0.02 (0.003) | 0.02 (0.002) | 0.172 | 0.315 |
| Glycerol | 8.16 (1.05) | 8.63 (0.66) | 8.33 (1.4) | 0.834 | 0.848 |
| Glucose | 0.25 (0.07) | 0.32 (0.12) | 0.21 (0.11) | 0.195 | 0.342 |
| Valine | 0.01 (0.001) | 0.01 (0.002) | 0.01 (0.002) | 0.595 | 0.694 |
| Pyruvate | 0.02 (0.01) | 0.02 (0.003) | 0.02 (0.004) | 0.393 | 0.533 |
| Succinate | 0.04 (0.003) | 0.04 (0.01) | 0.04 (0.01) | 0.733 | 0.791 |
| Galactose | 0.21 (0.13) a | 0.39 (0.12) b | 0.36 (0.10) b | 0.010 | 0.027 |
| Lactate | 0.23 (0.21) | 0.63 (0.84) | 0.81 (0.71) | 0.309 | 0.463 |
| Formate | 0.09 (0.07) | 0.26 (0.35) | 0.34 (0.33) | 0.305 | 0.463 |
| Lactose | 67.02 (11.36) | 70.14 (9.40) | 69.78 (5.96) | 0.540 | 0.648 |
| Taurine | 0.09 (0.01) | 0.09 (0.03) | 0.08 (0.03) | 0.468 | 0.578 |
| Ethanolamine | 0.05 (0.01) a | 0.08 (0.04) b | 0.09 (0.02) b | 0.016 | 0.039 |
| Glycine | 4.72 (0.48) | 4.88 (0.44) | 4.59 (0.78) | 0.733 | 0.791 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Connolly, C.; Yin, X.; Brennan, L. Impact of Lactation Stage on the Metabolite Composition of Bovine Milk. Molecules 2023, 28, 6608. https://doi.org/10.3390/molecules28186608
Connolly C, Yin X, Brennan L. Impact of Lactation Stage on the Metabolite Composition of Bovine Milk. Molecules. 2023; 28(18):6608. https://doi.org/10.3390/molecules28186608
Chicago/Turabian StyleConnolly, Claire, Xiaofei Yin, and Lorraine Brennan. 2023. "Impact of Lactation Stage on the Metabolite Composition of Bovine Milk" Molecules 28, no. 18: 6608. https://doi.org/10.3390/molecules28186608
APA StyleConnolly, C., Yin, X., & Brennan, L. (2023). Impact of Lactation Stage on the Metabolite Composition of Bovine Milk. Molecules, 28(18), 6608. https://doi.org/10.3390/molecules28186608