Possibilities and Limitations of ICP-Spectrometric Determination of the Total Content of Tin and Its Inorganic and Organic Speciations in Waters with Different Salinity Levels—Part 2: Separate Determination of Inorganic and Organic Speciations of Tin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Assessment of the Possibility of Tin Forms Separation by Precipitation from Aqueous Media
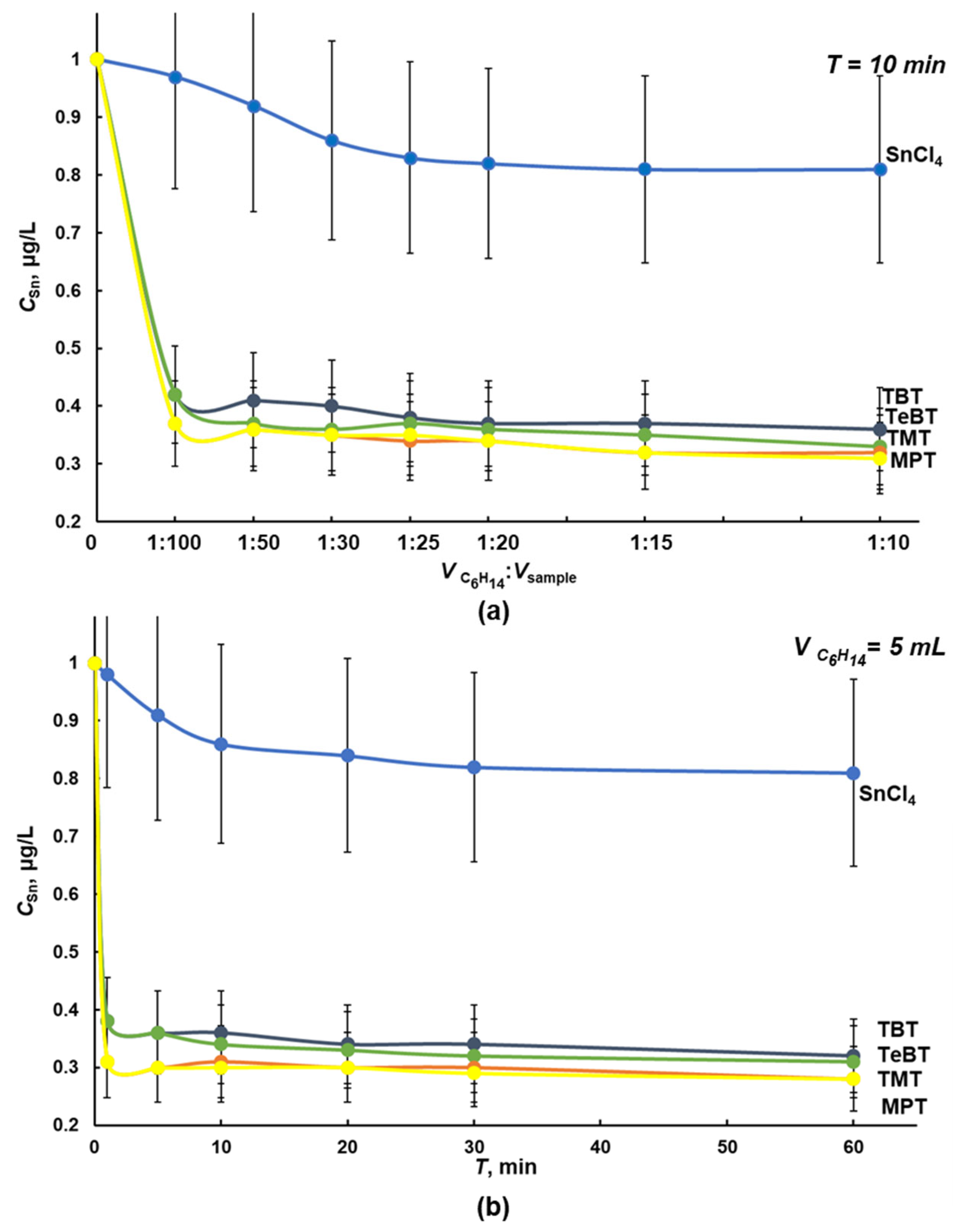
2.2. Liquid–Liquid Separation of Tin Forms in Water
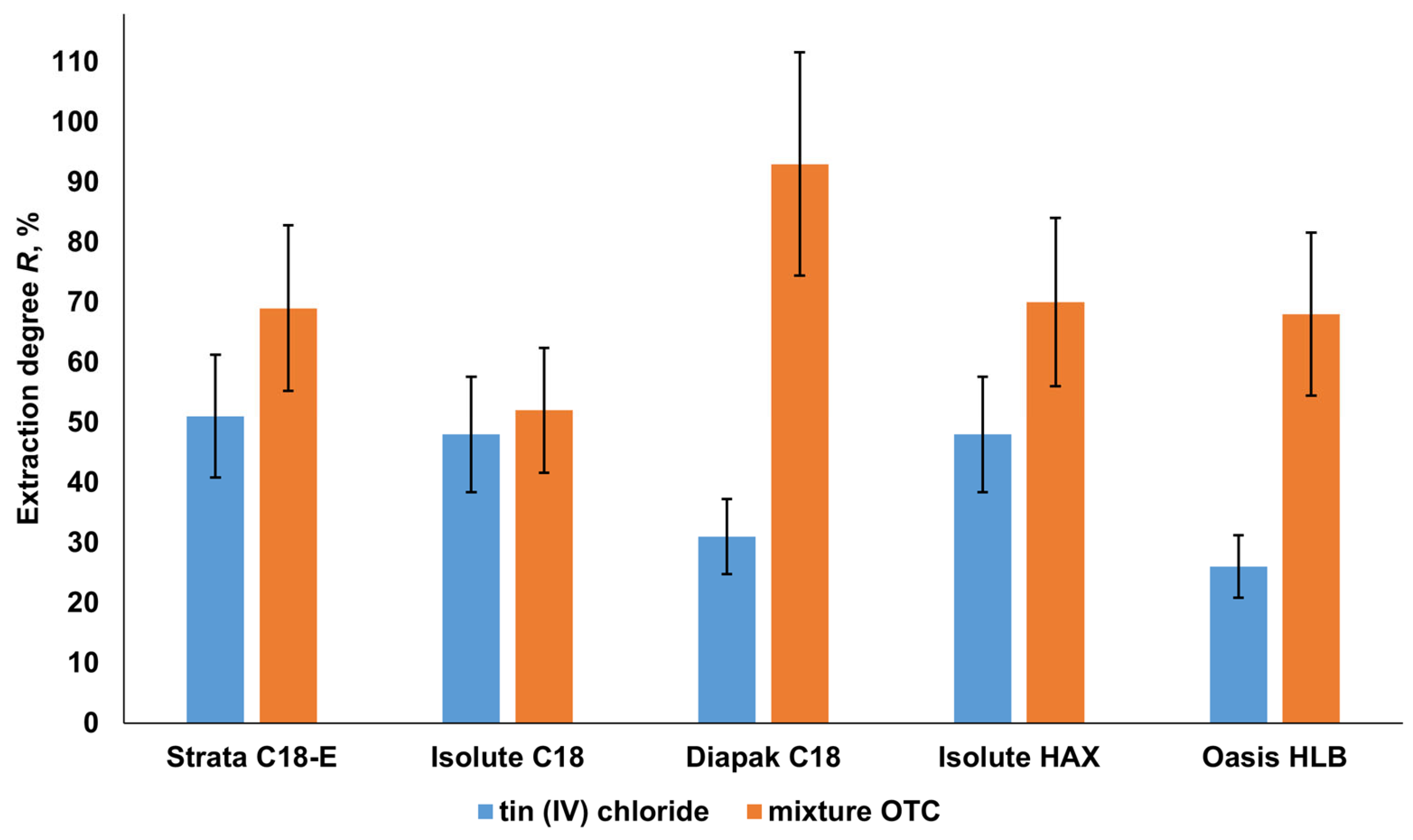
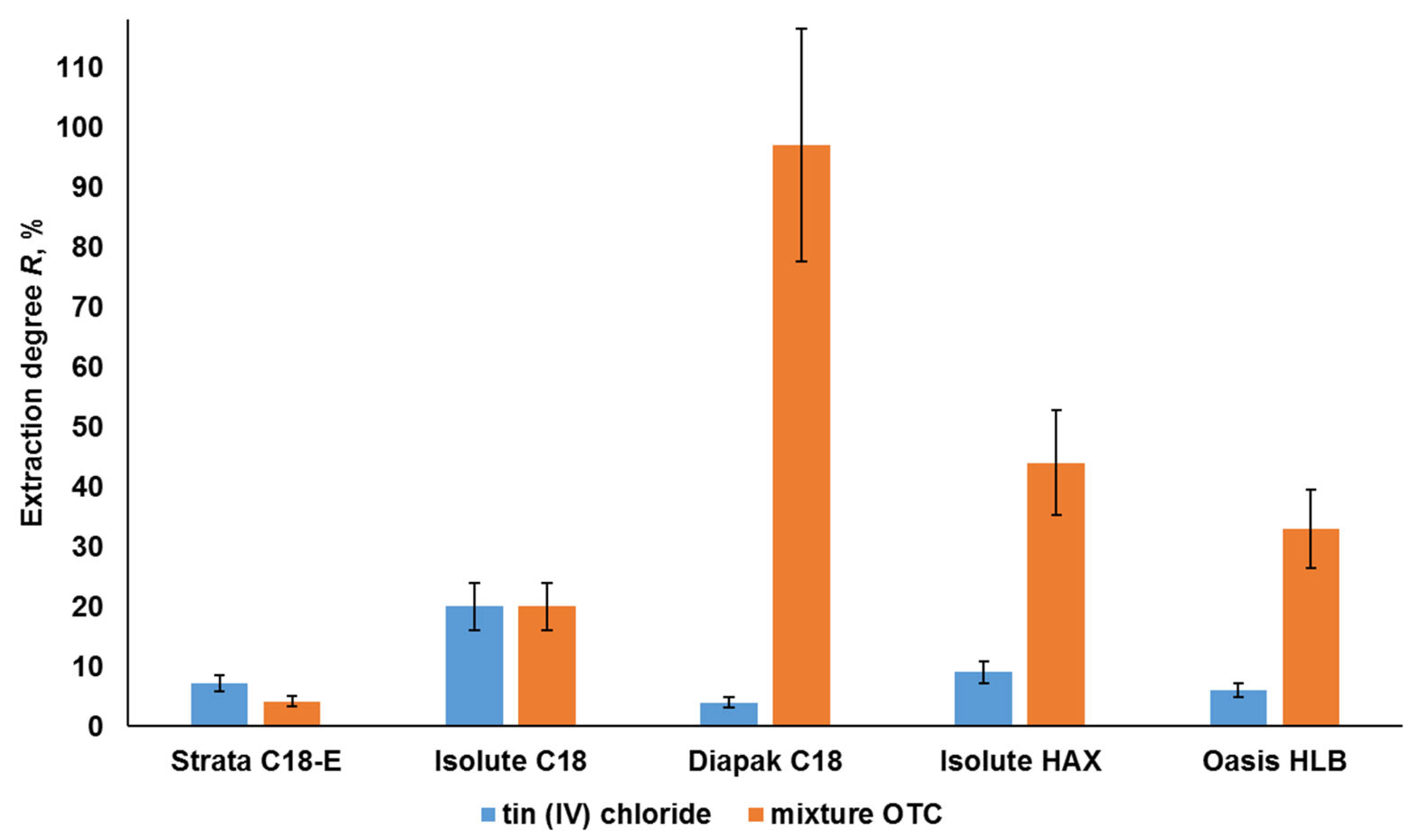
2.3. Solid-Phase Separation of Tin Forms in Water
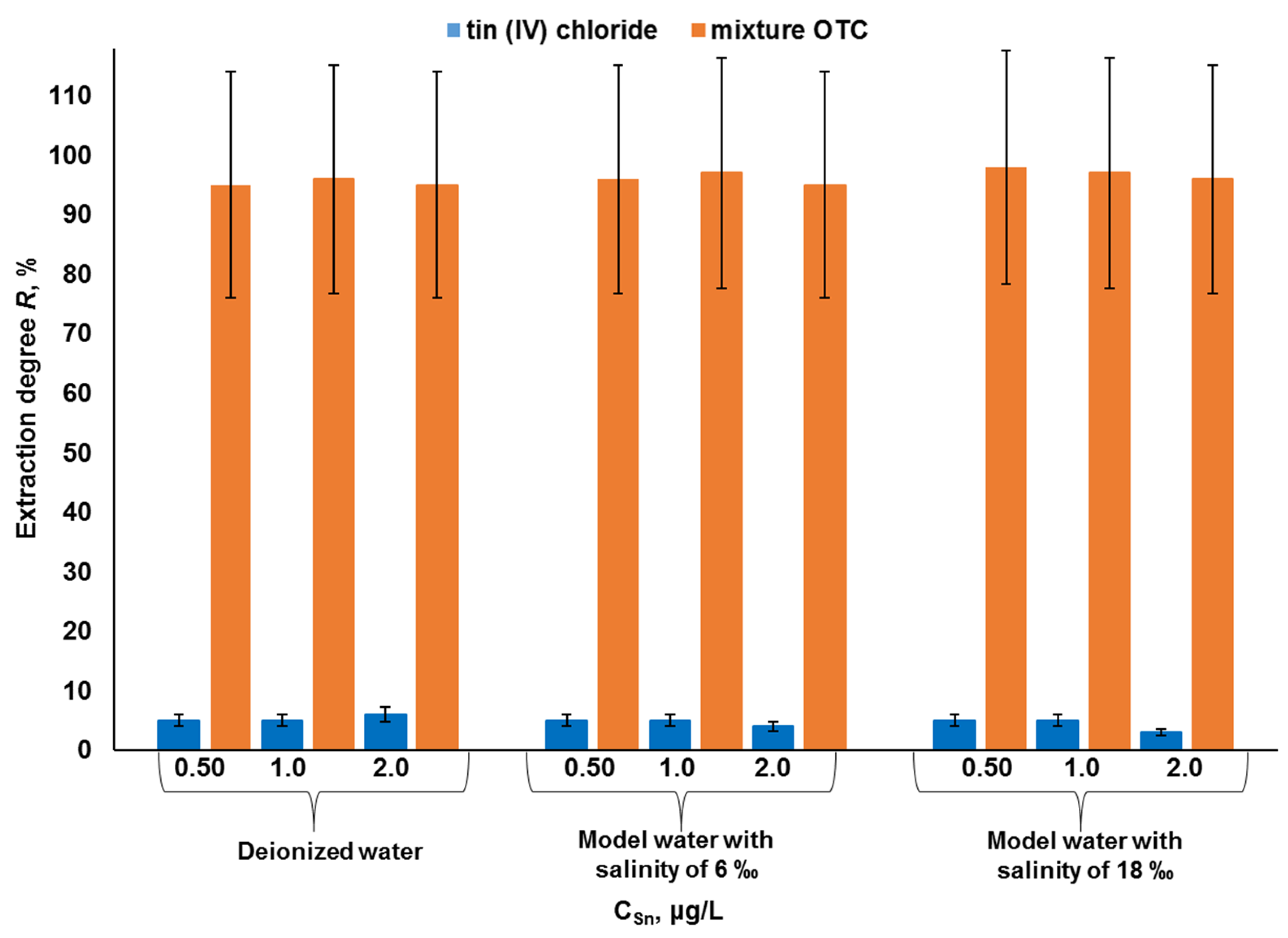
2.4. Separate Determination of Organic and Inorganic Forms of Tin in Water
3. Materials and Methods
3.1. Research Objects
3.2. Reagents
3.3. Instrumentation
3.3.1. Optimization of Operating Modes of Spectrometers
3.3.2. Conditions for the Generation of Tin Hydrides
3.3.3. Calculation of the Limit of Quantification of Tin
3.3.4. Separation of Inorganic and Organic Tin in Water
- i.
- Hydrophobic silica gel sorbent with grafted octadecyl groups (40–63 μm, pore diameter of 6 nm, sorbent weight of 600 mg) Diapak C18 (Biohimmak, Russia);
- ii.
- Polymeric sorbent with the reversed-phase retention mechanism with a hydrophilic-lipophilic balance (31.4 μm, pore diameter of 8.2 nm, sorbent weight of 100 mg) Waters Oasis HLB 1cc (Waters Corporation Milford, Milford, MA, USA);
- iii.
- Silica gel sorbent with a grafted octadecyl group with the reversed-phase retention mechanism Strata C18-E (Phenomenex, Torrance, CA, USA), acting due to strong hydrophobic and insignificant secondary polar interaction of active silanol groups (55 µm, pore diameter of 7.0 nm, sorbent weight of 100 mg);
- iv.
- Functionalized silicon dioxide based on the trifunctional silane Isolute C18 (EC) (BCM Diagnostics, San Diego, CA, USA), which provides the main non-polar retention of analytes and the secondary interaction of silanol groups (50 μm, pore diameter of 6.0 nm, sorbent mass of 100 mg);
- v.
- Sorbent with mixed action Isolute HAX (BCM Diagnostics, San Diego, CA, USA)—non-polar interaction due to octyl group C8 and strong anion-exchange mechanism of retention by quaternary amine -NR3+ (50 µm, pore diameter of 6.0 nm, sorbent mass of 200 mg).
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cima, F. Tin: Environmental pollution and health effects. In Encyclopedia of Environmental Health, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 351–359. [Google Scholar]
- Oliveira, R.C.; Santelli, R.E. Occurrence and chemical speciation analysis of organotin compounds in the environment: A review. Talanta 2010, 82, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addendaю WHO Library Cataloguing-in-Publication. 2022. Available online: https://www.who.int/publications/i/item/9789240045064 (accessed on 28 April 2023).
- Weber, G. The importance of tin in the environment and its determination at trace levels. Fresenius Z. Anal. Chem. 1985, 321, 217–224. [Google Scholar] [CrossRef]
- Howe, P.D.; Watts, P.; World Health Organization. Chemical Safety Team & International Programme on Chemical Safety. Tin and Inorganic Tin Compounds; World Health Organization: Geneva, Switzerland, 2005; 73p. [Google Scholar]
- Horiguchi, T. Biological Effects by Organotins, 1st ed.; Springer: Tokyo, Japan, 2017; 390p. [Google Scholar]
- Hoch, M. Organotin compounds in the environment—An overview. J. Appl. Geochem. 2001, 16, 719–743. [Google Scholar] [CrossRef]
- Kucklick, J.R.; Ellisor, M.D. A review of organotin contamination in arctic and subarctic regions. Emerg. Contam. 2019, 5, 150–156. [Google Scholar] [CrossRef]
- Okoro, H.K.; Fatoki, O.; Adekola, F.; Ximba, B.J. Sources, environmental levels and toxicity of organotin in marine environment. Chem. Asian J. 2011, 23, 473–482. [Google Scholar]
- Gianguzza, A.; Giuffrè, O.; Piazzese, D.; Sammartano, S. Aqueous solution chemistry of alkyltin (IV) compounds for speciation studies in biological fluids and natural waters. Coord. Chem. Rev. 2012, 256, 222–239. [Google Scholar] [CrossRef]
- Blunden, S.G.; Evans, C.J. Organotin compounds. In Handbook of Environmental Chemistry; Springer Science + Business Media: Berlin, Germany, 1990; pp. 1–44. [Google Scholar]
- Cole, R.F.; Mills, G.A.; Parker, R.; Bolam, T.; Birchenough, A.; Kröger, S.; Fones, G.R. Trends in the analysis and monitoring of organotins in the aquatic environment. Trends Environ. Anal. Chem. 2015, 8, 1–11. [Google Scholar] [CrossRef]
- International Marine Organization. Anti-Fouling Systems. 2002. Available online: https://www.imo.org/en/OurWork/Environment/Pages/Anti-fouling.aspx (accessed on 30 June 2022).
- European Community. Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC of the European Parliament and of the Council. 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008L0105 (accessed on 5 June 2023).
- OSPAR Convention for the Protection of the Marine Environment of the North East Atlantic OSPAR/ICES Workshop on the Evaluation and Update of Background Reference Concentrations (BRCS) and Ecotoxicological Assessment Criteria (EACS) and How These Assessment Tools Should Be Used in Assessing Contaminants in Water, Sediment, and Biota. 2004. 169p. Available online: https://www.ospar.org/documents?d=6989 (accessed on 5 June 2023).
- United States Environmental Protection Agency. Ambient Aquatic Life Water Quality Criteria for Tributyltin (TBT). 2003. Available online: https://www.epa.gov/sites/default/files/2019-02/documents/ambient-wqc-tributyltin-final.pdf (accessed on 5 June 2023).
- Australian and New Zealand Guidelines for Fresh and Marine Water Quality. 2000. Available online: https://www.gaea.ca/public/Regulations/anzecc-armcanz-2000-guidelines-vol1.pdf (accessed on 5 June 2023).
- Canadian Water Quality Guidelines for the Protection of Aquatic Life//Organotins: Tributyltin, Triphenyltin, and Tricyclohexyltin. 1999. Available online: https://ccme.ca/en/res/organotins-en-canadian-water-quality-guidelines-for-the-protection-of-aquatic-life.pdf (accessed on 5 June 2023).
- Russian Federation. On Approval of Water Quality Standards for Water Bodies of Fishery Significance, Including Standards for Maximum Permissible Concentrations of Harmful Substances in the Waters of Water Bodies of Fishery Significance: Order of the Ministry of Agriculture of the Russian Federation No. 552, Moscow, 2016. 215p. Available online: https://docs.cntd.ru/document/420389120 (accessed on 5 June 2023).
- Rosen, A.L.; Hieftje, G.M. Inductively coupled plasma mass spectrometry and electrospray mass spectrometry for speciation analysis: Applications and instrumentation. Spectrochim. Acta Part B At. Spectrosc. 2004, 59, 135–146. [Google Scholar] [CrossRef]
- Xiao, Q.; Hu, B.; He, M. Speciation of butyltin compounds in environmental and biological samples using headspace single drop microextraction coupled with gas chromatography-inductively coupled plasma mass spectrometry. J. Chromatogr. A 2008, 1211, 135–141. [Google Scholar] [CrossRef]
- Kurihara, R.; Rajendran, R.B.; Tao, H.; Yamamoto, I.; Hashimoto, S. Analysis of organotins in seawater of the Southern Ocean and Suruga Bay, Japan, by gas chromatography/inductively coupled plasma mass. Environ. Toxicol. Chem. 2007, 26, 647–654. [Google Scholar] [CrossRef]
- Ceulemans, M.; Łobiński, R.; Dirkx, W.M.R.; Adams, F.C. Rapid sensitive speciation analysis of butyl- and phenyltin compounds in water by capillary gas chromatography atomic emission spectrometry (GC-AES) after in-situ ethylation and in-liner preconcentration. Fresenius Z. Anal. Chem. 1993, 347, 256–262. [Google Scholar] [CrossRef]
- Shioji, H.; Tsunoi, S.; Harino, H.; Tanaka, M. Liquid-phase microextraction of tributyltin and triphenyltin coupled with gas chromatography-tandem mass spectrometry comparison between 4-fluorophenyl and ethyl derivatizations. J. Chromatogr. A 2004, 1048, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cea, A.; Rodríguez-González, P.; Cardona, N.F.; Mares, J.L.A.; Nebot, S.B.; Alonso, J.I.G. Determination of ultratrace levels of tributyltin in waters by isotope dilution and gas chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2015, 1425, 265–272. [Google Scholar] [CrossRef]
- Tsunoi, S.; Matoba, T.; Shioji, H.; Giang, L.T.H.; Harino, H.; Tanaka, M. Analysis of organotin compounds by grignard derivatization and gas chromatography-ion trap tandem mass spectrometry. J. Chromatogr. A 2002, 962, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.D. Comprehensive trace level determination of organotin compounds in environmental samples using high-resolution gas chromatography with flame photometric detection. Anal. Chem. 1987, 59, 617–623. [Google Scholar] [CrossRef]
- Spivakovsky, V.B. Analytical Chemistry of Tin; Science: Moskow, Russia, 1975; 252p. [Google Scholar]
- Cassol, A.; Margon, L.; Barbieri, R. Complexes of organometallic compounds. XVI. Stability constants of organotin (IV)-fluoride and -chloride complexes in aqueous solution. J. Inorg. Nucl. 1967, 3, 25–29. [Google Scholar] [CrossRef]
- Davies, A.G. Tin organometallics. In Comprehensive Organometallic Chemistry III; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA; Heidelberg, Germany, 2007; pp. 809–883. [Google Scholar]
- Temerdashev, Z.; Abakumov, P.; Bolshov, M.; Abakumova, D.; Pupyshev, A. Possibilities and Limitations of ICP-Spectrometric Determination of the Total Content of Tin, Its Inorganic and Organic Speciations in Waters with Different Salinity Levels—Part 1: Determination of the Total Tin Content. Molecules 2023, 28, 5967. [Google Scholar] [CrossRef]
- Cole, R.F.; Graham, A.; Mills, G.A.; Bakir, A.; Townsend, I.; Gravell, A.; Fones, G.R. A simple, low cost GC/MS method for the sub-nanogram per litre measurement of organotins in coastal water. MethodsX 2016, 3, 490–496. [Google Scholar] [CrossRef]
- Abalos, M.; Bayona, J.M.; Compañó, R.; Granados, M.; Leal, C.; Prat, M.D. Analytical procedures for the determination of organotin compounds in sediment and biota: A critical review. J. Chromatogr. A 1997, 788, 1–49. [Google Scholar] [CrossRef]
- Foti, C.; Gianguzza, A.; Piazzese, D.; Trifiletti, G. Inorganic speciation of organotin (IV) cations in natural waters with particular reference to seawater. Chem. Speciat. Bioavailab. 2000, 12, 41–52. [Google Scholar] [CrossRef]
- Telepchak, M.J.; August, T.F.; Chaney, G. Silica-based solid phase extraction. In Forensic and Clinical Applications of Solid Phase Extraction; Humana: New York, NY, USA, 2004; pp. 41–53. [Google Scholar]
- Kosyan, R.D.; Krylenko, M.V. Modern state and dynamics of the Sea of Azov coasts. Estuar. Coast. Shelf Sci. 2019, 224, 314–323. [Google Scholar] [CrossRef]
- Poulos, S.E. The Mediterranean and Black Sea marine system: An overview of its physico-geographic and oceanographic characteristics. Earth-Sci. Rev. 2020, 200, 103004. [Google Scholar] [CrossRef]
- ASTM D1141-98(2021); Standard Practice for the Preparation of Substitute Ocean Water. ASTM: West Conshohocken, PA, USA, 2021. Available online: https://www.astm.org/d1141-98r21.html (accessed on 28 April 2023).
- Horne, R.A. Marine Chemistry: The Structure of Water and the Chemistry of the Hydrosphere, 1st ed.; Wiley-Interscience, A Division of John Wiley & Sons: Hoboken, NJ, USA, 1969; 568р. [Google Scholar]
- Stauffer, M.T. Calibration and Validation of Analytical Methods—A Sampling of Current Approaches; IntechOpen: London, UK, 2018; 174p. [Google Scholar]
- Housecroft, C.E. Tin Organometallics. In Comprehensive Organometallic Chemistry III. Vol. 3: Compounds of Groups 13 to 15; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA; Heidelberg, Germany, 2007; pp. 809–882. [Google Scholar]
- Inagaki, K.; Takatsu, A.; Watanabe, T.; Aoyagi, Y.; Yarita, T.; Okamoto, K.; Chiba, K. Certification of butyltins and phenyltins in marine sediment certified reference material by species-specific isotope-dilution mass spectrometric analysis using synthesized Sn-118-enriched organotin compounds. Anal. Bioanal. Chem. 2007, 387, 2325–2334. [Google Scholar] [CrossRef] [PubMed]



| Organizations Regulating the Use of OTCs and the Name of the Document | Tributyltin Content | Source |
|---|---|---|
| European Community//Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 | 0.2 ng/L annual average and 1.5 ng/L maximum allowable concentration | [14] |
| OSPAR Convention for the protection of the marine environment of the North East Atlantic OSPAR/ICES Workshop on the evaluation and update of background reference concentrations (BRCS) and ecotoxicological assessment criteria (EACS) and how these assessment tools should be used in assessing contaminants in water, sediment, and biota (Update 2004) | 0.1 ng/L water concentration | [15] |
| United States Environmental Protection Agency. Ambient Aquatic Life. Water Quality Criteria for Tributyltin | One-hour average concentration not exceeding 420 ng/L more than once every three years (acute criterion). Four-day average concentration does not exceed 7.4 ng/L more than once every three years (chronic criterion) | [16] |
| Australia. Toxicant Guidelines for the Protection of Aquaculture Species (under review). Australian Sediment Quality Guidelines for TBT | Saltwater production: <10 ng/L | [17] |
| Canadian water quality guidelines for the protection of aquatic life//Organotins: tributyltin, triphenyltin, and tricyclohexyltin, 1999 | 1 ng/L | [18] |
| IMO’s Marine Environment Protection Committee (MEPC) | Prohibition of application | [13] |
| Deposition Method | Total Tin Concentration in Tested Samples/Mixtures, µg/L | Chalide ion, g/L | Found Analyte Concentration, µg/L | |
|---|---|---|---|---|
| ICP-OES | ICP-MS | |||
| OTC precipitation with fluorides | Mixture of OTC—1.0 µg/L | 0.5 | 0.9 ± 0.2 | 1.0 ± 0.2 |
| 1.0 | 0.9 ± 0.2 | 0.9 ± 0.2 | ||
| 2.0 | 1.0 ± 0.2 | 0.9 ± 0.2 | ||
| 3.0 | 0.9 ± 0.2 | 0.9 ± 0.2 | ||
| Mixture of tin (IV) chloride and OTC—1.0 µg/L | 0.5 | 0.9 ± 0.2 | 0.9 ± 0.2 | |
| 1.0 | 0.9 ± 0.2 | 0.9 ± 0.2 | ||
| 2.0 | 0.9 ± 0.2 | 0.9 ± 0.2 | ||
| 3.0 | 0.9 ± 0.2 | 0.9 ± 0.2 | ||
| OTC precipitation with iodides | Mixture of OTC—1.0 µg/L | 0.5 | 1.0 ± 0.2 | 0.9 ± 0.2 |
| 1.0 | 0.9 ± 0.2 | 1.0 ± 0.2 | ||
| 2.0 | 1.0 ± 0.2 | 0.9 ± 0.2 | ||
| Mixture of tin (IV) chloride and OTC—1.0 µg/L | 0.5 | 0.9 ± 0.2 | 0.9 ± 0.2 | |
| 1.0 | 1.0 ± 0.2 | 1.0 ± 0.2 | ||
| 2.0 | 0.9 ± 0.2 | 1.0 ± 0.2 | ||
| Precipitation of Sn4+ aqueous ammonia solution and iron (III) chloride | Tin (IV) chloride—1.0 µg/L | – | 0.9 ± 0.2 | 0.9 ± 0.2 |
| Mixture of tin (IV) chloride and OTC—1.0 µg/L | – | 0.9 ± 0.2 | 0.9 ± 0.2 | |
| Added Inorganic Form of Tin, µg/L | Added OTCs in Terms of Tin, µg/L | Ratio of Inorganic Tin to OTCs | Found Tin Content, µg/L | |
|---|---|---|---|---|
| ICP-OES | ICP-MS | |||
| 0.05 | 0.20 | 1:4 | 0.04 ± 0.01 | 0.05 ± 0.01 |
| 0.05 | 0.05 | 1:1 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| 0.10 | 0.05 | 2:1 | 0.10 ± 0.02 | 0.11 ± 0.02 |
| 0.25 | 0.05 | 5:1 | 0.24 ± 0.05 | 0.26 ± 0.05 |
| 0.50 | 0.05 | 10:1 | 0.51 ± 0.10 | 0.48 ± 0.10 |
| Type of Water | Introduced in Terms of Tin, µg/L | Found with ICP-MS, µg/L | Found with ICP-OES, µg/L | ||||
|---|---|---|---|---|---|---|---|
| CΣ | CIT | Calculated COTC | CΣ | CIT | Calculated COTC | ||
| Deionized water | 0.25 | 0.25 ± 0.05 | 0.04 ± 0.01 | 0.21 ± 0.04 | 0.25 ± 0.05 | 0.05 ± 0.01 | 0.20 ± 0.04 |
| 0.50 | 0.48 ± 0.10 | 0.09 ± 0.02 | 0.39 ± 0.08 | 0.51 ± 0.10 | 0.10 ± 0.02 | 0.41 ± 0.08 | |
| 1.00 | 1.01 ± 0.20 | 0.21 ± 0.04 | 0.80 ± 0.15 | 1.05 ± 0.20 | 0.22 ± 0.04 | 0.83 ± 0.15 | |
| Model seawater with salinity of 6 ‰ | 0.25 | 0.24 ± 0.05 | 0.05 ± 0.01 | 0.19 ± 0.04 | 0.26 ± 0.05 | 0.05 ± 0.01 | 0.21 ± 0.04 |
| 0.50 | 0.51 ± 0.10 | 0.11 ± 0.02 | 0.40 ± 0.08 | 0.54 ± 0.10 | 0.11 ± 0.02 | 0.43 ± 0.08 | |
| 1.00 | 1.01 ± 0.20 | 0.19 ± 0.04 | 0.82 ± 0.15 | 0.99 ± 0.20 | 0.19 ± 0.04 | 0.80 ± 0.15 | |
| Model seawater with salinity of 18 ‰ | 0.25 | 0.26 ± 0.05 | 0.04 ± 0.01 | 0.22 ± 0.04 | 0.26 ± 0.05 | 0.06 ± 0.01 | 0.20 ± 0.04 |
| 0.50 | 0.50 ± 0.10 | 0.10 ± 0.02 | 0.40 ± 0.08 | 0.49 ± 0.10 | 0.09 ± 0.02 | 0.40 ± 0.08 | |
| 1.00 | 0.99 ± 0.20 | 0.20 ± 0.04 | 0.79 ± 0.15 | 1.00 ± 0.20 | 0.21 ± 0.04 | 0.79 ± 0.15 | |
| Parameter | iCAP RQ Mass Spectrometer | iCAP-7400 Spectrometer (Axial Overview of Plasma) | |
|---|---|---|---|
| Analyte | 120Sn | Sn II 189.989 nm | |
| Applied power, W | 1300 | 1150 | |
| Argon flow rate, L/min | Plasma-forming (cooling) | 15 | 12 |
| Auxiliary | 0.80 | 0.50 | |
| Nebulizer | 1.10 | 0.50 | |
| Peristaltic pump speed, rpm | 40 | 50 | |
| Sample rate, mL/min | 0.4 | ||
| Parameter | iCAP RQ Mass Spectrometer | iCAP-7400 Spectrometer (Axial Overview of Plasma) | |
|---|---|---|---|
| Analyte | 120Sn | Sn II 189.989 nm | |
| Applied power, W | 1300 | 1150 | |
| Argon flow rate, L/min | Plasma-forming (cooling) | 15 | 12 |
| Auxiliary | 0.80 | 0.50 | |
| Nebulizer | 0.45 | ||
| Peristaltic pump speed, rpm | 60 | 30 | |
| Sample introduction | Hydride system: oxidizer—0.10 mol/L HCl; reducing agent—0.50 mol/L NaBH4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temerdashev, Z.; Abakumov, P.; Bolshov, M.; Abakumova, D.; Pupyshev, A. Possibilities and Limitations of ICP-Spectrometric Determination of the Total Content of Tin and Its Inorganic and Organic Speciations in Waters with Different Salinity Levels—Part 2: Separate Determination of Inorganic and Organic Speciations of Tin. Molecules 2023, 28, 6615. https://doi.org/10.3390/molecules28186615
Temerdashev Z, Abakumov P, Bolshov M, Abakumova D, Pupyshev A. Possibilities and Limitations of ICP-Spectrometric Determination of the Total Content of Tin and Its Inorganic and Organic Speciations in Waters with Different Salinity Levels—Part 2: Separate Determination of Inorganic and Organic Speciations of Tin. Molecules. 2023; 28(18):6615. https://doi.org/10.3390/molecules28186615
Chicago/Turabian StyleTemerdashev, Zaual, Pavel Abakumov, Mikhail Bolshov, Darya Abakumova, and Alexander Pupyshev. 2023. "Possibilities and Limitations of ICP-Spectrometric Determination of the Total Content of Tin and Its Inorganic and Organic Speciations in Waters with Different Salinity Levels—Part 2: Separate Determination of Inorganic and Organic Speciations of Tin" Molecules 28, no. 18: 6615. https://doi.org/10.3390/molecules28186615







