Ochratoxin A—The Current Knowledge Concerning Hepatotoxicity, Mode of Action and Possible Prevention
Abstract
1. Introduction
2. General Information
2.1. Chemical Structure and Physical and Chemical Properties
2.2. Biosynthesis
2.3. Toxicokinetics
2.4. Metabolism in the Liver
3. The Impact of OTA on Hepatocytes and Hepatotoxicity
4. The Mechanisms Responsible for Liver Damage
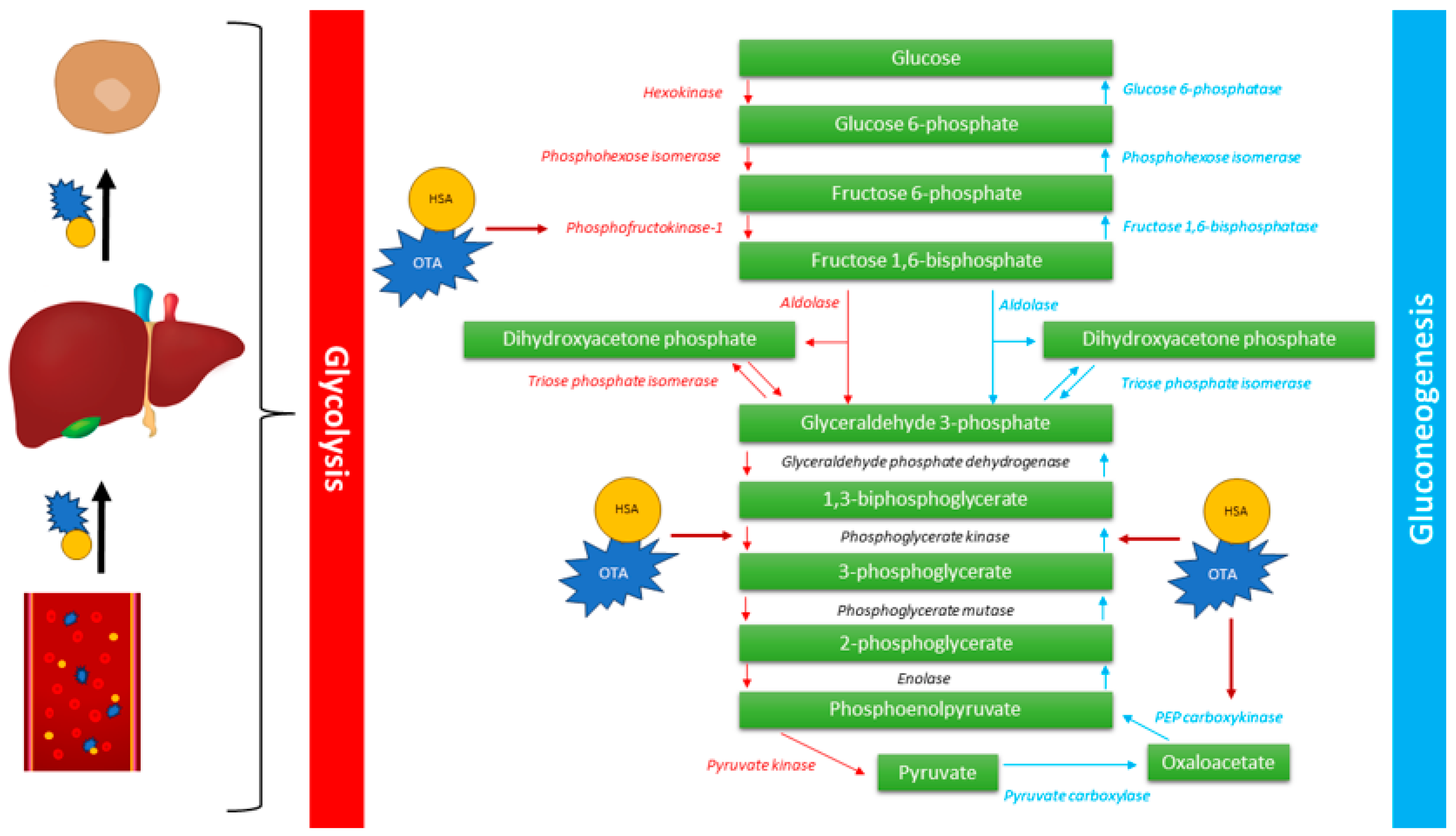
4.1. Gluconeogenesis
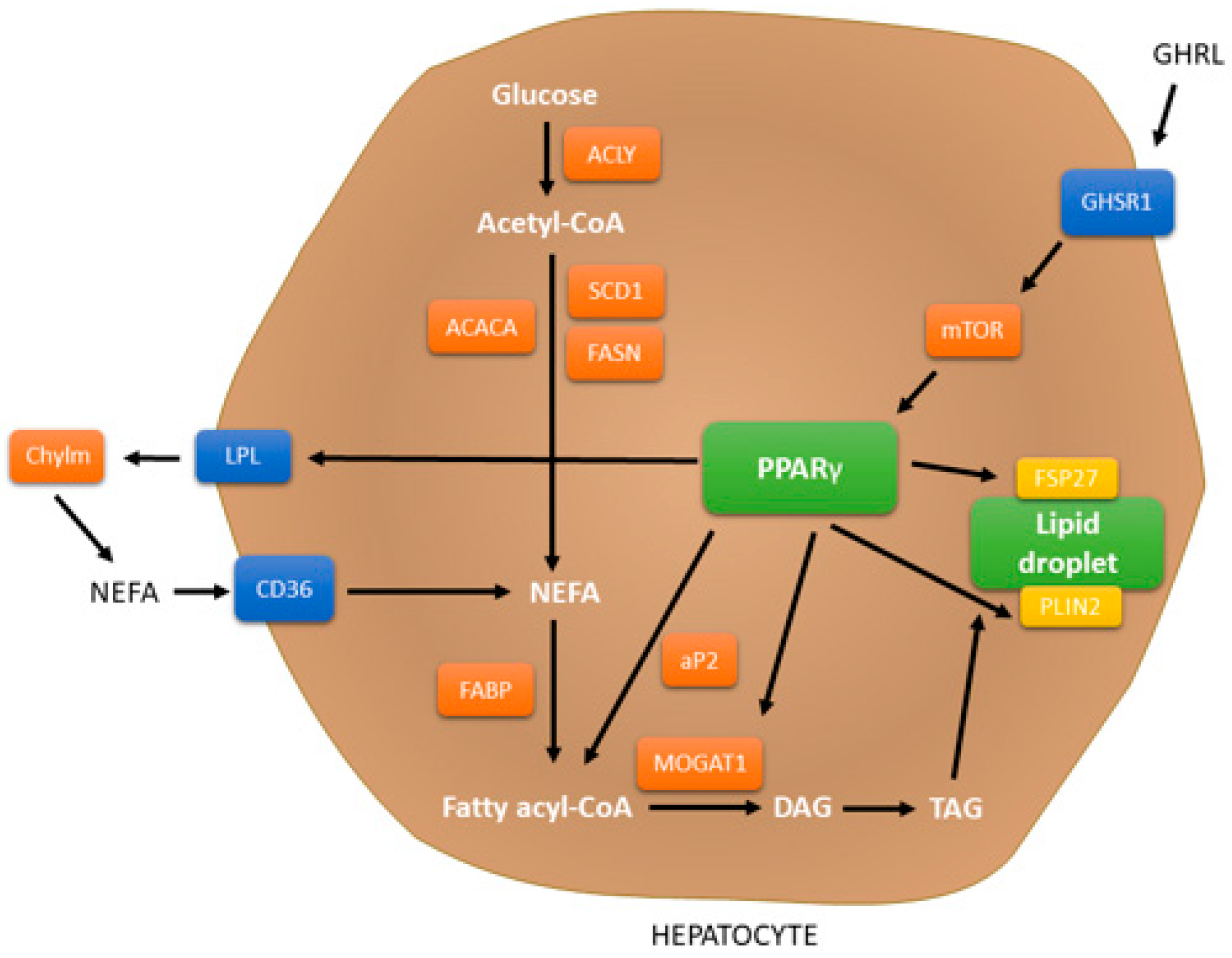
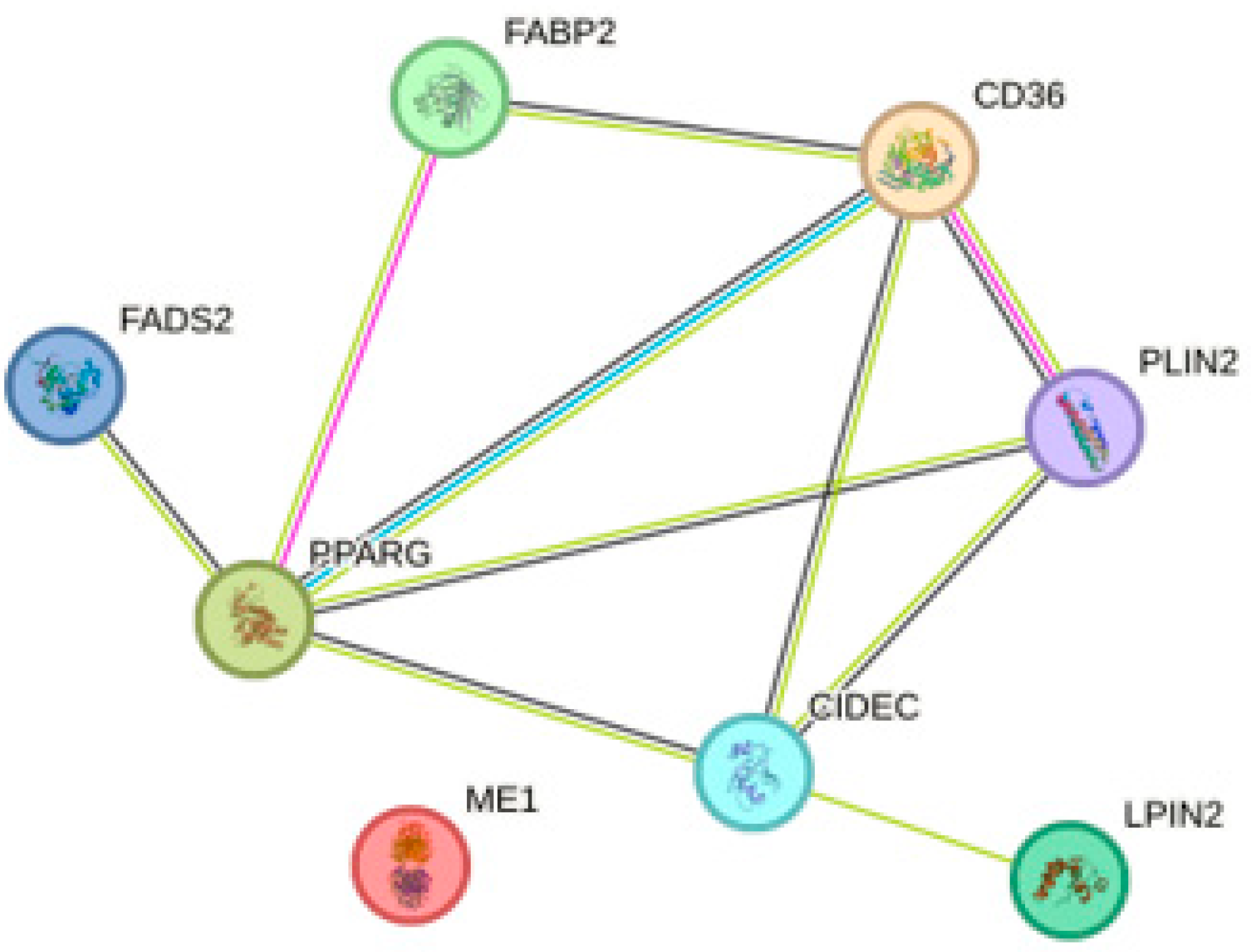
4.2. PPAR Signaling Pathway
4.3. Oxidative Stress
4.4. Apoptosis
5. Prevention
5.1. Antioxidant Substances
5.1.1. Vitamin E
5.1.2. Vitamin C
5.1.3. Quercetin
5.1.4. Melatonin (MEL)
5.1.5. Coenzyme Q10 (CoQ10)
5.2. Trace Elements
5.2.1. Zinc
5.2.2. Natural Form of Selenium–Selenomethionine
5.3. Plants Extracts
5.3.1. Emblica officinalis (amla)
5.3.2. Sea buckthorn (SBT)
5.3.3. Nigella sativa (Black Seed)
5.3.4. Curcuma longa Extract (Curcumin)
5.3.5. Fruit Anthocyanin
5.3.6. Yemeni Green Coffee
5.3.7. Green Tea
5.3.8. Lycopene
5.3.9. Silymarin (Sil)
5.3.10. Silibinin
5.4. Organic Substances
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ringot, D.; Chango, A.; Schneider, Y.J.; Larondelle, Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem. Biol. Interact. 2006, 159, 18–46. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, K.J.; Steyn, P.S.; Fourie, L.; Scott, D.B.; Theron, J.J. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature 1965, 205, 1112–1113. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, J.; Waśkiewicz, A. Ochratoxin A and citrinin production by Penicillium verrucosum on cereal solid substrates. Food Addit. Contam. Part A 2014, 31, 139–148. [Google Scholar] [CrossRef]
- Harwig, J.; Chen, Y.K. Some conditions favoring production of ochratoxin a and citrinin by penicillium viridicatum in wheat and barley. Can. J. Plant Sci. 1974, 54, 17–22. [Google Scholar] [CrossRef]
- Palacios-Cabrera, H.; Taniwaki, M.H.; Hashimoto, J.M.; Menezes, H.C.D. Growth of Aspergillus ochraceus, A. carbonarius and A. niger on culture media at different water activities and temperatures. Braz. J. Microbiol. 2005, 36, 24–28. [Google Scholar] [CrossRef]
- Oliveira, G.; Evangelista, S.R.; Passamani, F.R.F.; Santiago, W.D.; Cardoso, M.D.G.; Batista, L.R. Influence of temperature and water activity on Ochratoxin A production by Aspergillus strain in coffee south of Minas Gerais/Brazil. LWT 2019, 102, 1–7. [Google Scholar] [CrossRef]
- Alborch, L.; Bragulat, M.R.; Abarca, M.L.; Cabañes, F.J. Effect of water activity, temperature and incubation time on growth and ochratoxin A production by Aspergillus niger and Aspergillus carbonarius on maize kernels. Int. J. Food Microbiol. 2011, 147, 53–57. [Google Scholar] [CrossRef]
- Zebiri, S.; Mokrane, S.; Verheecke-Vaessen, C.; Choque, E.; Reghioui, H.; Sabaou, N.; Mathieu, F.; Riba, A. Occurrence of ochratoxin A in Algerian wheat and its milling derivatives. Toxin Rev. 2019, 38, 206–211. [Google Scholar] [CrossRef]
- Torović, L. Aflatoxins and ochratoxin A in flour: A survey of the Serbian retail market. Food Addit. Contam. Part B Surveill. 2018, 11, 26–32. [Google Scholar] [CrossRef]
- Kosicki, R.; Twarużek, M.; Dopierała, P.; Rudzki, B.; Grajewski, J. Occurrence of Mycotoxins in Winter Rye Varieties Cultivated in Poland (2017–2019). Toxins 2020, 12, 423. [Google Scholar] [CrossRef]
- Wajih Ul Hassan, S.; Sadef, Y.; Hussain, S.; Rafique Asi, M.; Ashraf, M.Y.; Anwar, S.; Malik, A. Unusual pattern of aflatoxins and ochratoxin in commercially grown maize varieties of Pakistan. Toxicon 2020, 182, 66–71. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Asi, M.R.; Hanif, U.; Zuber, M.; Jinap, S. The presence of aflatoxins and ochratoxin A in rice and rice products; and evaluation of dietary intake. Food Chem. 2016, 210, 135–140. [Google Scholar] [CrossRef]
- Lai, X.; Liu, R.; Ruan, C.; Zhang, H.; Liu, C. Occurrence of aflatoxins and ochratoxin A in rice samples from six provinces in China. Food Control 2015, 50, 401–404. [Google Scholar] [CrossRef]
- Leoni, L.A.; Soares, L.M.; Oliveira, P.L. Ochratoxin A in Brazilian roasted and instant coffees. Food Addit. Contam. 2000, 17, 867–870. [Google Scholar] [CrossRef]
- Galarce-Bustos, O.; Alvarado, M.; Vega, M.; Aranda, M. Occurrence of ochratoxin A in roasted and instant coffees in Chilean market. Food Control 2014, 46, 102–107. [Google Scholar] [CrossRef]
- Juan, C.; Raiola, A.; Mañes, J.; Ritieni, A. Presence of mycotoxin in commercial infant formulas and baby foods from Italian market. Food Control 2014, 39, 227–236. [Google Scholar] [CrossRef]
- Darouj, E.; Massouh, L.; Ghanem, I. Investigation of ochratoxin A in Syrian consumed baby foods. Food Control 2016, 62, 97–103. [Google Scholar] [CrossRef]
- Al-Taher, F.; Cappozzo, J.; Zweigenbaum, J.; Lee, H.J.; Jackson, L.; Ryu, D. Detection and quantitation of mycotoxins in infant cereals in the U.S. market by LC-MS/MS using a stable isotope dilution assay. Food Control 2017, 72, 27–35. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Mehmood, Z.; Asi, M.R.; Shahid, M.; Sehar, M.; Malik, N. Co-occurrence of aflatoxins and ochratoxin A in nuts, dry fruits, and nuty products. J. Food Saf. 2018, 38, e12462. [Google Scholar] [CrossRef]
- Silva, A.; Fungaro, M.H.P.; Silva, J.J.; Martins, L.M.; Taniwaki, M.H.; Iamanaka, B.T. Ochratoxin A and related fungi in Brazilian black pepper (Piper nigrum L.). Food Res. Int. 2021, 142, 110207. [Google Scholar] [CrossRef]
- El Darra, N.; Gambacorta, L.; Solfrizzo, M. Multimycotoxins occurrence in spices and herbs commercialized in Lebanon. Food Control 2019, 95, 63–70. [Google Scholar] [CrossRef]
- Nguegwouo, E.; Sone, L.E.; Tchuenchieu, A.; Tene, H.M.; Mounchigam, E.; Njayou, N.F.; Nama, G.M. Ochratoxin A in black pepper, white pepper and clove sold in Yaoundé (Cameroon) markets: Contamination levels and consumers’ practices increasing health risk. Int. J. Food Contam. 2018, 5, 1. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Asi, M.R.; Zuber, M.; Akhtar, J.; Jawwad Saif, M. Natural occurrence of aflatoxins and ochratoxin A in commercial chilli and chilli sauce samples. Food Control 2013, 30, 621–625. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Mumtaz, A.; Mahmood, Z.; Waqas, M.; Ghaffar, A.; Ismail, A.; Pervaiz, W. Assessment of aflatoxins and ochratoxin a in chili sauce samples and estimation of dietary intake. Food Control 2021, 121, 107621. [Google Scholar] [CrossRef]
- Remiro, R.; Irigoyen, A.; González-Peñas, E.; Lizarraga, E.; López de Cerain, A. Levels of ochratoxins in Mediterranean red wines. Food Control 2013, 32, 63–68. [Google Scholar] [CrossRef]
- Silva, L.J.G.; Teixeira, A.C.; Pereira, A.M.P.T.; Pena, A.; Lino, C.M. Ochratoxin A in Beers Marketed in Portugal: Occurrence and Human Risk Assessment. Toxins 2020, 12, 249. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, Z.; Nie, D.; Zhao, Z.; Han, Z. Analysis of the Carry-Over of Ochratoxin A from Feed to Milk, Blood, Urine, and Different Tissues of Dairy Cows Based on the Establishment of a Reliable LC-MS/MS Method. Molecules 2019, 24, 2823. [Google Scholar] [CrossRef]
- Armorini, S.; Altafini, A.; Zaghini, A.; Roncada, P. Ochratoxin A in artisan salami produced in Veneto (Italy). Food Addit. Contam. Part B Surveill. 2016, 9, 9–14. [Google Scholar] [CrossRef]
- Altafini, A.; Fedrizzi, G.; Roncada, P. Occurrence of ochratoxin A in typical salami produced in different regions of Italy. Mycotoxin Res. 2019, 35, 141–148. [Google Scholar] [CrossRef]
- Roncada, P.; Altafini, A.; Fedrizzi, G.; Guerrini, A.; Polonini, G.L.; Elisabetta, C. Ochratoxin A contamination of the casing and the edible portion of artisan salamis produced in two Italian regions. World Mycotoxin J. 2020, 13, 553–562. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Nisar, S.; Asi, M.R.; Jinap, S. Natural incidence of aflatoxins, ochratoxin A and zearalenone in chicken meat and eggs. Food Control 2014, 43, 98–103. [Google Scholar] [CrossRef]
- Turkoglu, C.; Keyvan, E. Determination of Aflatoxin M1 and Ochratoxin A in Raw, Pasteurized and UHT Milk in Turkey. Acta Sci. Vet. 2019, 47, 1626. [Google Scholar] [CrossRef]
- Altafini, A.; Roncada, P.; Guerrini, A.; Minkoumba Sonfack, G.; Fedrizzi, G.; Caprai, E. Occurrence of Ochratoxin A in Different Types of Cheese Offered for Sale in Italy. Toxins 2021, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Cramer, B.; Osteresch, B.; Muñoz, K.A.; Hillmann, H.; Sibrowski, W.; Humpf, H.U. Biomonitoring using dried blood spots: Detection of ochratoxin A and its degradation product 2’R-ochratoxin A in blood from coffee drinkers. Mol. Nutr. Food Res. 2015, 59, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Biasucci, G.; Calabrese, G.; Di Giuseppe, R.; Carrara, G.; Colombo, F.; Mandelli, B.; Maj, M.; Bertuzzi, T.; Pietri, A.; Rossi, F. The presence of ochratoxin A in cord serum and in human milk and its correspondence with maternal dietary habits. Eur. J. Nutr. 2011, 50, 211–218. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Gambacorta, L.; Visconti, A. Assessment of Multi-Mycotoxin Exposure in Southern Italy by Urinary Multi-Biomarker Determination. Toxins 2014, 6, 523–538. [Google Scholar] [CrossRef]
- Ali, N.; Muñoz, K.; Degen, G.H. Ochratoxin A and its metabolites in urines of German adults-An assessment of variables in biomarker analysis. Toxicol. Lett. 2017, 275, 19–26. [Google Scholar] [CrossRef]
- Muñoz, K.; Cramer, B.; Dopstadt, J.; Humpf, H.U.; Degen, G.H. Evidence of ochratoxin A conjugates in urine samples from infants and adults. Mycotoxin Res. 2017, 33, 39–47. [Google Scholar] [CrossRef]
- Klarić, M.Š.; Rašić, D.; Peraica, M. Deleterious Effects of Mycotoxin Combinations Involving Ochratoxin A. Toxins 2013, 5, 1965–1987. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- EC. Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Kőszegi, T.; Poór, M. Ochratoxin A: Molecular Interactions, Mechanisms of Toxicity and Prevention at the Molecular Level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, A.; Atoui, A. Ochratoxin A: General Overview and Actual Molecular Status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef] [PubMed]
- Ihlenfeldt, W.D.; Bolton, E.E.; Bryant, S.H. The PubChem chemical structure sketcher. J. Cheminform. 2009, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Maor, U.; Barda, O.; Sadhasivam, S.; Bi, Y.; Levin, E.; Zakin, V.; Prusky, D.B.; Sionov, E. Functional roles of LaeA, polyketide synthase, and glucose oxidase in the regulation of ochratoxin A biosynthesis and virulence in Aspergillus carbonarius. Mol. Plant Pathol. 2021, 22, 117–129. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.; Chen, H.; Li, M.; Zhu, X.; Gao, Q.; Wang, D.; Zhang, Y. A Polyketide Synthase Encoded by the Gene An15g07920 Is Involved in the Biosynthesis of Ochratoxin A in Aspergillus niger. J. Agric. Food Chem. 2016, 64, 9680–9688. [Google Scholar] [CrossRef]
- Bacha, N.; Atoui, A.; Mathieu, F.; Liboz, T.; Lebrihi, A. Aspergillus westerdijkiae polyketide synthase gene “aoks1” is involved in the biosynthesis of ochratoxin A. Fungal Genet. Biol. 2009, 46, 77–84. [Google Scholar] [CrossRef]
- Färber, P.; Geisen, R. Analysis of Differentially-Expressed Ochratoxin A Biosynthesis Genes of Penicillium Nordicum. Eur. J. Plant Pathol. 2004, 110, 661–669. [Google Scholar] [CrossRef]
- Rodríguez, A.; Medina, Á.; Córdoba, J.J.; Magan, N. The influence of salt (NaCl) on ochratoxin A biosynthetic genes, growth and ochratoxin A production by three strains of Penicillium nordicum on a dry-cured ham-based medium. Int. J. Food Microbiol. 2014, 178, 113–119. [Google Scholar] [CrossRef]
- Ferrara, M.; Gallo, A.; Cervini, C.; Gambacorta, L.; Solfrizzo, M.; Baker, S.E.; Perrone, G. Evidence of the Involvement of a Cyclase Gene in the Biosynthesis of Ochratoxin A in Aspergillus carbonarius. Toxins 2021, 13, 892. [Google Scholar] [CrossRef]
- Wei, S.; Hu, C.; Zhang, Y.; Lv, Y.; Zhang, S.; Zhai, H.; Hu, Y. AnAzf1 acts as a positive regulator of ochratoxin A biosynthesis in Aspergillus niger. Appl. Microbiol. Biotechnol. 2023, 107, 2501–2514. [Google Scholar] [CrossRef] [PubMed]
- Gerin, D.; Garrapa, F.; Ballester, A.-R.; González-Candelas, L.; De Miccolis Angelini, R.M.; Faretra, F.; Pollastro, S. Functional Role of Aspergillus carbonarius AcOTAbZIP Gene, a bZIP Transcription Factor within the OTA Gene Cluster. Toxins 2021, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Bruno, K.S.; Solfrizzo, M.; Perrone, G.; Mulè, G.; Visconti, A.; Baker, S.E. New insight into the ochratoxin A biosynthetic pathway through deletion of a nonribosomal peptide synthetase gene in Aspergillus carbonarius. Appl. Environ. Microbiol. 2012, 78, 8208–8218. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Sempere, A.; Marín, S.; Sanchis, V.; Ramos, A.J. VeA and LaeA transcriptional factors regulate ochratoxin A biosynthesis in Aspergillus carbonarius. Int. J. Food Microbiol. 2013, 166, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, H.; Sumarah, M.W.; Gao, Q.; Wang, D.; Zhang, Y. veA Gene Acts as a Positive Regulator of Conidia Production, Ochratoxin A Biosynthesis, and Oxidative Stress Tolerance in Aspergillus niger. J. Agric. Food Chem. 2018, 66, 13199–13208. [Google Scholar] [CrossRef]
- Hagelberg, S.; Hult, K.; Fuchs, R. Toxicokinetics of ochratoxin A in several species and its plasma-binding properties. J. Appl. Toxicol. 1989, 9, 91–96. [Google Scholar] [CrossRef]
- Studer-Rohr, I.; Schlatter, J.; Dietrich, D.R. Kinetic parameters and intraindividual fluctuations of ochratoxin A plasma levels in humans. Arch. Toxicol. 2000, 74, 499–510. [Google Scholar] [CrossRef]
- Kane, A.; Creppy, E.E.; Roth, A.; Röschenthaler, R.; Dirheimer, G. Distribution of the [3H]-label from low doses of radioactive ochratoxin A ingested by rats, and evidence for DNA single-strand breaks caused in liver and kidneys. Arch. Toxicol. 1986, 58, 219–224. [Google Scholar] [CrossRef]
- Zimmerli, B.; Dick, R. Determination of ochratoxin A at the ppt level in human blood, serum, milk and some foodstuffs by high-performance liquid chromatography with enhanced fluorescence detection and immunoaffinity column cleanup: Methodology and Swiss data. J. Chromatogr. B Biomed. Sci. Appl. 1995, 666, 85–99. [Google Scholar] [CrossRef]
- Jung, K.Y.; Takeda, M.; Kim, D.K.; Tojo, A.; Narikawa, S.; Yoo, B.S.; Hosoyamada, M.; Cha, S.H.; Sekine, T.; Endou, H. Characterization of ochratoxin A transport by human organic anion transporters. Life Sci. 2001, 69, 2123–2135. [Google Scholar] [CrossRef]
- Bahnemann, E.; Kerling, H.P.; Ensminger, S.; Schwerdt, G.; Silbernagl, S.; Gekle, M. Renal transepithelial secretion of ochratoxin A in the non-filtering toad kidney. Toxicology 1997, 120, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Leier, I.; Hummel-Eisenbeiss, J.; Cui, Y.; Keppler, D. ATP-dependent para-aminohippurate transport by apical multidrug resistance protein MRP2. Kidney Int. 2000, 57, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Kontaxi, M.; Echkardt, U.; Hagenbuch, B.; Stieger, B.; Meier, P.J.; Petzinger, E. Uptake of the mycotoxin ochratoxin A in liver cells occurs via the cloned organic anion transporting polypeptide. J. Pharmacol. Exp. Ther. 1996, 279, 1507–1513. [Google Scholar]
- Wang, J.; Gan, C.; Qi, X.; Lebre, M.C.; Schinkel, A.H. Human organic anion transporting polypeptide (OATP) 1B3 and mouse OATP1A/1B affect liver accumulation of Ochratoxin A in mice. Toxicol. Appl. Pharmacol. 2020, 401, 115072. [Google Scholar] [CrossRef]
- Størmer, F.C.; Hansen, C.E.; Pedersen, J.I.; Hvistendahl, G.; Aasen, A.J. Formation of (4R)- and (4S)-4-hydroxyochratoxin A from ochratoxin A by liver microsomes from various species. Appl. Environ. Microbiol. 1981, 42, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Størmer, F.C.; Støren, O.; Hansen, C.E.; Pedersen, J.I.; Aasen, A.J. Formation of (4R)- and (4S)-4-hydroxyochratoxin A and 10-hydroxyochratoxin A from Ochratoxin A by rabbit liver microsomes. Appl. Environ. Microbiol. 1983, 45, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Omar, R.F.; Gelboin, H.V.; Rahimtula, A.D. Effect of cytochrome P450 induction on the metabolism and toxicity of ochratoxin A. Biochem. Pharmacol. 1996, 51, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y. Biotransformation of Mycotoxins in the Reconstituted Cytochrome P-450 System. Mycotoxins 1985, 1985, 28–30. [Google Scholar] [CrossRef]
- Gross-Steinmeyer, K.; Weymann, J.; Hege, H.G.; Metzler, M. Metabolism and lack of DNA reactivity of the mycotoxin ochratoxin a in cultured rat and human primary hepatocytes. J. Agric. Food Chem. 2002, 50, 938–945. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, H.; De Saeger, S.; De Boevre, M.; Sun, F.; Zhang, S.; Cao, X.; Wang, Z. In vitro and in vivo metabolism of ochratoxin A: A comparative study using ultra-performance liquid chromatography-quadrupole/time-of-flight hybrid mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 3579–3589. [Google Scholar] [CrossRef]
- Gupta, R.C. Chapter 91—Ochratoxins and citrinin. In Veterinary Toxicology, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2012; pp. 1220–1226. [Google Scholar]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, L.; Dhivya, R.; Dhanasekaran, D.; Periasamy, V.S.; Alshatwi, A.A.; Akbarsha, M.A. Hepatotoxic effect of ochratoxin A and citrinin, alone and in combination, and protective effect of vitamin E: In vitro study in HepG2 cell. Food Chem. Toxicol. 2015, 83, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Lee, H.J.; Pyo, M.C.; Ryu, D.; Lee, K.-W. Ochratoxin A-Induced Hepatotoxicity through Phase I and Phase II Reactions Regulated by AhR in Liver Cells. Toxins 2019, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yang, X.; Chen, S.; He, X.; Dweep, H.; Guo, M.; Cheng, W.-H.; Xu, W.; Luo, Y.; Gretz, N.; et al. Ochratoxin A induced early hepatotoxicity: New mechanistic insights from microRNA, mRNA and proteomic profiling studies. Sci. Rep. 2014, 4, 5163. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2018, 47, D506–D515. [Google Scholar] [CrossRef]
- Wang, W.; Zhai, S.; Xia, Y.; Wang, H.; Ruan, D.; Zhou, T.; Zhu, Y.; Zhang, H.; Zhang, M.; Ye, H.; et al. Ochratoxin A induces liver inflammation: Involvement of intestinal microbiota. Microbiome 2019, 7, 151. [Google Scholar] [CrossRef]
- Nuttall, F.Q.; Ngo, A.; Gannon, M.C. Regulation of hepatic glucose production and the role of gluconeogenesis in humans: Is the rate of gluconeogenesis constant? Diabetes Metab. Res. Rev. 2008, 24, 438–458. [Google Scholar] [CrossRef]
- She, P.; Shiota, M.; Shelton, K.D.; Chalkley, R.; Postic, C.; Magnuson, M.A. Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Mol. Cell. Biol. 2000, 20, 6508–6517. [Google Scholar] [CrossRef]
- Meisner, H.; Meisner, P. Ochratoxin A, an in vivo inhibitor of renal phosphoenolpyruvate carboxykinase. Arch. Biochem. Biophys. 1981, 208, 146–153. [Google Scholar] [CrossRef]
- Il’ichev, Y.V.; Perry, J.L.; Simon, J.D. Interaction of Ochratoxin A with Human Serum Albumin. Preferential Binding of the Dianion and pH Effects. J. Phys. Chem. B 2002, 106, 452–459. [Google Scholar] [CrossRef]
- Il’ichev, Y.V.; Perry, J.L.; Rüker, F.; Dockal, M.; Simon, J.D. Interaction of ochratoxin A with human serum albumin. Binding sites localized by competitive interactions with the native protein and its recombinant fragments. Chem. Biol. Interact. 2002, 141, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Hundhausen, C.; Boesch-Saadatmandi, C.; Matzner, N.; Lang, F.; Blank, R.; Wolffram, S.; Blaschek, W.; Rimbach, G. Ochratoxin a lowers mRNA levels of genes encoding for key proteins of liver cell metabolism. Cancer Genom. Proteom. 2008, 5, 319–332. [Google Scholar]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef] [PubMed]
- Berthier, A.; Johanns, M.; Zummo, F.P.; Lefebvre, P.; Staels, B. PPARs in liver physiology. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166097. [Google Scholar] [CrossRef]
- Lee, Y.K.; Park, J.E.; Lee, M.; Hardwick, J.P. Hepatic lipid homeostasis by peroxisome proliferator-activated receptor gamma 2. Liver Res. 2018, 2, 209–215. [Google Scholar] [CrossRef]
- Zheng, Q.W.; Ding, X.F.; Cao, H.J.; Ni, Q.Z.; Zhu, B.; Ma, N.; Zhang, F.K.; Wang, Y.K.; Xu, S.; Chen, T.W.; et al. Ochratoxin A Induces Steatosis via PPARγ-CD36 Axis. Toxins 2021, 13, 802. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, T.H.; Hong, M.W.; Park, T.S.; Lee, H.; Lee, S.J. Transcriptomic alterations induced by aflatoxin B1 and ochratoxin A in LMH cell line. Poult. Sci. 2020, 99, 5265–5274. [Google Scholar] [CrossRef]
- Omar, R.F.; Hasinoff, B.B.; Mejilla, F.; Rahimtula, A.D. Mechanism of ochratoxin a stimulated lipid peroxidation. Biochem. Pharmacol. 1990, 40, 1183–1191. [Google Scholar] [CrossRef]
- Manderville, R.; Leszkowicz, A. Chapter 4 Genotoxicity of Chlorophenols and Ochratoxin A. Adv. Mol. Toxicol. 2006, 1, 85–138. [Google Scholar]
- Hoehler, D.; Marquardt, R.R.; McIntosh, A.R.; Hatch, G.M. Induction of free radicals in hepatocytes, mitochondria and microsomes of rats by ochratoxin A and its analogs. Biochim. Biophys. Acta Mol. Cell. Res. 1997, 1357, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Atroshi, F.; Biese, I.; Saloniemi, H.; Ali-Vehmas, T.; Saari, S.; Rizzo, A.; Veijalainen, P. Significance of apoptosis and its relationship to antioxidants after ochratoxin A administration in mice. J. Pharm. Pharm. Sci. 2000, 3, 281–291. [Google Scholar] [PubMed]
- Guerra, M.C.; Galvano, F.; Bonsi, L.; Speroni, E.; Costa, S.; Renzulli, C.; Cervellati, R. Cyanidin-3-O-β-glucopyranoside, a natural free-radical scavenger against aflatoxin B1- and ochratoxin A-induced cell damage in a human hepatoma cell line (Hep G2) and a human colonic adenocarcinoma cell line (CaCo-2). Br. J. Nutr. 2005, 94, 211–220. [Google Scholar] [CrossRef]
- Domijan, A.M.; Rudes, K.; Peraica, M. The effect of ochratoxin A on the concentration of protein carbonyls in rats. Arch. Ind. Hyg. Toxicol. 2005, 56, 311–315. [Google Scholar]
- Marin-Kuan, M.; Nestler, S.; Verguet, C.; Bezençon, C.; Piguet, D.; Mansourian, R.; Holzwarth, J.; Grigorov, M.; Delatour, T.; Mantle, P.; et al. A toxicogenomics approach to identify new plausible epigenetic mechanisms of ochratoxin a carcinogenicity in rat. Toxicol. Sci. 2006, 89, 120–134. [Google Scholar] [CrossRef]
- Cavin, C.; Delatour, T.; Marin-Kuan, M.; Holzhäuser, D.; Higgins, L.; Bezençon, C.; Guignard, G.; Junod, S.; Richoz-Payot, J.; Gremaud, E.; et al. Reduction in antioxidant defenses may contribute to ochratoxin A toxicity and carcinogenicity. Toxicol. Sci. 2007, 96, 30–39. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Zhang, X.; Wang, J.; Xie, H.; Sun, Y.; Zhang, Q.; Chang, Z.; Liu, Y. Protective Effect of SeMet on Liver Injury Induced by Ochratoxin A in Rabbits. Toxins 2022, 14, 628. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–w221. [Google Scholar] [CrossRef] [PubMed]
- Parvataneni, S.; Vemuri-Reddy, S. N-acetyl Cysteine Use in the Treatment of Shock Liver. Cureus 2020, 12, e7149. [Google Scholar] [CrossRef] [PubMed]
- Sueck, F.; Specht, J.; Cramer, B.; Humpf, H.U. Identification of ochratoxin-N-acetyl-L-cysteine as a new ochratoxin A metabolite and potential biomarker in human urine. Mycotoxin Res. 2020, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Shi, L.; Huang, K.; Xu, W. Protective effect of N-acetylcysteine against DNA damage and S-phase arrest induced by ochratoxin A in human embryonic kidney cells (HEK-293). Food Chem. Toxicol. 2014, 70, 40–47. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Manderville, R.A. An update on direct genotoxicity as a molecular mechanism of ochratoxin a carcinogenicity. Chem. Res. Toxicol. 2012, 25, 252–262. [Google Scholar] [CrossRef]
- Ma, T.-F.; Chen, Y.-P.; Shen, Y. Chapter Seven—Progress in the applications of surface plasmon resonance for food safety. In Comprehensive Analytical Chemistry; Chen, Y.-P., Ma, T.-F., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 95, pp. 237–275. [Google Scholar]
- Waidyanatha, S.; Lin, P.H.; Rappaport, S.M. Characterization of chlorinated adducts of hemoglobin and albumin following administration of pentachlorophenol to rats. Chem. Res. Toxicol. 1996, 9, 647–653. [Google Scholar] [CrossRef]
- Vaidyanathan, V.G.; Villalta, P.W.; Sturla, S.J. Nucleobase-dependent reactivity of a quinone metabolite of pentachlorophenol. Chem. Res. Toxicol. 2007, 20, 913–919. [Google Scholar] [CrossRef]
- Murray, A.R.; Kisin, E.; Castranova, V.; Kommineni, C.; Gunther, M.R.; Shvedova, A.A. Phenol-induced in vivo oxidative stress in skin: Evidence for enhanced free radical generation, thiol oxidation, and antioxidant depletion. Chem. Res. Toxicol. 2007, 20, 1769–1777. [Google Scholar] [CrossRef]
- Dai, J.; Park, G.; Wright, M.W.; Adams, M.; Akman, S.A.; Manderville, R.A. Detection and characterization of a glutathione conjugate of ochratoxin A. Chem. Res. Toxicol. 2002, 15, 1581–1588. [Google Scholar] [CrossRef]
- Gillman, I.G.; Clark, T.N.; Manderville, R.A. Oxidation of ochratoxin A by an Fe-porphyrin system: Model for enzymatic activation and DNA cleavage. Chem. Res. Toxicol. 1999, 12, 1066–1076. [Google Scholar] [CrossRef]
- Kamp, H.G.; Eisenbrand, G.; Janzowski, C.; Kiossev, J.; Latendresse, J.R.; Schlatter, J.; Turesky, R.J. Ochratoxin A induces oxidative DNA damage in liver and kidney after oral dosing to rats. Mol. Nutr. Food Res. 2005, 49, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Chopra, M.; Link, P.; Michels, C.; Schrenk, D. Characterization of ochratoxin A-induced apoptosis in primary rat hepatocytes. Cell Biol. Toxicol. 2010, 26, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, C.; Sharaf El Dein, O.; El Golli, E.; Abid-Essefi, S.; Brenner, C.; Lemaire, C.; Bacha, H. Different apoptotic pathways induced by zearalenone, T-2 toxin and ochratoxin A in human hepatoma cells. Toxicology 2008, 254, 19–28. [Google Scholar] [CrossRef]
- Al-Anati, L.; Essid, E.; Reinehr, R.; Petzinger, E. Silibinin protects OTA-mediated TNF-alpha release from perfused rat livers and isolated rat Kupffer cells. Mol. Nutr. Food Res. 2009, 53, 460–466. [Google Scholar] [CrossRef]
- Barré-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Future Sci. OA 2015, 1, Fso63. [Google Scholar] [CrossRef]
- Pound, P.; Ebrahim, S.; Sandercock, P.; Bracken, M.B.; Roberts, I. Where is the evidence that animal research benefits humans? BMJ 2004, 328, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Hoehler, D.; Marquardt, R.R. Influence of vitamins E and C on the toxic effects of ochratoxin A and T-2 toxin in chicks. Poult. Sci. 1996, 75, 1508–1515. [Google Scholar] [CrossRef]
- Shalaby, A.M.E. The opposing effect of ascorbic acid (vitamin C) on ochratoxin toxicity in nile tilapia (Oreochromis niloticus). In Proceedings of the 6th International Symposium on Tilapia in Aquaculture, Manila, Philippines, 12–16 September 2004; pp. 209–221. [Google Scholar]
- Badriyah, B.; Hastuti, U.S. The effect of pomelo citrus (Citrus maxima var. Nambangan), vitamin C and lycopene towards the number reduction of mice (Mus musculus) apoptotic hepatocyte caused of ochratoxin A. In Proceedings of the AIP Conference Proceedings, Surabaya, Indonesia, 15 October 2016. [Google Scholar]
- Ramyaa, P.; Krishnaswamy, R.; Padma, V.V. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells—Up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochim. Biophys. Acta 2014, 1840, 681–692. [Google Scholar] [CrossRef]
- Oršolić, N.; Jazvinšćak Jembrek, M.; Terzić, S. Honey and quercetin reduce ochratoxin A-induced DNA damage in the liver and the kidney through the modulation of intestinal microflora. Food Agric. Immunol. 2017, 28, 812–833. [Google Scholar] [CrossRef]
- Sutken, E.; Aral, E.; Ozdemir, F.; Uslu, S.; Alatas, O.; Colak, O. Protective role of melatonin and coenzyme Q10 in ochratoxin A toxicity in rat liver and kidney. Int. J. Toxicol. 2007, 26, 81–87. [Google Scholar] [CrossRef]
- Soyöz, M.; Ozçelik, N.; Kilinç, I.; Altuntaş, I. The effects of ochratoxin A on lipid peroxidation and antioxidant enzymes: A protective role of melatonin. Cell Biol. Toxicol. 2004, 20, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Meki, A.R.; Hussein, A.A. Melatonin reduces oxidative stress induced by ochratoxin A in rat liver and kidney. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahhab, M.A.; Abdel-Galil, M.M.; El-Lithey, M. Melatonin counteracts oxidative stress in rats fed an ochratoxin A contaminated diet. J. Pineal Res. 2005, 38, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Yang, L.; Li, Y.; Chen, J.; Zhang, X.; Wang, H.; Zhai, S.; Jiang, X.; Meca, G.; Wang, S.; et al. Melatonin alleviates Ochratoxin A-induced liver inflammation involved intestinal microbiota homeostasis and microbiota-independent manner. J. Hazard. Mater. 2021, 413, 125239. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Y.; Xu, W.; Luo, Y.; Hao, J.; Shen, X.L.; Yang, X.; Li, X.; Huang, K. Zinc protects HepG2 cells against the oxidative damage and DNA damage induced by ochratoxin A. Toxicol. Appl. Pharmacol. 2013, 268, 123–131. [Google Scholar] [CrossRef]
- Chakraborty, D.; Verma, R. Ameliorative effect of Emblica officinalis aqueous extract on ochratoxin-induced lipid peroxidation in the kidney and liver of mice. Int. J. Occup. Med. Environ. Health 2010, 23, 63–73. [Google Scholar] [CrossRef]
- Guliyev, V.B.; Gul, M.; Yildirim, A. Hippophae rhamnoides L.: Chromatographic methods to determine chemical composition, use in traditional medicine and pharmacological effects. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 812, 291–307. [Google Scholar] [CrossRef]
- Patial, V.; Asrani, R.; Patil, R.; Kumar, N.; Sharma, R. Protective Effect of Sea buckthorn (Hippophae rhamnoides L.) Leaves on Ochratoxin-A Induced Hepatic Injury in Japanese Quail. Vet. Res. Int. 2015, 3, 98–108. [Google Scholar]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Alhussaini, M.; Alyahya, A. Protective Role of Nigella Sativa Oil on Ochratoxin A Toxicity in Liver and Kidney of Male Albino Rats: Histological and Histochemical Studies. Wulfenia 2014, 21, 59–77. [Google Scholar]
- Damiano, S.; Longobardi, C.; Andretta, E.; Prisco, F.; Piegari, G.; Squillacioti, C.; Montagnaro, S.; Pagnini, F.; Badino, P.; Florio, S.; et al. Antioxidative Effects of Curcumin on the Hepatotoxicity Induced by Ochratoxin A in Rats. Antioxidants 2021, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, C.; Damiano, S.; Andretta, E.; Prisco, F.; Russo, V.; Pagnini, F.; Florio, S.; Ciarcia, R. Curcumin Modulates Nitrosative Stress, Inflammation, and DNA Damage and Protects against Ochratoxin A-Induced Hepatotoxicity and Nephrotoxicity in Rats. Antioxidants 2021, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Di Giacomo, C.; Acquaviva, R.; Bognanno, M.; Grilli, E.; D’Orazio, N.; Galvano, F. Dimethylarginine Dimethylaminohydrolase/Nitric Oxide Synthase Pathway in Liver and Kidney: Protective Effect of Cyanidin 3-O-β-D-Glucoside on Ochratoxin-A Toxicity. Toxins 2012, 4, 353–363. [Google Scholar] [CrossRef]
- Nogaim, Q.A.; Bugata, L.S.P.; Prabhakar, P.V.; Reddy, U.A.; Kumari, I.; Mahboob, M. Protective effect of Yemeni green coffee powder against the oxidative stress induced by Ochratoxin A. Toxicol. Rep. 2020, 7, 142–148. [Google Scholar] [CrossRef]
- Hassan, S.; Mujahid, H.; Ali, M.M.; Irshad, S.; Naseer, R.; Saeed, S.; Firyal, S.; Arooj, F. Synthesis, characterization and protective effect of green tea-mediated zinc oxide nanoparticles against ochratoxin A induced hepatotoxicity and nephrotoxicity in albino rats. Appl. Nanosci. 2021, 11, 2281–2289. [Google Scholar] [CrossRef]
- Palabiyik, S.S.; Erkekoglu, P.; Kızılgun, M.; Sahin, G.; Kocer-Gumusel, B. Lycopene restores trace element levels in ochratoxin A-treated rats. Arch. Ind. Hyg. Toxicol. 2017, 68, 135–141. [Google Scholar] [CrossRef][Green Version]
- Aydin, S.; Palabiyik, S.; Erkekoglu, P.; Sahin, G.; Başaran, N.; Giray, B.K. The carotenoid lycopene protects rats against DNA damage induced by Ochratoxin A. Toxicon 2013, 73, 96–103. [Google Scholar] [CrossRef]
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef]
- Stoev, S.; Mircheva, T.; Denev, S.; Chobanova, S.; Ivanov, V. The Protective Effect of Silymarin against Ochratoxin A Induced Histopathological and Biochemical Changes in Chicks. J. Adv. Vet. Res. 2021, 11, 1–8. [Google Scholar]
- Yu, Z.; Wu, F.; Tian, J.; Guo, X.; An, R. Protective effects of compound ammonium glycyrrhizin, L-arginine, silymarin and glucurolactone against liver damage induced by ochratoxin A in primary chicken hepatocytes. Mol. Med. Rep. 2018, 18, 2551–2560. [Google Scholar] [CrossRef]
- Essid, E.; Dernawi, Y.; Petzinger, E. Apoptosis Induction by OTA and TNF-α in Cultured Primary Rat Hepatocytes and Prevention by Silibinin. Toxins 2012, 4, 1139–1156. [Google Scholar] [CrossRef] [PubMed]
- Massolini, G.; De Lorenzi, E.; Ponci, M.C.; Caccialanza, G. Comparison of drug binding sites on rat and human serum albumins using immobilized-protein stationary phases as a tool for the selection of suitable animal models in pharmacological studies. Boll. Chim. Farm. 1996, 135, 382–386. [Google Scholar] [PubMed]
- Deguchi, S.; Takayama, K. State-of-the-art liver disease research using liver-on-a-chip. Inflamm. Regen. 2022, 42, 62. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Więckowska, M.; Szelenberger, R.; Niemcewicz, M.; Harmata, P.; Poplawski, T.; Bijak, M. Ochratoxin A—The Current Knowledge Concerning Hepatotoxicity, Mode of Action and Possible Prevention. Molecules 2023, 28, 6617. https://doi.org/10.3390/molecules28186617
Więckowska M, Szelenberger R, Niemcewicz M, Harmata P, Poplawski T, Bijak M. Ochratoxin A—The Current Knowledge Concerning Hepatotoxicity, Mode of Action and Possible Prevention. Molecules. 2023; 28(18):6617. https://doi.org/10.3390/molecules28186617
Chicago/Turabian StyleWięckowska, Magdalena, Rafał Szelenberger, Marcin Niemcewicz, Piotr Harmata, Tomasz Poplawski, and Michał Bijak. 2023. "Ochratoxin A—The Current Knowledge Concerning Hepatotoxicity, Mode of Action and Possible Prevention" Molecules 28, no. 18: 6617. https://doi.org/10.3390/molecules28186617
APA StyleWięckowska, M., Szelenberger, R., Niemcewicz, M., Harmata, P., Poplawski, T., & Bijak, M. (2023). Ochratoxin A—The Current Knowledge Concerning Hepatotoxicity, Mode of Action and Possible Prevention. Molecules, 28(18), 6617. https://doi.org/10.3390/molecules28186617




