Abstract
This study investigated the anticoccidial activity of spinach (Spinacia oleracea) whole-plant extract against Eimeria tenella, both in vitro and in vivo. For this purpose, one hundred 8-day-old broiler chicks of both sexes were divided into four groups (n = 25 in each group). Chicks in the first group served as the negative control (non-treated–non-infected). Chicks in the second group were challenged at 18 days old with 5 × 104 E. tenella sporulated oocysts. The third group was challenged with 5 × 104 sporulated E. tenella oocysts at 18 days old after receiving spinach extract at a dose of 50 mg/kg at 8 days old. The fourth group received 0.2 mg/kg diclazuril (Coxiril® 0.2%) in their diet two days before being orally infected with 5 × 104 sporulated E. tenella oocysts and this continued till day 10 post-infection (PI). The growth performance, clinical symptoms, oocyst shedding, histological findings, and biochemical parameters were used to evaluate the efficacy on day 8 PI when the infection was at its peak. A gas chromatography examination revealed that omega-3 fatty acids were the main constituents of the spinach extract, followed by oleic acid, palmitic acid, and phytol, with amounts of 23.37%, 17.53%, 11.26%, and 7.97%, respectively. The in vitro investigation revealed that the spinach extract at concentrations of 10% and 5% inhibited the oocyst sporulation by 52.1% and 45.1%, respectively. The 5% concentration was selected for the in vivo trial based on the results of the in vitro study. The infected–untreated group showed high levels of OPG; lower body weight; a greater number of parasite stages; few goblet cells; decreased SOD, CAT, and GPX levels; and increased MDA and NO levels. The spinach-treated group, on the other hand, showed a significant decrease in oocyst output per gram of feces (OPG), increased body weight, decreased parasitic stages, and a nearly normal number of goblet cells. Additionally, it reduced malondialdehyde (MDA) and nitric oxide (NO), while increasing superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX). In conclusion, spinach produced significant antioxidant effects, increased body weight, reduced the number of oocysts and parasite stages in the caecum, and restored the number of goblet cells relative to those of an uninfected control. Furthermore, spinach extract inhibits the sporulation percentage of E. tenella oocysts. The ethanolic extract of S. oleracea (whole plant) contained high concentrations of fatty acids, palmitic acid, Phytol, betulin, and ursolic aldehyde, all of which are known to regulate the antioxidant pathway and modulate inflammatory processes and may be the main reason for its anticoccidial activity.
1. Introduction
Members of the genus Eimeria are the most frequent parasites that infect chickens [1,2,3]. Eimeria is an apicomplexan parasite that comprises several species and is the causative agent of coccidiosis. Coccidiosis is common wherever chickens are raised (industrial, traditional, or organic/bio farms), and it remains one of the main illnesses that have a negative impact on bird performance in intensive production systems because it causes intestinal lesions (such as inflammation, diarrhea, and hemorrhage), growth impairment, poor feed utilization, non-homogenized flock weights, and increased mortality [4]. Avian coccidiosis causes enormous economic losses in poultry production worldwide [1]. According to estimates made by Rashid et al. [2], there are losses in a variety of poultry categories that vary from USD 104.74 to USD 2,750,779.00 due to the expense of the vaccine, treatment, and other preventative measures.
There are several drugs available to treat coccidiosis; however, their overuse has hastened the development of multidrug resistance and increased the residue in tissues. As a result, a global strategy is being developed to explore the antiparasitic capabilities of various herbal plants in order to alleviate the problems caused by coccidial illness in the chicken industry. The main advantages of using herbal-based therapies to treat coccidiosis are their low toxicity and inexpensive production costs [3]. Spinach (Spinacia oleracea) belongs to the family Amaranthaceae and is a highly significant and nutrient-dense plant. It contains numerous amounts of B vitamins, riboflavin, foliate, niacin, soluble dietary fiber, omega-3 fatty acids, and minerals. Spinach is also abundant in iron, which protects against iron-deficiency-related illnesses, such as osteoporosis and anemia [4]. Spinach contains a variety of active compounds, including flavonoids and other polyphenolic active ingredient molecules that act as antioxidants and anti-inflammatory agents. When ingested together, the flavonoids, carotenoids, vitamins (C, E), and phenolic components in spinach have an antioxidative effect that reduces the harmful effects of free radicals [5]. In addition, spinach leaf extract and its phytoconstituents were shown to have anti-inflammatory, antidiarrheal, antibacterial, antioxidant, and insecticidal properties [6]. This study therefore aimed to investigate the anticoccidial effect of spinach extract on Eimeria tenella in experimentally infected broiler chicks.
2. Results
2.1. GC-MS of Spinach Extract
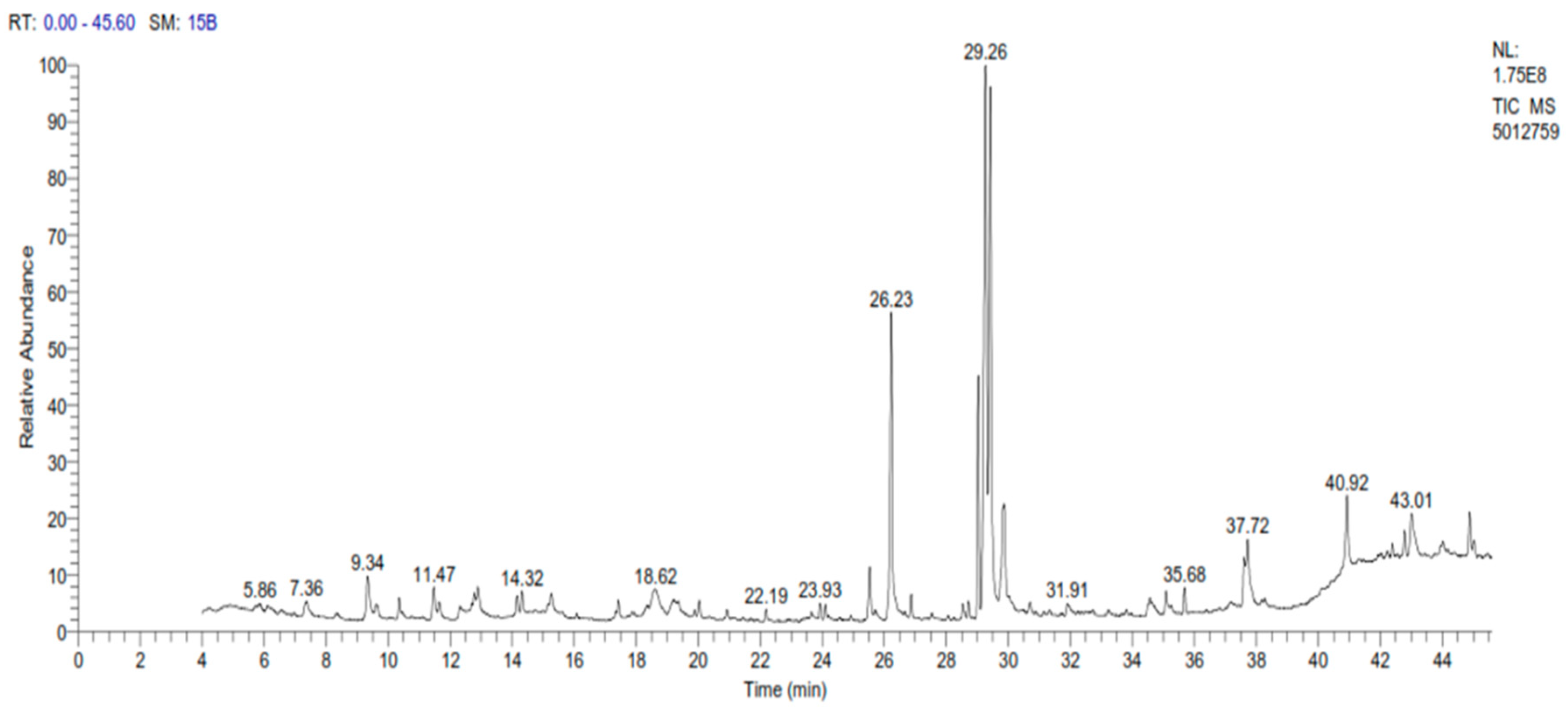
A sample of spinach extract was subjected to gas chromatography examination, which revealed a high number of elements, with omega-3 accounting for the greatest amount, followed by oleic acid, palmitic acid, and phytol with concentrations of 23.37%, 17.53%, 11.26%, and 7.97%, respectively, as shown in Table 1 and Figure 1.

Table 1.
Chemical composition of the spinach extract.
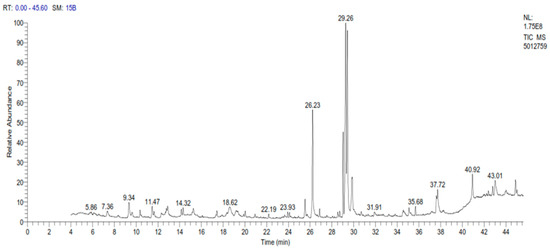
Figure 1.
GC-MS chromatogram of spinach extract.
2.2. In Vitro Studies of Spinach Extract on Eimeria tenella Oocysts
Using 10% and 5% spinach extracts achieved an inhibition of oocysts sporulation at rates of 52.1% and 45.1%, respectively, and the lowest concentration (0.625%) caused a sporulation inhibition percentage of 10.27% after 72 h (Figure 2). Meanwhile, 10% formalin (positive control) caused an estimated inhibition of 67.27% (Table 2).
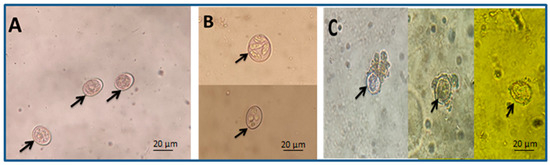
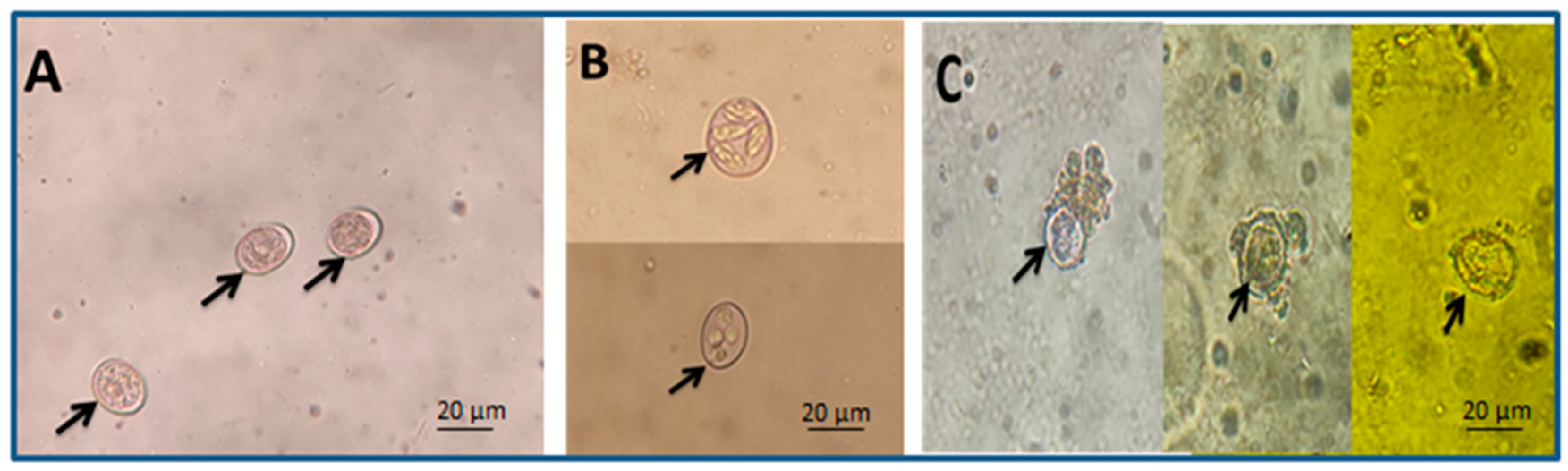
Figure 2.
Arrows refer to different oocyst shapes: (A) unsporulated oocysts, (B) sporulated oocysts, and (C) damaged oocysts (scale bar—20 μm, seen with a 40× lens).

Table 2.
In vitro evaluation of the effect of different concentrations of spinach extract compared with negative control on sporulation percentage of Eimeria tenella oocysts. The data are expressed as mean ± S.D.
2.3. Bloody Diarrhea
All infected groups were observed to have bloody diarrhea, but its severity varied from the highest in the positive control group, then the diclazuril group, to the lowest in the spinach group. The highest degree of bloody diarrhea in all infected groups was noticed on the fifth DPI and the spinach extract achieved a lower degree of bloody diarrhea than diclazuril (Table 3).

Table 3.
Bloody diarrhea in different groups, as noticed from day 4 to day 10 post-infection.
2.4. Survival Percentage and Lesion Scoring
It was noticed that the treated groups had a higher survival percentage than the infected–untreated group and the lesions had a low severity in the treated groups compared with the untreated group. In the diclazuril group, the number of deaths was lower than in the spinach group. Mild lesions were recorded in the diclazuril and spinach groups, but there were severe lesions in the infected–untreated group (Table 4).

Table 4.
Mortality, survival percentage, and lesion score in different groups throughout the experiment.
2.5. Oocysts per Gram of Feces
Fecal samples were collected from all groups for analysis from the 4th to the 10th DPI. The number of oocysts increased until they reached peaks in the diclazuril and spinach groups on the 8th DPI, achieving 3819 and 7906 oocysts/g, respectively, and then dramatically decreasing, achieving totals of 7504 and 18,894, respectively, after 10 days post-infection with Eimeria tenella oocysts; the values in the positive control group continued to increase until they reached the peak on the 9th DPI with 58,696 oocysts/g and achieved a total of 92,263 oocysts/g after 10 days post-infection (Table 5).

Table 5.
The number of oocysts shed per gram of feces from chickens of different groups from day 4 to day 10 PI.
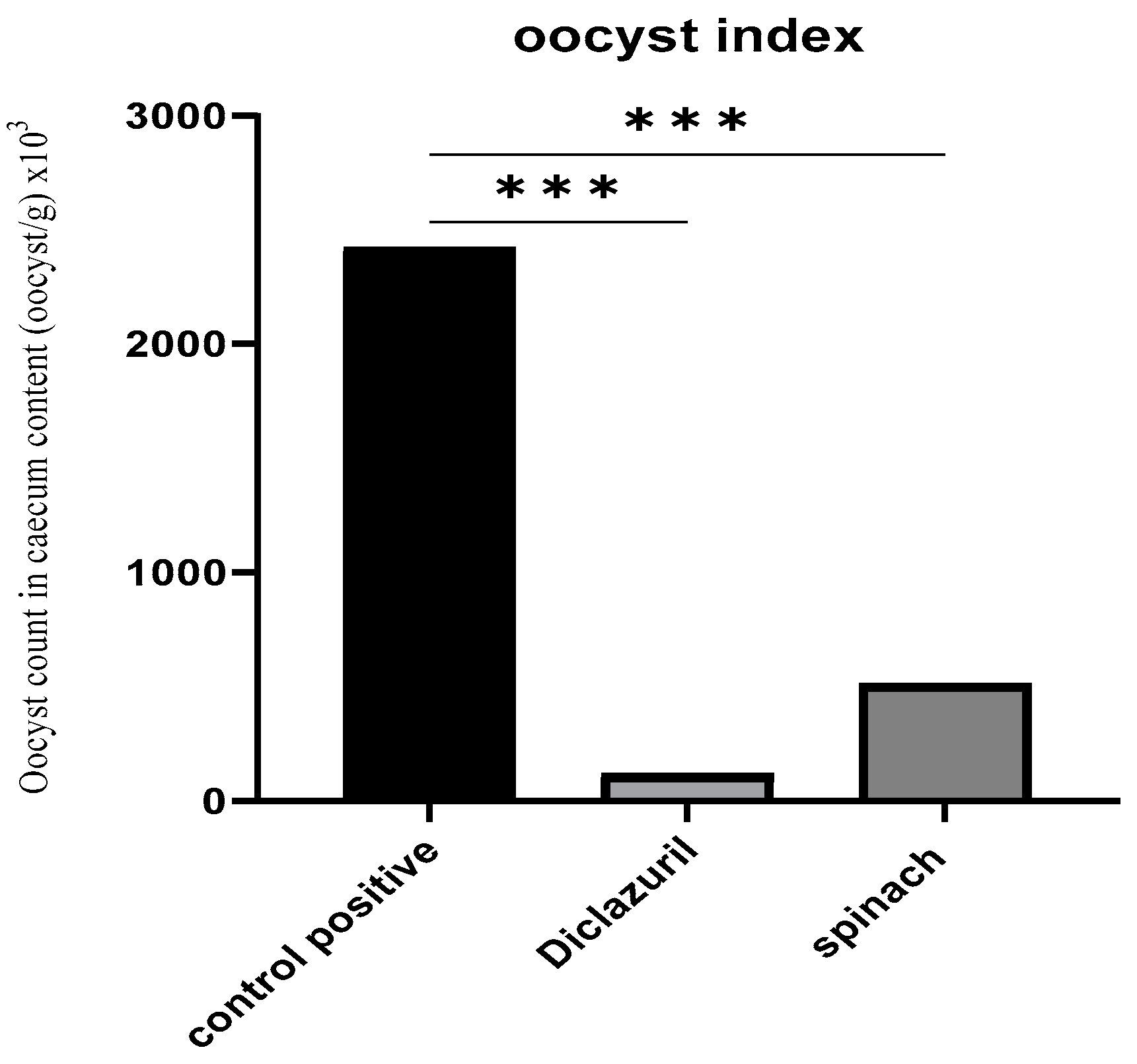
2.6. Oocyst Index
Following the examination of the cecum’s contents and the counting of the oocysts, it was found that the treated groups had a decreased number of oocysts by about 95.1% and 78.8% for the diclazuril group and spinach group, respectively, compared with the positive control (Figure 3).
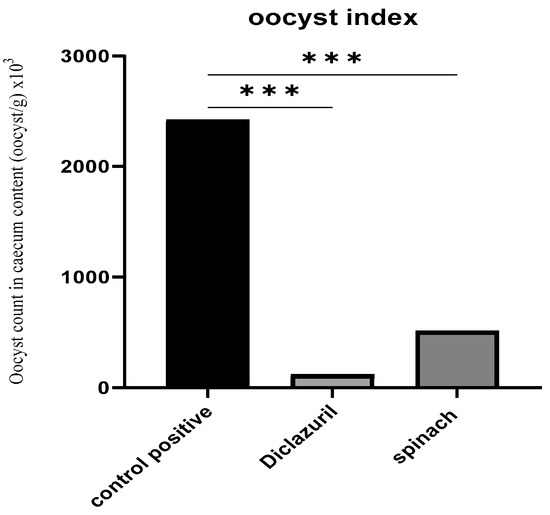
Figure 3.
Oocyst index of caecal content (oocyst/g). (***) The mean difference was significant at p < 0.001.
2.7. Feed Conversion Ratio
During the first 2 weeks of age, all groups had a close FCR because the infection had not occurred yet, but at the end of the third week, differences began to appear after infection on the 18th day of age: a significant difference was noticed between the untreated group and treated groups and the conversion rate was greater than the untreated groups. The results for all groups with p-values compared with the positive control are shown in Table 6.

Table 6.
Feed conversion ratio (FCR) was calculated by dividing feed intake (FI) by body weight gain (BWG). Data expressed as mean ± S.D.
2.8. Histopathological Studies
2.8.1. Number of Developmental Parasitic Stages and Goblet Cells in Ceca of Chickens
There was a significant difference in the number of parasitic stages and the number of goblet cells in the treated groups relative to the infected–untreated groups. The diclazuril group had a lower number of parasitic stages than the spinach group but the spinach group contained a higher number of goblet cells than the diclazuril group (Table 7).

Table 7.
Developmental stages of Eimeria tenella and goblet cells per 10 villi in ceca dissected on day 8 PI compared with the positive control. Data expressed as mean ± S.D.
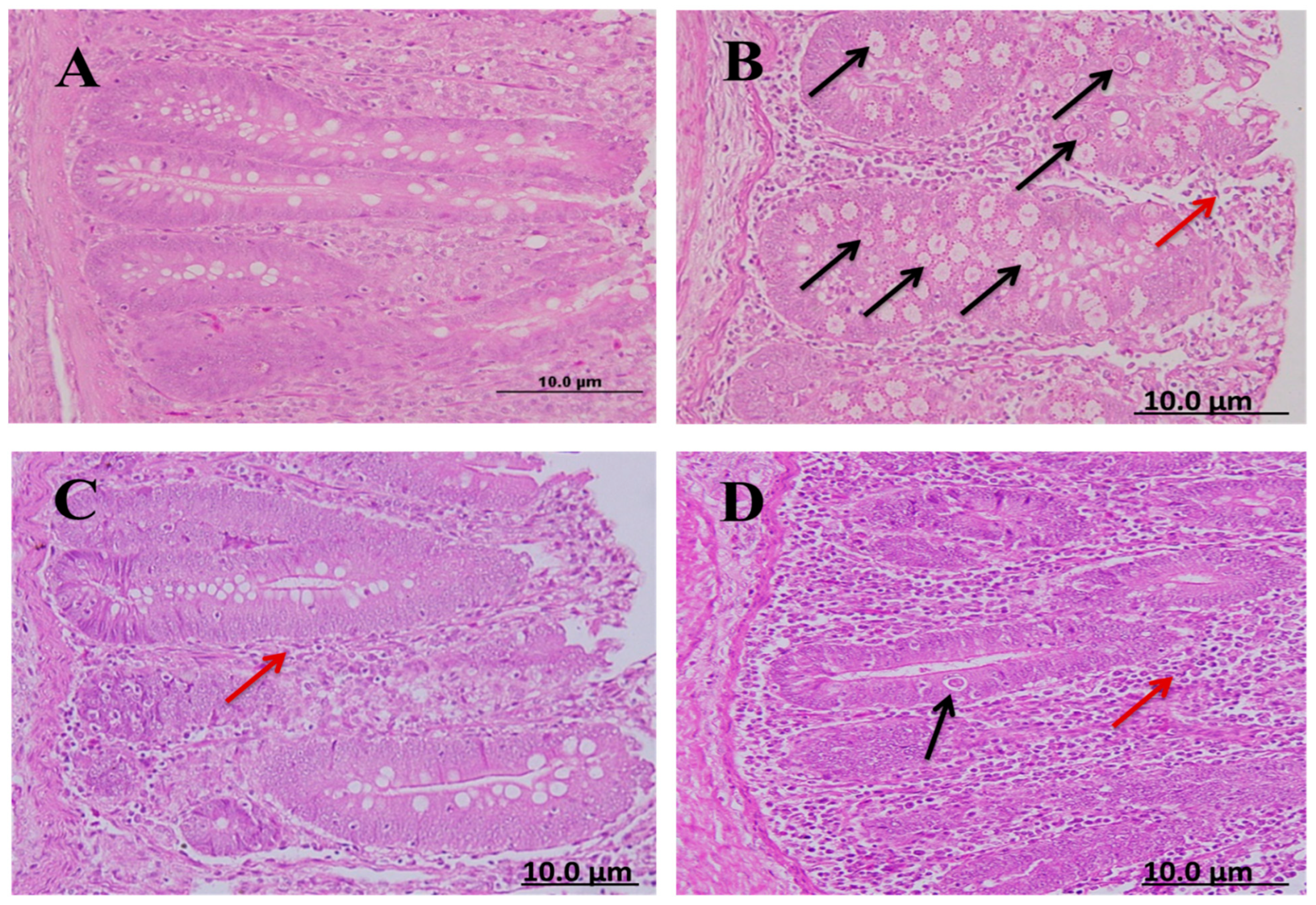
Cecal sections of different groups were collected on the 8th day after infecting chickens with 50,000 sporulated Eimeria tenella oocysts; the sections were stained with hematoxylin and eosin stain to determine the number of parasitic stages. It was found that the diclazuril group had a lower number of parasitic stages than the spinach group (Figure 4).
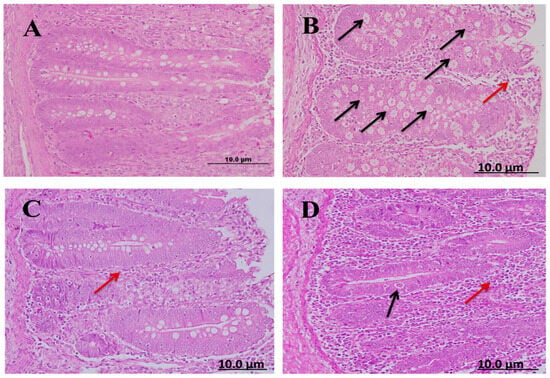
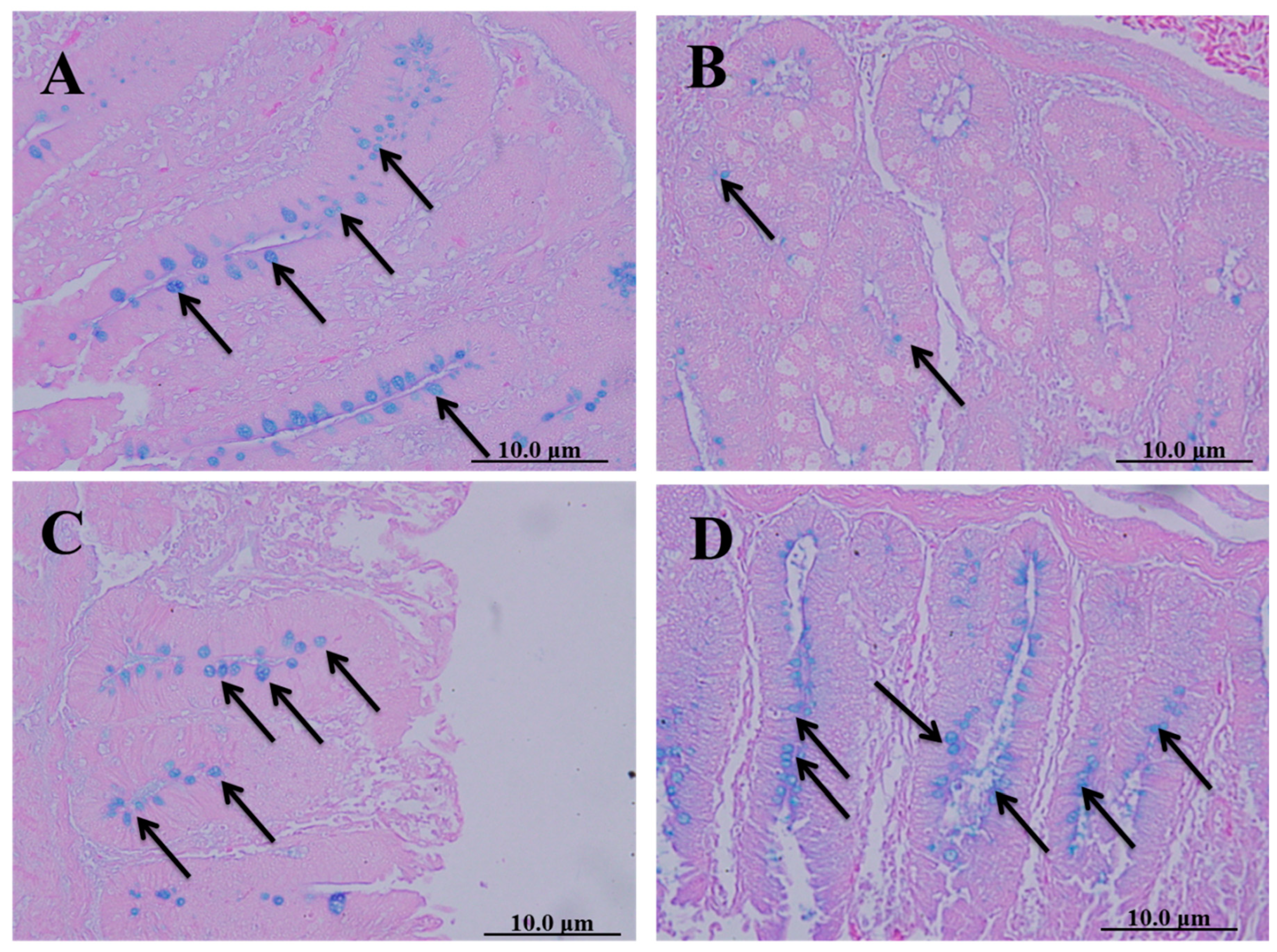
Figure 4.
Cecal sections of chickens in different groups showing the number of parasitic (H&E); (A) negative control group (noninfected–untreated), (B) positive control group (infected–untreated), (C) Diclazuril group (infected–treated with diclazuril 0.2 g/kg of food), and (D) spinach group (infected–treated with spinach extract 50 mg/kg of body). Black arrows refer to parasitic stage while red arrows refer to leukocytic infiltration.
2.8.2. Number of Goblet Cells in Ceca of Chickens
Cecal sections of different groups were collected on the 8th day after infecting chickens with 50,000 sporulated Eimeria tenella oocysts; they were stained with alcian blue stain to determine the number of goblet cells. The spinach extract achieved a better effect than diclazuril in returning the number of goblet cells close to normal (Figure 5).
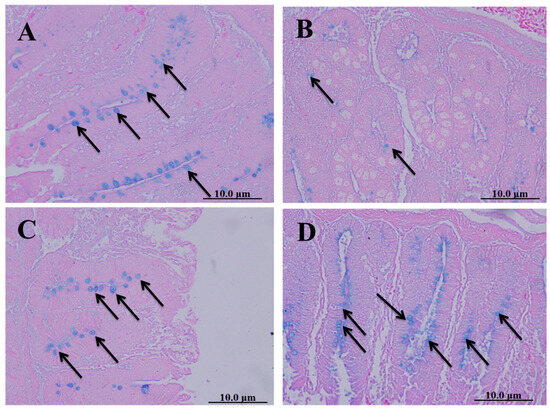
Figure 5.
Cecal sections of chickens in different groups stained with alcian blue stain to determine the number of goblet cells. (A) Negative control group (noninfected–untreated), (B) positive control group (infected–untreated), (C) Diclazuril group (infected–treated with diclazuril 0.2 g/kg of food), and (D) spinach group (infected–treated with spinach extract 50 mg/kg of body weight). Black arrows refer to goblet cells.
2.9. Biochemical Studies on the Ceca of Chickens Infected with Eimeria tenella
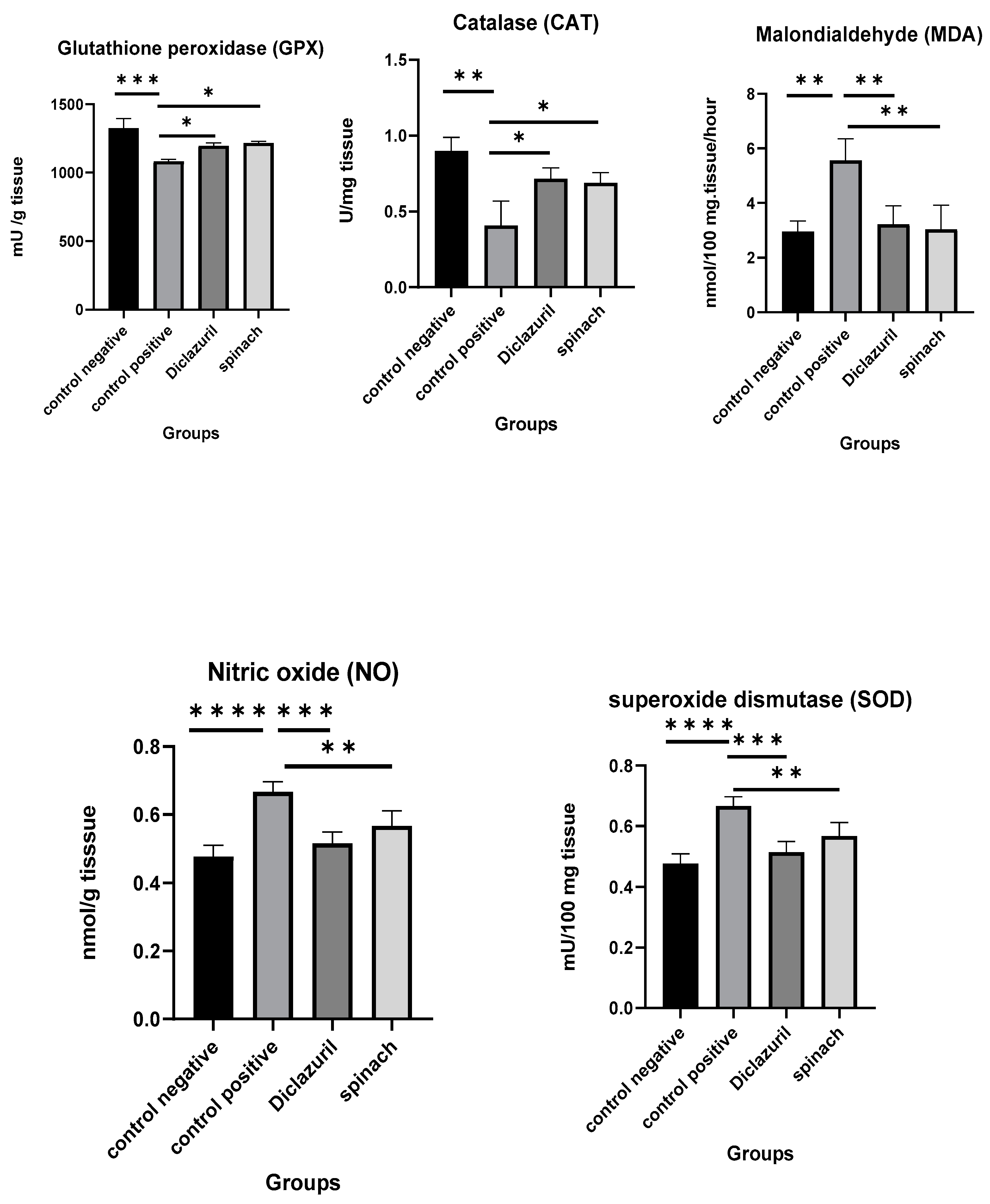
The effects of diclazuril and spinach extract on the levels of some oxidative stress markers in the ceca of chickens that were infected with 50,000 sporulated Eimeria tenella oocysts on the 18th day of age and then humanly killed on the 8th DPI was measured and compared with the positive control (infected–untreated). It was obvious that the diclazuril and spinach groups contained higher levels of GPX, CAT, and SOD than the positive control and lower MDA and NO than the positive control. This indicates that the spinach extract had a good effect on preserving cells from oxidative stress. There was no significant difference between the diclazuril and spinach groups (Figure 6).
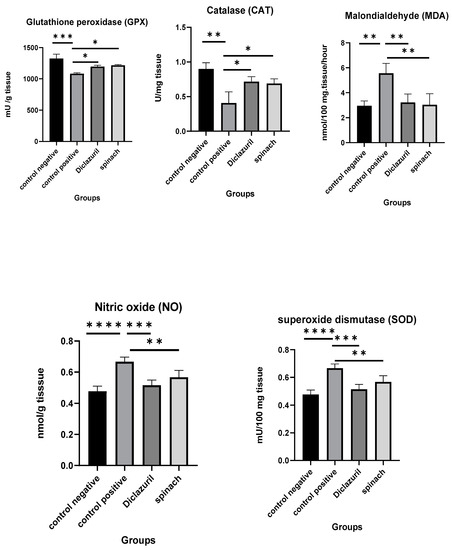
Figure 6.
Effect of diclazuril and spinach extract on levels of some oxidative stress markers in ceca of chickens in different groups. (*) the mean difference is significant, (**) Low significance, (***) moderate significance, and (****) very high significance at p-value < 0.05.
3. Discussion
Polyether ionophore antibiotics, chemically synthesized anticoccidial drugs, and vaccines are the main methods of preventing and controlling coccidiosis in chickens. However, resistance has developed for all of the drugs currently in use, which remains a significant limitation for their use [7]. Although vaccination is a viable alternative to the synthetic chemical anticoccidial drugs, its use is constrained by a laborious manufacturing process, the high expense of production and licensing of new vaccines, and the danger of pathogen spread (with the injection of attenuated coccidiosis vaccines) [8,9]. Therefore, interest in research on alternate anticoccidial treatments, such as plant extracts, has been substantially developed [10,11]. Following this approach, the current study compared the in vitro and in vivo efficacies of spinach extract and diclazuril against Eimeria tenella in experimentally infected chickens.
The GC-MS technique was employed in this investigation to determine the probable chemical compounds in an extract of Spinacia oleracea. According to the findings of this study, the ethanol extract of S. oleracea (whole plant) contained a high concentration of fatty acids, sterol, diterpenoids, and triterpenes, all of which are known to regulate the antioxidant pathway and modulate inflammatory processes with anti-cancer properties [12,13,14]. Similar components were reported by Abdelgawad et al. [15] in their phytochemical analysis of S. oleracea leaves from Egypt. According to the gas chromatography analysis, omega-3 made up the largest proportion of the spinach extract, which is consistent with the findings of Hetta et al. [16], who found that omega-3 is a major component of S. oleracea flowers (30.53%). Similar to our findings, it was found that the main fatty acids in Polyalthia longifolia seed oil were oleic, linoleic, and palmitic acids. It was also found that these acids had several biological properties, including anti-lipooxygenase, antioxidant, anti-inflammatory, anti-parasite, anti-microbial, and cytotoxicity properties. α-linolenic acid (ALA), which is an omega-3 fatty acid, and Linoleic acid (LA), which is an omega-6 fatty acid, are regarded as essential fatty acids that are known to have antibacterial properties [17]. Therefore, the South African plants Helichrysum pedunculatum (family: Asteraceae) and Schotia brachypetala (family: Fabaceae), which mostly consist of linoleic and oleic acids as main components, are used for wound healing [18,19]. C18 fatty acids, including oleic, linolenic, and linoleic acids, were demonstrated to have antimalarial effects by preventing the proliferation of malarial parasites in mice infected with Plasmodium vinckei petteri and Plasmodium yoelii nigeriensis [20]. Also, omega-3 fatty acids were found to provide health benefits in the treatment of depression and schizophrenia [21,22], as well as benefits for cancer, inflammatory bowel disease, rheumatoid arthritis, psoriasis, and mental health [23]. In addition, omega-3 polyunsaturated fatty acids (ω-3PUFAs) are hypothesized to have beneficial effects on reducing cytokines and inflammation-associated proteins by altering the signaling pathways that regulate gene expression in inflammatory cells [24]. One example of these pathways is nuclear factor kappa B (NF-B), which is linked to the activation of the genes encoding several cytokines, adhesion molecules, and COX-2 [25]. The proliferator-activated receptor (PPAR), which is activated by ω-3PUFAs, is an anti-inflammatory transcription factor that prevents NF-B from entering the nucleus. Allen et al. [26] evaluated the effects of ω-3PUFAs from animal or plant sources on cecal lesions caused by Eimeria tenella (80 strain) in chicken vs. medium chain triglycerides (MCTs) or a simple starter diet (SS). They found significant decreases in the mean cecal lesion scores, as well as decreases in bird mortality and significant increases in IL-6 blood levels. Choi et al. [27] discovered that ω-3PUFAs increased autophagy activation, resulting in decreased intracellular survival of T. gondii, suggesting that ω-3PUFAs could be used as a therapeutic candidate to prevent toxoplasmosis and infection with other intracellular protozoan parasites. According to Stok and Francis [28], the unsaturated fatty acid oleic acid was shown to be able to inactivate influenza type A virions. It was found that supplementation with omega-9, or oleic acid, is crucial in the metabolic dysfunction that occurs during sepsis because it increases the levels of the anti-inflammatory cytokine IL-10, lowers levels of the pro-inflammatory cytokines TNF- and IL-1, and inhibits neutrophil migration [29].
Through in vitro testing, it was discovered that the highest concentration of spinach extract produced the highest percentage of oocyst sporulation inhibition. This may have been due to the ability of the spinach extract to modify the permeability of the oocyst wall, penetrate the oocyst wall, and damage the oocyst’s contents, as indicated by the cracked oocysts subjected to 10% spinach extract. Molan et al. [30] found similar effects when they tested pine bark (Pious radiata, family: Pinaceae) extract on the sporulation of three species of avian coccidian oocysts. Fatemi et al. [31] also found that Artemisia annua (family: Asteraceae) extract has a significant effect on the sporulation rate of mixed oocysts of Eimeria acervulina, Eimeria necatrix, and Eimeria tenella. The number of oocysts per gram of feces and cecal content of oocysts was reduced in the spinach-treated chicks. It was discovered that spinach extract has a high concentration of phytonutrients, such as fatty acids, palmitic acid, Phytol, betulin, and ursolic aldehyde [32]. It was proposed that these compounds may change the permeability of the cytoplasmic membrane, which inhibits many physiological functions and results in a loss of membrane potential, allowing vital cellular components to leak out, inhibiting protein and ATP production, and causing cellular death [33,34,35]. The present study found that following infection with E. tenella, the infected chicks had a higher cecal lesion score and less weight gain than the chicks in the negative control group similar to those reported by Conway et al. [36]. According to Christaki et al. [33], the decrease in body weight in E.-tenella-infected chicks was attributed to the infection’s negative effects on food digestion, absorption, and metabolism. The spinach-treated chicks recovered rapidly from infection and displayed a low degree of lesions because spinach contains numerous potent chemicals, including phytol and palmitic acid, which can accelerate and enhance wound healing [37]. Additionally, phytol has been found to possess potential anti-schistosome, antinociceptive, antioxidant, anti-inflammatory, and antiallergenic properties. Phytol is a frequently used food additive and serves as a precursor in the synthesis of vitamins E and K. Also, phytol possesses antibacterial properties that are effective against Staphylococcus aureus and Mycobacterium tuberculosis [38]. Vitamin K has been used to treat hemorrhages associated with coccidiosis and other diseases due to its coagulation-promoting properties [39,40].
The body weight of the chicks in the spinach group significantly increased. Similarly, Wagde et al. [41] found a significant increase in the body weight of ornamental fish (Xiphophorus hellerii) fed a spinach-extract-supplemented diet. These findings are in agreement with those of Abbas et al. [42], who noted a marked increase in body weight in the chicks treated with turmeric compared with the infected-untreated group. The diclazuril-treated group exhibited a significant increase in body weight and a decrease in oocyst count; this finding was also consistent with that of Abbas et al. [43].
Because spinach extract is high in fatty acids, it may have an inhibitory effect on different intracellular developmental stages of Eimeria, similar to their effect on Giardia duodenalis in vitro, which is severely impacted by media supplemented with fatty acids [44]. The two primary immune-competent cells in the intestine are known to be mast cells and goblet cells. According to some studies, the mucus that goblet cells secrete can serve as a protective barrier [45]. Mucus serves as the first line of defense against infections by protecting the gut epithelial layer from pathogens [46]. The decrease in goblet cells may indicate damage to the stem cell population. Goblet cells are produced through mitosis by multipotent stem cells located close to the base of the crypt [47]. Changes in the number of goblet cells can hinder the parasite-infected host’s ability to prevent opportunistic infections from spreading or penetrating the local epithelium [48]. The increase in goblet cells after infection may be due to the active compounds in spinach due to its strong anticoccidial properties and capacity to modify the goblet cell response after infection. This result is similar to that reported by Ramirez et al. [49], who found that neem increased the number of goblet cells in the mouse jejunum after infection with E. papillata. Our results suggest that spinach may be useful as a food supplement for animals with an Eimeria infection.
Spinach extract also contains ursolic aldehyde and betulin which have antiparasitic properties against Trypanosoma cruzi, as indicated by [50,51].
Spinach extract was discovered to have a powerful effect on free radical reduction because it includes palmitic acid and phytol, both of which have a substantial antioxidant effect in trapping free radicals [25,52,53]. Their antioxidative properties are enhanced by suppressing reactive oxidant species (ROS) accumulation while recovering antioxidant enzymes, including superoxide dismutase and catalase [53]. Several studies indicated that omega-3 PUFAs are essential for improving broiler immunity [54,55]. Highly unsaturated omega-3 fatty acids can permeate the parasite’s tissues. After invasion, they are more vulnerable to oxidative assault by phagocytic cells. This oxidative stress has a deleterious impact on coccidian development [56,57]. Oxidative stress, which is hypothesized to be caused by highly unsaturated omega-3 fatty acids, is likely to be deleterious to coccidia growth. According to certain theories, the membranes of coccidia contain unsaturated fatty acids. During development, their membranes undergo continual turnover, and dietary fatty acids influence the composition of the membranes. There, free radical-producing leucocytes will attack the coccidia, making them more vulnerable to oxidation [56,57].
4. Materials and Methods
This experiment was approved by the Beni-suef University Institutional Animal Care and Use Committee (BSU-IACUC), and the approval number is (022-237). Ethanol 70%, formalin, potassium dichromate, and phosphate buffer saline were among the chemicals used in this experiment; they were purchased from PIOCHEM chemical company in 6 October City, Egypt, while Diclazuril (Coxiril ® 0.2%) was obtained from Huvepharma N.V. Uitbreidingstraat 80, 2600 Antwerp, Belgium.
4.1. Botanical Extract Preparation
Spinach (whole plant) was purchased from a local market and identified by specialists in the Botany Department, Faculty of Science, Beni-suef University, Egypt. The ethanol leaf extract of spinach was prepared according to Okechukwui et al. [58] with some modifications. Briefly, the plant was cleansed with distilled water and air dried for one week before being ground into powder in an electrical blender. This powder was soaked in 70% alcohol for three days at room temperature in a ratio of 1:2 w/v (plant powder to 70% alcohol). During this period, the mixture was agitated three times per day. Finally, it was filtered through filter paper, and the filtrate was concentrated and dried in a rotatory evaporator at 40–50 °C. A total of 15 g of greenish viscous extract was produced for every 100 g of spinach powder. The extracted material was kept in the refrigerator at 4 °C until usage, as phytochemicals’ bioavailability is impacted by the matrix and microstructure of the food they are present in, as well as the storage circumstances and temperature range [59]. Cold storage at temperatures below 5 °C is recommended for spinach to maintain its quality and improve its shelf life [60].
Gas Chromatography–Mass Spectrometry (GC-MS) of Spinach Extract
The spinach was analyzed at the Nawah Scientific Educational Research Centre in Egypt “(https://nawah-scientific.com/) accessed on 26 September 2023” using a Trace Ultra gas chromatograph (GC) combined with a Thermo Scientific DSQ II mass spectrometer (MS). The chemicals were separated on a TR-5MS (30 m × 0.25 mm × 0.25 m) capillary column (Thermo Scientific) with helium flowing at a rate of 1 mL/min and a temperature controlled from 60 to 250 °C at 3 °C/min. Temperatures of 220 and 250 °C were chosen for the MS transfer line and injector, respectively. One milligram of spinach extract was diluted in one milliliter of acetone to make the sample. Manual splitless injection of the diluted material was performed in a volume of one liter. Mass spectra were acquired with the ion source temperature set at 240 °C and the MS set to EI mode at 70 eV. The compounds’ relative retention indices and mass spectra were compared with relevant information in the literature and databases [61]. The relative retention index (RRI) was constructed using a series of n-alkanes (C8–C24). The relative percentages of the chemicals were determined through the use of area percentage data.
4.2. Eimeria tenella Oocysts Collection
The coccidian oocysts of Eimeria tenella were collected from naturally infected chicks. The oocysts were concentrated, sporulated in 2.5% potassium dichromate, and then orally inoculated in five healthy chicks to propagate the oocysts [62]. Eight days post-inoculation, the birds were euthanized and the cecal contents were obtained. The oocysts were concentrated, then sporulated as previously mentioned, and stored in a refrigerator (2–5 °C) until use [63].
4.3. In Vitro Evaluation of Spinach Extract against E. tenella Oocysts
In a 96-well ELISA plate, the anticoccidial activity of spinach extract against E. tenella oocysts in vitro was evaluated. The extract was dissolved in potassium dichromate 2.5% (w/v) to prepare five concentrations of the extract: 10%, 5%, 2.5%, 1.25%, and 0.625. Each concentration was then placed in a well and 1200 oocysts were added. Then, 2.5% potassium dichromate was used as a negative control and 10% formalin served as the positive control. Each concentration was used in three replicates. The plate was incubated at 30 °C and after 24, 48, and 72 h, 25 µL from each well was placed on a glass slide and inspected under a microscope to determine the percentages of sporulated oocysts [64]. The sporulation inhibition effect of the extract was then estimated using the formula: (% of inhibition = sporulation% of negative control − sporulation% of spinach extract). The concentration of spinach extract that had the greatest effect was utilized again to examine its effect in vivo.
4.4. Experimental Design of In Vivo Evaluation of Spinach Extract against Eimeria tenella Oocysts on Broiler Chickens
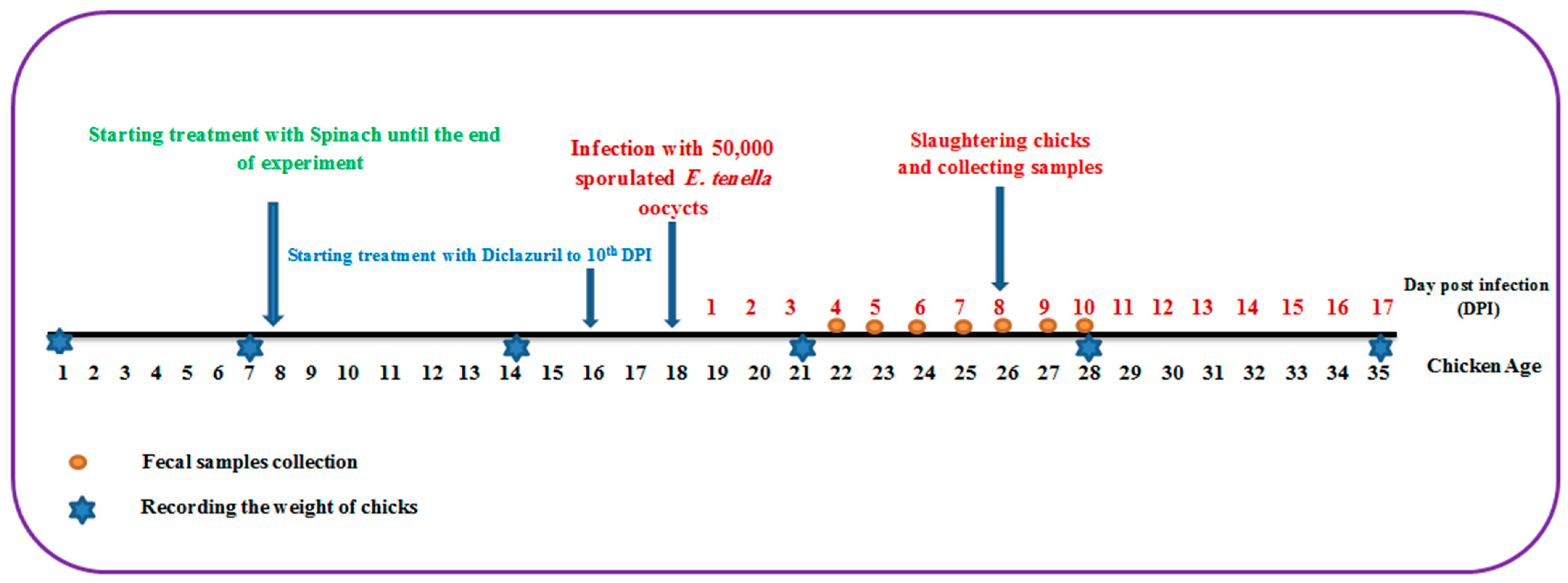
One hundred one-day-old broiler chicks of both sexes were purchased from El-Abd local company. These chicks were reared in wire-mesh batteries with free access to water and food, and the temperature was set at 34 °C, then decreased by 2 °C per week. At the age of 8 days, these chicks were divided into four groups of 25 chickens each as follows: The first group comprised uninfected chicks and served as the negative control group. The second group was infected orally with 5 × 104 E. tenella sporulated oocysts at the age of 18 days and acted as the infected control group. The third group was the spinach-treated group, which began receiving spinach extract at a dose of 50 mg/kg supplemented with water at the age of 8 days as a prophylactic treatment and continued until the end of the experiment while being orally infected with 5 × 104 sporulated oocysts of E. tenella at the age of 18 days and continuing the spinach treatment. The fourth group was the drug-treated group, which received 0.2 mg/kg diclazuril (Coxiril ® 0.2%) in food two days before being orally infected with 5 × 104 E. tenella sporulated oocysts till day 10 post-infection (PI). At day 8 PI, five chickens from each group were sacrificed and ceca were retrieved for further studies (Figure 7).
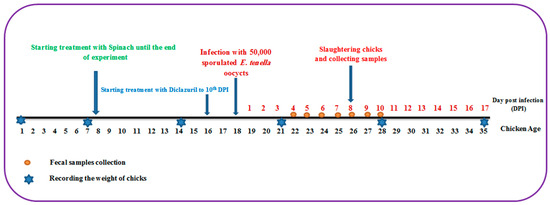
Figure 7.
The experimental design depicts the entire course of the experiment, from the first day to the end.
4.5. Evaluation of Spinach Extract Anticoccidial Activity
4.5.1. Clinical Examination, Clinical Symptoms, Mortality, and Bloody Diarrhea
The chicks were examined daily for clinical signs, such as anorexia, ruffled feathers, diarrhea, crowding, loose droppings, difficulty breathing, and bloody droppings. The number of deaths was also recorded on a daily basis. From day 4 to day 10 PI, fecal samples were collected from each group and grossly inspected for blood.
4.5.2. Lesion Scoring and Cecal Core
On day 8 PI, five birds were randomly selected from each group and inspected for lesion scoring. The 0–4 lesion-scoring categorization was used to classify the lesions, which included petechial hemorrhages, bloody fecal contents, cecal wall thickening, and mucoid discharge. Based on the severity of the lesions, no lesions (0), mild lesions (1), moderate lesions (2), severe lesions (3), or very severe lesions (4) were determined for each bird [65].
4.5.3. Parasitological Examination (Oocyst Count per Gram of Feces)
From day 4 PI to day 10 PI, randomly selected fecal samples from each group were collected and prepared for examination according to the methods indicated by Hodgson [66] and Long and Rowell [67]. In brief, 10 g of feces were dissolved in 100 mL of water and left at room temperature in well-covered cups for 24 h. After carefully mixing and sieving the solution, 15 mL of it was centrifuged for 5 min. After removing the supernatant, the pellet was resuspended in a few milliliters of saturated salt solution (NaCl) using a vortex or by tapping the tube. More salt solution was added to the initial 15 mL volume, and the tube was repeatedly turned upside down. Once a sample was added to the McMaster counting chamber by a Pasteur pipette, oocysts floated to the top of the solution, the number of oocysts was counted, and the total number of oocysts per gram was estimated using the equation below:
where “n”—number of counted oocysts, “0.1”—correction for 10 g originally taken from the litter, “0.15”—volume of the McMaster counting chamber, and “Volume”—100 mL of water in which the litter was soaked.
4.5.4. Oocyst Index
The ceca of the five chicks previously selected on the day 8 PI were dissected and their contents were collected and prepared for counting their oocyst content using the previously mentioned McMaster chamber method [66,67,68].
4.6. Growth Performance
The chicks were weighed once a week from the start of the experiment to the end. The weekly increase in body weight was calculated by subtracting the starting weight from the total weight. From day 1 to day 20, the birds were fed a coccidiostat-free commercial starter food. When the birds were 21 days old, the starting diet was replaced with a finisher diet, which was fed till the end of the experiment. During the pre-infection and post-infection periods, daily feed and water intake were recorded. The total feed intake for each week was calculated by deducting the amount of feed rejected from the feed supplied. Finally, the feed conversion ratio (FCR) was determined for each week using this formula: .
4.7. Histological Studies
Parts of the ceca from the previously selected five chicks on day 8 PI were separated and fixed in 10% phosphate-buffered formalin. Then, these samples were processed for normal histology, as described by Tanweer et al. [65]. Sections were stained with hematoxylin and eosin (H&E) to estimate the number of parasitic stages while other sections were stained with Alcian blue to assess the number of goblet cells. The number of goblet cells in each animal’s cecum was counted in well-oriented villous crypt units (VCUs). The results were expressed as the average number of goblet cells per 10 VCUs [69]. In addition, H&E sections were examined for inflammation, necrosis, degeneration, and other pathological alterations.
4.8. Biochemical Studies (Oxidative Markers) on the Ceca of Infected Birds
Parts of the selected ceca were washed in 10% phosphate-buffered solution before being homogenized with 0.9% saline at a 1:10 g/mL ratio and centrifuged at 2000 rpm for 10 min. The resultant supernatant was separated, divided into aliquots, and stored at −80 °C in eppendorf tubes to determine some oxidative stress markers. The level of lipid peroxidation was determined by measuring the amount of malondialdehyde (MDA) produced by the breakdown of polysaturated fatty acids. The interaction of lipid peroxidation products with thiobarbituric acid quantifies the products of lipid peroxidation [70]. Superoxide-Dismutase (SOD) activity was determined using pyrogallol autoxidation inhibition as described by Marklund and Marklund [71]. Peroxidase activity (GPX) was measured spectrophotometrically according to the procedure suggested by Manoranjan and Mishra [72], catalase (CAT) activity was estimated in the tissue extract according to the method described by Cohen et al. [73], and the nitric oxide (NO) concentration was determined using the method outlined by James and Glaven [74].
4.9. Statistical Analysis
Data was statistically analyzed using SPSS version 17.0 and graph pad prism 8 software. One-way ANOVA, followed by least significant difference (LSD) was used for the comparison between the test and control group and data are expressed as the mean ± standard deviation (SD); p-values <0.05 (p < 0.05) were considered statistically significant.
5. Conclusions
Spinach has an anticoccidial impact because it inhibits the sporulation percentage of Eimeria tenella oocysts, increases body weight, decreases the count of oocysts and parasitic stages in the cecum, restores the number of goblet cells to that of an uninfected control, and has a substantial antioxidant effect. In the present work, we used 5% spinach extract in vivo because it had a high sporulation inhibition effect relative to 10% spinach extract in vitro, but we recommend using higher concentrations to study its effect on coccidiosis models and other species that infect chickens and other animals.
Author Contributions
Conceptualization: S.M.A. and A.-A.S.A.-B.; data curation: S.A.-Q. and A.-A.S.A.-B.; formal analysis: H.E.-F. and H.A.-T.; funding acquisition: A.-A.S.A.-B. and S.A.-Q.; investigation: O.E. and H.A.-T.; methodology: O.E., H.A.-T. and P.F.; supervision: S.M.A. and A.-A.S.A.-B.; validation: P.F., S.A.-Q. and H.E.-F.; visualization: O.E.; roles/writing—original draft: O.E. and H.A.-T.; writing—review and editing: A.-A.S.A.-B. and S.M.A. All authors read and agreed to the published version of the manuscript.
Funding
This work was supported by a Researcher-Supporting Project (RSP-2023/3), King Saud University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Beni-suef University Institutional Animal Care and Use Committee (BSU-IACUC), and the approval number is (022-237).
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data to this work are available in this manuscript.
Acknowledgments
The authors appereciated the assistant of zoology department members in helping for supplling some cheemicals.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships.
Sample Availability
Not applicable.
References
- Allen, P.C.; Fetterer, R. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin. Microbiol. Rev. 2002, 15, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Akbar, H.; Bakhsh, A.; Rashid, M.I.; Hassan, M.A.; Ullah, R.; Hussain, T.; Manzoor, S.; Yin, H. Assessing the prevalence and economic significance of coccidiosis individually and in combination with concurrent infections in Pakistani commercial poultry farms. Poult. Sci. 2019, 98, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.Z.; Iqbal, Z.; Akhtar, M.S.; Khan, M.N.; Jabbar, A.; Sandhu, Z. Anticoccidial screening of Azadirachta indica (Neem) in broilers. Pharmacologyonline 2006, 3, 365–371. [Google Scholar]
- Patricia, O.; Zoue, L.; Megnanou, R.-M.; Doue, R.; Niamke, S. Proximate composition and nutritive value of leafy vegetables consumed in Northern Cote d’Ivoire. Eur. Sci. J. 2014, 10. [Google Scholar]
- Bergquist, S. Bioactive Compounds in Baby Spinach (Spinacia oleracea L.); Department of Crop Science, Swedish University of Agricultural Sciences: Alnarp, Sweden, 2006; Volume 2006. [Google Scholar]
- Olagoke, O. Phytochemical analysis and antibacterial activities of spinach leaf. Am. J. Phytomed. Clin. Ther. Vol. 2018, 6, 8. [Google Scholar]
- Han, M.; Hu, W.; Chen, T.; Guo, H.; Zhu, J.; Chen, F. Anticoccidial activity of natural plants extracts mixture against Eimeria tenella: An in vitro and in vivo study. Front. Vet. Sci. 2022, 9, 1066543. [Google Scholar] [CrossRef]
- Witcombe, D.M.; Smith, N.C. Strategies for anti-coccidial prophylaxis. Parasitology 2014, 141, 1379–1389. [Google Scholar] [CrossRef]
- Khater, H.F.; Ziam, H.; Abbas, A.; Abbas, R.Z.; Raza, M.A.; Hussain, K.; Younis, E.; Radwan, I.; Selim, A. Avian coccidiosis: Recent advances in alternative control strategies and vaccine development. Agrobiol. Rec. 2020, 1, 11–25. [Google Scholar] [CrossRef]
- Alzahrani, F.; Al-Shaebi, E.M.; Dkhil, M.A.; Al-Quraishy, S. In Vivo anti-eimeria and in vitro anthelmintic activity of Ziziphus spina-christi leaf extracts. Pakistan Journal of Zoology 2016, 48. [Google Scholar]
- Metwaly, M.S.; Dkhil, M.A.; Gewik, M.M.; Al-Ghamdy, A.O.; Al-Quraishy, S. Induced metabolic disturbance and growth depression in rabbits infected with Eimeria coecicola. Parasitol. Res. 2013, 112, 3109–3114. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: An update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Torequl Islam, M.; Quispe, C.; Herrera-Bravo, J.; Rahaman, M.M.; Hossain, R.; Sarkar, C.; Raihan, M.A.; Chowdhury, M.M.; Uddin, S.J.; Shilpi, J.A. Activities and molecular mechanisms of diterpenes, diterpenoids, and their derivatives in rheumatoid arthritis. Evid.-Based Complement. Altern. Med. 2022, 2022, 4787643. [Google Scholar] [CrossRef] [PubMed]
- Arru, L.; Mussi, F.; Forti, L.; Buschini, A. Biological effect of different spinach extracts in comparison with the individual components of the phytocomplex. Foods 2021, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, S.M.; Hetta, M.H.; Ibrahim, M.A.; Balachandran, P.; Zhang, J.; Wang, M.; Fawzy, G.A.; El-Askary, H.I.; Ross, S.A. Phytochemical Investigation of Egyptian Spinach Leaves, a Potential Source for Antileukemic Metabolites: In Vitro and In Silico Study. Rev. Bras. Farmacogn. 2022, 32, 774–785. [Google Scholar] [CrossRef]
- Hetta, M.H.; Moawad, A.S.; Hamed, M.A.-A.; Sabri, A.I. In-Vitro and in-vivo hypolipidemic activity of spinach roots and flowers. Iran. J. Pharm. Res. IJPR 2017, 16, 1509. [Google Scholar]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol. J. Healthc. Nutr. Technol. 2006, 1, 420–439. [Google Scholar] [CrossRef]
- Dilika, F.; Bremner, P.; Meyer, J. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia 2000, 71, 450–452. [Google Scholar] [CrossRef]
- McGaw, L.; Jäger, A.; Van Staden, J. Isolation of antibacterial fatty acids from Schotia brachypetala. Fitoterapia 2002, 73, 431–433. [Google Scholar] [CrossRef]
- Krugliak, M.; Deharo, E.; Shalmiev, G.; Sauvain, M.; Moretti, C.; Ginsburg, H. Antimalarial effects of C18 fatty acids on Plasmodium falciparum in culture and on Plasmodium vinckei petteri and Plasmodium yoelii nigeriensis in vivo. Exp. Parasitol. 1995, 81, 97–105. [Google Scholar] [CrossRef]
- Peet, M.; Stokes, C. Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs 2005, 65, 1051–1059. [Google Scholar] [CrossRef]
- Schram, L.B.; Nielsen, C.J.; Porsgaard, T.; Nielsen, N.S.; Holm, R.; Mu, H. Food matrices affect the bioavailability of (n − 3) polyunsaturated fatty acids in a single meal study in humans. Food Res. Int. 2007, 40, 1062–1068. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Novak, T.E.; Babcock, T.A.; Jho, D.H.; Helton, W.S.; Espat, N.J. NF-κB inhibition by ω-3 fatty acids modulates LPS-stimulated macrophage TNF-α transcription. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2003, 284, L84–L89. [Google Scholar] [CrossRef]
- ALLEN, P.C.; DANFORTH, H.D.; LEVANDER, O.A. Diets high in n-3 fatty acids reduce cecal lesion scores in chickens infected with Eimeria tenella. Poult. Sci. 1996, 75, 179–185. [Google Scholar] [CrossRef]
- Choi, J.-W.; Lee, J.; Lee, J.-H.; Park, B.-J.; Lee, E.J.; Shin, S.; Cha, G.-H.; Lee, Y.-H.; Lim, K.; Yuk, J.-M. Omega-3 polyunsaturated fatty acids prevent Toxoplasma gondii infection by inducing autophagy via AMPK activation. Nutrients 2019, 11, 2137. [Google Scholar] [CrossRef]
- Stock, C.C.; Francis, T., Jr. The inactivation of the virus of epidemic influenza by soaps. J. Exp. Med. 1940, 71, 661. [Google Scholar] [CrossRef]
- Araújo, C.V.; Campbell, C.; Gonçalves-de-Albuquerque, C.F.; Molinaro, R.; Cody, M.J.; Yost, C.C.; Bozza, P.T.; Zimmerman, G.A.; Weyrich, A.S.; Castro-Faria-Neto, H.C. A PPARγ agonist enhances bacterial clearance through neutrophil extracellular trap formation and improves survival in sepsis. Shock 2016, 45, 393. [Google Scholar] [CrossRef]
- Molan, A.L.; Liu, Z.; De, S. Effect of pine bark (Pious radiata) extracts on sporulation of coccidian oocysts. Folia Parasitol. 2009, 56, 1. [Google Scholar] [CrossRef]
- Fatemi, A.; Razavi, S.M.; Asasi, K.; Torabi Goudarzi, M. Effects of Artemisia annua extracts on sporulation of Eimeria oocysts. Parasitol. Res. 2015, 114, 1207–1211. [Google Scholar] [CrossRef]
- Aldred, E.M. Pharmacology: A Handbook for Complementary Healthcare Professionals; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Christaki, E.; Florou-Paneri, P.; Giannenas, I.; Papazahariadou, M.; Botsoglou, N.A.; Spais, A.B. Effect of a mixture of herbal extracts on broiler chickens infected with Eimeria tenella. Anim. Res. 2004, 53, 137–144. [Google Scholar] [CrossRef]
- Giannenas, I.; Florou-Paneri, P.; Papazahariadou, M.; Christaki, E.; Botsoglou, N.; Spais, A. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch. Anim. Nutr. 2003, 57, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Kets, E.; Smid, E. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.; Mathis, G.; Lang, M. The use of diclazuril in extended withdrawal anticoccidial programs: 1. Efficacy against Eimeria spp. in broiler chickens in floor pens. Poult. Sci. 2002, 81, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R.; Fleta-Soriano, E.; Buezo, J.; Míguez, F.; Becerril, J.M.; García-Plazaola, J.I. Enhancement of zeaxanthin in two-steps by environmental stress induction in rocket and spinach. Food Res. Int. 2014, 65, 207–214. [Google Scholar] [CrossRef]
- de Moraes, J.; de Oliveira, R.N.; Costa, J.P.; Junior, A.L.; de Sousa, D.P.; Freitas, R.M.; Allegretti, S.M.; Pinto, P.L. Phytol, a diterpene alcohol from chlorophyll, as a drug against neglected tropical disease Schistosomiasis mansoni. PLoS Negl. Trop. Dis. 2014, 8, e2617. [Google Scholar] [CrossRef]
- Baldwin, F.M.; Wiswell, O.B.; Jankiewicz, H.A. Hemorrhage control in Eimeria tenella infected chicks when protected by anti-hemorrhagic factor, vitamin K. Proc. Soc. Exp. Biol. Med. 1941, 48, 278–280. [Google Scholar] [CrossRef]
- Ryley, J.F.; Hardman, L. The use of vitamin K deficient diets in the screening and evaluation of anticoccidial drugs. Parasitology 1978, 76, 11–20. [Google Scholar] [CrossRef]
- Wagde, M.S.; Sharma, S.K.; Sharma, B.K.; Shivani, A.P.; Keer, N.R. Effect of natural β-carotene from-carrot (Daucus carota) and Spinach (Spinacia oleracea) on colouration of an ornamental fish-swordtail (Xiphophorus hellerii). J. Entomol. Zool. Stud. 2018, 6, 699–705. [Google Scholar]
- Abbas, R.Z.; Iqbal, Z.; Khan, M.N.; Zafar, M.A.; Zia, M.A. Anticoccidial activity of Curcuma longa L. in broilers. Braz. Arch. Biol. Technol. 2010, 53, 63–67. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Iqbal, Z.; Khan, M.N.; Hashmi, N.; Hussain, A. Prophylactic efficacy of diclazuril in broilers experimentally infected with three field isolates of Eimeria tenella. Int. J. Agric. Biol. 2009, 11, 606–610. [Google Scholar]
- Rayan, P.; Stenzel, D.; McDonnell, P.A. The effects of saturated fatty acids on Giardia duodenalis trophozoites in vitro. Parasitol. Res. 2005, 97, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Deplancke, B.; Gaskins, H.R. Microbial modulation of innate defense: Goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001, 73, 1131S–1141S. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Leslie, J.L.; Petri, W.A. Host protective mechanisms to intestinal amebiasis. Trends Parasitol. 2021, 37, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine II. Mucous cells. Am. J. Anat. 1974, 141, 481–501. [Google Scholar] [CrossRef]
- Yunus, M.; Horii, Y.; Makimura, S.; Smith, A.L. Murine goblet cell hypoplasia during Eimeria pragensis infection is ameliorated by clindamycin treatment. J. Vet. Med. Sci. 2005, 67, 311–315. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Al-Quraishy, S.; Abdel Moneim, A.E.; Delic, D. Protective effect of Azadirachta indica extract against Eimeria papillata-induced coccidiosis. Parasitol. Res. 2013, 112, 101–106. [Google Scholar] [CrossRef]
- Leite, J.P.V.; Oliveira, A.B.; Lombardi, J.A.; S Filho, J.D.; Chiari, E. Trypanocidal activity of triterpenes from Arrabidaea triplinervia and derivatives. Biol. Pharm. Bull. 2006, 29, 2307–2309. [Google Scholar] [CrossRef]
- Meira, C.S.; Barbosa-Filho, J.M.; Lanfredi-Rangel, A.; Guimaraes, E.T.; Moreira, D.R.M.; Soares, M.B.P. Antiparasitic evaluation of betulinic acid derivatives reveals effective and selective anti-Trypanosoma cruzi inhibitors. Exp. Parasitol. 2016, 166, 108–115. [Google Scholar] [CrossRef]
- Alnahdi, A.; John, A.; Raza, H. Augmentation of glucotoxicity, oxidative stress, apoptosis and mitochondrial dysfunction in HepG2 cells by palmitic acid. Nutrients 2019, 11, 1979. [Google Scholar] [CrossRef]
- Kim, E.-N.; Trang, N.M.; Kang, H.; Kim, K.H.; Jeong, G.-S. Phytol Suppresses Osteoclast Differentiation and Oxidative Stress through Nrf2/HO-1 Regulation in RANKL-Induced RAW264. 7 Cells. Cells 2022, 11, 3596. [Google Scholar] [CrossRef] [PubMed]
- Jameel, Y.J.; Sahib, A.M. Effect of In Ovo Injection with Newcastle Disease Vaccine, Multivitamins AD3E, and Omega-3 on Carcass Characteristics of Broilers. Mirror Res. Vet. Sci. Anim. 2014, 3, 23–30. [Google Scholar]
- Jameel, Y.J. Effect of the content of fish oil, L-carnitine and their combination in diet on immune response and some blood parameters of broilers. Int. J. Sci. Nat. 2014, 5, 501–504. [Google Scholar]
- Spurney, R.F.; Coffman, T.M.; Ruiz, P.; Albrightson, C.R.; Pisetsky, D.S. Fish oil feeding modulates leukotriene production in murine lupus nephritis. Prostaglandins 1994, 48, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.C. Production of free radical species during Eimeria maxima infections in chickens. Poult. Sci. 1997, 76, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Okechukwu, P.U.; Okwesili, F.N.; Parker, E.J.; Abubakar, B.; Emmanuel, C.O.; Christian, E.O. Phytochemical and acute toxicity studies of Moringa oleifera ethanol leaf extract. Int. J. Life Sci. BiotechNol. Pharma Res. 2013, 2, 66–71. [Google Scholar]
- Bunea, A.; Andjelkovic, M.; Socaciu, C.; Bobis, O.; Neacsu, M.; Verhé, R.; Van Camp, J. Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea L.). Food Chem. 2008, 108, 649–656. [Google Scholar] [CrossRef]
- Rodríguez-Hidalgo, S.; Artés-Hernández, F.; Gómez, P.A.; Fernández, J.A.; Artés, F. Quality of fresh-cut baby spinach grown under a floating trays system as affected by nitrogen fertilisation and innovative packaging treatments. J. Sci. Food Agric. 2010, 90, 1089–1097. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- Thienpont, D.; Rochette, F.; Vanparijs, O. Diagnosing helminthiasis through coprological examination. In Diagnosing Helminthiasis through Coprological Examination; Janssen Research Foundation: Beerse, Belgium, 1979. [Google Scholar]
- Davies, S.F.M.; Joyner, L.P.; Kendall, S.B. Coccidiosis; Oliver & Boyd: London, UK, 1963. [Google Scholar]
- Habibi, H.; Firouzi, S.; Nili, H.; Razavi, M.; Asadi, S.; Daneshi, S. Anticoccidial effects of herbal extracts on Eimeria tenella infection in broiler chickens. J. Parasit. Dis. 2016, 40, 401–407. [Google Scholar] [CrossRef]
- Tanweer, A.J.; Saddique, U.; Bailey, C.; Khan, R. Antiparasitic effect of wild rue (Peganum harmala L.) against experimentally induced coccidiosis in broiler chicks. Parasitol. Res. 2014, 113, 2951–2960. [Google Scholar] [CrossRef]
- Hodgson, J. Coccidiosis: Oocyst counting technique for coccidiostat evaluation. Exp. Parasitol. 1970, 28, 99–102. [Google Scholar] [CrossRef]
- Long, P.; Rowell, J. Counting oocysts of chicken coccidia. Lab. Pract. 1958, 7, 534. [Google Scholar]
- Long, P.; Millard, B.; Joyner, L.; Norton, C. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet. Lat. 1976, 6, 201–217. [Google Scholar] [PubMed]
- Allen, A. The role of mucus in the protection of the gastroduodenal mucosa. Scand. J. Gastroenterol. Suppl. 1987, 128, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Jarrell, S.T.; Scheckenbach, R.; Lieberman, S.; Anderson, R.A. Comparative effects of chromium, vanadium and Gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J. Am. Coll. Nutr. 1998, 17, 116–123. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Manoranjan, K.; Mishra, D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar]
- Cohen, G.; Dembree, D.; Marcus, J. An assay to analyse the catalase level in tissues. Anal. Biochem. 1970, 34, 30–38. [Google Scholar] [CrossRef]
- James, S.; Glaven, J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J. Immunol. 1989, 143, 4208–4212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).