Hexamethyldisiloxane Removal from Biogas Using a Fe3O4–Urea-Modified Three-Dimensional Graphene Aerogel
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Modifier Fe(NO3)3·9H2O on Texture Properties
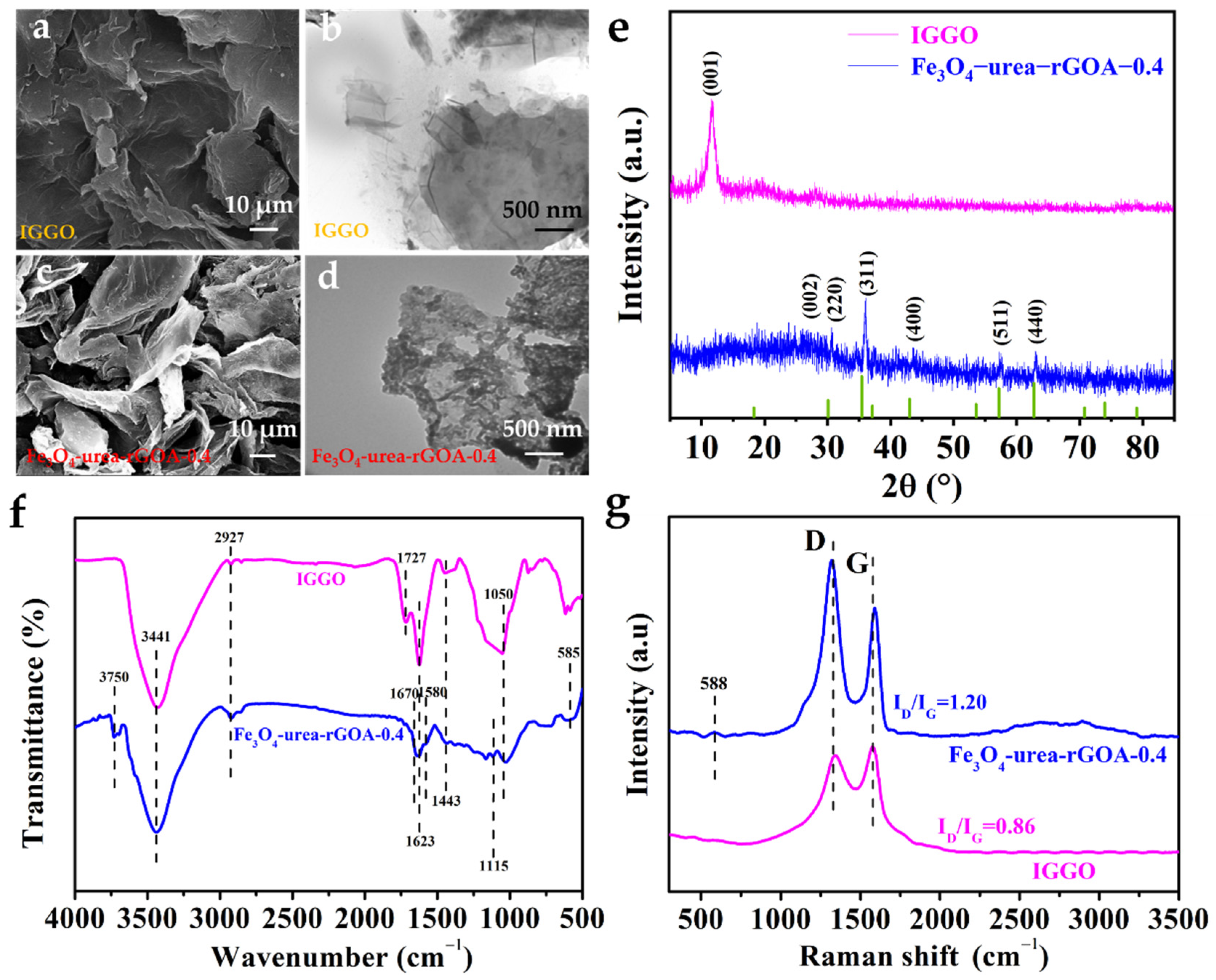
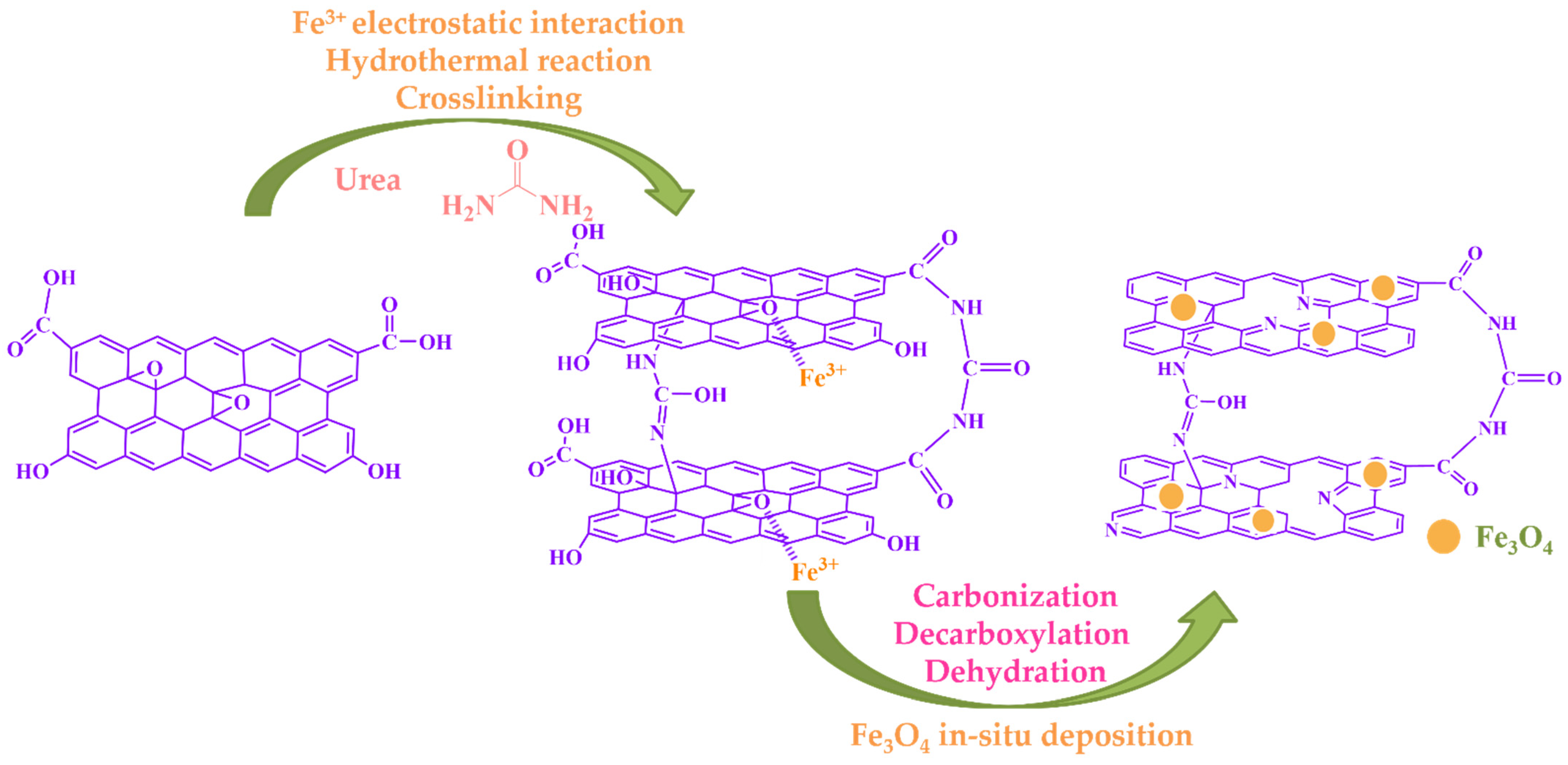
2.2. Characterization of Adsorbents
2.3. Comparison of Dynamic Adsorption Performances
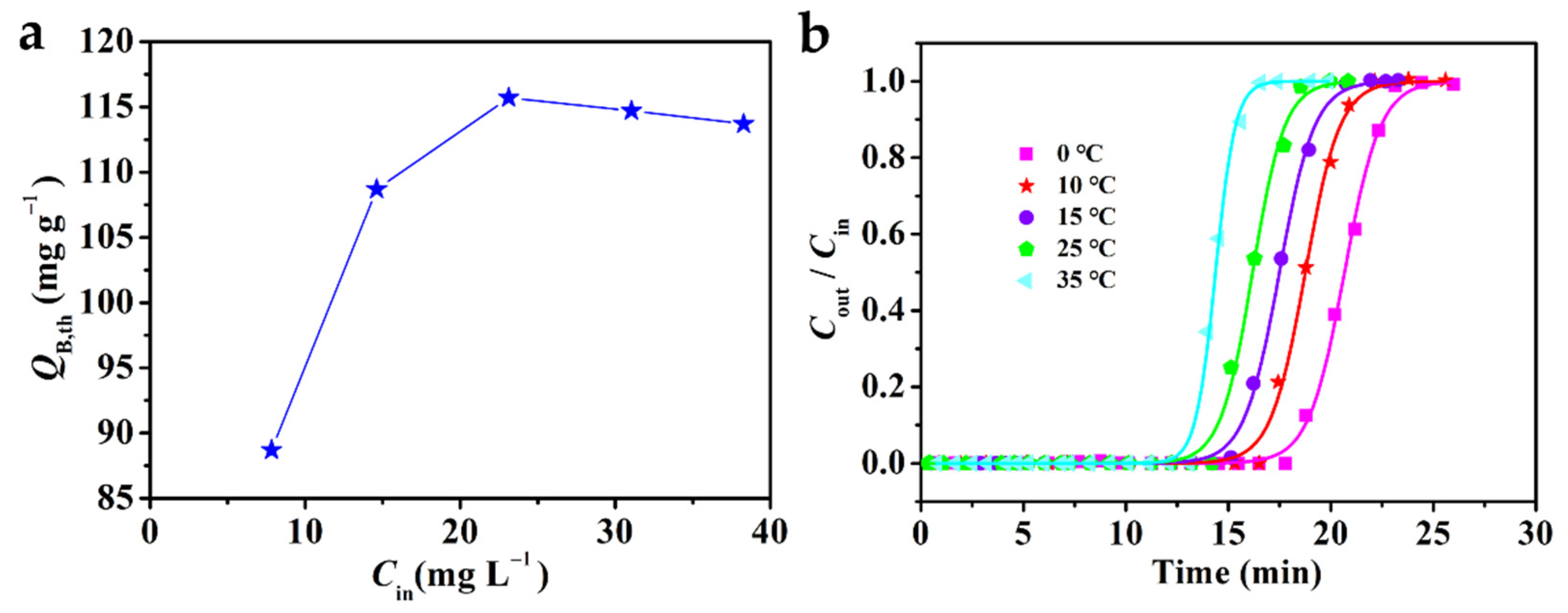
2.4. Effect of Process Conditions on Adsorption
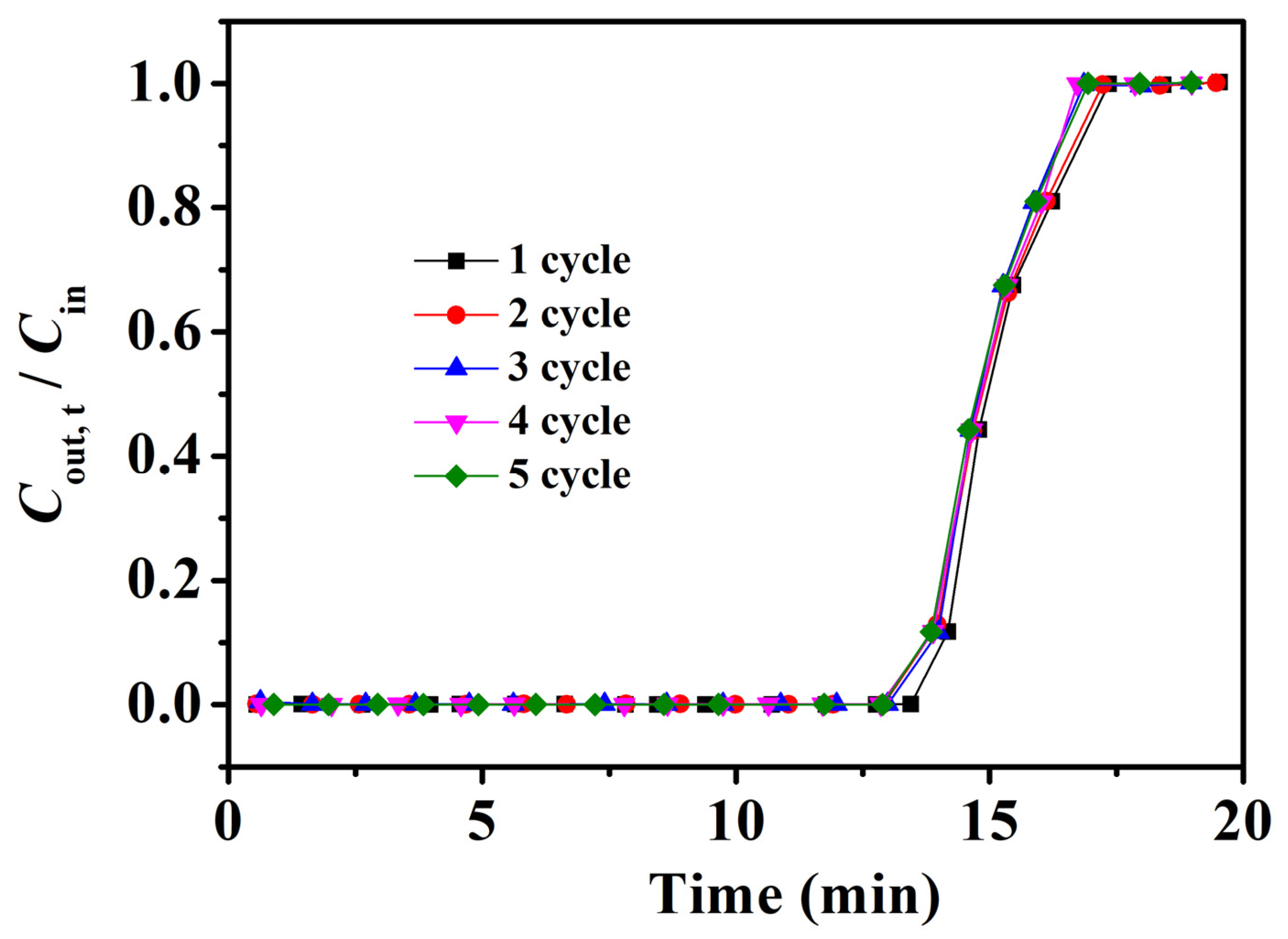
2.5. Recycling Performance of Fe3O4–Urea–rGOA–0.4
3. Materials and Methods
3.1. Materials and Chemicals
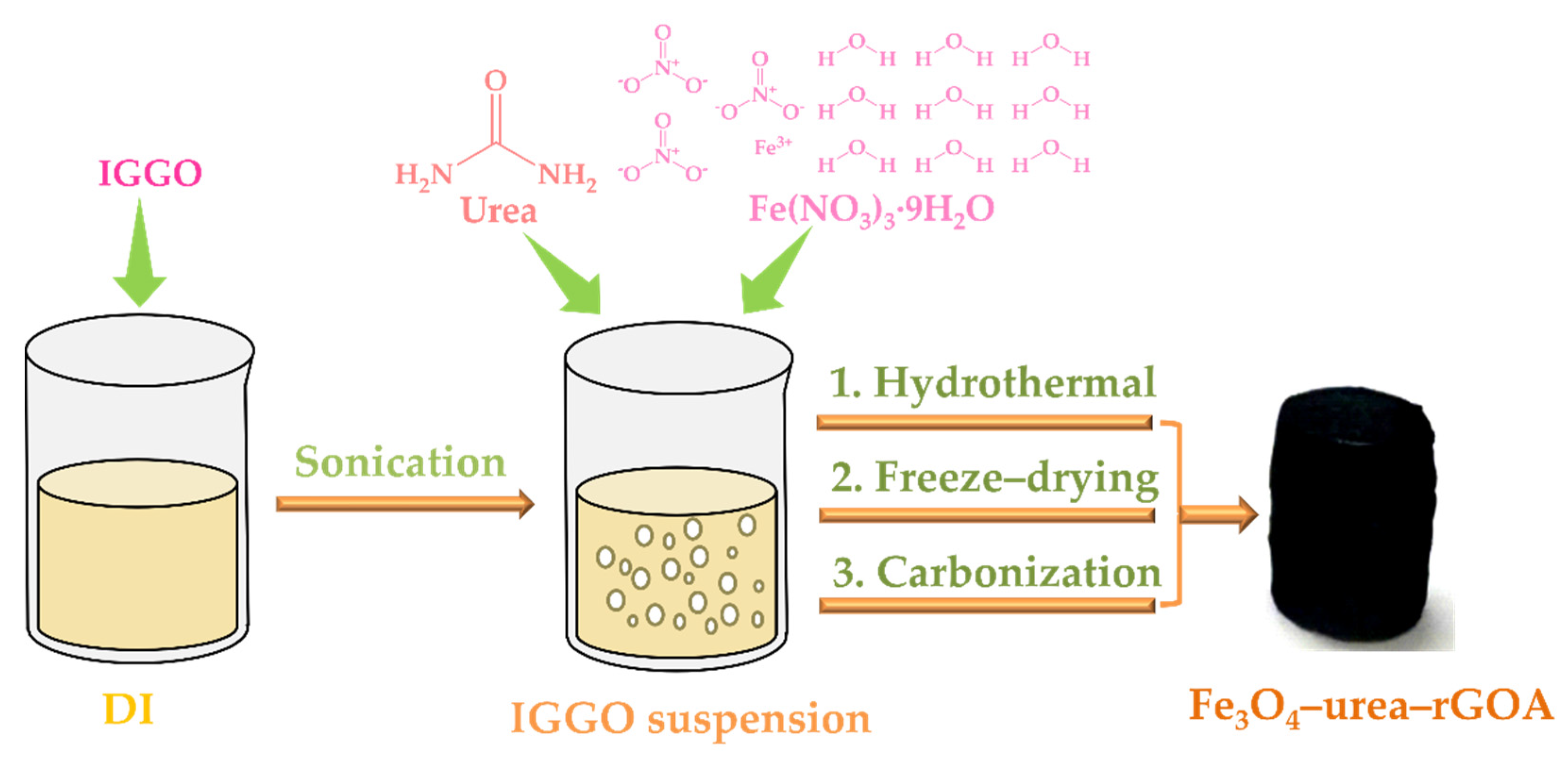
3.2. Preparation of Fe3O4–Urea–rGOAs
3.3. Adsorption Experiments of L2 Gas
3.4. Model for the Breakthrough Curves
3.5. Regeneration of the Spent Fe3O4–Urea–rGOAs
3.6. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- De Arespacochaga, N.; Valderrama, C.; Raich–Montiu, J.; Crest, M.; Mehta, S.; Cortina, J.L. Understanding the effects of the origin, occurrence, monitoring, control, fate and removal of siloxanes on the energetic valorization of sewage biogas—A review. Renew. Sust. Energy Rev. 2015, 52, 366–381. [Google Scholar]
- Rucker, C.; Kummerer, K. Environmental chemistry of organosiloxanes. Chem. Rev. 2015, 115, 466–524. [Google Scholar] [PubMed]
- Chuah, C.Y.; Goh, K.; Yang, Y.; Gong, H.; Li, W.; Karahan, H.E.; Guiver, M.D.; Wang, R.; Bae, T.H. Harnessing filler materials for enhancing biogas separation membranes. Chem. Rev. 2018, 118, 8655–8769. [Google Scholar] [PubMed]
- Gao, R.; Cheng, S.; Li, Z. Research progress of siloxane removal from biogas. Int. J. Agric. Biol. Eng. 2017, 10, 30–39. [Google Scholar]
- Pascual, C.; Cantera, S.; Lebrero, R. Volatile siloxanes emissions: Impact and sustainable abatement perspectives. Trends Biotechnol. 2021, 39, 1245–1248. [Google Scholar]
- Rivera-Montenegro, L.; Valenzuela, E.I.; González-Sánchez, A.; Muñoz, R.; Quijano, G. Volatile Methyl siloxanes as key biogas pollutants: Occurrence, impacts and treatment technologies. Bioenergy Res. 2022, 16, 801–816. [Google Scholar]
- Shen, M.; Zhang, Y.; Hu, D.; Fan, J.; Zeng, G. A review on removal of siloxanes from biogas: With a special focus on volatile methylsiloxanes. Environ. Sci. Pollut. Res. 2018, 25, 30847–30862. [Google Scholar]
- Alves, C.M.A.C.; Abreu, F.O.M.S.; Araújo, R.S.; Oliveira, M.L.M. Recent advances in siloxanes removal from biogas and their efficiency: A short review. Chem. Pap. 2022, 77, 1–9. [Google Scholar]
- Piechota, G. Removal of siloxanes from biogas upgraded to biomethane by Cryogenic Temperature Condensation System. J. Clean. Prod. 2021, 308, 127404. [Google Scholar]
- Papat, S.C.; Deshusses, M.A. Biological removal of siloxanes from landfill and digester gases: Opportunities and challenges. Environ. Sci. Technol. 2008, 42, 8510–8515. [Google Scholar]
- Ajhar, M.; Bannwarth, S.; Stollenwerk, K.-H.; Spalding, G.; Yüce, S.; Wessling, M.; Melin, T. Siloxane removal using silicone–rubber membranes. Sep. Purif. Technol. 2012, 89, 234–244. [Google Scholar] [CrossRef]
- Sigot, L.; Ducom, G.; Benadda, B.; Labouré, C. Adsorption of octamethylcyclotetrasiloxane on silica gel for biogas purification. Fuel 2014, 135, 205–209. [Google Scholar] [CrossRef]
- Yang, L.; Corsolini, S.I. Online removal of volatile siloxanes in solid-state anaerobic digester biogas using a biofilter and an activated carbon filter. J. Environ. Chem. Eng. 2019, 7, 103284. [Google Scholar] [CrossRef]
- Hou, X.; Zheng, Y.; Ma, X.; Liu, Y.; Ma, Z. The effects of hydrophobicity and textural properties on hexamethyldisiloxane adsorption in reduced graphene oxide aerogels. Molecules 2021, 26, 1130. [Google Scholar] [CrossRef] [PubMed]
- Elwell, A.C.; Elsayed, N.H.; Kuhn, J.N.; Joseph, B. Design and analysis of siloxanes removal by adsorption from landfill gas for waste–to–energy processes. Waste Manag. 2018, 73, 189–196. [Google Scholar] [CrossRef]
- Yu, M.; Gong, H.; Chen, Z.; Zhang, M. Adsorption characteristics of activated carbon for siloxanes. J. Environ. Chem. Eng. 2013, 1, 1182–1187. [Google Scholar] [CrossRef]
- Wang, G.; Li, N.; Xing, X.; Sun, Y.; Zhang, Z.; Hao, Z. Gaseous adsorption of hexamethyldisiloxane on carbons: Isotherms, isosteric heats and kinetics. Chemosphere 2020, 247, 125862. [Google Scholar] [CrossRef]
- Molino, G.; Gandiglio, M.; Fiorilli, S.; Lanzini, A.; Drago, D.; Papurello, D. Design and performance of an adsorption bed with activated carbons for biogas purification. Molecules 2022, 27, 7882. [Google Scholar] [CrossRef]
- Sigot, L.; Ducom, G.; Germain, P. Adsorption of octamethylcyclotetrasiloxane (D4) on silica gel (SG): Retention mechanism. Micropor. Mesopor. Mater. 2015, 213, 118–124. [Google Scholar] [CrossRef]
- Jiang, T.; Zhong, W.; Jafari, T.; Du, S.; He, J.; Fu, Y.-J.; Singh, P.; Suib, S.L. Siloxane D4 adsorption by mesoporous aluminosilicates. Chem. Eng. J. 2016, 289, 356–364. [Google Scholar] [CrossRef]
- Zhong, W.; Jiang, T.; Jafari, T.; Poyraz, A.S.; Wu, W.; Kriz, D.A.; Du, S.; Biswas, S.; Thompson Pettes, M.; Suib, S.L. Modified inverse micelle synthesis for mesoporous alumina with a high D4 siloxane adsorption capacity. Micropor. Mesopor. Mater. 2017, 239, 328–335. [Google Scholar] [CrossRef]
- Jung, H.; Lee, D.-Y.; Jurng, J. Low-temperature regeneration of novel polymeric adsorbent on decamethylcyclopentasiloxane (D5) removal for cost–effective purification of biogases from siloxane. Renew. Energy 2017, 111, 718–723. [Google Scholar] [CrossRef]
- Cabrera-Codony, A.; Santos-Clotas, E.; Ania, C.O.; Martín, M.J. Competitive siloxane adsorption in multicomponent gas streams for biogas upgrading. Chem. Eng. J. 2018, 344, 565–573. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, X.; Liu, Y.; Ma, Z. Hexamethyldisiloxane removal from biogas using reduced graphene-oxide aerogels as adsorbents. Renew. Energy 2021, 178, 153–161. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, X.; Ma, X.; Hao, Z.; Ma, Z. Vitamin C-assisted fabrication of aerogels from industrial graphene oxide for gaseous hexamethyldisiloxane adsorption. Appl. Sci. 2021, 11, 8486. [Google Scholar] [CrossRef]
- Hu, J.; Shi, Z.; Wang, X.; Qiao, H.; Huang, H. Silver–modified porous 3D nitrogen-doped graphene aerogel: Highly efficient oxygen reduction electrocatalyst for Zn–Air battery. Electrochim. Acta 2019, 302, 216–224. [Google Scholar] [CrossRef]
- Wuamprakhon, P.; Krittayavathananon, A.; Ma, N.; Phattharasupakun, N.; Maihom, T.; Limtrakul, J.; Sawangphruk, M. Layered manganese oxide nanosheets coated on N-doped graphene aerogel for hydrazine detection: Reaction mechanism investigated by in situ electrochemical X-ray absorption spectroscopy. J. Electroanal. Chem. 2018, 808, 124–132. [Google Scholar] [CrossRef]
- Ranku, M.N.; Uwaya, G.E.; Fayemi, O.E. Electrochemical detection of dopamine at Fe3O4/SPEEK modified electrode. Molecules 2021, 26, 5357. [Google Scholar] [CrossRef]
- Li, J.; Ji, H.; Xu, Y.; Zhang, J.; Yan, Y. Three–dimensional graphene supported Fe3O4 coated by polypyrrole toward enhanced stability and microwave absorbing properties. J. Mater. Res. Technol. 2020, 9, 762–772. [Google Scholar] [CrossRef]
- Vinodhkumar, G.; Wilson, J.; Inbanathan, S.S.R.; Potheher, I.V.; Ashokkumar, M.; Peter, A.C. Solvothermal synthesis of magnetically separable reduced graphene oxide/Fe3O4 hybrid nanocomposites with enhanced photocatalytic properties. Phys. B Condens. Matter 2020, 580, 411752. [Google Scholar] [CrossRef]
- Lu, K.-Q.; Xin, X.; Zhang, N.; Tang, Z.-R.; Xu, Y.-J. Photoredox catalysis over graphene aerogel–supported composites. J. Mater. Chem. A 2018, 6, 4590–4604. [Google Scholar] [CrossRef]
- Cheng, S.; Guo, X.; Tan, P.; Yan, B.; Lin, M.; Cai, J.; Zhang, Y.; Cai, W.; Zhang, X.-A. A graphene aerogel with reversibly tunable thermal resistance for battery thermal management. J. Mater. Chem. A 2023, 11, 17779–17786. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Zhang, X.; Zhang, J.; Duan, Y.; Li, X.; Jiang, D.; Kozawa, T.; Naito, M. Development of graphene aerogels with high strength and ultrahigh adsorption capacity for gas purification. Mater. Des. 2021, 208, 109903. [Google Scholar] [CrossRef]
- Castaldo, R.; Avolio, R.; Cocca, M.; Errico, M.E.; Lavorgna, M.; Šalplachta, J.; Santillo, C.; Gentile, G. Hierarchically porous hydrogels and aerogels based on reduced graphene oxide, montmorillonite and hyper–crosslinked resins for water and air remediation. Chem. Eng. J. 2022, 430, 133162. [Google Scholar] [CrossRef]
- Shu, R.; Yang, X.; Zhao, Z. Fabrication of core-shell structure NiFe2O4@SiO2 decorated nitrogen–doped graphene composite aerogels towards excellent electromagnetic absorption in the Ku band. Carbon 2023, 210, 118047. [Google Scholar] [CrossRef]
- Xu, J.; Shu, R.; Wan, Z.; Shi, J. Construction of three–dimensional hierarchical porous nitrogen-doped reduced graphene oxide/hollow cobalt ferrite composite aerogels toward highly efficient electromagnetic wave absorption. J. Mater. Sci. Technol. 2023, 132, 193–200. [Google Scholar] [CrossRef]
- Shu, R.; Xu, J.; Shi, J. Construction of nitrogen-doped graphene-based ternary magnetic composite aerogel towards excellent electromagnetic absorption in the Ku-band. J. Alloys Compd. 2023, 956, 170349. [Google Scholar] [CrossRef]
- Wu, L.; Qin, Z.; Zhang, L.; Meng, T.; Yu, F.; Ma, J. CNT-enhanced amino-functionalized graphene aerogel adsorbent for highly efficient removal of formaldehyde. New J. Chem. 2017, 41, 2527–2533. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems—with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wang, Y.; Berthon-Fabry, S. One-pot synthesis of Fe-N-containing carbon aerogel for oxygen reduction reaction. Electrocatalysis 2020, 12, 78–90. [Google Scholar] [CrossRef]
- Meng, Z.; Hou, X.; Liu, Y.; Ma, Z.; Shen, H. Facile fabrication of iron–modified biochar as a renewable adsorbent for efficient siloxane (L2) removal. J. Environ. Chem. Eng. 2021, 9, 105799. [Google Scholar] [CrossRef]
- Bagheri, S.; Esrafili, A.; Kermani, M.; Mehralipour, J.; Gholami, M. Performance evaluation of a novel rGO–Fe0/Fe3O4–PEI nanocomposite for lead and cadmium removal from aqueous solutions. J. Mol. Liq. 2020, 320, 114422. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, J.; Singh, R.C. Graphene aerogel based room temperature chemiresistive detection of hydrogen peroxide: A key explosive ingredient. Sens. Actuators A 2018, 282, 97–113. [Google Scholar] [CrossRef]
- Rahmani, Z.; Rashidi, A.M.; Kazemi, A.; Samadi, M.T.; Rahmani, A.R. N-doped reduced graphene oxide aerogel for the selective adsorption of oil pollutants from water: Isotherm and kinetic study. J. Ind. Eng. Chem. 2018, 61, 416–426. [Google Scholar] [CrossRef]
- Ren, X.; Guo, H.; Feng, J.; Si, P.; Zhang, L.; Ci, L. Synergic mechanism of adsorption and metal–free catalysis for phenol degradation by N–doped graphene aerogel. Chemosphere 2018, 191, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, Y.; Chen, S.; Zhang, Y.; Li, J.; Hong, J. Construction of three–dimensional nitrogen–doped graphene aerogel (NGA) supported cobalt catalysts for Fischer-Tropsch synthesis. Catal. Today 2020, 355, 10–16. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, L.; Xu, M.; Li, Y.; Song, X.; Yin, L. Difference between ammonia and urea on nitrogen doping of graphene quantum dots. Colloids Surf. A 2021, 610, 125703. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, R.; Sun, D.; Li, Y.; Wu, T. Sustainable fabrication of graphene oxide/manganese oxide composites for removing phenolic compounds by adsorption-oxidation process. J. Clean. Prod. 2019, 215, 165–174. [Google Scholar] [CrossRef]
- Cong, H.P.; Ren, X.C.; Wang, P.; Yu, S.H. Macroscopic multifunctional graphene–based hydrogels and aerogels by a metal ion induced self–assembly process. ACS Nano 2012, 6, 2693–2703. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Z.; Ji, X.; Wang, Y.; Zhang, Y.; Sun, H.; Li, W.; Wang, L.; Duan, J. Efficient adsorption of tebuconazole in aqueous solution by calcium modified water hyacinth–based biochar: Adsorption kinetics, mechanism and feasibility. Molecules 2023, 28, 3478. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Z.; Hao, Z. Recent advances in technologies for the removal of volatile methylsiloxanes: A case in biogas purification process. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2257–2313. [Google Scholar] [CrossRef]
- Wang, Z.; Muhammad, Y.; Tang, R.; Lu, C.; Yu, S.; Song, R.; Tong, Z.; Han, B.; Zhang, H. Dually organic modified bentonite with enhanced adsorption and desorption of tetracycline and ciprofloxacine. Sep. Purif. Technol. 2021, 274, 119059. [Google Scholar] [CrossRef]
- Darwish, A.A.A.; Rashad, M.; Al-Aoh, H.A. Methyl orange adsorption comparison on nanoparticles: Isotherm, kinetics, and thermodynamic studies. Dyes Pigment. 2019, 160, 563–571. [Google Scholar] [CrossRef]
- Finocchio, E.; Montanari, T.; Garuti, G.; Pistarino, C.; Federici, F.; Cugino, M.; Busca, G. Purification of biogases from siloxanes by adsorption: On the regenerability of activated carbon sorbents. Energy Fuel 2009, 23, 4156–4159. [Google Scholar] [CrossRef]
- Gislon, P.; Galli, S.; Monteleone, G. Siloxanes removal from biogas by high surface area adsorbents. Waste Manag. 2013, 33, 2687–2693. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Codony, A.; Gonzalez-Olmos, R.; Martin, M.J. Regeneration of siloxane–exhausted activated carbon by advanced oxidation processes. J. Hazard. Mater. 2015, 285, 501–508. [Google Scholar] [CrossRef]



| Samples | SBET (m2 g−1) | Vtot (cm3 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) a | Daver (nm) |
|---|---|---|---|---|---|
| IGGO | 7 | 0.23 | 0.01 | 0.22 | 13.18 |
| Fe3O4–urea–rGOA–0.12 | 124 | 0.59 | 0.06 | 0.53 | 5.02 |
| Fe3O4–urea–rGOA–0.24 | 160 | 0.67 | 0.11 | 0.56 | 4.95 |
| Fe3O4–urea–rGOA–0.4 | 188 | 0.77 | 0.19 | 0.58 | 3.94 |
| Fe3O4–urea–rGOA–0.8 | 178 | 0.74 | 0.16 | 0.58 | 4.88 |
| Fe3O4–urea–rGOA–1 | 162 | 0.68 | 0.12 | 0.56 | 4.95 |
| Adsorbents | Experimental | Model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| tB/ min | QB/ mg g−1 | Qm/ mg g−1 | tB,th/ min | QB,th/ mg g−1 | Qm,th/ mg g−1 | KYN | τ/ min | R2 | |
| IGGO | 0.53 | 3.9 | 5.8 | 0.65 | 4.8 | 5.2 | 34.06 | 0.72 | 0.9999 |
| Fe3O4–urea–rGOA–0.12 | 8.00 | 58.4 | 69.4 | 7.60 | 55.6 | 68.7 | 1.59 | 9.42 | 0.9945 |
| Fe3O4–urea–rGOA–0.24 | 11.94 | 87.2 | 98.2 | 11.60 | 84.8 | 97.7 | 1.63 | 13.38 | 0.9962 |
| Fe3O4–urea–rGOA–0.4 | 13.77 | 100.5 | 110.9 | 13.88 | 101.5 | 112.4 | 1.77 | 15.07 | 0.9945 |
| Fe3O4–urea–rGOA–0.8 | 12.48 | 91.2 | 103.8 | 11.86 | 86.7 | 102.7 | 1.31 | 14.10 | 0.9906 |
| Fe3O4–urea–rGOA–1 | 11.73 | 85.7 | 96.3 | 11.48 | 83.9 | 95.4 | 1.70 | 13.10 | 0.9946 |
| Statistical Parameters | QB,th–SBET | QB,th–Vmeso | QB,th–Vtot | QB,th–Vmicro | |
|---|---|---|---|---|---|
| Number of Points | 5 | 5 | 5 | 5 | |
| Equation | y = a + bx | ||||
| Residual Sum of Squares | 14.6870 | 7.8656 | 33.8747 | 55.5914 | |
| Standard Deviation | 1.9162 | 1.4023 | 2.9101 | 3.7280 | |
| R-Square | 0.9838 | 0.9913 | 0.9626 | 0.9386 | |
| Intercept | Value | −27.6825 | −455.2833 | −87.0625 | 45.3823 |
| Standard Error | 7.4994 | 25.4242 | 17.2712 | 5.7807 | |
| Slope | Value | 0.7141 | 961.8600 | 253.7942 | 341.0927 |
| Standard Error | 0.04575 | 44.9644 | 24.8926 | 43.2595 | |
| Term | Value | tB,th/ min | QB,th/ mg g−1 | Qm,th/ mg g−1 | KYN | τ/ min | R2 |
|---|---|---|---|---|---|---|---|
| T/°C | 0 | 18.08 | 132.2 | 146.5 | 1.1431 | 20.66 | 0.9982 |
| 10 | 16.28 | 119.0 | 131.8 | 1.1901 | 18.75 | 0.9979 | |
| 15 | 14.97 | 109.4 | 121.3 | 1.1705 | 17.49 | 0.9989 | |
| 25 | 13.88 | 101.5 | 112.4 | 1.2814 | 16.18 | 0.9963 | |
| 35 | 12.95 | 94.7 | 105.0 | 2.0786 | 14.37 | 0.9981 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Hou, X.; Zheng, Y.; Ma, Z. Hexamethyldisiloxane Removal from Biogas Using a Fe3O4–Urea-Modified Three-Dimensional Graphene Aerogel. Molecules 2023, 28, 6622. https://doi.org/10.3390/molecules28186622
Lv S, Hou X, Zheng Y, Ma Z. Hexamethyldisiloxane Removal from Biogas Using a Fe3O4–Urea-Modified Three-Dimensional Graphene Aerogel. Molecules. 2023; 28(18):6622. https://doi.org/10.3390/molecules28186622
Chicago/Turabian StyleLv, Siqi, Xifeng Hou, Yanhui Zheng, and Zichuan Ma. 2023. "Hexamethyldisiloxane Removal from Biogas Using a Fe3O4–Urea-Modified Three-Dimensional Graphene Aerogel" Molecules 28, no. 18: 6622. https://doi.org/10.3390/molecules28186622
APA StyleLv, S., Hou, X., Zheng, Y., & Ma, Z. (2023). Hexamethyldisiloxane Removal from Biogas Using a Fe3O4–Urea-Modified Three-Dimensional Graphene Aerogel. Molecules, 28(18), 6622. https://doi.org/10.3390/molecules28186622








