Emerging Applications of Nanotechnology in Healthcare and Medicine
Abstract
:1. Introduction
2. Results and Discussion—Applications of Nanotechnology in the Medical Field
2.1. Applications of Nanotechnology in Diagnostics
2.2. Nanotechnology and Lab-on-Chip Technology
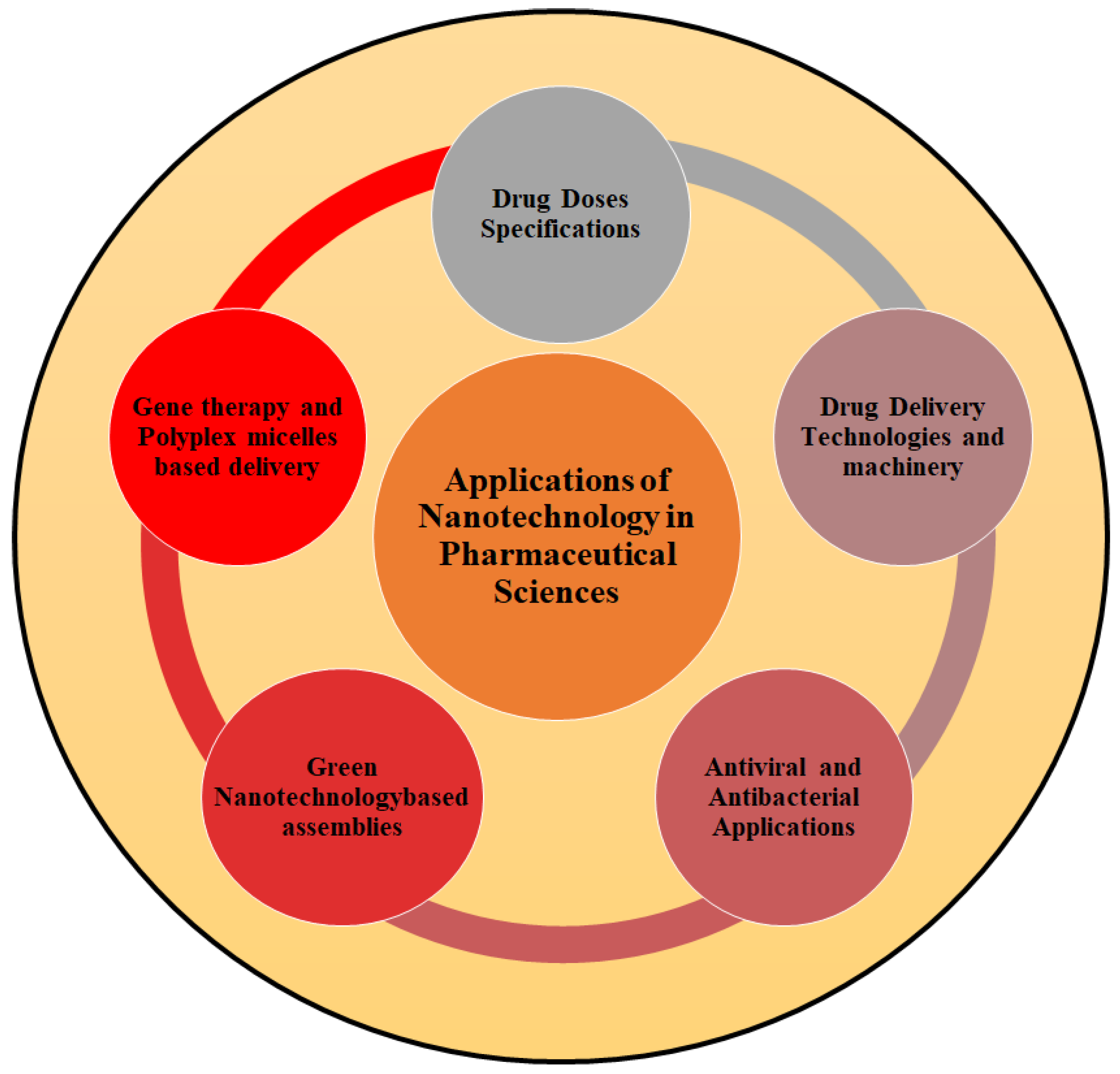
2.3. Applications of Nanotechnology in Pharmaceutical Sciences
2.3.1. Nanoscience and Drug Dose Specifications
2.3.2. Nanotechnology and Drug Delivery Technologies
2.3.3. DNA Nanotechnology and Drug Delivery System
2.3.4. Nanobiotechnology and Gene Therapy
Gene Therapy Approaches via Polyplex Micelles
2.3.5. Green Nanotechnology-Driven Drug Delivery Assemblies
2.3.6. Nanotechnology—Antiviral and Antibacterial Applications
2.3.7. Barriers Associated with Nanoparticle-Based Delivery Efficiency and Clinical Translation
2.4. Applications of Nanotechnology in Regenerative Medical Sciences
2.4.1. Nanotechnology and Bone Regeneration Technology
2.4.2. Nanotechnology and Regenerative Medicine
2.5. Applications of Nanotechnology in Surgery
2.5.1. Surgical Nanorobotics and Nano-Bioelectric Medicine
2.5.2. Implantable Medical Nanogenerators
2.5.3. Nanotechnology and Anesthesia Induction
2.6. Applications of Nanotechnology in Dentistry
2.6.1. Nanotechnologies, Tooth Repair, and Hypersensitivity Treatment
2.6.2. Tooth Repositioning and Renaturalization
2.6.3. Nanotechnology and Dental Durability
2.7. Applications of Nanotechnology in Oncology Field
2.7.1. Nanotechnology and Cancer Treatment Strategies
2.7.2. Nanotechnology in Cancer Diagnosis
2.7.3. Multifunctional, Multimodal, Theranostics-Based Anticancer Therapy
2.7.4. Targeted Nano Drug Delivery Technology for Cancer Therapy
2.7.5. Nanotech Based Magnetic Drug Delivery Technology and Cancer Therapy
2.8. Other Applications of Nanotechnology in the Medical Field
2.8.1. Applications of Nanotechnology in Medical Machinery
2.8.2. Nanotechnology and Veterinary Medicine
2.8.3. Nano Sensors, Nano Microbivores and Chemical Warfare Technology
2.8.4. Nanomedicine and COVID-19
2.9. Toxicology and Safety Analyses of Nanotechnologies
2.10. Future Prospects Regarding Nano-Medical Applications
3. Materials and Methods
3.1. Search Strategy
3.2. Inclusion and Exclusion Criteria
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nikalje, A.P. Nanotechnology and Its Applications in Medicine. Med. Chem. 2015, 5, 81–89. [Google Scholar] [CrossRef]
- Thakur, A.; Thakur, P.; Khurana, S.M.P. Synthesis and Applications of Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Modi, S.; Prajapati, R.; Inwati, G.K.; Deepa, N.; Tirth, V.; Yadav, V.K.; Yadav, K.K.; Islam, S.; Gupta, P.; Kim, D.-H. Recent Trends in Fascinating Applications of Nanotechnology in Allied Health Sciences. Crystals 2022, 12, 39. [Google Scholar] [CrossRef]
- Avula, L.R.; Grodzinski, P. Nanotechnology-Aided Advancement in the Combating of Cancer Metastasis. Cancer Metastasis Rev. 2022, 41, 383–404. [Google Scholar] [CrossRef] [PubMed]
- Das Talukdar, A.; Sarker, S.D.; Patra, J.K. Advances in Nanotechnology-Based Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Erkoc, P.; Ulucan-Karnak, F. Nanotechnology-based antimicrobial and antiviral surface coating strategies. Prosthesis 2021, 3, 25–52. [Google Scholar] [CrossRef]
- Hulla, J.E.; Sahu, S.C.; Hayes, A.W. Nanotechnology: History and Future. Hum. Exp. Toxicol. 2015, 34, 1318–1321. [Google Scholar] [CrossRef]
- Wong, I.Y.; Bhatia, S.N.; Toner, M. Nanotechnology: Emerging Tools for Biology and Medicine. Genes. Dev. 2013, 27, 2397–2408. [Google Scholar] [CrossRef]
- Misra, R.; Acharya, S.; Sahoo, S.K. Cancer Nanotechnology: Application of Nanotechnology in Cancer Therapy. Drug Discov. Today 2010, 15, 842–850. [Google Scholar] [CrossRef]
- Bhushan, B. Introduction to Nanotechnology. In Springer Handbook of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–19. [Google Scholar]
- Chakravarthi, V.P.; Balaji, N. Applications of Nanotechnology in Veterinary Medicine. Vet. World 2010, 3, 477. [Google Scholar]
- Pramanik, P.K.D.; Solanki, A.; Debnath, A.; Nayyar, A.; El-Sappagh, S.; Kwak, K.S. Advancing modern healthcare with nanotechnology, nanobiosensors, and internet of nano things: Taxonomies, applications, architecture, and challenges. IEEE Access 2020, 8, 65230–65266. [Google Scholar] [CrossRef]
- Badrunnisa, S.; Menghani, S.S. Review on Health Sciences Applications of Nanotechnology. J. Coast. Life Med. 2023, 11, 94–100. [Google Scholar]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Vaishampayan, V.; Kapoor, A.; Gumfekar, S.P. Enhancement in the limit of detection of lab-on-chip microfluidic devices using functional nanomaterials. Can. J. Chem. Eng. 2023, 101, 5208. [Google Scholar] [CrossRef]
- Dessale, M.; Mengistu, G.; Mengist, H.M. Nanotechnology: A Promising Approach for Cancer Diagnosis, Therapeutics and Theragnosis. Int. J. Nanomed. 2022, 17, 3735–3749. [Google Scholar] [CrossRef] [PubMed]
- Mbunge, E.; Muchemwa, B.; Batani, J. Sensors and healthcare 5.0: Transformative shift in virtual care through emerging digital health technologies. Glob. Health J. 2021, 5, 169–177. [Google Scholar] [CrossRef]
- Fox, K.E.; Tran, N.L.; Nguyen, T.A.; Nguyen, T.T.; Tran, P.A. Surface modification of medical devices at nanoscale—Recent development and translational perspectives. In Biomaterials in Translational Medicine; Academic Press: Cambridge, MA, USA, 2019; pp. 163–189. [Google Scholar]
- Singh, A.; Amiji, M.M. Application of nanotechnology in medical diagnosis and imaging. Curr. Opin. Biotechnol. 2022, 74, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.Y.; Tan, Z.; Liao, X.W.; Wang, C. Recent advances in nanoscale metal-organic frameworks biosensors for detection of biomarkers. Chin. Chem. Lett. 2022, 33, 22–32. [Google Scholar] [CrossRef]
- Sadeghi, M.; Sadeghi, S.; Naghib, S.M.; Garshasbi, H.R. A Comprehensive Review on Electrochemical Nano Biosensors for Precise Detection of Blood-Based Oncomarkers in Breast Cancer. Biosensors 2023, 13, 481. [Google Scholar] [CrossRef]
- Rajput, A.; Sevalkar, G.; Pardeshi, K.; Pingale, P. Computational Nanoscience and Technology. OpenNano 2023, 12, 100147. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Bollas, A.; Wang, Y.; Au, K.F. Nanopore sequencing technology, bioinformatics and applications. Nat. Biotechnol. 2021, 39, 1348–1365. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-Based Drug Delivery Systems for Cancer Therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A revolution in modern industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef] [PubMed]
- Welch, E.C.; Powell, J.M.; Clevinger, T.B.; Fairman, A.E.; Shukla, A. Advances in biosensors and diagnostic technologies using nanostructures and nanomaterials. Adv. Funct. Mater. 2021, 31, 2104126. [Google Scholar] [CrossRef]
- Patel, S.S.; Patel, P.N. A Brief Review on Nanorobotics Applications in Medicine and Future Prospects. Asian J. Res. Pharm. Sci. 2023, 13, 198. [Google Scholar] [CrossRef]
- Wang, W.; Ye, Z.; Gao, H.; Ouyang, D. Computational Pharmaceutics-A New Paradigm of Drug Delivery. J. Control. Release 2021, 338, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Du, J.; Wang, L.; Wang, Y. Nanocrystals Technology for Improving Bioavailability of Poorly Soluble Drugs: A Mini-Review. J. Nanosci. Nanotechnol. 2017, 17, 18–28. [Google Scholar] [CrossRef]
- Thwala, L.N.; Ndlovu, S.C.; Mpofu, K.T.; Lugongolo, M.Y.; Mthunzi-Kufa, P. Nanotechnology-Based Diagnostics for Diseases Prevalent in Developing Countries: Current Advances in Point-of-Care Tests. Nanomaterials 2023, 13, 1247. [Google Scholar] [CrossRef]
- Kumari, S.; Goyal, A.; Sönmez Gürer, E.; Algın Yapar, E.; Garg, M.; Sood, M.; Sindhu, R.K. Bioactive Loaded Novel Nano-Formulations for Targeted Drug Delivery and Their Therapeutic Potential. Pharmaceutics 2022, 14, 1091. [Google Scholar] [CrossRef]
- Shen, L.; Wang, P.; Ke, Y. DNA nanotechnology-based biosensors and therapeutics. Adv. Healthcare Mater. 2021, 10, 2002205. [Google Scholar] [CrossRef]
- Babu, N.A.; Sridevi Anjuga, E.P.; Nagarajan, K.; Masthan, K.M.K. Nanotechnology in Detection of Oral Cancer. Indian J. Public Health Res. Dev. 2019, 10, 3205–3207. [Google Scholar] [CrossRef]
- Souri, M.; Soltani, M.; Kashkooli, F.M.; Shahvandi, M.K.; Chiani, M.; Shariati, F.S.; Mehrabi, M.R.; Munn, L.L. Towards Principled Design of Cancer Nanomedicine to Accelerate Clinical Translation. Mater. Today Bio 2022, 13, 100208. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; van Hoogevest, P.; Storm, G. The Role of Liposomes in Clinical Nanomedicine Development. What Now? Now What? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Tahir, A.; Wang, H.; Chang, J. Applications of nanotechnology in virus detection, tracking, and infection mechanisms. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1700. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Facing the Truth about Nanotechnology in Drug Delivery. ACS Nano 2013, 7, 7442–7447. [Google Scholar] [CrossRef] [PubMed]
- Mazayen, Z.M.; Ghoneim, A.M.; Elbatanony, R.S.; Basalious, E.B.; Bendas, E.R. Pharmaceutical nanotechnology: From the bench to the market. Future J. Pharm. Sci. 2022, 8, 12. [Google Scholar] [CrossRef]
- Adir, O.; Poley, M.; Chen, G.; Froim, S.; Krinsky, N.; Shklover, J.; Shainsky-Roitman, J.; Lammers, T.; Schroeder, A. Integrating artificial intelligence and nanotechnology for precision cancer medicine. Adv. Mater. 2020, 32, 1901989. [Google Scholar] [CrossRef] [PubMed]
- Buya, A.B.; Beloqui, A.; Memvanga, P.B.; Préat, V. Self-nano-emulsifying drug-delivery systems: From the development to the current applications and challenges in oral drug delivery. Pharmaceutics 2020, 12, 1194. [Google Scholar] [CrossRef]
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA Nanotechnology-Enabled Drug Delivery Systems. Chem. Rev. 2018, 119, 6459–6506. [Google Scholar] [CrossRef]
- Amna, T.; Hassan, M.S.; Gharsan, F.N.; Rehman, S.; Sheikh, F.A. Nanotechnology in Drug Delivery Systems: Ways to Boost Bioavailability of Drugs. In Nanotechnology for Infectious Diseases; Springer: Berlin/Heidelberg, Germany, 2022; pp. 223–236. [Google Scholar]
- Iravani, S.; Varma, R.S. Nanosponges for drug delivery and cancer therapy: Recent advances. Nanomaterials 2022, 12, 2440. [Google Scholar] [CrossRef]
- Baroud, M.; Lepeltier, E.; Thepot, S.; El-Makhour, Y.; Duval, O. The evolution of nucleosidic analogues: Self-assembly of prodrugs into nanoparticles for cancer drug delivery. Nanoscale Adv. 2021, 3, 2157–2179. [Google Scholar] [CrossRef]
- Dong, P.; Rakesh, K.P.; Manukumar, H.M.; Mohammed, Y.H.E.; Karthik, C.S.; Sumathi, S.; Mallu, P.; Qin, H.-L. Innovative Nano-Carriers in Anticancer Drug Delivery-a Comprehensive Review. Bioorg. Chem. 2019, 85, 325–336. [Google Scholar] [CrossRef]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.V.K.S.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Oroojalian, F.; Charbgoo, F.; Hashemi, M.; Amani, A.; Yazdian-Robati, R.; Mokhtarzadeh, A.; Ramezani, M.; Hamblin, M.R. Recent advances in nanotechnology-based drug delivery systems for the kidney. J. Control. Release 2020, 321, 442–462. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Niazi, M.; Khan, M.; Rauff, B.; Anwar, S.; Amin, F.; Hanif, R. Cytotoxicity Study of Gold Nanoparticle Synthesis Using Aloe Vera, Honey, and Gymnema Sylvestre Leaf Extract. ACS Omega 2023, 8, 6325–6336. [Google Scholar] [CrossRef] [PubMed]
- Enrico, C. Nanotechnology-based drug delivery of natural compounds and phytochemicals for the treatment of cancer and other diseases. Stud. Nat. Prod. Chem. 2019, 62, 91–123. [Google Scholar]
- Suhail, M.; Khan, A.; Rahim, M.A.; Naeem, A.; Fahad, M.; Badshah, S.F.; Jabar, A.; Janakiraman, A.K. Micro and nanorobot-based drug delivery: An overview. J. Drug Target. 2022, 30, 349–358. [Google Scholar] [CrossRef]
- Liu, J.; Xie, G.; Lv, S.; Xiong, Q.; Xu, H. Recent Applications of Rolling Circle Amplification in Biosensors and DNA Nanotechnology. TrAC Trends Anal. Chem. 2023, 160, 116953. [Google Scholar] [CrossRef]
- Chouhan, A.S.; Rangi, N. A Research on Future Scenario in the Field of Role of Nanorobotics a Device for Diagnosis and Treatment. Glob. Acad. J. Med. Sci. 2023, 5, 85–95. [Google Scholar]
- DeLuca, M.; Shi, Z.; Castro, C.E.; Arya, G. Dynamic DNA nanotechnology: Toward functional nanoscale devices. Nanoscale Horiz. 2020, 5, 182–201. [Google Scholar] [CrossRef]
- Kim, J.; Franco, E. RNA nanotechnology in synthetic biology. Curr. Opin. Biotechnol. 2020, 63, 135–141. [Google Scholar] [CrossRef]
- Yu, C.; Li, L.; Hu, P.; Yang, Y.; Wei, W.; Deng, X.; Wang, L.; Tay, F.R.; Ma, J. Recent Advances in Stimulus-responsive Nanocarriers for Gene Therapy. Adv. Sci. 2021, 8, 2100540. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, M.; Yang, Z.; Liu, X.; Xu, Z.; Liu, S.; Feng, G.; Tang, S.; Li, Z.; Zhang, Y. Recent Advances in Nanotechnology Approach for Non-Viral Gene Therapy. Biomater. Sci. 2022, 10, 6862–6892. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, L.; Lou, J. Nanotechnology strategies for the analysis of circulating tumor DNA: A review. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e921040-1–e921040-9. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Haleem, A.; Singh, R.P.; Rab, S.; Suman, R. Exploring the potential of nanosensors: A brief overview. Sens. Int. 2021, 2, 100130. [Google Scholar] [CrossRef]
- Cheng, M.; Dou, H. Nano-assemblies based on biomacromolecules to overcome cancer drug resistance. Polym. Int. 2022, 71, 371–378. [Google Scholar] [CrossRef]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.U. A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef]
- Salameh, J.W.; Zhou, L.; Ward, S.M.; Santa Chalarca, C.F.; Emrick, T.; Figueiredo, M.L. Polymer-mediated gene therapy: Recent advances and merging of delivery techniques. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1598. [Google Scholar] [CrossRef]
- Li, Q.; Hu, Z.; Rong, X.; Chang, B.; Liu, X. Multifunctional polyplex micelles for efficient microRNA delivery and accelerated osteogenesis. Nanoscale 2021, 13, 12198–12211. [Google Scholar] [CrossRef]
- Uchida, S.; Kataoka, K. Design concepts of polyplex micelles for in vivo therapeutic delivery of plasmid DNA and messenger RNA. J. Biomed. Mater. Res. A 2019, 107, 978–990. [Google Scholar] [CrossRef]
- Koji, K.; Yoshinaga, N.; Mochida, Y.; Hong, T.; Miyazaki, T.; Kataoka, K.; Osada, K.; Cabral, H.; Uchida, S. Bundling of mRNA strands inside polyion complexes improves mRNA delivery efficiency in vitro and in vivo. Biomaterials 2020, 261, 120332. [Google Scholar] [CrossRef]
- Tockary, T.A.; Foo, W.; Dirisala, A.; Chen, Q.; Uchida, S.; Osawa, S.; Mochida, Y.; Liu, X.; Kinoh, H.; Cabral, H.; et al. Single-stranded DNA-packaged polyplex micelle as adeno-associated-virus-inspired compact vector to systemically target stroma-rich pancreatic cancer. ACS Nano 2019, 13, 12732–12742. [Google Scholar] [CrossRef]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A Review of Drug Delivery Systems Based on Nanotechnology and Green Chemistry: Green Nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, R.; Rathee, J.; Salunke, D.B.; Mehta, S.K. Green nanotechnology-driven drug delivery assemblies. ACS Omega 2019, 4, 8804–8815. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Green nanotechnology. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 145–198. [Google Scholar]
- Amiri, M.R.; Alavi, M.; Taran, M.; Kahrizi, D. Antibacterial, Antifungal, Antiviral, and Photocatalytic Activities of TiO2 Nanoparticles, Nanocomposites, and Bio-Nanocomposites: Recent Advances and Challenges. J. Public. Health Res. 2022, 11, 22799036221104150. [Google Scholar] [CrossRef]
- Lal, H.M.; Uthaman, A.; Thomas, S. Silver Nanoparticle as an Effective Antiviral Agent. In Polymer Nanocomposites Based on Silver Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2021; pp. 247–265. [Google Scholar]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Nandi, A.; Simnani, F.Z.; Saha, U.; Ghosh, A.; Sinha, A.; Sahay, A.; Samal, S.K.; Panda, P.K.; Verma, S.K. Emerging trends in advanced translational applications of silver nanoparticles: A progressing dawn of nanotechnology. J. Funct. Biomater. 2023, 14, 47. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Li, J.; Nie, Y.; Liao, G.; Yu, Y.; Li, C. Overcoming the reticuloendothelial system barrier to drug delivery with a “don’t-eat-us” strategy. ACS Nano 2019, 13, 13015–13026. [Google Scholar] [CrossRef]
- Bao, L.; Cui, X.; Chen, C. Toxicology for Nanotechnology. In Nanomedicine; Springer Nature: Singapore, 2023; pp. 157–177. [Google Scholar]
- Wolfram, J.; Nizzero, S.; Liu, H.; Li, F.; Zhang, G.; Li, Z.; Shen, H.; Blanco, E.; Ferrari, M. A chloroquine-induced macrophage-preconditioning strategy for improved nanodelivery. Sci. Rep. 2017, 7, 13738. [Google Scholar] [CrossRef]
- Saunders, N.R.; Paolini, M.S.; Fenton, O.S.; Poul, L.; Devalliere, J.; Mpambani, F.; Darmon, A.; Bergère, M.; Jibault, O.; Germain, M.; et al. A nanoprimer to improve the systemic delivery of siRNA and mRNA. Nano Lett. 2020, 20, 4264–4269. [Google Scholar] [CrossRef]
- Dirisala, A.; Uchida, S.; Toh, K.; Li, J.; Osawa, S.; Tockary, T.A.; Liu, X.; Abbasi, S.; Hayashi, K.; Mochida, Y.; et al. Transient stealth coating of liver sinusoidal wall by anchoring two-armed PEG for retargeting nanomedicines. Sci. Adv. 2020, 6, eabb8133. [Google Scholar] [CrossRef]
- Dirisala, A.; Osada, K.; Chen, Q.; Tockary, T.A.; Machitani, K.; Osawa, S.; Liu, X.; Ishii, T.; Miyata, K.; Oba, M.; et al. Optimized rod length of polyplex micelles for maximizing transfection efficiency and their performance in systemic gene therapy against stroma-rich pancreatic tumors. Biomaterials 2014, 35, 5359–5368. [Google Scholar] [CrossRef]
- Dirisala, A.; Uchida, S.; Li, J.; Van Guyse, J.F.; Hayashi, K.; Vummaleti, S.V.; Kaur, S.; Mochida, Y.; Fukushima, S.; Kataoka, K. Effective mRNA Protection by Poly (l-ornithine) Synergizes with Endosomal Escape Functionality of a Charge-Conversion Polymer toward Maximizing mRNA Introduction Efficiency. Macromol. Rapid Commun. 2020, 43, 2100754. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression Kinetics of Nucleoside-Modified MRNA Delivered in Lipid Nanoparticles to Mice by Various Routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Agrawal, P.; Zhang, Y.S. Nanotechnologies and nanomaterials in 3D (Bio) printing toward bone regeneration. Adv. NanoBiomed. Res. 2021, 1, 2100035. [Google Scholar] [CrossRef]
- Hajiali, H.; Ouyang, L.; Llopis-Hernandez, V.; Dobre, O.; Rose, F.R. Review of emerging nanotechnology in bone regeneration: Progress, challenges, and perspectives. Nanoscale 2021, 13, 10266–10280. [Google Scholar] [CrossRef]
- Gu, W.; Wu, C.; Chen, J.; Xiao, Y. Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Int. J. Nanomed. 2013, 8, 2305–2317. [Google Scholar] [CrossRef]
- Wen, J.; Cai, D.; Gao, W.; He, R.; Li, Y.; Zhou, Y.; Klein, T.; Xiao, L.; Xiao, Y. Osteoimmunomodulatory Nanoparticles for Bone Regeneration. Nanomaterials 2023, 13, 692. [Google Scholar] [CrossRef]
- Engel, E.; Michiardi, A.; Navarro, M.; Lacroix, D.; Planell, J.A. Nanotechnology in Regenerative Medicine: The Materials Side. Trends Biotechnol. 2008, 26, 39–47. [Google Scholar] [CrossRef]
- Kubinová, Š.; Syková, E. Nanotechnologies in Regenerative Medicine. Minim. Invasive Ther. Allied Technol. 2010, 19, 144–156. [Google Scholar] [CrossRef]
- Yoon, H.-J.; Kim, S.-W. Nanogenerators to Power Implantable Medical Systems. Joule 2020, 4, 1398–1407. [Google Scholar] [CrossRef]
- Wang, W.; Pang, J.; Su, J.; Li, F.; Li, Q.; Wang, X.; Wang, J.; Ibarlucea, B.; Liu, X.; Li, Y. Applications of Nanogenerators for Biomedical Engineering and Healthcare Systems. InfoMat 2022, 4, e12262. [Google Scholar] [CrossRef]
- Li, M.; Hu, X.; Zhao, Y.; Jiao, N. An overview of recent progress in Micro/Nanorobots for biomedical applications. Adv. Mater. Nanotechnol. 2023, 8, 2201928. [Google Scholar] [CrossRef]
- Buniyamin, I.; Akhir, R.M.; Asli, N.A.; Khusaimi, Z.; Malek, M.F.; Mahmood, M.R. Nanotechnology Applications in Biomedical Systems. Curr. Nanomater. 2022, 7, 167–180. [Google Scholar] [CrossRef]
- Mazumder, S.; Biswas, G.R.; Majee, S.B. Applications of nanorobots in medical techniques. IJPSR 2020, 11, 3150. [Google Scholar]
- Zhang, Y.; Zhang, Y.; Han, Y.; Gong, X. Micro/Nanorobots for Medical Diagnosis and Disease Treatment. Micromachines 2022, 13, 648. [Google Scholar] [CrossRef]
- Quan, Y.; Wu, X.; Zhu, S.; Zeng, X.; Zeng, Z.; Zheng, Q. Triboelectric nanogenerators for clinical diagnosis and therapy: A report of recent progress. Med. Novel Technol. Devices 2022, 16, 100195. [Google Scholar] [CrossRef]
- Liu, S.; Tong, W.; Gao, C.; Liu, Y.; Li, X.; Zhang, Y. Environmentally friendly natural materials for triboelectric nanogenerators: A review. J Materials Chem A 2023, 11, 9270–9299. [Google Scholar] [CrossRef]
- Ryu, H.; Park, H.M.; Kim, M.K.; Kim, B.; Myoung, H.S.; Kim, T.Y.; Yoon, H.J.; Kwak, S.S.; Kim, J.; Hwang, T.H.; et al. Self-rechargeable cardiac pacemaker system with triboelectric nanogenerators. Nat. Commun. 2021, 12, 4374. [Google Scholar] [CrossRef]
- Naaz, S.; Asghar, A. Artificial intelligence, nano-technology and genomic medicine: The future of anaesthesia. J. Anaesthesiol. Clin. Pharmacol. 2022, 38, 11. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Zhang, S.; Huang, C.; Ding, Q.; Xia, J.; Wu, D.; Gao, W. Emerging Anesthetic Nanomedicines: Current State and Challenges. Int. J. Nanomed. 2023, 18, 3913–3935. [Google Scholar] [CrossRef]
- Bhansali, D.; Teng, S.L.; Lee, C.S.; Schmidt, B.L.; Bunnett, N.W.; Leong, K.W. Nanotechnology for pain management: Current and future therapeutic interventions. Nano Today 2021, 39, 101223. [Google Scholar] [CrossRef]
- Haridas, S.; Harish Kumar, V.V.; Sreekanth, P.; Sanara, P.P. Nanorobots in Periodontics. Dental Bites 2015, 2, 17–23. [Google Scholar]
- Wang, Q.; Zhang, Y.; Li, Q.; Chen, L.; Liu, H.; Ding, M.; Dong, H.; Mou, Y. Therapeutic Applications of Antimicrobial Silver-Based Biomaterials in Dentistry. Int. J. Nanomed. 2022, 17, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, S.; Racovita, S.; Gugoasa, I.A.; Lungan, M.-A.; Popa, M.; Desbrieres, J. The Benefits of Smart Nanoparticles in Dental Applications. Int. J. Mol. Sci. 2021, 22, 2585. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, R.; Gaur, S.; Albin, S. Nanometals in Dentistry: Applications and Toxicological Implications—A Systematic Review. Biol. Trace Elem. Res. 2020, 197, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Jandt, K.D.; Watts, D.C. Nanotechnology in Dentistry: Present and Future Perspectives on Dental Nanomaterials. Dent. Mater. 2020, 36, 1365–1378. [Google Scholar] [CrossRef]
- Chandra Mouli, P.E.; Manoj Kumar, S.; Parthiban, S. Nanotechnology in Dentistry-a Review. Int. J. Biol. Med. Res. 2012, 3, 1550–1553. [Google Scholar]
- Kasimoglu, Y.; Tabakcilar, D.; Guclu, Z.A.; Yamamoto-Nemoto, S.; Tuna, E.B.; Ozen, B.; Tuzuner, T.; Ince, G. Nanomaterials and Nanorobotics in Dentistry: A Review. J. Dent. Indones 2020, 27, 77–84. [Google Scholar] [CrossRef]
- Kochan, O.; Boitsaniuk, S.; Levkiv, M.; Przystupa, K.; Manashchuk, N.; Pohoretska, K.; Chornij, N.; Tsvyntarna, I.; Patskan, L. Emergence of Nano-Dentistry as a Reality of Contemporary Dentistry. Appl. Sci. 2022, 12, 2008. [Google Scholar] [CrossRef]
- Moradpoor, H.; Safaei, M.; Mozaffari, H.R.; Sharifi, R.; Imani, M.M.; Golshah, A.; Bashardoust, N. An overview of recent progress in dental applications of zinc oxide nanoparticles. RSC Adv. 2021, 11, 21189–21206. [Google Scholar] [CrossRef]
- Foong, L.K.; Foroughi, M.M.; Mirhosseini, A.F.; Safaei, M.; Jahani, S.; Mostafavi, M.; Ebrahimpoor, N.; Sharifi, M.; Varma, R.S.; Khatami, M. Applications of Nano-Materials in Diverse Dentistry Regimes. RSC Adv. 2020, 10, 15430–15460. [Google Scholar] [CrossRef]
- Prabakar, J. Current Applications of Nanoparticles in Preventive Dentistry–A Literature Review. J. Surv. Fish. Sci. 2023, 10, 460–467. [Google Scholar]
- Samanta, S. Periodontics: Original review Nanoperiodontics—A Futuristic Trend in Periodontal Management. Clin. Dent. 2022, 16, 12–20. [Google Scholar]
- Joseph, B. Nanotechnology in Oral and Dental Diagnosis. In Nanomaterials in Dental Medicine; Springer: Berlin/Heidelberg, Germany, 2023; pp. 33–49. [Google Scholar]
- Sen, S.; Singh, G. Finding Hidden Gems: Nanoparticles in Oral Health—A Review. Int. J. Drug Res. Dent. Sci. 2020, 2, 24–28. [Google Scholar]
- Koyande, N.P.; Srivastava, R.; Padmakumar, A.; Rengan, A.K. Advances in Nanotechnology for Cancer Immunoprevention and Immunotherapy: A Review. Vaccines 2022, 10, 1727. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.; Stewart, S.; White, A.; Kwizera, E.A.; Xu, J.; Fang, Y.; Shamul, J.G.; Xie, C.; Nurudeen, S.; Tirada, N.P. In-Situ Cryo-Immune Engineering of Tumor Microenvironment with Cold-Responsive Nanotechnology for Cancer Immunotherapy. Nat. Commun. 2023, 14, 392. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Khurana, S.; Choudhari, R.; Kesari, K.K.; Kamal, M.A.; Garg, N.; Ruokolainen, J.; Das, B.C.; Kumar, D. Specific Targeting Cancer Cells with Nanoparticles and Drug Delivery in Cancer Therapy. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 69, pp. 166–177. [Google Scholar]
- Kandasamy, G.; Maity, D. Multifunctional Theranostic Nanoparticles for Biomedical Cancer Treatments-A Comprehensive Review. Mater. Sci. Eng. C 2021, 127, 112199. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Jha, K.; Barman, D. Nanotechnology in Oral Cancer Prevention and Therapeutics: A Literature Review. Indian. J. Med. Paediatr. Oncol. 2021, 42, 146–152. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. 2019, 98, 1252–1276. [Google Scholar] [CrossRef]
- Khan, M.I.; Hossain, M.I.; Hossain, M.K.; Rubel, M.H.K.; Hossain, K.M.; Mahfuz, A.; Anik, M.I. Recent Progress in Nanostructured Smart Drug Delivery Systems for Cancer Therapy: A Review. ACS Appl. Bio Mater. 2022, 5, 971–1012. [Google Scholar] [CrossRef]
- Kher, C.; Kumar, S. The Application of Nanotechnology and Nanomaterials in Cancer Diagnosis and Treatment: A Review. Cureus 2022, 14, e29059. [Google Scholar] [CrossRef]
- Jing, X.; Meng, L.; Fan, S.; Yang, T.; Zhang, N.; Xu, R.; Zhao, X.; Yang, H.; Yang, Z.; Wang, D. Tumor Microenvironment Self-Regulation: Bimetallic Metal Nanozyme-Derived Multifunctional Nanodrug for Optimizable Cascade Catalytic Reaction-Synergetic Anti-Tumor Theranostics. Chem. Eng. J. 2022, 442, 136096. [Google Scholar] [CrossRef]
- Fathi, M.; Majidi, S.; Zangabad, P.S.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Chitosan-based Multifunctional Nanomedicines and Theranostics for Targeted Therapy of Cancer. Med. Res. Rev. 2018, 38, 2110–2136. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Cao, Z.; Liu, H.; Chen, L.; Bai, Y.; Wu, Q.; Yu, X.; Wei, W.; Wang, M. Multifunctional nanomedicines-enabled chemodynamic-synergized multimodal tumor therapy via Fenton and Fenton-like reactions. Theranostics 2023, 13, 1974–2016. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, L.; Sobhana, S.; Yasothamani, V.; Gowsalya, K.; Vivek, R. Multifunctional Theranostic Nanomedicines for Cancer Treatment: A Recent Progress and Challenges. Biomed. Eng. Adv. 2023, 5, 100082. [Google Scholar] [CrossRef]
- Sakthi Devi, R.; Girigoswami, A.; Siddharth, M.; Girigoswami, K. Applications of Gold and Silver Nanoparticles in Theranostics. Appl. Biochem. Biotechnol. 2022, 194, 4187–4219. [Google Scholar] [CrossRef]
- Ho, B.N.; Pfeffer, C.M.; Singh, A.T.K. Update on Nanotechnology-Based Drug Delivery Systems in Cancer Treatment. Anticancer. Res. 2017, 37, 5975–5981. [Google Scholar]
- Hani, U.; Osmani, R.A.M.; Yasmin, S.; Gowda, B.H.J.; Ather, H.; Ansari, M.Y.; Siddiqua, A.; Ghazwani, M.; Al Fatease, A.; Alamri, A.H. Novel Drug Delivery Systems as an Emerging Platform for Stomach Cancer Therapy. Pharmaceutics 2022, 14, 1576. [Google Scholar] [CrossRef]
- Huda, S.; Alam, M.A.; Sharma, P.K. Smart Nanocarriers-Based Drug Delivery for Cancer Therapy: An Innovative and Developing Strategy. J. Drug Deliv. Sci. Technol. 2020, 60, 102018. [Google Scholar] [CrossRef]
- Shen, X.; Li, T.; Xie, X.; Feng, Y.; Chen, Z.; Yang, H.; Wu, C.; Deng, S.; Liu, Y. PLGA-Based Drug Delivery Systems for Remotely Triggered Cancer Therapeutic and Diagnostic Applications. Front. Bioeng. Biotechnol. 2020, 8, 381. [Google Scholar] [CrossRef]
- Pusta, A.; Tertis, M.; Craciunescu, I.; Turcu, R.; Mirel, S.; Cristea, C. Recent advances in the development of drug delivery applications of magnetic nanomaterials. Pharmaceutics 2023, 15, 1872. [Google Scholar] [CrossRef]
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-mediated drug delivery systems for anticancer agents: An overview and perspectives. Int. J. Nanomed. 2021, 16, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P. Nanobased nano drug delivery: A comprehensive review. Appl. Targeted Nano Drugs Deliv. Syst. 2019, 4, 69–92. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drug delivery. Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef]
- Nikolova, M.; Slavchov, R.; Nikolova, G. Nanotechnology in medicine. In Drug Discovery and Evaluation: Methods in Clinical Pharmacology; Springer: Cham, Switzerland, 2020; pp. 533–546. [Google Scholar]
- Bodunde, O.P.; Ikumapayi, O.M.; Akinlabi, E.T.; Oladapo, B.I.; Adeoye, A.O.; Fatoba, S.O. A futuristic insight into a “nano-doctor”: A clinical review on medical diagnosis and devices using nanotechnology. Mater. Today Proc. 2021, 44, 1144–1153. [Google Scholar] [CrossRef]
- Tan, P.; Chen, X.; Zhang, H.; Wei, Q.; Luo, K. Artificial intelligence aids in development of nanomedicines for cancer management. Semin. Cancer Biol. 2023, 89, 61–75. [Google Scholar] [CrossRef]
- Zafar, M.S.; Alnazzawi, A.A.; Alrahabi, M.; Fareed, M.A.; Najeeb, S.; Khurshid, Z. Nanotechnology and nanomaterials in dentistry. In Advanced Dental Biomaterials; Woodhead Publishing: Sawston, UK, 2019; pp. 477–505. [Google Scholar]
- He, L.; Dai, D.; Xie, L.; Chen, Y.; Zhang, C. Biological Effects, Applications and Strategies of Nanomodification of Dental Metal Surfaces. Mater. Des. 2021, 207, 109890. [Google Scholar] [CrossRef]
- Jafary, F.; Motamedi, S.; Karimi, I. Veterinary Nanomedicine: Pros and Cons. Vet. Med. Sci. 2023, 9, 494–506. [Google Scholar] [CrossRef]
- Rajwade, J.M. An Overview of Myconanoparticles Applications in Veterinary Medicine. In Fungal Cell Factories for Sustainable Nanomaterials Productions and Agricultural Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 657–691. [Google Scholar]
- Prasad, R.D.; Charmode, N.; Shrivastav, O.P.; Prasad, S.R.; Moghe, A.; Sarvalkar, P.D.; Prasad, N.R. A review on concept of nanotechnology in veterinary medicine. ES Food Agrofor. 2021, 4, 28–60. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Advanced applications of nanotechnology in veterinary medicine. Environ. Sci. Pollut. Res. 2020, 27, 19073–19086. [Google Scholar] [CrossRef]
- Ianiski, L.B.; Rodrigues, F.D.S.; Stibbe, P.C.; Weiblen, C.; Pereira, D.I.B.; Santurio, J.M.; Silva, C.D.B.D.; Sangioni, L.A.; Vogel, F.S.F.; Costa, M.M.D.; et al. Nanotechnology in veterinary medicine: A review. Ciênc. Rural 2021, 52, e20210195. [Google Scholar] [CrossRef]
- Dedeoglu, A.; Kaya, S.I.; Bakirhan, N.K.; Ozkan, S.A. Nanotechnological Approaches and Materials in Commercial Biosensors. In Commercial Biosensors and Their Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 301–353. [Google Scholar]
- Thangavelu, L.; Veeraragavan, G.R.; Mallineni, S.K.; Devaraj, E.; Parameswari, R.P.; Syed, N.H.; Dua, K.; Chellappan, D.K.; Balusamy, S.R.; Bhawal, U.K. Role of Nanoparticles in Environmental Remediation: An Insight into Heavy Metal Pollution from Dentistry. Bioinorg. Chem. Appl. 2022, 2022, 1946724. [Google Scholar] [CrossRef] [PubMed]
- Comini, E.; Baratto, C.; Concina, I.; Faglia, G.; Falasconi, M.; Ferroni, M.; Galstyan, V.; Gobbi, E.; Ponzoni, A.; Vomiero, A. Metal Oxide Nanoscience and Nanotechnology for Chemical Sensors. Sens. Actuators B Chem. 2013, 179, 3–20. [Google Scholar] [CrossRef]
- Abed, S.M.; Reda, S.E.M.; Muhsin, N.M.B. Review on Nanotechnology Applications in Removing Chemical Pollutants and Their Application in Health. Trends Pharm. Nanotechnol. 2022, 4, 31–38. [Google Scholar]
- Sharon, M. Nanotechnology to Aid Biological and Chemical Warfare Defense. In Nanotechnology in the Defense Industry: Advances, Innovation, and Practical Applications; Wiley: Hoboken, NJ, USA, 2019; pp. 165–234. [Google Scholar]
- Sharma, S. The Role of Nanomedicine in COVID-19 Therapeutics. Nanomedicine 2022, 17, 133–136. [Google Scholar] [CrossRef]
- Gupta, N.; Bahl, S.; Bagha, A.K.; Vaid, S.; Javaid, M.; Haleem, A. Nanomedicine Technology and COVID-19 Outbreak: Applications and Challenges. J. Ind. Integr. Manag. 2021, 6, 161–174. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-based nanoparticles in the clinic and clinical trials: From cancer nanomedicine to COVID-19 vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- Vahedifard, F.; Chakravarthy, K. Nanomedicine for COVID-19: The role of nanotechnology in the treatment and diagnosis of COVID-19. Emergent Mater. 2021, 4, 75–99. [Google Scholar] [CrossRef]
- Mainardes, R.M.; Diedrich, C. The potential role of nanomedicine on COVID-19 therapeutics. Ther. Deliv. 2020, 11, 411–414. [Google Scholar] [CrossRef]
- Abd Ellah, N.H.; Gad, S.F.; Muhammad, K.; Batiha, G.E.; Hetta, H.F. Nanomedicine as a promising approach for diagnosis, treatment and prophylaxis against COVID-19. Nanomedicine 2020, 15, 2085–2102. [Google Scholar] [CrossRef]
- Sharma, A.; Kontodimas, K.; Bosmann, M. Nanomedicine: A diagnostic and therapeutic approach to COVID-19. Front. Med. 2021, 8, 648005. [Google Scholar] [CrossRef]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T. Nanotechnology in Bone Tissue Engineering. Nanomedicine 2015, 11, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Margel, S. Bioimaging probes based on Magneto-Fluorescent nanoparticles. Pharmaceutics 2023, 15, 686. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fu, S.; Liu, L.; Cai, Z.; Xia, C.; Song, B.; Gong, Q.; Lu, Z.; Ai, H. Tetraphenylethylene-Conjugated Polycation Covered Iron Oxide Nanoparticles for Magnetic Resonance/Optical Dual-Mode Imaging. Regen. Biomater. 2021, 8, rbab023. [Google Scholar] [CrossRef]
- Sharifi, S.; Hajipour, M.J.; Gould, L.; Mahmoudi, M. Nanomedicine in healing chronic wounds: Opportunities and challenges. Mol. Pharm. 2020, 18, 550–575. [Google Scholar] [CrossRef]
- Wu, W.; Pu, Y.; Shi, J. Nanomedicine-enabled chemotherapy-based synergetic cancer treatments. J. Nanobiotechnol. 2020, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Kumari, G.; Abhishek, K.; Singh, S.; Hussain, A.; Altamimi, M.A.; Madhyastha, H.; Webster, T.J.; Dev, A. A voyage from 3D to 4D printing in nanomedicine and healthcare: Part II. Nanomedicine 2022, 17, 255–270. [Google Scholar] [CrossRef]
- Halamoda-Kenzaoui, B.; Rolland, E.; Piovesan, J.; Puertas Gallardo, A.; Bremer-Hoffmann, S. Toxic Effects of Nanomaterials for Health Applications: How Automation Can Support a Systematic Review of the Literature? J. Appl. Toxicol. 2022, 42, 41–51. [Google Scholar] [CrossRef]
- Oberdörster, G. Safety Assessment for Nanotechnology and Nanomedicine: Concepts of Nanotoxicology. J. Intern. Med. 2010, 267, 89–105. [Google Scholar] [CrossRef]
- Hemmrich, E.; McNeil, S. Active ingredient vs excipient debate for nanomedicines. Nat. Nanotechnol. 2023, 18, 692–695. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, N.; Kumar, M. 2 Environmentally Friendly Green Approaches and Applications of Nanoparticles. In Metal Oxide–Based Carbon Nanocomposites for Environmental Remediation and Safety; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Adetunji, C.O.; Olaniyan, O.T.; Anani, O.A.; Olisaka, F.N.; Inobeme, A.; Bodunrinde, R.E.; Adetunji, J.B.; Singh, K.R.B.; Palnam, W.D.; Singh, R.P. Current Scenario of Nanomaterials in the Environmental, Agricultural, and Biomedical Fields. In Nanomaterials in Bionanotechnology; CRC Press; Boca Raton, FL, USA, 2021; pp. 129–158.
- Kalola, U.K.; Pellegrini, M.V. Patisiran. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Thapa, R.K.; Kim, J.O. Nanomedicine-based commercial formulations: Current developments and future prospects. J. Pharm. Investig. 2023, 53, 19–33. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, L.; Wang, X.; Zhu, S.; Chen, C.; Gu, Z.; Zhao, Y. Progress, challenges, and future of nanomedicine. Nano Today 2020, 35, 101008. [Google Scholar] [CrossRef]
- Jansma, S.R.; Dijkstra, A.M.; de Jong, M.D.T. Co-Creation in Support of Responsible Research and Innovation: An Analysis of Three Stakeholder Workshops on Nanotechnology for Health. J. Responsible Innov. 2022, 9, 28–48. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Hartshorn, C.M.; Bradbury, M.S.; Lanza, G.M.; Nel, A.E.; Rao, J.; Wang, A.Z.; Wiesner, U.B.; Yang, L.; Grodzinski, P. Nanotechnology Strategies to Advance Outcomes in Clinical Cancer Care. ACS Nano 2018, 12, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.Y.Y.; Lin, B.J.Y.; Liu, J.S.; Yu, C.-Y. Ethics in Nanotechnology: What’s Being Done? What’s Missing? J. Bus. Ethics 2012, 109, 583–598. [Google Scholar] [CrossRef]
- Younis, M.A.; Tawfeek, H.M.; Abdellatif, A.A.H.; Abdel-Aleem, J.A.; Harashima, H. Clinical Translation of Nanomedicines: Challenges, Opportunities, and Keys. Adv. Drug Deliv. Rev. 2022, 181, 114083. [Google Scholar] [CrossRef]
- Sadiku, M.N.; Ashaolu, T.J.; Ajayi-Majebi, A.; Musa, S.M. Future of nanotechnology. Int. J. Sci. Adv. 2021, 2, 131–134. [Google Scholar] [CrossRef]




| Sr. No. | Barriers Associated with Nano-Based Drug Delivery | Brief Explanation | Examples | References |
|---|---|---|---|---|
| 1. | Size and stability | The size of nanoparticles plays a crucial role in their delivery efficiency. Very small nanoparticles may be cleared quickly from the bloodstream, while larger nanoparticles may have limited tissue penetration. Additionally, maintaining the stability of nanoparticles during storage and delivery can be challenging. | Liposomal nanoparticles although initially promising for drug delivery, have faced challenges due to their size variability and instability, leading to a limited clinical translation. | [15,16,35] |
| 2. | Controlled release | Efficient release of the encapsulated drug or payload at target site is critical. It requires precise control over the release mechanism, kinetics, and release triggers (pH, temperature, and enzymes) to ensure optimal therapeutic effects. | Researchers working on tumor-targeted drug delivery have faced challenges in achieving the controlled release of drugs from nanoparticles thereby reducing their therapeutic efficacy. | [16,17] |
| 3. | Targeting specificity | Nanoparticles often require functionalization with targeting ligands to enhance their specificity toward diseased cells or tissues. Achieving selective targeting while minimizing off-target effects remains a significant challenge | Gold nanoparticles functionalized with antibodies for cancer-targeted photothermal therapy have faced issues related to non-specific accumulation and targeting of healthy tissues, leading to toxicity concerns | [66,74] |
| 4. | Biocompatibility and toxicity | Nanoparticles should be biocompatible to avoid adverse reactions and toxicity. This includes minimizing immune responses, toxicity to healthy tissues, and ensuring nanoparticles do not accumulate excessively in the body. | Carbon nanotubes showed significant toxicity concerns due to inflammation and tissue damage, limiting their clinical translation despite their potential applications. | [74] |
| 5. | Scalability and manufacturing | The development of nanoparticles suitable for large-scale production and manufacturing can be challenging. Ensuring reproducibility and stability across batches is crucial for clinical translation. | Promising nanoparticle formulations have failed to progress to clinical trials due to scalability issues and the inability to reproduce them consistently at larger scale. | [68,74] |
| 6. | Regulatory approval | Nanoparticles as drug delivery systems require rigorous regulatory assessment for safety, efficacy, and quality control. Meeting the extensive regulatory requirements demanded for clinical translation can be lengthy and resource-intensive. | The regulatory approval process for nanoparticles such as liposomes or polymeric nanoparticles often involves long and complex pathways, delaying their clinical translation. | [35] |
| Sr. No. | Examples of Nanotechnological Applications and Their Commercialized Cases in Medical Field | Brief Explanation | References |
|---|---|---|---|
| 1 | Drug delivery systems | Nanoparticles can be used to deliver drugs directly to targeted areas, improving their efficacy and reducing side effects. Examples include Abraxane (paclitaxel nanoparticles) and Doxil (liposomal doxorubicin). | [5,15,24,28] |
| 2 | Cancer diagnostics | Nanotechnology-based platforms can detect cancer biomarkers with high sensitivity and specificity. One example is MagArray, a magnetic nanotechnology-based biosensor for breast cancer diagnosis. | [113,114,115] |
| 3 | Tissue engineering | Nanomaterials such as nanofibers and nanocomposites can be used to construct artificial tissues and scaffolds to promote tissue regeneration and repair, as seen in the commercialized case of CardioCel for cardiovascular tissue repair. | [16,17,156] |
| 4 | Imaging agents | Nanoparticles can enhance the contrast of medical imaging techniques such as MRI, CT, and PET scans. Feridex (iron oxide nanoparticles) is an example of a commercialized MRI contrast agent. | [19,133,157,158] |
| 5 | Antibacterial coatings | Nanoscale antibacterial agents can be incorporated into medical devices such as catheters to prevent infections. The commercial product Nano-Silver Catheter is one such example. | [6,69,70,77] |
| 6 | Diagnostic nanoparticles | Quantum dots or gold nanoparticles can be engineered to detect and quantify target molecules, enabling highly sensitive medical diagnostics. ClearLight™ Diagnostics uses quantum dots for molecular imaging in tissue diagnostics. | [118,129,155] |
| 7 | Biosensors | Nanofabricated sensors can detect disease-related biomarkers and monitor conditions in real-time, such as glucose monitoring devices for diabetes management, for example, FreeStyle Libre. | [12,17,20,21,26] |
| 8 | Wound healing | Nanofiber-based dressings and coatings can accelerate wound healing by promoting | [159] |
| 9 | Chemotherapy | Nanoparticles can be loaded with therapeutic agents such as chemotherapy drugs or gene therapies, allowing targeted treatment of cancer cells. Examples include Doxil (liposomal doxorubicin) and Onivyde (nanoliposomal irinotecan). | [4,16,32,39,40,160] |
| 10 | Regenerative medicine | Nanomaterials can stimulate tissue regeneration and repair, such as the commercialized product BioCartilage for osteochondral defects. | [73,79,85] |
| 11 | Artificial organs | Nanotechnology can assist in designing and fabricating artificial organs with improved functional properties. The HeartWare™ Ventricular Assist System is a commercialized example for heart failure patients. | [85,161] |
| 12 | Early disease detection | Nanosensors can detect early-stage diseases through biomarker analysis, potentially enabling early intervention and improved outcomes. CarisomeOvarian is a nanosensor-based test for early detection of ovarian cancer. | [16,19,52,92,93] |
| 13 | Nanorobots for targeted therapy | Tiny nanorobots can be engineered to perform specific medical tasks, such as delivering drugs or unclogging. | [91,92,99] |
| 14 | Dental applications | Nanomaterials are used in dental restoration materials, such as nanocomposites | [99,100,101,102,103,104,105] |
| 15 | Drug discovery | Nanotechnology enables high-throughput screenings and drug design methods, accelerating the discovery of new therapeutic compounds. The commercialized product Nanotax uses nanotechnology for drug discovery. | [112,115,116,117,118,119,120] |
| Sr. No. | Names of Products | Brief Explanation | References |
|---|---|---|---|
| 1. | Patisiran (Onpattro®)—FDA-approved lipid nanoparticles (LNPs) are a type of delivery system used in RNA interference (RNAi) drugs. RNAi is a biological process that regulates gene expression by silencing specific genes. | Patisiran is used to treat hereditary transthyretin-mediated amyloidosis (hATTR), a rare genetic disease. It works by targeting and silencing the gene responsible for producing transthyretin, a protein that forms abnormal amyloid deposits in tissues such as the nerves and heart. LNPs are crucial in delivering a small piece of RNA (siRNA) that specifically binds to and prevents the production of the disease-causing transthyretin protein. | [164,167,168,169] |
| 2. | Comirnaty® and Spikevax®—both are mRNA-LNPs, based COVID-19 vaccines | Comirnaty® is the brand name for the mRNA-LNP vaccine developed by Pfizer-BioNTech, while Spikevax® is the mRNA-LNP vaccine produced by Moderna. These vaccines use messenger RNA (mRNA) technology to provide instructions to the body’s cells to produce a harmless piece of the spike protein found on the SARS-CoV-2 virus. The LNPs in these vaccines act as delivery vehicles to protect and transport the mRNA into the cells. The cells then use these instructions to produce the spike protein, enabling the immune system to recognize and mount a defense against the spike protein. This immune response prepares the body to defend against a subsequent infection with the actual SARS-CoV-2 virus. | [160,161,164,167,168,169] |
| Sr. No. | Steps Needed for Industrial Regulation | Brief Explanation | References |
|---|---|---|---|
| 1 | Risk assessment: | There is need to conduct a comprehensive risk assessment to understand the potential risks associated with the use of nanomaterials in medical applications. Evaluate the toxicity, exposure pathways, and potential environmental impacts of these materials. | [170] |
| 2 | Regulatory framework | Develop a regulatory framework specifically tailored to govern the industrialization of nanomaterials in medical sciences. This framework should consider existing regulations and guidelines but also address the unique properties and potential risks posed by nanomaterials. | [170,171,172] |
| 3 | Classification and characterization | Establish criteria for classifying and characterizing different types of nanomaterials used in medical sciences. This should include their physical and chemical properties, intended uses, and potential risks. This information will assist in determining appropriate regulations and handling requirements | [170,173] |
| 4 | Product registration and safety assessment | Introduce a registration or approval process for nanomaterials used in medical applications. Manufacturers must submit detailed information about the materials, including their synthesis methods, intended applications, potential hazards, and safety data. Conduct a thorough safety assessment based on this information before granting approvals. | [173,174] |
| 5 | Labeling and traceability | Implement labeling requirements to ensure proper identification and traceability of medical products that contain nanomaterials. Labels should provide clear information about the presence of nanomaterials, their type, concentration, and any potential risks associated with their use. | [174,175] |
| 6 | Manufacturing standards | Establish manufacturing standards and best practices specifically for nanomaterials used in medical applications. These standards should address issues such as quality control, handling, storage, transportation, and waste management, considering the unique properties of nanomaterials. | [170,173] |
| 7 | Monitoring and surveillance | Develop a surveillance system to monitor the usage, performance, and safety of nanomaterials in medical sciences. Regularly review and update regulations based on emerging scientific evidence, advancements in technology, and any new risks identified. | [174] |
| 8 | Collaboration and international harmonization | Foster collaboration and information-sharing initiatives with other regulatory bodies and international organizations to harmonize standards and regulations for nanomaterials used in medical sciences. This will help avoid duplication of efforts, facilitate global trade, and ensure a consistent level of safety worldwide. | [175] |
| 9 | Public engagement and communication | Engage the public, stakeholders, and healthcare professionals in the regulatory process. Conduct public consultations, disseminate information about regulations, and address concerns raised by various stakeholders. Effective communication will help build trust and ensure transparency in the regulation of nanomaterials in medical sciences. | [175] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, S.; Muhammad, K.; Waheed, Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules 2023, 28, 6624. https://doi.org/10.3390/molecules28186624
Malik S, Muhammad K, Waheed Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules. 2023; 28(18):6624. https://doi.org/10.3390/molecules28186624
Chicago/Turabian StyleMalik, Shiza, Khalid Muhammad, and Yasir Waheed. 2023. "Emerging Applications of Nanotechnology in Healthcare and Medicine" Molecules 28, no. 18: 6624. https://doi.org/10.3390/molecules28186624
APA StyleMalik, S., Muhammad, K., & Waheed, Y. (2023). Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules, 28(18), 6624. https://doi.org/10.3390/molecules28186624








