Tuning the Acid–Base Properties of Lignin-Derived Carbon Modulated ZnZr/SiO2 Catalysts for Selective and Efficient Production of Butadiene from Ethanol
Abstract
:1. Introduction
2. Results
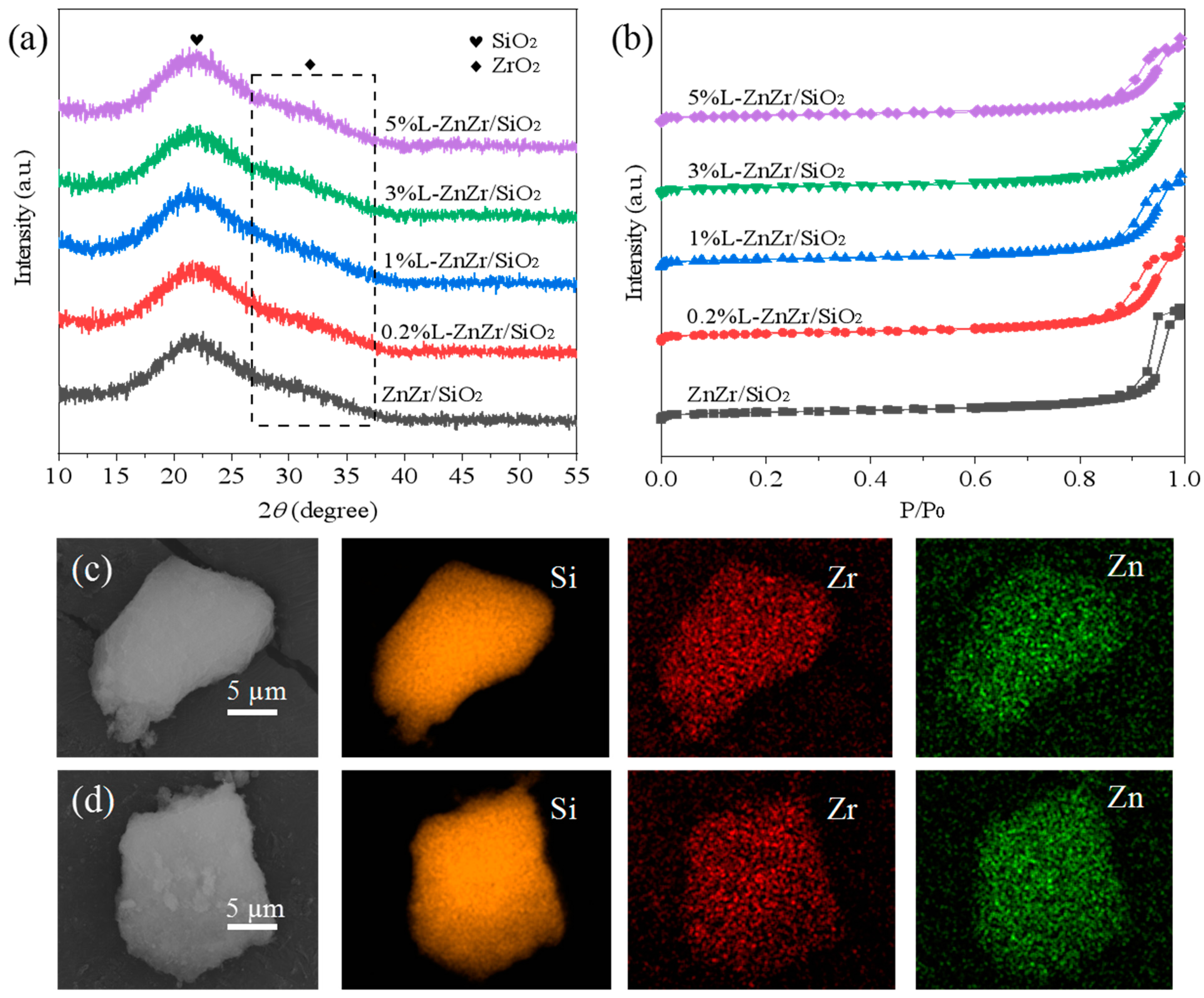
2.1. Structural Characterization
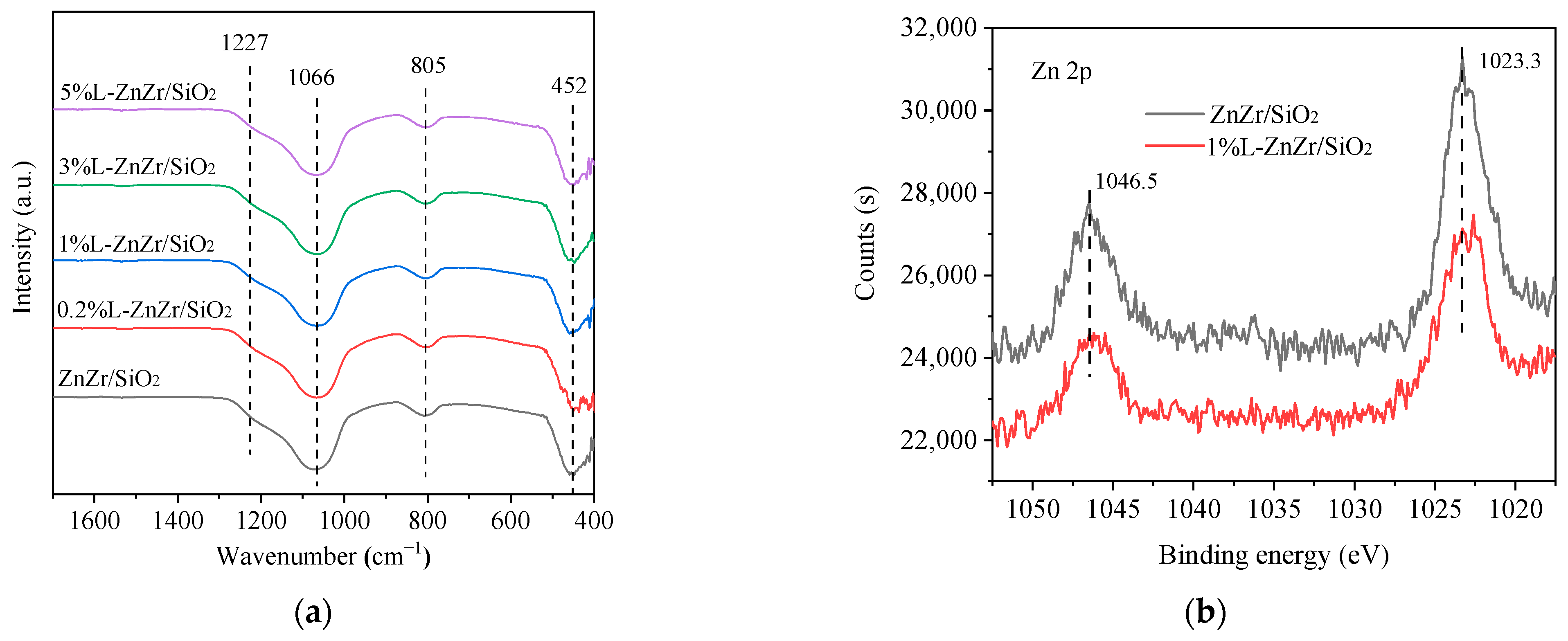
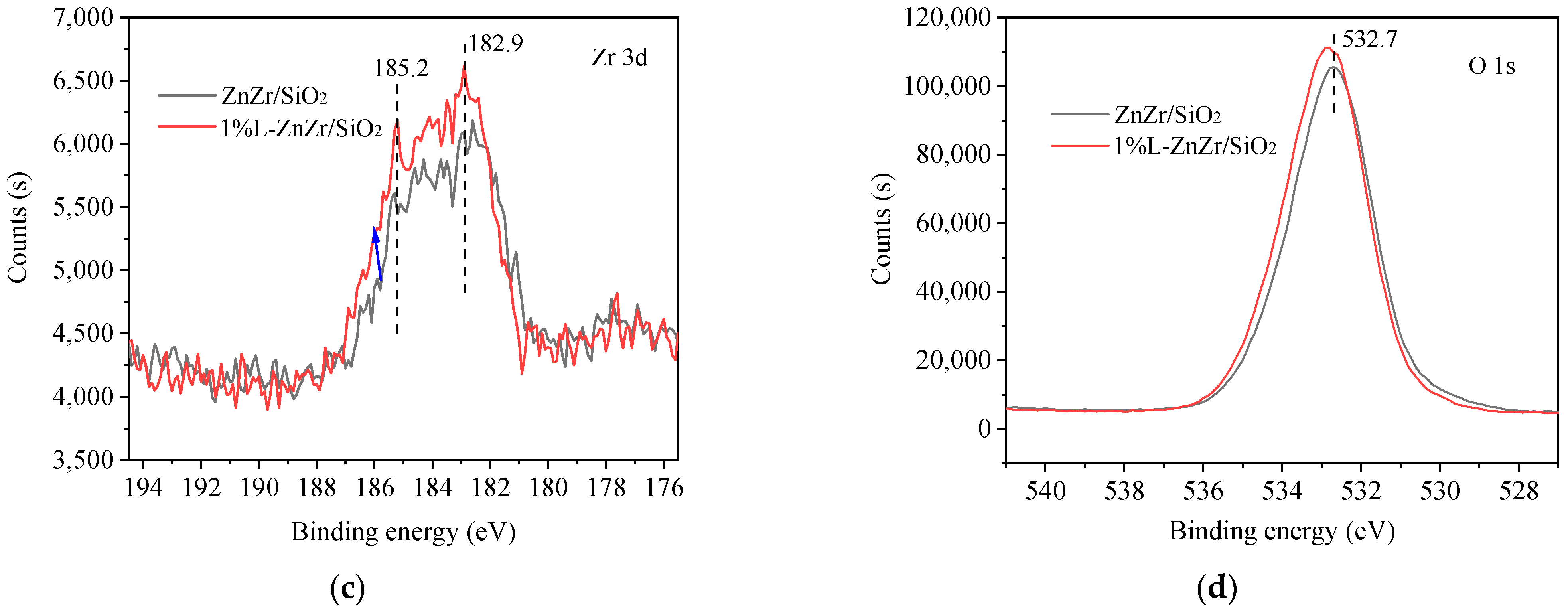
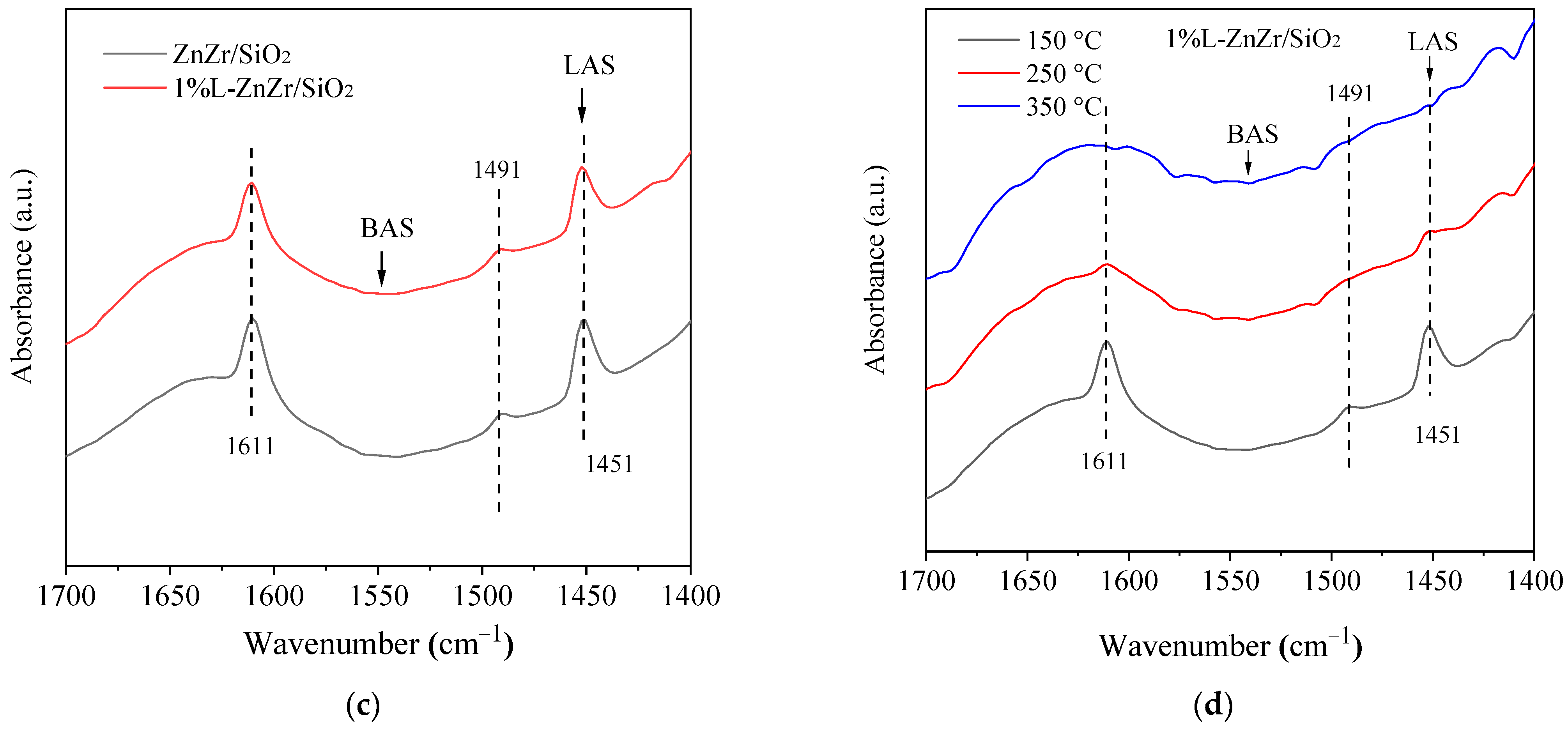
2.2. Functional Group Properties
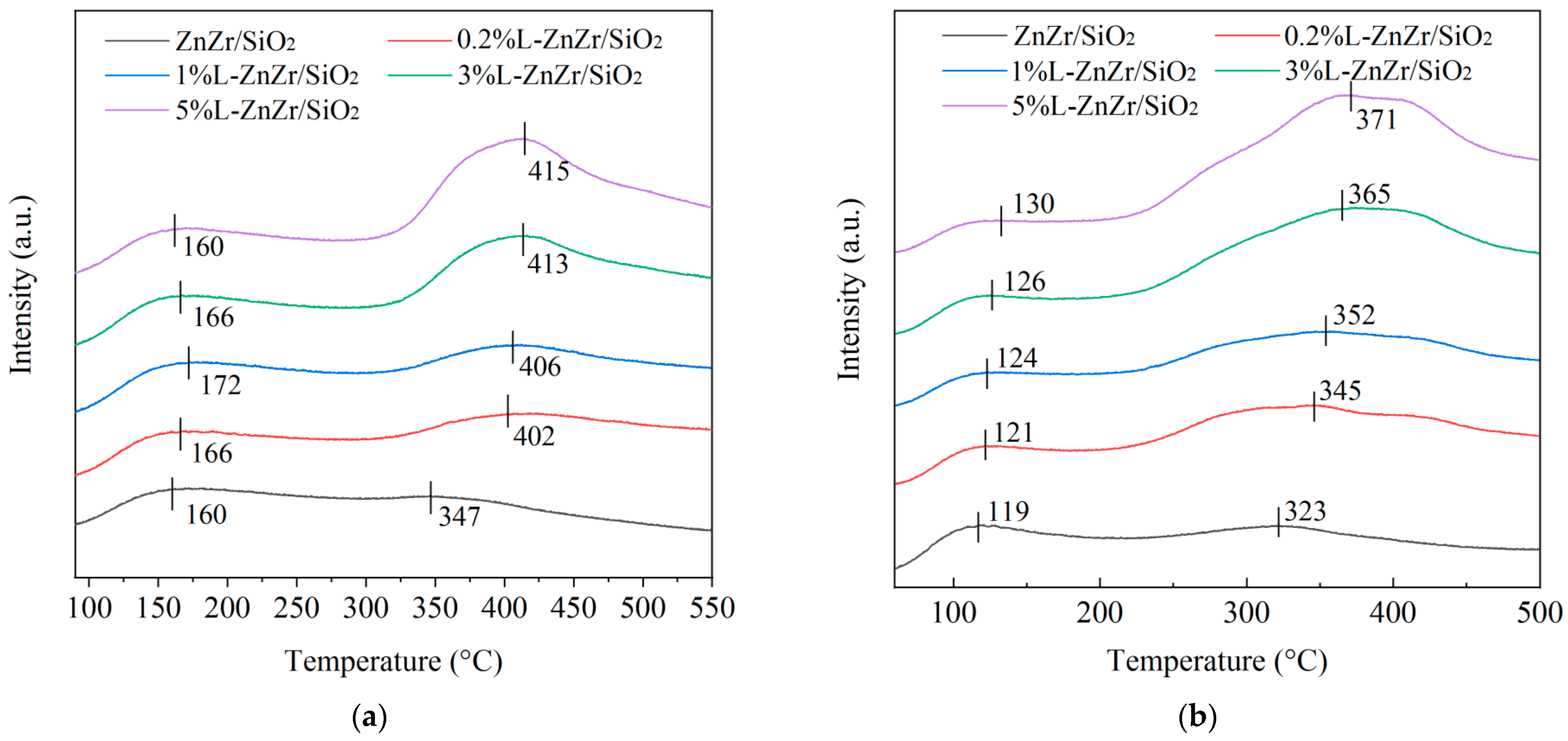
2.3. Acid–Base Properties
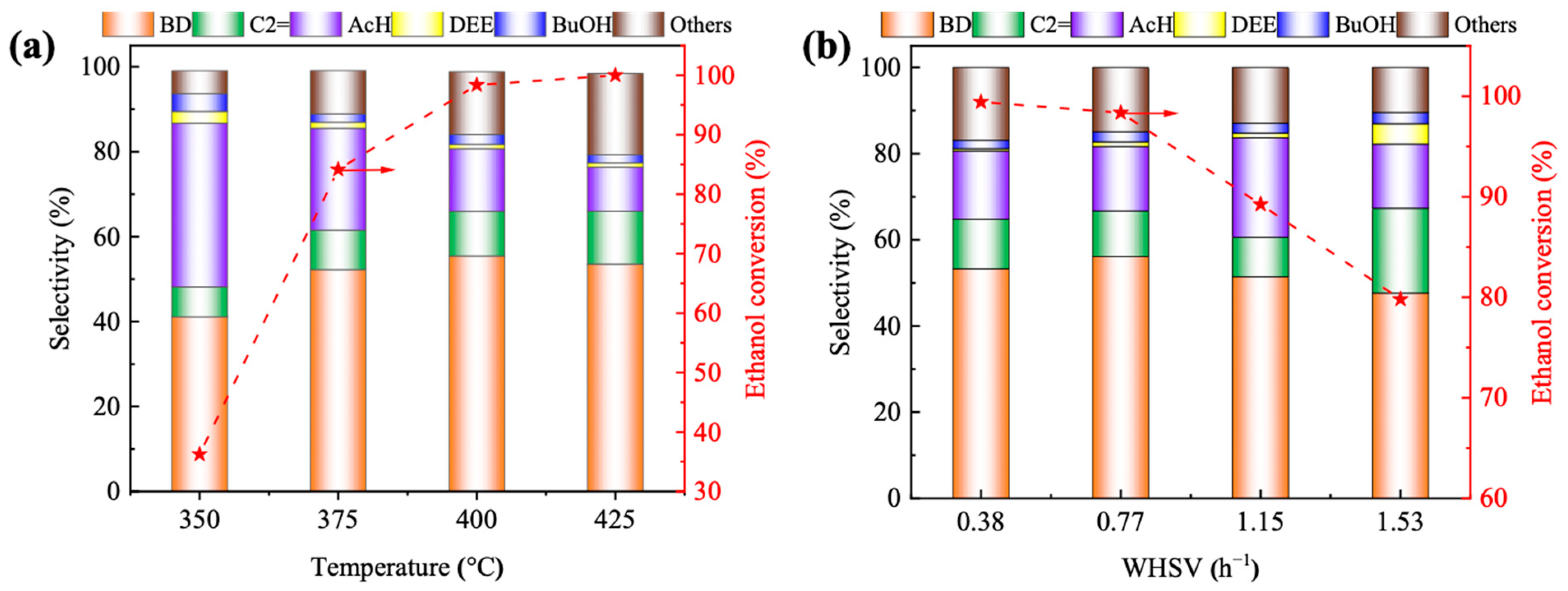
2.4. Catalytic Performance
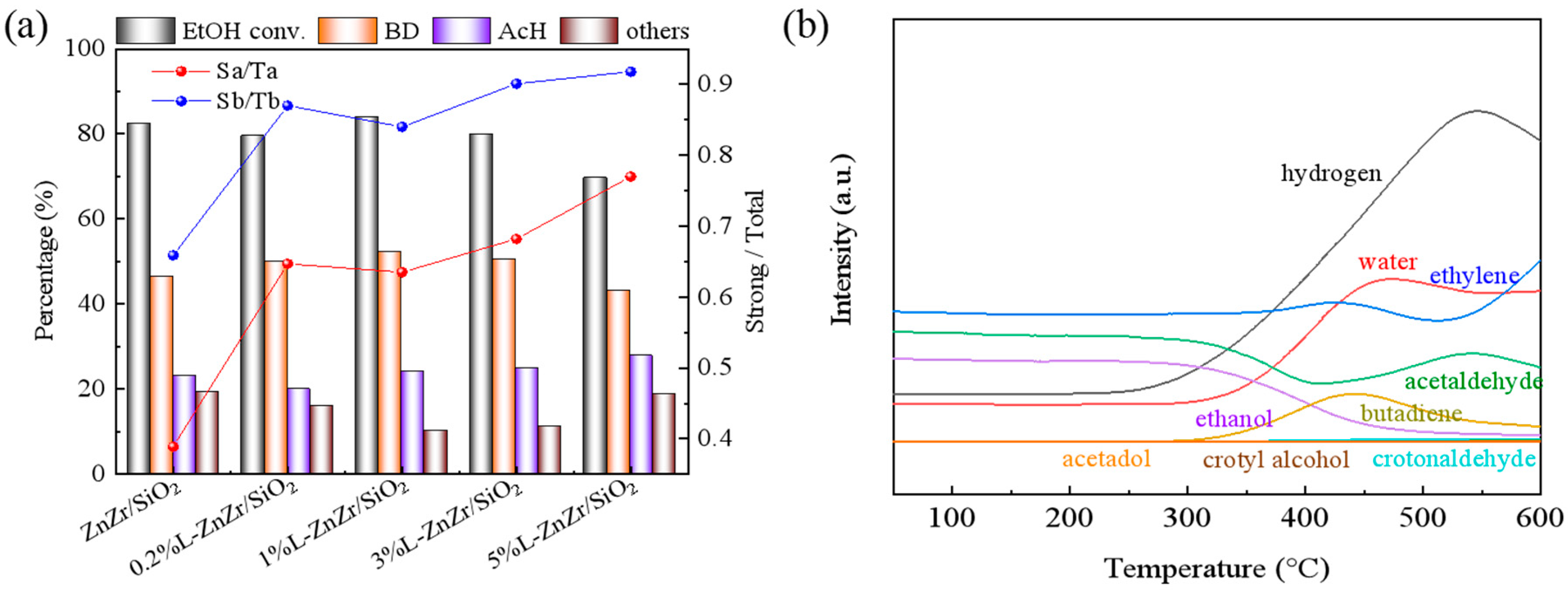
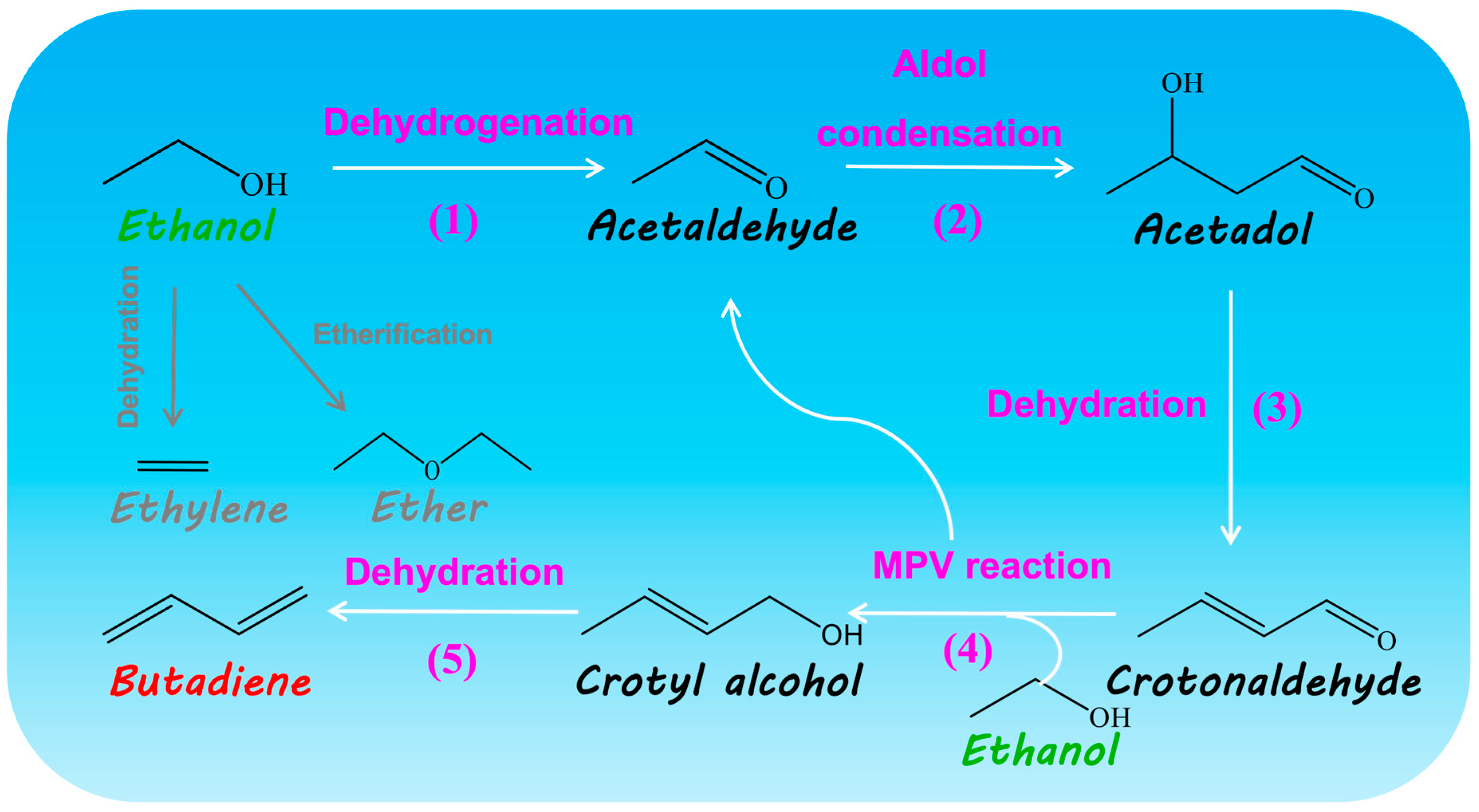
2.5. Relationship between Acid–Base Properties and Catalytic Performance
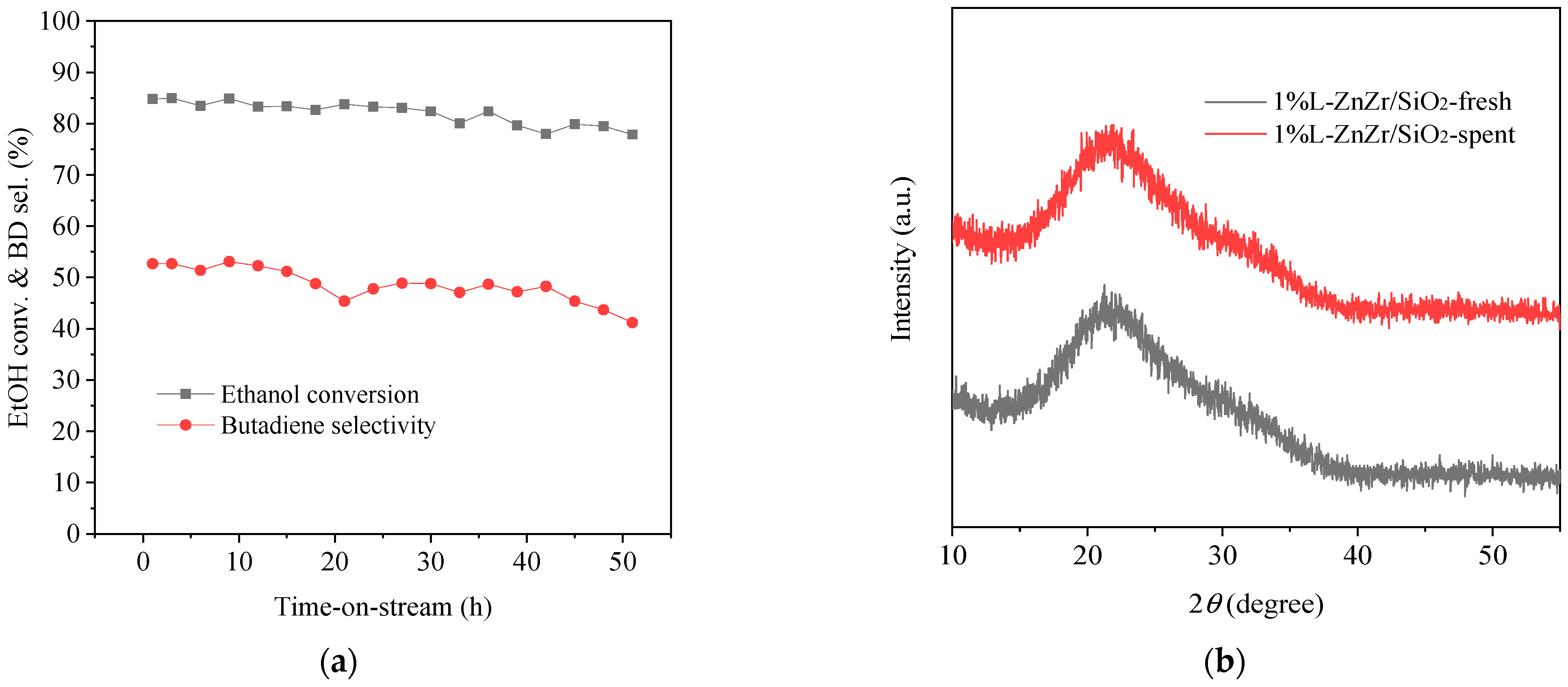
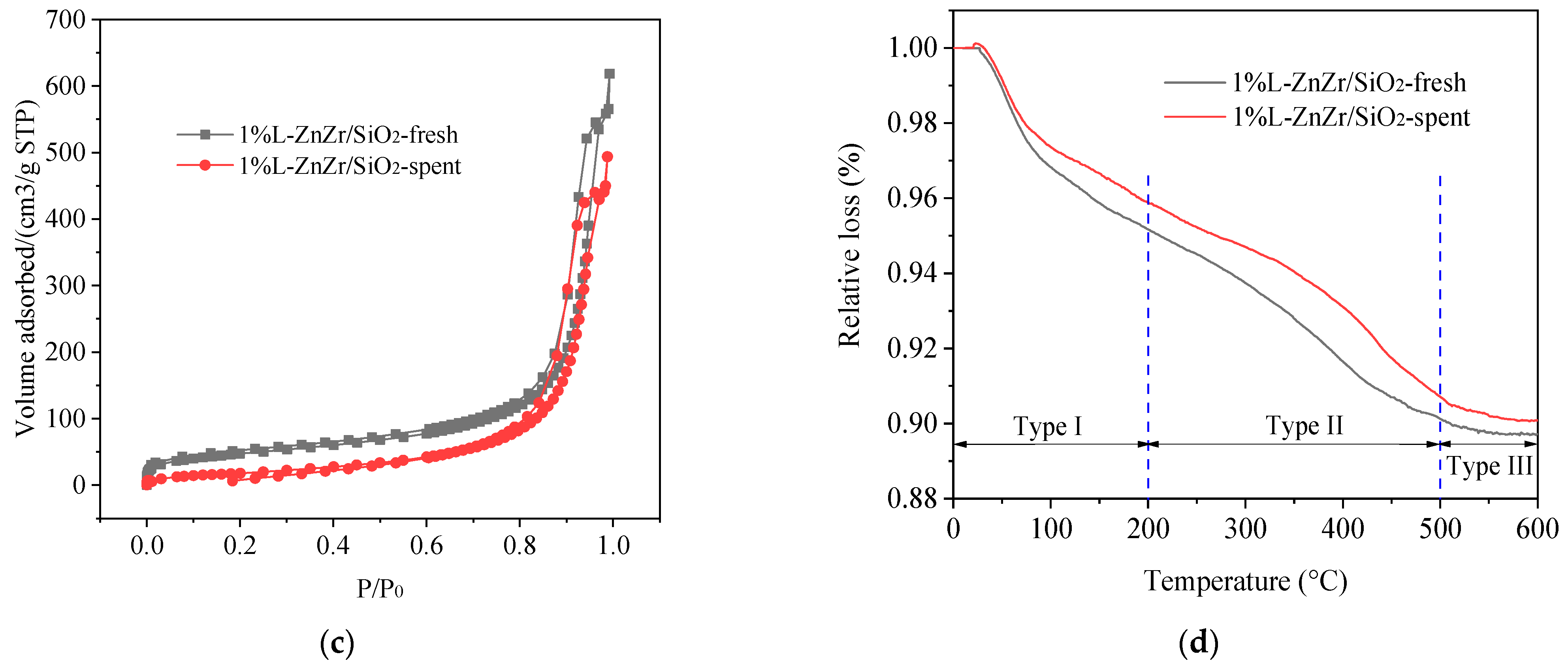
2.6. Stability and Spent Catalyst Characterization
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalyst Characterization
3.3. Catalytic Activity Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lu, Y.; Zhang, Z.; Wang, H.; Wang, Y. Toward Efficient Single-Atom Catalysts for Renewable Fuels and Chemicals Production from Biomass and CO2. Appl. Catal. B-Environ. 2021, 292, 120162. [Google Scholar] [CrossRef]
- Chen, X.; Song, S.; Li, H.; Gozaydin, G.; Yan, N. Expanding the Boundary of Biorefinery: Organonitrogen Chemicals from Biomass. Acc. Chem. Res. 2021, 54, 1711–1722. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, B.; Luo, L.; Zhang, F.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Wang, X.; Lü, X. A Review on Recycling Techniques for Bioethanol Production from Lignocellulosic Biomass. Renew. Sustain. Energ. Rev. 2021, 149, 111370. [Google Scholar] [CrossRef]
- Dagle, R.A.; Winkelman, A.D.; Ramasamy, K.K.; Dagle, V.L.; Weber, R.S. Ethanol as a Renewable Building Block for Fuels and Chemicals. Ind. Eng. Chem. Res. 2020, 59, 4843–4853. [Google Scholar] [CrossRef]
- Cespi, D.; Passarini, F.; Vassura, I.; Cavani, F. Butadiene from Biomass, A Life Cycle Perspective to Address Sustainability in the Chemical Industry. Green Chem. 2016, 18, 1625–1638. [Google Scholar] [CrossRef]
- Yang, M.; You, F. Comparative Techno-Economic and Environmental Analysis of Ethylene and Propylene Manufacturing from Wet Shale Gas and Naphtha. Ind. Eng. Chem. Res. 2017, 56, 4038–4051. [Google Scholar] [CrossRef]
- Pomalaza, G.; Ponton, P.A.; Capron, M.; Dumeignil, F. Ethanol-to-Butadiene: The Reaction and Its Catalysts. Catal. Sci. Technol. 2020, 10, 4860–4911. [Google Scholar] [CrossRef]
- Cai, D.; Zhu, Q.; Chen, C.; Hu, S.; Qin, P.; Wang, B.; Tan, T. Fermentation-Pervaporation-Catalysis Integration Process for Bio-Butadiene Production Using Sweet Sorghum Juice as Feedstock. J. Taiwan Inst. Chem. Eng. 2018, 82, 137–143. [Google Scholar] [CrossRef]
- Farzad, S.; Mandegari, M.A.; Görgens, J.F. Integrated Techno-Economic and Environmental Analysis of Butadiene Production from Biomass. Bioresour. Technol. 2017, 239, 37–48. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Ivanova, I.I. Mechanistic Study of Ethanol Conversion into Butadiene over Silver Promoted Zirconia Catalysts. Appl. Catal. B-Environ. 2017, 215, 36–49. [Google Scholar] [CrossRef]
- Gholipour, Z.N.; Kamran, P.A.; Yazdanian, E. Biodiesel Production in the Presence of Heterogeneous Catalyst of Alumina: Study of Kinetics and Thermodynamics. Int. J. Chem. Kinet. 2020, 52, 472–484. [Google Scholar] [CrossRef]
- Fallah, A.; Kamran-Pirzaman, A.; Gholipour, Z.N. The Sono-physical Effect of Cavitation Bubbles on Homogeneous Catalyzed Biodiesel Production. Biomass Convers. Bior. 2023, 13, 5339–5351. [Google Scholar] [CrossRef]
- Zanjani, N.G.; Kamran-Pirzaman, A.; Khalajzadeh, M. Synthesis of Modified Layered Double Hydroxide of MgAl Catalyst with Ba and Li for the Biodiesel Production. Clean Technol. Environ. 2020, 22, 1173–1185. [Google Scholar] [CrossRef]
- Raynes, S.J.; Taylor, R.A. Zinc Oxide-Modified Mordenite as an Effective Catalyst for the Dehydrogenation of (Bio) Ethanol to Acetaldehyde. Sustain. Energy Fuels 2021, 5, 2136–2148. [Google Scholar] [CrossRef]
- Kyriienko, P.I.; Larina, O.V.; Balakin, D.Y.; Stetsuk, A.O.; Nychiporuk, Y.M.; Soloviev, S.O.; Orlyk, S.M. 1,3-Butadiene Production from Aqueous Ethanol over ZnO/MgO-SiO2 Catalysts: Insight into H2O Effect on Catalytic Performance. Appl. Catal. A-Gen. 2021, 616, 118081. [Google Scholar] [CrossRef]
- Larina, O.V.; Shcherban, N.D.; Kyriienko, P.I.; Remezovskyi, I.M.; Yaremov, P.S.; Khalakhan, I.; Mail, G.; Soloviev, S.O.; Orlyk, S.M.; Dzwigaj, S. Design of Effective Catalysts Based on ZnLaZrSi Oxide Systems for Obtaining 1,3-Butadiene from Aqueous Ethanol. ACS Sustain. Chem. Eng. 2020, 8, 16600–16611. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Ivanova, I.I.; Ordomsky, V.V.; Taarning, E. Design of a Metal-Promoted Oxide Catalyst for the Selective Synthesis of Butadiene from Ethanol. ChemSusChem 2014, 7, 2527–2536. [Google Scholar] [CrossRef]
- Wen, G.; Wang, B.; Wang, C.; Wang, J.; Tian, Z.; Schlogl, R.; Su, D.S. Hydrothermal Carbon Enriched with Oxygenated Groups from Biomass Glucose as An Efficient Carbocatalyst. Angew. Chem. Int. Ed. Engl. 2017, 129, 615–619. [Google Scholar] [CrossRef]
- Wen, G.; Diao, J.; Wu, S.; Yang, W.; Schlogl, R.; Su, D.S. Acid Properties of Nanocarbons and Their Application in Oxidative Dehydrogenation. ACS Catal. 2015, 5, 3600–3608. [Google Scholar] [CrossRef]
- Jing, Q.; Zhu, J.; Wei, X.; Lin, Y.; Wang, X.; Wu, Z. An Acid-Base Molecular Assembly Strategy Toward N-Doped Mo2C@C Nanowires with Mesoporous Mo2C Cores and Ultrathin Carbon Shells for Efficient Hydrogen Evolution. J. Colloid Interf. Sci. 2021, 602, 520–533. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in The Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Lou, H.; Liu, W.; Yang, D.; Ouyang, X.; Li, Y.; Qiu, X. Lignin-A Promising Biomass Resource. Tappi J. 2018, 17, 125–141. [Google Scholar] [CrossRef]
- Liu, N.; Chen, J.; Wu, Z.; Zhan, P.; Zhang, L.; Wei, Q.; Wang, F.; Shao, L. Construction of Microporous Lignin-Based Hypercross-Linked Polymers with High Surface Areas for Enhanced Iodine Capture. ACS Appl. Polym. Mater. 2021, 3, 2178–2188. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Li, H.; Lu, J.; Liu, M.; Wang, F. Carbon Modification of Nickel Catalyst for Depolymerization of Oxidized Lignin to Aromatics. ACS Catal. 2018, 8, 1614–1620. [Google Scholar] [CrossRef]
- Higgins, L.J.R.; Brown, A.P.; Harrington, J.P.; Ross, A.B.; Kaulich, B.; Mishra, B. Evidence for a Core-shell Structure of Hydrothermal Carbon. Carbon 2020, 161, 423–431. [Google Scholar] [CrossRef]
- Ahn, C.I.; Kim, C.; Bae, J.W.; Jeon, J.; Jung, H.S.; Kim, Y.; Lee, S.; Lee, J.; Ha, K. Ethanol Conversion into 1,3-Butadiene over ZnZr Mixed Oxide Catalysts Supported on Ordered Mesoporous Materials. Fuel Process. Technol. 2020, 200, 106317. [Google Scholar] [CrossRef]
- Ramezani, F.; Zare-Dorabei, R. Simultaneous Ultrasonic-Assisted Removal of Malachite Green and Methylene Blue from Aqueous Solution by Zr-SBA-15. Polyhedron 2019, 166, 153–161. [Google Scholar] [CrossRef]
- Wang, K.; Gao, W.; Chen, F.; Liu, G.; Wu, j.; Liu, N.; Kawabata, Y.; Guo, X.; He, Y.; Zhang, P. Hierarchical Nano-Sized ZnZr-Silicalite-1 Multifunctional Catalyst for Selective Conversion of Ethanol to Butadiene. Appl. Catal. B-Environ. 2022, 301, 120822. [Google Scholar] [CrossRef]
- Dagle, V.L.; Flake, M.D.; Lemmon, T.L.; Lopez, J.S.; Kovarik, L.; Dagle, R.A. Effect of The SiO2 Support on The Catalytic Performance of Ag/ZrO2/SiO2 Catalysts for the Single-Bed Production of Butadiene from Ethanol. Appl. Catal. B-Environ. 2018, 236, 576–587. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Thunyaratchatanon, C.; Luengnaruemitchai, A.; Chaisuwan, T.; Chollacoop, N.; Chen, S.; Yoshimura, Y. Synthesis and Characterization of Zr Incorporation into Highly Ordered Mesostructured SBA-15 Material and Its Performance for CO2 Adsorption. Micropor. Mesopor. Mat. 2017, 253, 18–28. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Reliable Determination of Chemical State in X-ray Photoelectron Spectroscopy Based on Sample-Work-Function Referencing to Adventitious Carbon: Resolving the Myth of Apparent Constant Binding Energy of the C 1s Peak. Appl. Surf. Sci. 2018, 451, 99–103. [Google Scholar] [CrossRef]
- Larina, O.V.; Kyriienko, P.I.; Balakin, D.Y.; Vorokhta, M.; Khalakhan, I.; Nychiporuk, Y.M.; Matolin, V.; Soloviev, S.O.; Orlyk, S.M. Effect of ZnO on Acid–Base Properties and Catalytic Performances of ZnO/ZrO2-SiO2 Catalysts in 1,3-Butadiene Production from Ethanol-Water Mixture. Catal. Sci. Technol. 2019, 9, 3964–3978. [Google Scholar] [CrossRef]
- Rakshe, B.; Ramaswamy, V.; Hegde, S.G.; Vetrivel, R.; Ramaswamy, A.V. Crystalline, Microporous Zirconium Silicates with MFI Structure. Catal. Lett. 1997, 45, 41–50. [Google Scholar] [CrossRef]
- Wang, K.; Peng, X.; Gao, X.; Araki, Y.; Zhao, H.; Liang, J.; Xiao, L.; Chen, J.; Liu, G.; Wu, J. Insights Into The Synergistic Effect of Active Centers over ZnMg/SBA-15 Catalysts in Direct Synthesis of Butadiene from Ethanol. React. Chem. Eng. 2021, 6, 548–558. [Google Scholar] [CrossRef]
- Su, C.; Acik, M.; Takai, K.; Lu, J.; Hao, S.; Zheng, Y.; Wu, P.; Bao, Q.; Enoki, T.; Chabal, Y.J. Probing The Catalytic Activity of Porous Graphene Oxide and the Origin of This Behaviour. Nat. Commun. 2012, 3, 1298. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Fan, S.; Peng, X.; Tsubaki, N.; Zhao, T. Transformation of LPG to Light Olefins on Composite HZSM-5/SAPO-5. New J. Chem. 2021, 45, 4860–4866. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, C.; Wang, Q.; Zhang, L.; Huang, A.; Ke, M.; Song, Z. Direct Dehydrogenation of Isobutane to Isobutene over Zn-Doped ZrO2 Metal Oxide Heterogeneous Catalysts. Catal. Sci. Technol. 2018, 8, 4916–4924. [Google Scholar] [CrossRef]
- Huang, X.; Men, Y.; Wang, J.; An, W.; Wang, Y. Highly Active and Selective Binary MgO-SiO2 Catalysts for The Production of 1,3-Butadiene from Ethanol. Catal. Sci. Technol. 2017, 7, 168–180. [Google Scholar] [CrossRef]
- Da, R.S.; Jones, M.D.; Mattia, D.; Schwaab, M.; Noronha, F.B.; Pinto, J.C. Modelling The Effects of Reaction Temperature and Flow Rate on the Conversion of Ethanol to 1,3-Butadiene. Appl. Catal. A-Gene. 2017, 530, 37–47. [Google Scholar] [CrossRef]
- Wang, K.; Peng, X.; Wang, C.; Gao, W.; Liu, N.; Guo, X.; He, Y.; Yang, G.; Jiang, L.; Tsubaki, N. Selectively Direct Conversion of Aqueous Ethanol into Butadiene via Rational Design of Multifunctional Catalysts. Catal. Sci. Technol. 2022, 12, 2210–2222. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, L.; Wang, K.; Shao, L.; Guo, X.; He, Y.; Wu, Z.; Zhan, P.; Liu, G.; Wu, J. Selective synthesis of butadiene directly from aqueous ethanol over high performance multifunctional catalyst based on ZnZrSi oxide system. Appl. Surf. Sci. 2022, 602, 154299. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, Y.; Conrad, M.A.; Russell, C.K.; Miller, J.; Bell, A.T. Ethanol Conversion to Butadiene over Isolated Zinc and Yttrium Sites Grafted onto Dealuminated Beta Zeolite. J. Am. Chem. Soc. 2020, 142, 14674–14687. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, N.; Ma, Q.; Kawabata, Y.; Wang, F.; Gao, W.; Zhang, B.; Guo, X.; He, Y.; Yang, G. Probing the promotional roles of lanthanum in physicochemical properties and performance of ZnZr/Si-beta catalyst for direct conversion of aqueous ethanol to butadiene. Catal. Today 2023, 411, 11380. [Google Scholar] [CrossRef]
- Wang, K.; Guo, L.; Gao, W.; Zhang, B.; Zhao, H.; Liang, J.; Liu, N.; He, Y.; Zhang, P.; Yang, G. One-Pot Hydrothermal Synthesis of Multifunctional ZnZrTUD-1 Catalysts for Highly Efficient Direct Synthesis of Butadiene from Ethanol. ACS Sustain. Chem. Eng. 2021, 9, 10569–10578. [Google Scholar] [CrossRef]
- Yan, T.; Dai, W.; Wu, G.; Lang, S.; Hunger, M.; Guan, N.; Li, L. Mechanistic Insights into One-Step Catalytic Conversion of Ethanol to Butadiene over Bifunctional Zn–Y/beta Zeolite. ACS Catal. 2018, 8, 2760–2773. [Google Scholar] [CrossRef]
| Catalysts | Acid Amount (mmol/g) | LAS Amount(μmol·g−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | Wa | T (°C) | Sa | Ta | Wa/Ta | Sa/Ta | ||
| ZnZr/SiO2 | 160 | 0.15 | 347 | 0.10 | 0.25 | 0.60 | 0.39 | 85.0 |
| 0.2%L-ZnZr/SiO2 | 166 | 0.19 | 402 | 0.34 | 0.53 | 0.36 | 0.65 | - |
| 1%L-ZnZr/SiO2 | 172 | 0.20 | 406 | 0.34 | 0.54 | 0.37 | 0.64 | 65.7 |
| 3%L-ZnZr/SiO2 | 166 | 0.19 | 413 | 0.42 | 0.61 | 0.31 | 0.68 | - |
| 5%L-ZnZr/SiO2 | 162 | 0.18 | 415 | 0.59 | 0.76 | 0.24 | 0.77 | - |
| Catalysts | Base Amount (mmol/g) | ||||||
|---|---|---|---|---|---|---|---|
| T (°C) | Wb | T (°C) | Sb | Tb | wb/Tb | Sb/Tb | |
| ZnZr/SiO2 | 119 | 0.13 | 323 | 0.25 | 0.38 | 0.34 | 0.66 |
| 0.2%L-ZnZr/SiO2 | 121 | 0.08 | 345 | 0.56 | 0.64 | 0.13 | 0.87 |
| 1%L-ZnZr/SiO2 | 124 | 0.10 | 352 | 0.52 | 0.61 | 0.16 | 0.84 |
| 3%L-ZnZr/SiO2 | 126 | 0.08 | 365 | 0.74 | 0.82 | 0.10 | 0.90 |
| 5%L-ZnZr/SiO2 | 130 | 0.08 | 371 | 0.84 | 0.92 | 0.09 | 0.92 |
| Catalysts | EtOH Conv. (%) | Product Sel. (%) | BD Yield(%) | STY (g·kgcat−1·h−1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| BD | C2= | AcH | DEE | BuOH | Others | ||||
| ZnZr/SiO2 | 82.5 | 46.4 | 6.4 | 23.2 | 2.4 | 2.3 | 19.3 | 38.3 | 173 |
| 0.2%L-ZnZr/SiO2 | 79.6 | 49.9 | 10.1 | 19.9 | 2.3 | 1.9 | 15.9 | 39.7 | 180 |
| 1%L-ZnZr/SiO2 | 84.1 | 52.2 | 9.2 | 24.1 | 1.9 | 2.4 | 10.2 | 43.9 | 198 |
| 3%L-ZnZr/SiO2 | 80.0 | 50.4 | 8.9 | 24.9 | 2 | 2.7 | 11.1 | 40.3 | 182 |
| 5%L-ZnZr/SiO2 | 69.7 | 43.1 | 5.9 | 27.8 | 1.6 | 2.9 | 18.7 | 30.0 | 136 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; He, Y.; Wang, K.; Chen, F.; Yao, J.; Yang, G.; Huang, S.; Shao, L.; Tsubaki, N. Tuning the Acid–Base Properties of Lignin-Derived Carbon Modulated ZnZr/SiO2 Catalysts for Selective and Efficient Production of Butadiene from Ethanol. Molecules 2023, 28, 6632. https://doi.org/10.3390/molecules28186632
Liu N, He Y, Wang K, Chen F, Yao J, Yang G, Huang S, Shao L, Tsubaki N. Tuning the Acid–Base Properties of Lignin-Derived Carbon Modulated ZnZr/SiO2 Catalysts for Selective and Efficient Production of Butadiene from Ethanol. Molecules. 2023; 28(18):6632. https://doi.org/10.3390/molecules28186632
Chicago/Turabian StyleLiu, Na, Yingluo He, Kangzhou Wang, Fei Chen, Jie Yao, Guohui Yang, Shufang Huang, Lishu Shao, and Noritatsu Tsubaki. 2023. "Tuning the Acid–Base Properties of Lignin-Derived Carbon Modulated ZnZr/SiO2 Catalysts for Selective and Efficient Production of Butadiene from Ethanol" Molecules 28, no. 18: 6632. https://doi.org/10.3390/molecules28186632






